Abstract
The osteoblast-secreted molecule osteocalcin favors insulin secretion, but how this function is regulated in vivo by extracellular signals is for now unknown. In this study, we show that leptin, which instead inhibits insulin secretion, partly uses the sympathetic nervous system to fulfill this function. Remarkably, for our purpose, an osteoblast-specific ablation of sympathetic signaling results in a leptin-dependent hyperinsulinemia. In osteoblasts, sympathetic tone stimulates expression of Esp, a gene inhibiting the activity of osteocalcin, which is an insulin secretagogue. Accordingly, Esp inactivation doubles hyperinsulinemia and delays glucose intolerance in ob/ob mice, whereas Osteocalcin inactivation halves their hyperinsulinemia. By showing that leptin inhibits insulin secretion by decreasing osteocalcin bioactivity, this study illustrates the importance of the relationship existing between fat and skeleton for the regulation of glucose homeostasis.
Introduction
Our understanding of the mechanisms regulating insulin secretion at the organismal level improved greatly recently with the identification of several novel hormones (Herman and Kahn, 2006; Gesta et al., 2007; Lee et al., 2007). It was shown, for instance, that skeleton acts as an endocrine regulator of energy metabolism through the osteoblast-specific secreted molecule osteocalcin that favors insulin secretion by β cells, insulin sensitivity in fat, liver, and muscle, and energy expenditure (Lee et al., 2007). Although osteocalcin bioactivity is regulated by at least two gene products within the osteoblast, Esp and γ-carboxylase (Bügel, 2008), it remains unknown whether extracellular cues regulate its secretion or function.
In contrast to osteocalcin, leptin inhibits insulin secretion in part through a direct effect on β cells (Covey et al., 2006; Morioka et al., 2007) and, as is the case for most of its functions, in part through indirect mechanisms (Friedman and Halaas, 1998; Kieffer and Habener, 2000). Leptin affects osteoblast functions, raising the testable hypothesis that it could inhibit insulin secretion by decreasing osteocalcin activity (Ducy et al., 2000; Takeda et al., 2002).
In this study, we show that one important mechanism whereby leptin inhibits insulin secretion is by inhibiting the bioactivity of osteocalcin. These results provide in vivo evidence of the importance of the cross talk existing between osteoblasts and adipocytes in glucose homeostasis.
Results and discussion
Regulation of insulin secretion by leptin
The fact that leptin and osteocalcin exert opposite functions on insulin secretion prompted us to test whether they act independently of each other or not. To avoid the confounding issue of insulin resistance, we analyzed insulin secretion in leptin-deficient (ob/ob) mice at birth and 1 and 2 wk of age because at those ages body weight, abdominal and total fat mass, triglyceride level, and insulin sensitivity are not altered by the absence of leptin (Fig. 1 A and Fig. S1, A–D, available at http://www.jcb.org/cgi/content/full/jcb.200809113/DC1).
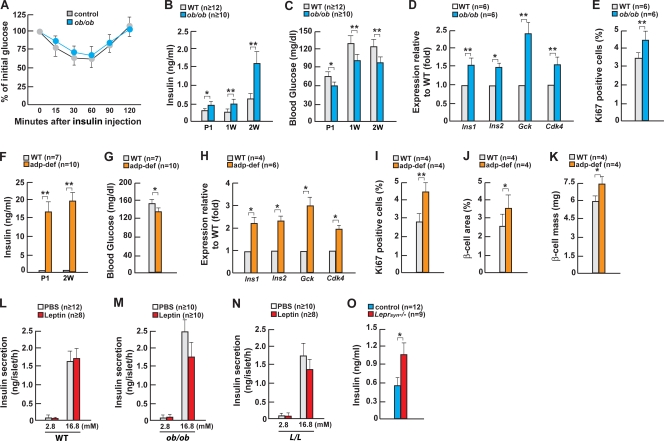
Figure 1.
Leptin regulates insulin secretion in part through the neuronal pathway. (A) ITT in 2-wk-old ob/ob mice. (B and C) Serum insulin and blood glucose in ob/ob mice. (D) Gene expression in pancreas or islets of 2-wk-old ob/ob mice. (E) Quantification of insulin/Ki67 immunoreactive cells in islets of 2-wk-old ob/ob mice. (F and G) Serum insulin and blood glucose in adipocyte-deficient (adp-def) mice. (H) Gene expression in pancreas or islets of 2-wk-old adipocyte-deficient mice. (I–K) Quantification of insulin/Ki67 immunoreactive cells in islets, β-cell area, and β-cell mass of 2-wk-old adipocyte-deficient mice. (L–N) Glucose-stimulated insulin secretion by leptin in islets from WT, ob/ob, and L/L mice. (O) Serum insulin levels in 1-mo-old Leprsyn−/− mice. Error bars indicate mean + SEM. *, P < 0.05; **, P < 0.01; P1, newborn; 1W, 1 wk old; 2W, 2 wk old. Control in O indicates Synapsin-Cre mice. In L–N, the concentration of glucose in the culture media is indicated in millimolars.
In 2-wk-old ob/ob mice serum, insulin levels were 2.5-fold higher than in wild-type (WT) littermates, resulting in a >30% decrease of blood glucose levels after feeding. Remarkably, hyperinsulinemia and low blood glucose levels were also present in newborn and 1-wk-old ob/ob mice (Fig. 1, B and C). To understand how this marked hyperinsulinemia develops in mice that are otherwise metabolically normal, we studied islet gene expression and β-cell proliferation in WT and ob/ob mice. Expression of the Insulin genes and of Glucokinase, a central component of the glucose-sensing machinery of β cells (Grupe et al., 1995), was increased 50% and 140%, respectively, in ob/ob mice (Fig. 1 D), at least partly explaining the aforementioned increased insulin levels. Serum c-peptide levels were increased 2.5-fold in ob/ob mice (Fig. S1 E). There was also a small but detectable and reproducible increase in insulin content in ob/ob pancreata (Fig. S1 F). In addition, expression of Cdk4, a gene favoring β-cell proliferation in vivo (Rane et al., 1999), was up-regulated 50% in ob/ob islets, and Ki67 immunostaining showed a significant increase in β-cell proliferation in ob/ob compared with WT mice (Fig. 1, D and E). Undetectable increases in β-cell area and β-cell mass (Fig. S1, G and H) indicate that the absence of leptin affects circulating insulin levels primarily by regulating insulin expression and secretion in addition to the possibility of changes of cell survival. Nevertheless, these results establish that leptin is a physiological regulator of serum insulin levels independent of the influence it may have on insulin sensitivity.
We asked whether similar abnormalities were present in mice lacking adipocytes altogether. 2-wk-old adipocyte-deficient mice were also markedly hyperinsulinemic and had a significant drop in blood glucose levels; this hyperinsulinemia was also observed at birth (Fig. 1, F and G; and Fig. S1, I and J). Expression of Insulin, Glucokinase, and Cdk4 was up-regulated to the same extent in islets of adipocyte-deficient and ob/ob mice (Fig. 1 H), and insulin content was significantly increased in islets of adipocyte-deficient mice (Fig. S1 K). β-cell proliferation, area, and mass were all significantly increased in adipocyte-deficient mice (Fig. 1, I–K; and Fig. S1 L). Collectively, and although insulin resistance may have favored β-cell proliferation in the adipocyte-deficient mice, the data presented here show that hyperinsulinemia and lower blood glucose levels precede the appearance of obesity and hyperglycemia in ob/ob and adipocyte-deficient mice, respectively, and can be ascribed in both cases to an increase in Insulin and Glucokinase expression and, to a lesser extent, to an increase in β-cell proliferation. More severe hyperinsulinemia in adipocyte-deficient mice than in ob/ob mice indicates that in addition to leptin deficiency, other mechanisms affect insulin secretion in the former model.
Multiple mechanisms contribute to leptin regulation of insulin secretion
Next, we studied leptin influence on isolated islets. When using WT islets cultured in low or high glucose concentration, we failed to observe a decrease in the amount of insulin secreted in the medium after leptin treatment. In contrast, leptin did decrease insulin secretion by ob/ob islets (Fig. 1, L and M). This result supports the notion that this hormone acts directly on β cells to regulate insulin secretion; however, the decrease observed was too modest to explain why the absence of leptin would result in a 2.5-fold increase in circulating insulin levels (Fig. 1 B). In the face of these results, we asked whether islets isolated from mice harboring an activating mutation in the leptin receptor (L/L mice; Björnholm et al., 2007) might be a better tool to uncover a more significant direct effect of leptin on β cells. In this study, again we observed only a modest decrease in insulin secretion after leptin treatment (Fig. 1 N). The marked increase in insulin secretion by high glucose concentration served as an internal positive control and indicated that the islets used in all of these experiments were in good condition. Collectively, data obtained using islets isolated from WT, ob/ob, and L/L mice verified that leptin acts locally on β cells to inhibit insulin secretion but were also consistent with the hypothesis that in addition to this direct effect, leptin may use indirect mechanisms to regulate insulin secretion.
Leptin-dependent sympathetic regulation of insulin secretion
Given what is known about the mediation of most of its functions, the aforementioned results prompted us to ask whether leptin also uses a neuronal relay to inhibit insulin secretion. Thus, we crossed mice harboring a floxed allele of the leptin receptor gene Lepr (Lepr-flox/flox) with Synapsin-Cre transgenic mice to remove the leptin receptor in all neurons (van de Wall et al., 2008). 1-mo-old Synapsin-Cre;Lepr-flox/flox (Leprsyn−/−) mice had a normal body weight but harbored a significant increase in serum insulin levels, indicating that in vivo there is a significant neuronal contribution to the leptin regulation of insulin secretion (Fig. 1 O and Fig. S1 M).
We noted that in Leprsyn−/− mice, sympathetic tone, as measured by Ucp1 expression in brown fat and serum levels of epinephrine and norepinephrine, was low (Fig. 2, A–C). This decrease, similar to the one observed in ob/ob mice, led us to ask whether the sympathetic tone could be a mediator of the leptin-dependent neuronal regulation of insulin secretion (Berthoud and Jeanrenaud, 1979). WT, ob/ob, and adipocyte-deficient newborn pups were treated for 2 wk with sympathomimetics acting through β-adrenergic receptors (isoproterenol) or through the Adrα2 (clonidine) or Adrα1 (phenylephrine) receptors. Neither phenylephrine nor clonidine affected serum insulin levels in any of the mouse models tested; in contrast, isoproterenol used at a moderate dose (3 mg/kg) significantly decreased circulating insulin levels in ob/ob and adipocyte-deficient mice (Fig. 2, D and E). Isoproterenol increased Ucp1 expression in brown fat, supporting the notion that there was an increase in sympathetic tone following this treatment (Fig. 2 F). Isoproterenol did not affect circulating insulin in WT mice, probably because it was used at a dose that did not cause a massive increase in sympathetic tone. Indeed, Ucp1 expression was not significantly increased in WT mice by isoproterenol (Fig. 2 F). Collectively, these observations establish that the sympathetic tone acting through β-adrenergic receptors regulates insulin secretion.
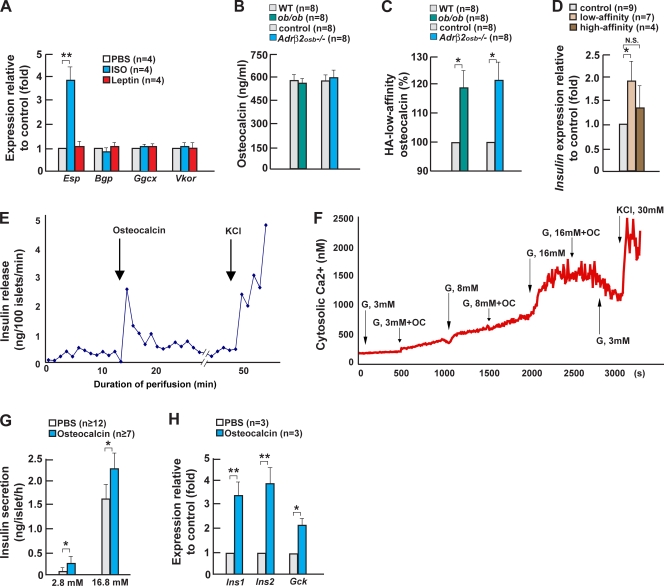
Figure 2.
Sympathetic signaling in osteoblasts regulates insulin expression and secretion. (A) Ucp1 expression in brown fat of Leprsyn−/− mice. (B and C) Serum epinephrine and norepinephrine levels in Leprsyn−/− mice. (D and E) Serum insulin levels in ob/ob and adipocyte-deficient (adp-def) mice after daily isoproterenol (+ISO), phenylephrine (+Phe), or clonidine (+Clo) injection for 2 wk. (F) Ucp1 expression in brown fat of ob/ob and adipocyte-deficient mice after daily isoproterenol injection. (G) Serum insulin levels in 2-wk-old Adrβ2osb−/− mice. (H) Serum insulin levels in WT and Adrβ2osb−/− mice after daily propranolol injection for 2 wk. (I and J) Blood glucose levels and gene expression in islets of 2-wk-old Adrβ2osb−/− mice. (K–M) Serum insulin, blood glucose, and Insulin expression in the pancreas of 2-wk-old ob/+;Adrβ2osb+/− mice. (N) Delta insulin after glucose-stimulated insulin secretion in WT and Adrβ2osb−/− mice treated with central leptin infusion at 4 ng/h for 1 wk. (O and P) Plasma insulin levels during hyperglycemic clamps in WT and Adrβ2osb−/− mice treated with central leptin infusion at 4 ng/h for 1 wk. Error bars indicate mean + SEM. *, P < 0.05; **, P < 0.01. Control in A–C indicates Synapsin-Cre mice. Control in G–J and M indicates α1(I)Collagen-Cre mice.
Leptin-dependent sympathetic regulation of insulin secretion occurs through the osteoblasts
Although isoproterenol can affect secretion of insulin by isolated islets (Iversen, 1973), two reasons led us to test the hypothesis that it was also through the osteoblasts that the sympathetic tone, under the control of leptin, regulates insulin secretion. One is the functional link existing between leptin, sympathetic tone, and bone mass (Takeda et al., 2002), and another one is the recently uncovered role of osteoblasts in regulating insulin secretion (Lee et al., 2007). To test this hypothesis, we deleted Adrβ2, the only adrenergic receptor expressed in osteoblasts, from these cells (Adrβ2osb−/− mice).
Consistent with our hypothesis, serum insulin levels were markedly higher in Adrβ2osb−/− mice than in WT mice (Fig. 2 G and Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200809113/DC1). Accordingly, propranolol, a β blocker acting through Adrβ2, increased insulin serum levels in WT but not in Adrβ2osb−/− mice (Fig. 2 H). The increase in serum insulin levels developed in Adrβ2osb−/− mice in the absence of any evidence of insulin resistance as determined by insulin tolerance test (ITT), serum adiponectin level, and Adiponectin expression in fat (Fig. S2, F–H). This was specific of Adrβ2 deletion because serum insulin levels were normal in Adrβ1−/− and Adrα2A−/− mice (unpublished data). Adrβ2osb−/− mice also had an 80–150% increase in Insulin and Glucokinase expression and exhibited postprandial low blood glucose levels (Fig. 2, I and J), and Cdk4 expression was increased 50% in Adrβ2osb−/− mice (Fig. 2 J). It is worthwhile to note that these abnormalities were similar to the ones observed in ob/ob mice (compare with Fig. 1 D).
If sympathetic signaling in osteoblasts and leptin are in the same genetic pathway, genetic epistasis predicts that mice heterozygous for Adrβ2 deficiency and Leptin deficiency should harbor the same defect in insulin secretion than the one observed in Adrβ2osb−/− and ob/ob mice. Indeed, although both Adrβ2osb+/− and ob/+ mice had normal serum insulin levels, ob/+;Adrβ2osb+/− mice displayed a hyperinsulinemia and lower blood glucose level after feeding (Fig. 2, K and L). The severity of these two abnormalities was only slightly milder than in ob/ob mice (compare with Fig. 1, B and C). At the molecular level, Insulin expression was elevated to the same degree in ob/ob and ob/+;Adrβ2osb+/− mice (Fig. 2 M).
To add further credence to the notion that sympathetic signaling in osteoblasts contributes to the leptin regulation of insulin secretion, we performed two other experiments. Long-term (1 wk) intracerebroventricular (ICV) infusion of 4 ng/h leptin decreased glucose-stimulated insulin secretion in WT but not in Adrβ2osb−/− mice (Fig. 2 N). We also used a hyperglycemic clamp to assess glucose-stimulated insulin secretion in vivo. Leptin ICV infusion also decreased circulating insulin levels in WT but not Adrβ2osb−/− mice (Fig. 2, O and P). Collectively, these observations identify sympathetic signaling in osteoblasts as a significant mediator of leptin's regulation of insulin secretion.
The sympathetic tone in osteoblasts inhibits osteocalcin activity
How does sympathetic signaling in osteoblasts regulate insulin secretion in β cells? Osteoblasts secrete osteocalcin, a molecule stimulating β-cell proliferation and Insulin expression whose biological activity is decreased by the product of the Esp gene (Lee et al., 2007; Ferron et al., 2008). Multiple observations indicate that the leptin-dependent sympathetic regulation of insulin secretion occurs in part by modulating osteocalcin bioactivity.
Esp expression was up-regulated fourfold by isoproterenol in osteoblasts, whereas expression of Osteocalcin and of other enzymes modifying it was not (Fig. 3 A). Accordingly, and although the serum level of total osteocalcin was normal (Fig. 3 B), the amount of osteocalcin binding to hydroxyapatite (HA) with low affinity, i.e., undercarboxylated and biologically active (Lee et al., 2007), was increased in both ob/ob and Adrβ2osb−/− mice (Fig. 3 C). Two control experiments validated this finding. First, when using serum containing only uncarboxylated osteocalcin as a negative control, osteocalcin eluted in the same fractions as the ones harboring an increased content of osteocalcin in ob/ob serum (unpublished data). Second, the low affinity fractions regulated Insulin expression in isolated islets, whereas the ones binding to HA with high affinity did not (Fig. 3 D).
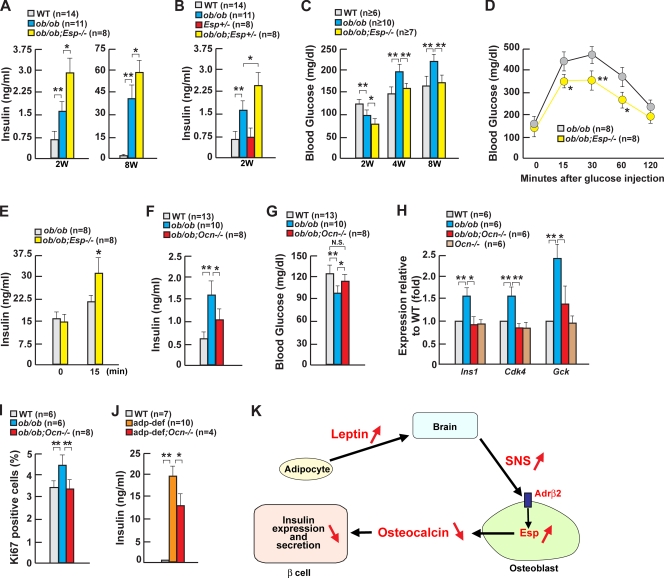
Figure 3.
Osteocalcin directly regulates insulin secretion in a Ca2+-dependent manner. (A) Gene expression in isoproterenol (ISO)- or leptin-treated osteoblasts. (B and C) Levels of total and uncarboxylated osteocalcin (HA low affinity) in ob/ob and Adrβ2osb−/− mice. (D) Insulin expression by HA affinity fraction in islets. (E) Perifusion of WT islets in the presence of 0.03 ng/ml osteocalcin under a 3-mM glucose condition. (F) Intracellular calcium imaging assay using WT islets in the presence of osteocalcin under the different concentrations of glucose. (G) Insulin secretion by osteocalcin in WT islets maintained in either low or high glucose. (H) Gene expression in osteocalcin-treated islets. Error bars indicate mean + SEM. *, P < 0.05; **, P < 0.01. Control in B and C indicates α1(I)Collagen-Cre mice. In G, the concentration of glucose in the culture media is indicated in millimolars.
Next, we tested whether osteocalcin regulates insulin secretion directly. In an islets perifusion assay, 0.03 ng/ml uncarboxylated osteocalcin was a powerful insulin secretagogue (Fig. 3 E) with a strong first phase and a sustained but lower second phase of insulin secretion in all experiments performed (n = 3). Cytosolic Ca2+ imaging was also performed on untreated and osteocalcin-treated WT mouse islets at 3-, 8-, and 16-mM glucose levels. 30 mM KCl was used as a positive control. At 3 mM glucose, osteocalcin initiated a steady increase of basal Ca2+ concentration, whereas at 8 and 16 mM glucose, osteocalcin notably enhanced the cytosolic Ca2+ concentration as compared with control untreated islets (n = 3; Fig. 3 F and Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200809113/DC1). Osteocalcin also augmented glucose-stimulated insulin secretion from isolated islets maintained in either low or high glucose concentrations (Fig. 3 G). In addition, expression of Insulin and Glucokinase in isolated islets (Fig. 3 H) was induced by osteocalcin in a time- and dose-dependent manner.
The relevance of osteocalcin in the leptin-dependent sympathetic regulation of insulin secretion was also verified in vivo. First, increasing osteocalcin bioactivity should worsen hyperinsulinemia in ob/ob mice. To test this hypothesis, we generated ob/ob mice lacking Esp (ob/ob;Esp−/− mice). Ob/ob;Esp−/− mice were twofold more hyperinsulinemic than ob/ob mice at 2 wk of age (Fig. 4 A). Remarkably, similar abnormalities were observed in ob/ob mice lacking only one allele of Esp (Fig. 4 B). Ob/ob;Esp−/− mice also had low blood glucose levels and remained significantly less glucose intolerant than ob/ob mice until 8 wk of age, thus illustrating the importance of this pathway in adult mouse physiology (Fig. 4, A, C, and D). An improved glucose tolerance was demonstrated by the ability of a glucose challenge to increase insulin secretion in ob/ob;Esp−/− but not in ob/ob mice (Fig. 4 E). Insulin sensitivity, as measured by hyperinsulinemic-euglycemic clamps, was similar in ob/ob;Esp−/− and ob/ob mice (Table S1, available at http://www.jcb.org/cgi/content/full/jcb.200809113/DC1).
Figure 4.
Leptin regulates insulin secretion through osteoblasts. (A) Serum insulin in 2- and 8-wk-old ob/ob;Esp−/− mice. (B) Serum insulin in 2-wk-old ob/ob;Esp−/− mice. (C) Blood glucose in 2–8-wk-old ob/ob;Esp−/− mice. (D) Glucose tolerance tests in 2-mo-old ob/ob;Esp−/− mice. (E) Serum insulin after glucose challenge in 2-mo-old ob/ob;Esp−/− mice. (F and G) Serum insulin and blood glucose in 2-wk-old ob/ob;Ocn−/− mice. (H) Gene expression in pancreas or islets of 2-wk-old ob/ob;Ocn−/− mice. (I) Quantification of insulin/Ki67-positive cell islets of 2-wk-old ob/ob;Ocn−/− mice. (J) Serum insulin in 2-wk-old adipocyte-deficient;Ocn−/− mice. adp-def, adipocyte deficient. (K) In the presence of leptin, sympathetic tone is high; thus, Esp expression in the osteoblast is high, and osteocalcin bioactivity is low. This contributes to the inhibition of insulin secretion by leptin. Error bars indicate mean + SEM. *, P < 0.05; **, P < 0.01. 2W, 2 wk old; 4W, 4 wk old; 8W, 8 wk old; SNS, sympathetic nervous system.
Second, Osteocalcin inactivation decreased serum insulin levels 50% and normalized blood glucose levels in ob/ob mice (ob/ob;Osteocalcin−/−; Fig. 4, F and G). Expression of Insulin, Glucokinase, and Cdk4 was decreased 50–100% in ob/ob;Osteocalcin−/− mice compared with ob/ob mice, and β-cell proliferation was normalized in ob/ob;Osteocalcin−/− mice (Fig. 4, H and I). The similarity of correction of hyperinsulinemia in ob/ob;Osteocalcin−/− mice and in ob/ob mice treated with the sympathomimetic isoproterenol supports the view that osteocalcin is in vivo a significant mediator of the leptin-dependent sympathetic regulation of insulin secretion. Osteocalcin inactivation also decreased serum insulin levels by half in adipocyte-deficient mice (Fig. 4 J). Collectively, the results presented in this study support the hypothesis that the leptin-dependent sympathetic inhibition of insulin secretion occurs in part via the osteoblast and acts by modulating osteocalcin activity.
Our results reveal that leptin inhibits the endocrine function of the skeleton. Indeed, to a substantial extent, leptin's inhibition of insulin secretion relies on a three-step cascade, including (1) leptin's up-regulation of sympathetic tone, (2) sympathetic enhancement of Esp expression in osteoblasts, and (3) decrease in osteocalcin bioactivity. Osteocalcin acts in this context primarily by regulating insulin expression and secretion. These results do not contradict that leptin acts locally on β cells to inhibit insulin secretion (Covey et al., 2006; Morioka et al., 2007); rather, they reveal an additional pathway whereby leptin fulfills this function. Beyond linking two secreted molecules, i.e., leptin and osteocalcin, these observations establish that the metabolic connection between osteoblasts and adipocytes is tighter than originally thought (Karsenty, 2006; Rosen, 2008). This cross talk includes an important functional component because osteoblasts mediate a significant part of one of the endocrine functions on adipocytes. This unexpected functional relationship between adipocytes, neurons, sympathetic tone, and osteoblasts illustrates in vivo the importance of the skeleton in the regulation of glucose homeostasis and provides an example of how critical the interplay is between multiple organs in the establishment and regulation of major physiological functions in vertebrates.
Materials and methods
Animals, treatments, and surgical procedures
Adrα2A−/− (Q. Wang, University of Alabama at Birmingham, Birmingham, AL), Osteocalcin−/−, Esp−/−, Lepr-flox/flox, and α1(I)Collagen-Cre mice were previously described (Altman et al., 1999; Dacquin et al., 2002; Lee et al., 2007; van de Wall et al., 2008). Osteoblast-specific Adrβ2-deficient (Adrβ2osb−/−) mice were generated through homologous recombination in embryotic stem cells and through the use of α1(I)Collagen-Cre mice. B6.V-Lepob/J and FVB-Tg(AZIP/F)1Vsn/J mice were purchased from The Jackson Laboratory. 3 mg/kg isoproterenol was injected i.p. once daily for 2 wk. For ICV infusion, a 28-gauge cannula (brain infusion kit II; Alza) was implanted into the third ventricle as previously described, infusing human leptin (Sigma-Aldrich) at 4 ng/h for 7 d (Ducy et al., 2000). The cannula was connected to an osmotic pump (Alza) placed in the dorsal subcutaneous space of the animal. Genotyping was performed by PCR analysis of genomic DNA. All procedures involving animals were approved by the Institutional Animal Care and Use Committee and conform to the relevant regulatory standards.
Metabolic studies
For glucose tolerance tests, 0.625 or 2 g/kg glucose was injected i.p. after an overnight fast, and blood glucose was monitored using blood glucose strips and the Accu-Check glucometer (Roche) at the indicated times. For ITT, mice were injected i.p. with 0.25 or 0.5 U/kg insulin, and blood glucose levels were measured at the indicated times. ITT data are presented as a percentage of initial blood glucose concentration. Hyperinsulinemic-euglycemic clamps and hyperglycemic clamps were performed at the Pennsylvania State Diabetes and Obesity Mouse Phenotyping Center. In brief, ob/ob, ob/ob;Esp−/−, and WT littermate mice (n = 4 for each group) were fasted overnight, and a 2-h hyperinsulinemic (2.5 mU/kg/min)-euglycemic clamp was performed after intravenous administration of [3-3H] glucose and 2-deoxy-d-[1-14C] glucose as previously described (Kim et al., 2004). Adrβ2osb−/− and control mice (n = 4 for each group) were fasted overnight, and a 2-h hyperglycemic clamp was conducted with a variable infusion of 20% glucose to maintain hyperglycemia at ∼300 mg/dl. Blood samples were taken at 10–20-min intervals for the measurement of plasma insulin levels (Cho et al., 2007).
Islet perifusion
Islet perifusion was performed as described previously (Gao et al., 2007). In brief, islets were isolated from WT mice using standard collagenase digestion followed by purification through a Ficoll gradient. 100 islets were hand picked under a light microscope and placed into a perifusion chamber (Millipore). A computer-controlled high performance liquid chromatography system (625 LC; Waters Corporation) allowed programmable rates of flow and concentration of the appropriate solutions held in a 37°C water bath. Islets were perifused with Krebs bicarbonate buffer to reach baseline hormone secretion values before the addition of the appropriate secretagogues. Samples were collected at regular intervals with a fraction collector (Waters Corporation).
Ca2+ imaging
Cytosolic Ca2+ imaging was performed as described previously (Gao et al., 2007). Mouse islets cultured for 3–4 d in 10 mM glucose were loaded with 1 mmol/liter fura 2-AM (Invitrogen) during a 40-min pretreatment at 37°C in Krebs buffer, transferred to the perifusion chamber, and placed on the homeothermic platform of an inverted microscope (Axio Observer Z1; Carl Zeiss, Inc.). Islets were perfused with Krebs buffer at 37°C while various treatments were applied. The microscope was used with a 40× oil immersion objective. Intracellular Ca2+ was determined by the ratio of the excitation of fura 2-AM at 334 and 380 nm. Emission was measured at 520 nm with a charge-coupled device camera (Attofluor; Invitrogen) and calibrated using the Ratio Vision software (Attofluor; Invitrogen).
In vitro glucose-stimulated insulin secretion
For assay of insulin release (Kitamura et al., 2001), five islets were manually selected, incubated in Krebs-Ringer solution, and stimulated at 37°C with various concentrations of glucose in the presence or absence of osteocalcin for 1 h. The islets were collected by centrifugation, and the supernatant was assayed for insulin content by ELISA (Crystal Chem).
Cell and molecular studies
Primary osteoblasts were prepared from calvaria of 5-d-old pups as previously described (Takeda et al., 2002). Real-time PCR was performed on Dnase I–treated total RNA converted to cDNA using iTaq SYBR Green Supermix with ROX (6-carboxy-X-rhodamine; Bio-Rad Laboratories) on an MX3000 instrument; β-actin amplification was used as an internal reference for each sample.
Biochemistry
Serum levels of insulin (Crystal Chem) and c-peptide (Yanaihara Institute) were measured using commercial kits. For quantification of uncarboxylated levels of osteocalcin, sera were incubated with HA slurry for 1 h. The quantity of osteocalcin present in the unbound fraction and in the fractions eluted at 0.02 and 1 M sodium phosphate, pH 6.8, was measured by an immunoradiometric assay (Immunotopics).
Histology
Pancreas was fixed in 10% neutral formalin, embedded in paraffin, and sectioned at 5 μm; sections were stained with hematoxylin and eosin. Immunohistochemistry was performed using rabbit antiinsulin (Santa Cruz Biotechnology, Inc.) and mouse anti-Ki67 (Vector Laboratories) antibodies. β-cell area represents the surface positive for insulin immunostaining divided by the total pancreatic surface. β-cell mass was calculated as β-cell area multiplied by pancreatic weight as described previously (Lee et al., 2007).
Statistical analyses
Results are given as means ± SEM. Statistical analyses were performed using unpaired two-tailed Student's t or analysis of variance tests.
Online supplemental material
Fig. S1 shows the characterization of ob/ob and adipocyte-deficient mice. Fig. S2 shows the strategy for generation of conditional Adrβ2-deficient mice and the characterization of Adrβ2osb−/− mice. Fig. S3 shows the intracellular calcium imaging performed on untreated WT mice islets at different concentrations of glucose. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200809113/DC1.
Supplementary Material
Acknowledgments
We thank Dr. Q. Wang for mice and Dr. M. Ferron, P. Ducy, H. Liu, and G. Ren for technical assistance.
This work was supported by the Japan Society for the Promotion of Science, the Uehara Memorial Foundation, the Kanae Foundation for the Promotion of Medical Science (grant to E. Hinoi), the National Institutes of Health (grants to G. Karsenty, K.H. Kaestner, and J.K. Kim), and the American Diabetes Association (grant to J.K. Kim). G. Karsenty is the founder of Escoublac, Inc.
Abbreviations used in this paper: HA, hydroxyapatite; ICV, intracerebroventricular; ITT, insulin tolerance test; WT, wild type.
References
- Altman, J.D., A.U. Trendelenburg, L. MacMillan, D. Bernstein, L. Limbird, K. Starke, B.K. Kobilka, and L. Hein. 1999. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol. Pharmacol. 56:154–161. [DOI] [PubMed] [Google Scholar]
- Berthoud, H.R., and B. Jeanrenaud. 1979. Acute hyperinsulinemia and its reversal by vagotomy after lesions of the ventromedial hypothalamus in anesthetized rats. Endocrinology. 105:146–151. [DOI] [PubMed] [Google Scholar]
- Björnholm, M., H. Münzberg, R.L. Leshan, E.C. Villanueva, S.H. Bates, G.W. Louis, J.C. Jones, R. Ishida-Takahashi, C. Bjørbaek, and M.G. Myers Jr. 2007. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J. Clin. Invest. 117:1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bügel, S. 2008. Vitamin K and bone health in adult humans. Vitam. Horm. 78:393–416. [DOI] [PubMed] [Google Scholar]
- Cho, Y.R., H.J. Kim, S.Y. Park, H.J. Ko, E.G. Hong, T. Higashimori, Z. Zhang, D.Y. Jung, M.S. Ola, K.F. Lanoue, et al. 2007. Hyperglycemia, maturity-onset obesity, and insulin resistance in NONcNZO10/LtJ males, a new mouse model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 293:E327–E336. [DOI] [PubMed] [Google Scholar]
- Covey, S.D., R.D. Wideman, C. McDonald, S. Unniappan, F. Huynh, A. Asadi, M. Speck, T. Webber, S.C. Chua, and T.J. Kieffer. 2006. The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab. 4:291–302. [DOI] [PubMed] [Google Scholar]
- Dacquin, R., M. Starbuck, T. Schinke, and G. Karsenty. 2002. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev. Dyn. 224:245–251. [DOI] [PubMed] [Google Scholar]
- Ducy, P., M. Amling, S. Takeda, M. Priemel, A.F. Schilling, F.T. Beil, J. Shen, C. Vinson, J.M. Rueger, and G. Karsenty. 2000. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 100:197–207. [DOI] [PubMed] [Google Scholar]
- Ferron, M., E. Hinoi, G. Karsenty, and P. Ducy. 2008. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. USA. 105:5266–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, J.M., and J.L. Halaas. 1998. Leptin and the regulation of body weight in mammals. Nature. 395:763–770. [DOI] [PubMed] [Google Scholar]
- Gao, N., P. White, N. Doliba, M.L. Golson, F.M. Matschinsky, and K.H. Kaestner. 2007. Foxa2 controls vesicle docking and insulin secretion in mature beta cells. Cell Metab. 6:267–279. [DOI] [PubMed] [Google Scholar]
- Gesta, S., Y.H. Tseng, and C.R. Kahn. 2007. Developmental origin of fat: tracking obesity to its source. Cell. 131:242–256. [DOI] [PubMed] [Google Scholar]
- Grupe, A., B. Hultgren, A. Ryan, Y.H. Ma, M. Bauer, and T.A. Stewart. 1995. Transgenic knockouts reveal a critical requirement for pancreatic beta cell glucokinase in maintaining glucose homeostasis. Cell. 83:69–78. [DOI] [PubMed] [Google Scholar]
- Herman, M.A., and B.B. Kahn. 2006. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 116:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen, J. 1973. Adrenergic receptors and the secretion of glucagon and insulin from the isolated, perfused canine pancreas. J. Clin. Invest. 52:2102–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty, G. 2006. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 4:341–348. [DOI] [PubMed] [Google Scholar]
- Kieffer, T.J., and J.F. Habener. 2000. The adipoinsular axis: effects of leptin on pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 278:E1–E14. [DOI] [PubMed] [Google Scholar]
- Kim, H.J., T. Higashimori, S.Y. Park, H. Choi, J. Dong, Y.J. Kim, H.L. Noh, Y.R. Cho, G. Cline, Y.B. Kim, and J.K. Kim. 2004. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 53:1060–1067. [DOI] [PubMed] [Google Scholar]
- Kitamura, T., Y. Kido, S. Nef, J. Merenmies, L.F. Parada, and D. Accili. 2001. Preserved pancreatic beta-cell development and function in mice lacking the insulin receptor-related receptor. Mol. Cell. Biol. 21:5624–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, N.K., H. Sowa, E. Hinoi, M. Ferron, J.D. Ahn, C. Confavreux, R. Dacquin, P.J. Mee, M.D. McKee, D.Y. Jung, et al. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell. 130:456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka, T., E. Asilmaz, J. Hu, J.F. Dishinger, A.J. Kurpad, C.F. Elias, H. Li, J.K. Elmquist, R.T. Kennedy, and R.N. Kulkarni. 2007. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J. Clin. Invest. 117:2860–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane, S.G., P. Dubus, R.V. Mettus, E.J. Galbreath, G. Boden, E.P. Reddy, and M. Barbacid. 1999. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat. Genet. 22:44–52. [DOI] [PubMed] [Google Scholar]
- Rosen, C.J. 2008. Bone remodeling, energy metabolism, and the molecular clock. Cell Metab. 7:7–10. [DOI] [PubMed] [Google Scholar]
- Takeda, S., F. Elefteriou, R. Levasseur, X. Liu, L. Zhao, K.L. Parker, D. Armstrong, P. Ducy, and G. Karsenty. 2002. Leptin regulates bone formation via the sympathetic nervous system. Cell. 111:305–317. [DOI] [PubMed] [Google Scholar]
- van de Wall, E., R. Leshan, A.W. Xu, N. Balthasar, R. Coppari, S.M. Liu, Y.H. Jo, R.G. MacKenzie, D.B. Allison, N.J. Dun, et al. 2008. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 149:1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.