As one of the founding members of the European Molecular Biology Laboratory (EMBL) and a leading voice in European and American science advocacy organizations, Kai Simons has had an enduring influence on generations of scientists. His work on membrane organization has also made a deep impact in cell biology.
Figure 1.
Kai Simons
Simons, who is now based at the Max Planck Institute in Dresden, credits much of his career success to others (1), beginning with his father, who set him on the path to biomedical research, and then Leevi Kääriäinen, who introduced him to Semliki Forest Virus—which would subsequently become a focus of his early work on cell membrane trafficking pathways (2, 3). Later, at the newly formed EMBL, Simons assembled a multidisciplinary team of researchers to study how certain proteins, together with certain lipids, dynamically segregate into subdomains in the plasma membrane (4, 5). These subdomains, termed “lipid rafts,” serve as platforms for protein transport and launching pads for signaling pathways.
The lipid raft concept revolutionized our understanding of the role of membranes in cell biology, but it has suffered its share of controversy. Simons and his colleagues in the field have nonetheless persevered, and continue to refine their understanding of these important structures (6). He talked to us about his rafting adventures, and where he and his colleagues are now headed.
A PRACTICAL MAN
Where did you grow up? I grew up in Helsinki, Finland. My father was a farmer's son, and he was the first person to get out of his farming community, which was 500 km north of Helsinki. He became a professor of physics, so I grew up with a lot of physicists around me; my father's department would host seminars, and the speakers would often be invited for dinner at our home. So some very famous people came to our house.
What famous people did you meet? Well, the most famous person was Albert Einstein. When I was 11 years old, my father spent a sabbatical in Princeton at the Institute for Advanced Study, and he took us with him. We saw Einstein around the campus a lot; I remember that he always had an umbrella with him, no matter what the weather was like. And one day, there he was: a smiling man standing right in front of us. We asked to take a picture of him, and he said yes. I still have that picture that my brother and I took.
So did you originally want to be a physicist? Oh, yes. I wanted to follow in my father's footsteps. But my father—ever the farmer's son—was a practical man. He told me he didn't think I was up to becoming a physicist. He advised me to study medicine instead, because then I could always do research if I didn't like practicing medicine.
It turns out that was good advice, although it was definitely a struggle at first. One of my early research projects involved purifying a protein called intrinsic factor—which is needed for vitamin B12 absorption—from gastric juice. We needed about 30 liters of gastric juice for this, so I volunteered to be intubated for the gastric juice collection, but the first time I was intubated I nearly died because I got a laryngeal edema.
WILLING TO SACRIFICE
It sounds like you've made sacrifices for your research. Lots of people are willing to sacrifice for research. Once, I had my whole medical class, 60 or 70 people, donate saliva for me so that I could purify vitamin B12, binding proteins from it. I got permission from the professor, during a lecture in obstetrics, to have everyone chew paraffin and spit their saliva into a bowl. There they all were, discussing obstetrics, and interrupting themselves to spit [laughs]. It was quite a scene!
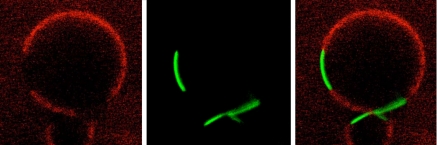
Figure 2.
Transferrin receptor (green) phase separates from GM1 glycolipids (red) in membrane spheres derived from cells.
A real team effort! You could say that. But actually my entire career in science has been based on working together with others, and trying to put together teams. As a postdoc at the Rockefeller University, I saw this incredible density of talent and resources—but I also saw that there was very little collaboration, even within laboratories.
When I returned to Finland I was convinced that the only way to survive doing research there was to pool my resources with others. I got together with two other researchers, Leevi Kääriäinen and Ossi Renkonen, to form a kind of research troika. Each of us brought specific strengths to the team to further our interests. I still think this kind of collaboration is essential if you want to do groundbreaking work.
Your work from this time provided fundamental information on lipid membranes. Yes. Our research troika—together with Ari Helenius—was able to do a methodical study about how different detergents solubilize membranes, because we had this extremely simple system in which to study it: Semliki Forest Virus. This virus's membrane had only one viral protein. If we had been using a complex cellular membrane, the data would have been uninterpretable.
But then you moved to Germany. I'd been given the opportunity to join the new European Molecular Biology Laboratory, which was being formed under John Kendrew. I went there on a three-year contract together with Ari Helenius—who was married to my sister—with the idea that we would return to Finland afterward. I did eventually get the job I wanted in Finland, but the funding situation there was so bad—on a level with Portugal—that I decided to stay at EMBL. Now, of course, Finland spends more per capita on scientific funding than the United States; only Sweden spends more. But I am glad I stayed at EMBL, because I had the opportunity to help influence the institute's direction as it grew.
DYNAMIC FUTURE
And this was when you first started formalizing your ideas about lipid rafts. By that time I had gotten interested in looking at how membranes and proteins are distributed to the apical and basolateral surfaces of MDCK cells. I became enamored with the concept of membrane compartmentalization and recruited Gerrit van Meer from Utrecht [University] to work with me on it. Our early studies showed that lipids and proteins are sorted together in the trans-Golgi, and that the glycolipids and apical proteins segregate into a platform that buds off from the Golgi and is brought by a carrier to the apical membrane. This was our first conceptualization of lipid rafts.
Figure 3.
Simons takes a firm position on lipid rafts.
The lipid raft concept became enormously popular, then equally controversial. Yes. The methodology everyone initially used to study lipid rafts was detergent resistance: if you put Triton on a membrane, any material that was insoluble at four degrees was considered a part of a lipid raft. Of course this was too simple-minded. People would try to manipulate things to get the protein they worked on to be insoluble. This was easy to do; you just had to use a little less detergent. So this led to an adverse reaction and the whole idea of lipid rafts became controversial. For a long time it was difficult to publish, and I thought I would never see the light at the end of the tunnel. There are quite a number of people out there still who think rafts are an artifact.
What are your thoughts on lipid rafts today? Over the last two years our methodology has improved so much. Those who work in the field now know that there is no current alternative explanation for the segregation behavior that is the basis for the raft concept. What has changed is that we now understand rafts to be more dynamic. The resting state is a fluctuating state where the lipid and protein raft components continually come together, and dissociate. Rafts coalesce into a platform by oligomerizing, but even these platforms are transitory entities.
The problem we now face is how to describe this collective behavior; there are many participants in the oligomerization reaction—thousands of lipids and proteins moving together at the same time. How do you study such a phenomenon? It's not simple. But with the infusion of biophysics into this field, we have many new tools—such as single molecule and STED spectroscopy—that are beginning to give us glimpses of the dynamics of membrane subcompartmentalization.
We have also recently shown that plasma membranes can phase separately into micrometer subdomains just as simple lipid model systems do, but that such phase separation is prevented in living cells…so we're still learning about rafts, and refining our understanding of their roles in cells. We're excited about where the field is headed. 
References
- 1.Simons, K., 2000. J. Cell Sci. 113:567–568. [DOI] [PubMed] [Google Scholar]
- 2.Renkonen, O., et al. 1972. Biochem. Soc. Symp. 35:407–422. [PubMed] [Google Scholar]
- 3.Helenius and Simons. 1975. Biochim. Biophys. Acta. 415:29–79. [DOI] [PubMed] [Google Scholar]
- 4.Simons, K., et al. 1982. Sci. Am. 246:58–66. [DOI] [PubMed] [Google Scholar]
- 5.van Meer, G., et al. 1987. J. Cell Biol. 105:1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lingwood, D., et al. 2008. Proc. Natl. Acad. Sci. USA. 105:10005–10010. [DOI] [PMC free article] [PubMed] [Google Scholar]