Abstract
Degradation of the extracellular matrix (ECM) protein laminin contributes to excitotoxic cell death in the hippocampus, but the mechanism of this effect is unknown. To study this process, we disrupted laminin γ1 (lamγ1) expression in the hippocampus. Lamγ1 knockout (KO) and control mice had similar basal expression of kainate (KA) receptors, but the lamγ1 KO mice were resistant to KA-induced neuronal death. After KA injection, KA1 subunit levels increased in control mice but were unchanged in lamγ1 KO mice. KA1 levels in tissue plasminogen activator (tPA)–KO mice were also unchanged after KA, indicating that both tPA and laminin were necessary for KA1 up-regulation after KA injection. Infusion of plasmin-digested laminin-1 into the hippocampus of lamγ1 or tPA KO mice restored KA1 up-regulation and KA-induced neuronal degeneration. Interfering with KA1 function with a specific anti-KA1 antibody protected against KA-induced neuronal death both in vitro and in vivo. These results demonstrate a novel pathway for neurodegeneration involving proteolysis of the ECM and KA1 KA receptor subunit up-regulation.
Introduction
Excitotoxicity is the main mechanism underlying neuronal death in stroke, anoxia, and seizure. The extracellular serine protease tissue plasminogen activator (tPA) and its zymogen substrate plasminogen are critical to excitotoxic neuronal death because mice deficient in either of these genes are resistant to excitotoxic neurodegeneration (Tsirka et al., 1995, 1997). Further study showed that the tPA/plasmin proteolytic cascade participates in excitotoxic neuronal death by degrading the ECM protein laminin (Chen and Strickland, 1997; Nagai et al., 1999).
Laminins are heterotrimeric ECM glycoproteins that play important roles in the nervous system. Laminins are expressed in the mouse hippocampus and disappear after excitotoxin injection (Hagg et al., 1989, 1997; Jucker et al., 1996; Chen and Strickland, 1997; Tian et al., 1997; Nagai et al., 1999; Indyk et al., 2003). Laminin disappearance precedes neuronal death, is spatially coincident with regions that exhibit neuronal loss, and is blocked by either tPA deficiency or infusion of a plasmin inhibitor, both of which also prevent neuronal degeneration. These studies indicate that laminin is a key player in excitotoxic neuronal degeneration. However, the mechanism of how laminin degradation participates in neuronal death is not clear.
To study the mechanistic role of laminin in excitotoxic neuronal death, we generated a laminin γ1 (lamγ1) conditional knockout (KO) mouse line using the Cre/loxP system (Chen and Strickland, 2003) and disrupted laminin expression in the hippocampus (hereafter referred to as lamγ1 KO mice). Analysis of these mice revealed that they were resistant to excitotoxic neuronal death. We established that laminin degradation products, which are produced via the tPA/plasmin system, lead to up-regulation of the KA1 subunit of the kainate (KA) receptor and subsequent neuronal death. Consistent with this conclusion, specific interference of KA1 subunit function rendered wild-type mice resistant to excitotoxic degeneration.
Our results illuminate a novel excitotoxic pathway in which KA up-regulates tPA, leading to laminin degradation by plasmin. The products of laminin proteolysis up-regulate a key KA receptor, which increases the sensitivity to excitotoxins and eventually causes neuronal death. This pathway suggests new approaches to countering the neuronal loss associated with excitotoxic injury in disorders like stroke.
Results
Lamγ1 depletion in the hippocampus renders neurons resistant to KA-induced neuronal cell death
Injection of excitotoxins into the hippocampus causes massive cell death in the cornu ammonis regions of the hippocampus (Coyle et al., 1978). Previous studies have implicated laminin in this process (Chen and Strickland, 1997; Chen et al., 2003). To further study the role of laminin in excitotoxic neuronal degeneration, we created a mouse line in which the lamγ1 gene is floxed (Chen and Strickland, 2003) and disrupted lamγ1 expression in the hippocampus using Cre recombinase controlled by calcium/calmodulin-dependent protein kinase II α (CaMKII) promoter (Dragatsis and Zeitlin, 2000). To analyze where Cre was expressed in the adult hippocampus, we created mice containing the CaMKII-Cre transgene and a double reporter gene in which GFP expression is activated by Cre-dependent excision of the lacZ gene together with a stop codon (lacZ/EGFP reporter mice; Novak et al., 2000). In these mice, GFP was expressed in the CA1 neuronal layers and dentate gyrus (DG) in the hippocampus (Fig. 1 C), indicating Cre expression in these regions.
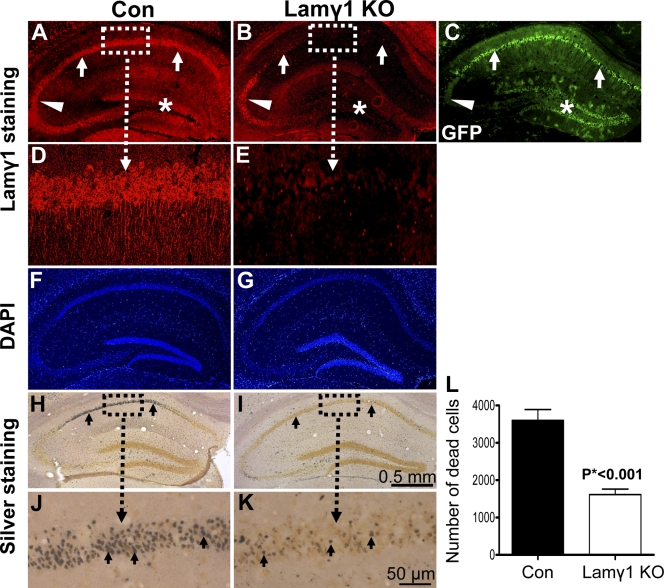
Figure 1.
Lamγ1 KO mice were resistant to KA-induced neuronal death in the hippocampus. (A and B) Lamγ1 was expressed in the hippocampal neuronal layers CA1 (A, arrows), CA2/3 (A, arrowhead), and DG (A, asterisk) of control (Con) mice (floxed lamγ1 mice) but was dramatically decreased in the CA1 and DG regions of the lamγ1 KO mice (B, arrows and asterisk). In the CA2 region, lamγ1 was still expressed (A and B, arrowheads). Higher magnification of boxed areas in A and B are shown in D and E, respectively. (C) In the lacZ/EGFP reporter mice that also carry the CaMKII-Cre transgene, GFP (indication of Cre activity) was expressed in the hippocampal neuronal layers CA1 (arrows) and DG (asterisk), whereas GFP was not expressed in the CA2 region (arrowhead indicates background GFP activity). The GFP expression regions correlated well with the regions of lamγ1 disruption in the KO mice. (F and G) DAPI staining revealed a similar pattern of hippocampal neuronal layers between control and lamγ1 KO mice. (H and I) Silver staining shows that intrahippocampal KA injection–induced neuronal death in the CA1 region of lamγ1 KO mice (I) was much less than that of controls (H; H–K, arrows). Higher magnification of boxed areas in H and I are shown in J and K, respectively. (L) Quantitative analysis of KA-induced neuronal death in control and KO mice (seven mice in each genotype). Error bars indicate SEM. Bars: (A–C and F–I) 0.5 mm; (D, E, J, and K) 50 μm.
In mice homozygous for the floxed lamγ1 gene but not containing CaMKII-Cre (control mice), lamγ1 was expressed in the hippocampus as shown previously (Fig. 1, A and D; Indyk et al., 2003; Yin et al., 2003). However, in mice homozygous for the floxed lamγ1 gene and containing the CaMKII-Cre transgene (lamγ1 KO mice), lamγ1 expression was disrupted in the CA1 (Fig. 1 B, arrows; magnified in E) and DG regions (Fig. 1 B, asterisk) where Cre was expressed. In the CA2 region, where GFP is not expressed (Fig. 1 C, arrowheads), lamγ1 was still expressed in the KO mice (Fig. 1 B, arrowheads). DAPI staining showed that the neuronal cell layers were similar between control and lamγ1 KO hippocampi (Fig. 1, F and G). Thus, this method depletes lamγ1 in the CA1 and DG regions.
Although lamγ1 expression was disrupted both in the CA1 and DG regions, intrahippocampal KA injection only induces neuronal cell death in the cornu ammonis region (Coyle et al., 1978). Therefore, we focused on how the lack of laminin expression in CA1 affects excitotoxic neuronal cell death. 2 d after intrahippocampal KA injection, there was substantial neuronal cell death in the CA1 of control mice (floxed lamγ1 mice) as revealed by silver staining (Fig. 1, H and J). In contrast, there was little neuronal death in the CA1 region of the lamγ1 KO mice (Fig. 1, I and K). Quantitative analysis from control and lamγ1 KO mice (n = 7 per group) showed a significant difference in KA-induced neuronal death in the CA1 region (Fig. 1 L).
In the CA2 and CA3 regions, the numbers of dead neurons between control and lamγ1 KO mice were similar (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200803107/DC1). Consistent with a previous study (Salles and Strickland, 2002), KA-induced neuronal death was greatest in the CA1 region, although there was substantial neuronal death in the CA3 region.
To investigate the time course of neuroprotection after lamγ1 depletion, we compared neuronal death between control and lamγ1 KO mice 6 d after KA injection. Lamγ1 KO mice still exhibited resistance to neuronal death (Fig. S1 B). This result demonstrates that the neuroprotective effect in lamγ1 KO mice is a long-term effect.
KA-induced c-fos protein expression in the hippocampus of lamγ1 KO mice is impaired
Because there was little neuronal cell death after KA injection in the hippocampus of the lamγ1 KO mice, it was possible that the neurons were not stimulated by KA. c-fos is an immediate early gene that is induced after KA injection into the hippocampus (Le Gal La Salle, 1988; Lerea and McNamara, 1993; Kasof et al., 1995) and can be used to monitor neuronal response. To investigate whether neurons were stimulated by KA, we compared c-fos expression at 0, 2, 4, and 6 h after KA injection in the hippocampus of control and lamγ1 KO mice by immunohistochemistry. Without KA injection, there was no c-fos expression in the hippocampus of either control (floxed lamγ1 mice) or lamγ1 KO mice (Fig. 2 A, a and c). c-fos was induced similarly in the DG of both control and lamγ1 KO mice 1 (not depicted) and 2 h after KA injection (Fig. 2 A, e and g). 4 h after KA injection, c-fos expression spread to CA1–3 regions in control mice but did not spread to CA1 and only minimally to CA3 in lamγ1 KO mice (Fig. 2 A, i and k). 6 h after KA injection, c-fos expression was decreased in the control mice compared with 4 h (Fig. 2 A, i and m). However, in lamγ1 KO mice, c-fos expression disappeared 6 h after KA injection (Fig. 2 A, o). DAPI staining showed similar patterns of hippocampal neuronal layers between control and lamγ1 KO mice at early time points after KA injections (Fig. 2 A, b, d, f, h, j, l, n, and p). Quantitative analyses by Western blotting showed significantly less c-fos induction in the whole hippocampus of lamγ1 KO mice compared with control mice 6 h after KA injection (n = 7 per group; Fig. 2 B). These results demonstrate that KA-induced c-fos expression in the DG region of lamγ1 KO mice is initially identical to wild-type mice, but the induction fails to propagate to other regions, notably CA1. The failure of c-fos expression to spread in the lamγ1 KO mice could be related to the neuroprotection observed in the absence of laminin.
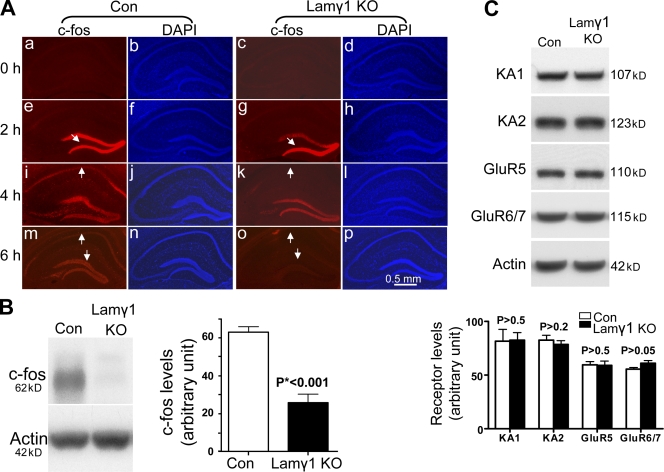
Figure 2.
KA-induced c-fos protein expression in the hippocampus of lamγ1 KO mice was impaired. (A) Time course of c-fos expression in the hippocampus of control (Con) and lamγ1 KO mice after KA injection. Under basal conditions, c-fos was not expressed in the hippocampus of either control (floxed lamγ1 mice) or lamγ1 KO mice (a and c). 2 h after KA injection, c-fos was similarly induced in the DG of either control or lamγ1 KO mice (e and g, arrows). 4 h after KA injection, c-fos was expressed in the CA1, CA3, and DG regions of control mice (i, arrow indicates c-fos expression in the CA1 region) but was absent in the CA1 region of lamγ1 KO mice (k, arrow). 6 h after KA injection, c-fos expression in the whole hippocampus of control mice was decreased compared with 4 h and had almost completely disappeared in lamγ1 KO (m and o, arrows). DAPI staining showed that the hippocampal neuronal layers at early time points after KA injection were similar between control and lamγ1 KO mice (b, d, f, h, j, l, n, and p). (B) Western blot analysis also showed that c-fos expression in the whole hippocampus of lamγ1 KO mice was much less than that of controls 6 h after KA injection. Quantitative analysis of Western blots of c-fos expression in control and lamγ1 KO mice after KA injection are shown on the right of the Western blot (n = 7 per genotype; Western blot signal intensities were normalized to actin). (C) Western blot analysis showed that under basal conditions, all five subunits of the KA receptor were expressed in the hippocampus of control and lamγ1 KO mice and that the expression levels were similar (signal intensities were normalized to actin). Error bars indicate SEM.
Because c-fos induction in the hippocampus of lamγ1 KO mice after KA injection was impaired, it was possible that the expression of KA receptors was affected. To investigate this possibility, we compared the expression of KA receptor subunits in the hippocampus of control and lamγ1 KO mice. There are five KA receptor subunits: KA1, KA2, GluR5, GluR6, and GluR7 (Lerma et al., 2001; Lerma, 2006). KA1 and KA2 subunits are high affinity receptors, whereas GluR5, GluR6, and GluR7 are low affinity receptors (London and Coyle, 1979; Unnerstall and Wamsley, 1983; Hampson et al., 1987). Western blot analysis revealed that all five subunits were expressed in the hippocampus of control and lamγ1 KO mice, and the expression levels of all five subunits were similar between these mice (Fig. 2 C).
Activation of intracellular death signaling pathways is impaired in lamγ1 KO mice
c-fos was not induced in the CA1 region of lamγ1 KO mice after KA injection, indicating that the excitotoxic resistance phenotype could be caused by a failure of intracellular death signaling pathway activation. The JNK intracellular signaling pathway plays an important role in mediating excitotoxic neuronal cell death (Yang et al., 1997; Saporito et al., 1998; Behrens et al., 1999; Wu et al., 2000; Savinainen et al., 2001; Borsello et al., 2003; Kuan et al., 2003; Zhang et al., 2006). Therefore, we tested whether activation of the JNK signaling pathway was affected in lamγ1 KO mice. Because JNK can be activated by MAPK kinase 4 (MKK4; Cuenda, 2000), we analyzed MKK4 phosphorylation levels in control and lamγ1 KO mice after KA injection. Phosphorylation of MKK4 was dramatically reduced in lamγ1 KO mice compared with control mice after KA injection (Fig. 3 A). Phosphorylated JNK was detected in control but not in lamγ1 KO mice (Fig. 3 B).
Figure 3.
Activation of intracellular death signaling pathways was impaired in lamγ1 KO mice. (A–C) The phosphorylation levels of MKK4 (A), JNK (B), and c-Jun (C) were significantly less in the hippocampus of lamγ1 KO mice compared with those of control (Con) mice 2 h after KA injection. Quantitative analyses are shown as bar graphs directly below the respective Western blots (n = 7 per genotype; signal intensities were normalized to actin). Error bars indicate SEM.
Another component of this pathway is c-Jun, which can be phosphorylated by activated JNK (Manning and Davis, 2003). After KA injection, phosphorylated c-Jun was reduced in lamγ1 KO mice compared with control mice (Fig. 3 C). These results demonstrate that activation of multiple components of the intracellular death signaling pathway was impaired in lamγ1 KO mice after KA injection.
KA specifically induces up-regulation of the KA1 subunit of the KA receptor in the hippocampus in control but not in lamγ1 KO mice
To analyze how KA injection affects the expression of KA receptors, we compared the expression levels of the five subunits between PBS- and KA-injected hippocampi. The KA1 subunit was dramatically increased after KA injection; however, all other subunits were unchanged (Fig. 4 A). These results demonstrate that all five subunits of the KA receptors were expressed in the mouse hippocampus, but after KA injection, only the KA1 subunit was significantly increased.
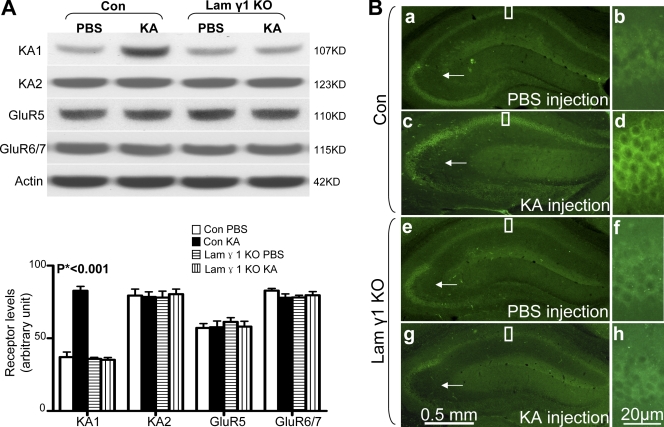
Figure 4.
KA specifically up-regulates the KA1 subunit of the KA receptor in the hippocampus in control but not in lamγ1 KO mice. (A) After KA injection, only the KA1 subunit was significantly higher in the hippocampus of control (Con) mice compared with PBS-injected control mice and lamγ1 KO mice injected with PBS or KA. All other subunits' expression levels remained similar between PBS- or KA-injected control and KO mice. Quantitative analyses were performed by one-way ANOVA and are shown as bar graphs (n = 7 per group for each experiment; Western blot signal intensities were normalized to actin). (B) Immunohistochemistry of KA1 subunit in PBS- or KA-injected control and lamγ1 KO mice. (a and b) In PBS-injected control mice, KA1 was expressed in the CA1 and CA3 regions but was not detected in the DG. (c and d) However, 2 h after KA injection, KA1 expression was dramatically increased in the CA1 region. KA1 expression in CA3 and mossy fiber pathway was slightly decreased (arrows), which may suggest early neuronal damage. (e–h) In lamγ1 KO mice, the expression levels of KA1 between PBS- and KA-injected mice were similar. Higher magnification of boxed areas in a, c, e, and g are shown in b, d, f, and h, respectively. Error bars indicate SEM. Bars: (a, c, e, and g) 0.5 mm; (b, d, f, and h) 20 μm.
To investigate how KA injection affects KA receptor expression in lamγ1 KO mice, we compared the expression levels of KA receptor subunits in lamγ1 KO mice after PBS or KA injection. KA1 subunit expression in the hippocampus of PBS- and KA-injected lamγ1 KO mice was similar and also similar to PBS-injected control mice, demonstrating a lack of KA1 subunit up-regulation by KA (Fig. 4 A). Analyses of other KA receptor subunit expression levels revealed that none are significantly changed in lamγ1 KO mice after KA injection (Fig. 4 A).
Because KA could also activate AMPA (α-amino-3-hydroxy-5-methyl-isoxazolepropionic acid) receptors (Arundine and Tymianski, 2003) and affect their expression, we compared AMPA receptor subunit expression between control and lamγ1 KO mice after KA injection. Our results show there were no significant differences in the expression levels of any AMPA receptor subunits (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200803107/DC1).
To determine which regions of the hippocampus and what cell types exhibit increased KA1 expression, we performed KA1 immunohistochemistry (Fig. 4 B). In PBS-injected control mice, KA1 immunoreactivity was detected in the CA1 and CA3 regions but not the DG, which agrees with a previous study (Darstein et al., 2003). KA1 immunoreactivity in CA3 was stronger than in the CA1 region (Fig. 4 B, a and b). However, after KA injection, KA1 immunoreactivity in the CA1 region was stronger than that of PBS-injected mice. In contrast to PBS-injected mice, the KA1 immunoreactivity in the CA1 region of KA-injected mice was stronger than in the CA3 region (Fig. 4 B, c and d). The cell types that exhibited increased KA1 expression in the CA1 region were pyramidal neurons (unpublished data). To confirm the specificity of the KA1 antibodies used for immunohistochemistry, we used a KA1 antibody–blocking peptide. Our results showed that the blocking peptide completely eliminated the KA1 immunoreactivity in the hippocampus (Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200803107/DC1). These results demonstrate that after KA injection, the KA1 subunit is up-regulated mainly in the pyramidal neurons of the CA1 region.
We compared KA1 expression patterns between control and lamγ1 KO mice after KA injection by immunohistochemistry. The KA1 expression pattern between PBS-injected control and lamγ1 KO mice was similar (Fig. 4 B, a, b, e, and f). However, 2 h after KA injection, the pattern between these mice was different; in KA-injected mice, KA1 was increased in the CA1 region of controls but not in lamγ1 KO mice (Fig. 4 B, g and h). In the CA3 region of both control and lamγ1 KO mice, KA1 expression was slightly decreased (Fig. 4 B, c and g [arrows] compared with a and e). All of these experiments indicate that KA-induced KA1 subunit up-regulation was impaired in lamγ1 KO mice.
KA-induced up-regulation of KA1 receptor expression in the hippocampus of tPA KO mice is also impaired
Previous studies showed that the tPA/plasmin proteolytic cascade is involved in laminin degradation and neuronal death (Tsirka et al., 1995, 1997; Chen and Strickland, 1997). To determine whether KA1 is up-regulated in tPA KO mice after KA injection, we analyzed KA1 expression in these mice under basal conditions and after KA injection. Under basal conditions, the expression of KA1 was similar in the hippocampus of control and tPA KO mice (Fig. 5). However, after KA injection, KA1 levels were up-regulated in the hippocampus of control mice but not in that of tPA KO mice. The KA1 expression levels in tPA KO mice after KA injection were similar to PBS-injected tPA KO mice or PBS-injected control animals and were also similar to the basal expression levels (Fig. 5). These results indicate that KA-induced up-regulation of the KA1 subunit is impaired in both lamγ1 KO and tPA KO mice, suggesting that tPA/plasmin/laminin molecules form a network that participates in excitotoxicity by regulating KA1 subunit expression.
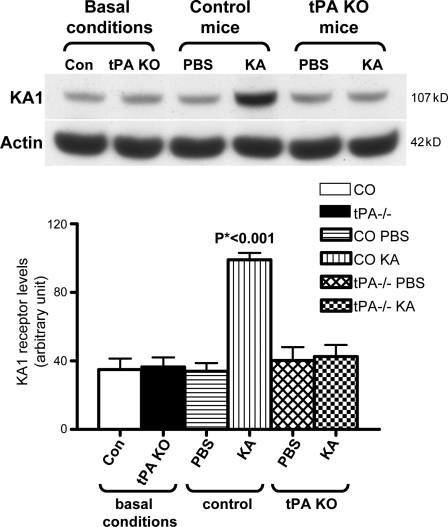
Figure 5.
KA-induced up-regulation of KA1 subunit expression in the hippocampus of tPA KO mice was also impaired. Western blot analyses showed that KA1 subunit expression in the hippocampi of control and tPA KO mice was similar under basal conditions. However, 2 h after KA injection, the expression levels of KA1 were significantly higher in the hippocampi of control mice compared with tPA KO mice injected with KA or PBS or control mice injected with PBS. Quantitative analysis from Western blots was performed by one-way ANOVA and is shown below the panel (n = 7 per group; Western blot signal intensities were normalized to actin). Con and CO, control. Error bars indicate SEM.
Plasmin-digested laminin restores KA1 subunit expression after KA injection in both tPA and lamγ1 KO mice, whereas intact laminin only restores KA1 subunit expression in lamγ1 KO mice but not tPA KO mice
tPA, plasminogen, and lamγ1 KO mice are all resistant to excitotoxic neuronal degeneration (Tsirka et al., 1995, 1997), and the KA1 subunit is not up-regulated after KA injection in either tPA KO or lamγ1 KO mice. An explanation for this observation could be that plasmin-catalyzed degradation of laminin generates fragments that cooperate with KA to stimulate KA1 expression. To test this hypothesis, we infused plasmin-digested laminin-1 into lamγ1 KO mice (plasmin was heat inactivated before infusion). 6 d later, either KA or PBS (for control) was injected. Infusion of plasmin-digested laminin followed by the injection of PBS had no effect on KA1 levels (Fig. 6), indicating that this material alone does not stimulate KA1 subunit expression. However, 2 h after KA injection, the KA1 levels in plasmin-digested laminin-infused mice were significantly higher than control buffer–infused mice (Fig. 6). Similar results were obtained when the same experiment was performed in tPA KO mice; after KA injection, the KA1 levels in the plasmin-digested laminin-infused mice were significantly higher than control buffer–infused mice (Fig. 6).
Figure 6.
Infusion of plasmin-digested laminin restores KA1 subunit up-regulation in both tPA and lamγ1 KO mice after KA injection, whereas infusion of intact laminin only restores KA1 levels in lamγ1 but not tPA KO mice. Lamγ1 or tPA KO mice were infused with plasmin-digested laminin, control buffer, intact laminin, or PBS for 6 d. Mice were intrahippocampally injected with PBS or KA. Infusion of plasmin-digested laminin up-regulated KA1 in both tPA and lamγ1 KO mice after KA injection, but infusion of intact laminin only up-regulated KA1 expression in lamγ1 KO mice, not tPA KO mice, after KA injection. In all other treatments, KA1 expression levels are similar. Quantitative analysis of Western blots was performed by one-way ANOVA and is shown as a bar graph (n = 7 per genotype; Western blot signal intensities were normalized to actin). Buf, buffer; Ln-P, plasmin-digested laminin-1; Ln, laminin-1. Error bars indicate SEM.
We further investigated whether this effect is dependent on degradation of laminin by comparing intact laminin and plasmin-digested laminin intrahippocampally infused into lamγ1 KO or tPA KO mice. Infusion of intact laminin into lamγ1 KO mice also up-regulated KA1 expression after KA injection (Fig. 6) because these mice have an intact tPA/plasmin system and can degrade the intact laminin. In contrast, intact laminin had no significant effect on KA1 expression in tPA KO mice because in the absence of tPA, this infused material cannot be degraded into the active degradation products (Fig. 6). We also compared KA1 expression levels between plasmin-digested fibronectin-infused mice and plasmin-digested laminin- or control buffer–infused mice. Our results showed that plasmin-digested fibronectin did not up-regulate the KA1 expression level (Fig. S3 C). These results indicate that digestion of laminin by plasmin generated fragments that can preferentially up-regulate KA1 subunit expression after KA exposure.
Plasmin-digested laminin restores neuronal sensitivity to excitotoxicity in both lamγ1 and tPA KO mice
Because infusion of plasmin-digested laminin restored KA1 up-regulation in both lamγ1 and tPA KO mice after KA injection, we further investigated whether restoration of KA1 subunit up-regulation in these KO mice is sufficient to rescue neuronal sensitivity to KA-induced neuronal death. After infusion, the lamγ1 KO mice were injected with KA. Mice infused with control buffer showed very little neuronal death 2 d after KA injection (Fig. 7, A and B). However, plasmin-digested laminin-infused mice exhibited much more neuronal loss than control buffer–infused mice (Fig. 7, C and D). The difference in the number of dead neurons between buffer-infused and plasmin-digested laminin-infused lamγ1 KO mice was significant (Fig. 7 G). The numbers of dead neurons in plasmin-digested laminin-infused lamγ1 KO were similar to control mice (Fig. 7, C–G). Furthermore, infusion of intact laminin in lamγ1 KO mice also restored neuronal sensitivity to excitotoxins (unpublished data). These results demonstrate that infusion of either intact or plasmin-digested laminin into the hippocampus of lamγ1 KO mice restores KA1 subunit up-regulation and neuronal sensitivity to excitotoxins.
Figure 7.
Infusion of plasmin-digested laminin into lamγ1 KO mice restores neuronal sensitivity to excitotoxins. (A–D) Lamγ1 KO mice were infused with control buffer (A and B) or plasmin-digested laminin (C and D) for 5 d. Control mice were infused with buffer for 5 d and intrahippocampally injected with KA. Silver staining shows that lamγ1 KO mice infused with plasmin-digested laminin (C and D) exhibited much more neuronal death than control buffer–infused mice (A and B), which is comparable with control (Con) mice (E and F). A higher magnification of the boxed areas in A, C, and E are shown in B, D, and F, respectively. (G) Bar graph shows the quantitative result of silver-positive cells in these three groups (n = 7 per group). Ln, laminin-1. Error bars indicate SEM. Bars: (A, C, and E) 0.5 mm; (B, D, and F) 25 μm.
We have previously reported that infusion of plasmin-digested laminin into the hippocampus of tPA KO mice can influence KA-induced neuronal death (Chen et al., 2003). We have reinvestigated this result in light of the aforementioned experiments. In tPA KO mice infused with control buffer, there was very little neuronal death 2 d after KA injection (Fig. S4, A and B, available at http://www.jcb.org/cgi/content/full/jcb.200803107/DC1). However, tPA KO mice infused with plasmin-digested laminin exhibited much more neuronal loss than animals infused with control buffer (Fig. S4, C and D) and were comparable with control mice (Fig. S4, C–G). The difference in the number of dead neurons between plasmin-digested laminin-infused and buffer-infused tPA KO mice was significant (Fig. S4 G). Because infusion of intact laminin into the hippocampus of tPA KO mice did not up-regulate KA1 expression (Fig. 6), we analyzed whether intact laminin could restore neuronal sensitivity to excitotoxins in tPA KO mice. In contrast to our previous findings (Chen et al., 2003), this intact laminin did not rescue KA-induced neurodegeneration in tPA KO mice (unpublished data). One possible explanation for the difference between our previous and present results is in the batch of laminin used. If our previous batch of intact laminin (no longer available) had some degradation, it could have facilitated KA-induced neuronal death in tPA KO mice. These results demonstrate that only infusion of plasmin-digested laminin into the hippocampus of tPA KO mice restores neuronal sensitivity to excitotoxins.
Interfering with KA1 function in control mice desensitizes neurons to KA-induced neuronal cell death in vitro and in vivo
To investigate the relationship between KA1 subunit up-regulation and excitotoxic neuronal death, we compared the time course of KA-induced KA1 up-regulation and KA-induced neuronal death (Andersson et al., 1991) using Fluoro Jade B (FJB) staining to label degenerating cells (Schmued and Hopkins, 2000). A time course of KA1 expression after KA injection showed that KA1 increased at 1 h, peaked at 2 h, returned to normal levels at 6 h, and then decreased (Fig. S5 A, available at http://www.jcb.org/cgi/content/full/jcb.200803107/DC1), presumably as a result of neuronal loss. A time-course analysis of KA-induced neuronal cell death showed that neuronal loss was first detected 6 h after KA injection and gradually increased over the next 2 d (Fig. S5 B). The CA1 region showed the greatest neuronal death. Because KA1 expression is up-regulated in a 1–4-h time frame and is mostly increased in CA1 after KA injection, whereas neuronal death begins at 6 h and is most obvious in the CA1 region, these results demonstrate that KA1 up-regulation precedes neuronal death and is spatially correlated to the regions that show the most profound degeneration.
The aforementioned experiments established a correlation between KA1 up-regulation and excitotoxic neuronal death. To further investigate this correlation, we investigated the effect of interfering with KA1 function on KA-induced neuronal cell death. We screened five different anti-KA1 antibodies for their potential binding capacity to KA1 subunit by immunohistochemistry using fresh frozen brain sections. Among the five antibodies, three showed staining in the hippocampal CA1 and CA3 regions. We analyzed the effect of these three anti-KA1 antibodies on KA-induced neuronal death in vitro. One of the antibodies, which was raised against a domain of the extracellular region of KA1 subunit, exhibited a significant neuronal protective effect (see following paragraph; Fig. 8). The other two showed no significant effect (unpublished data).
Figure 8.
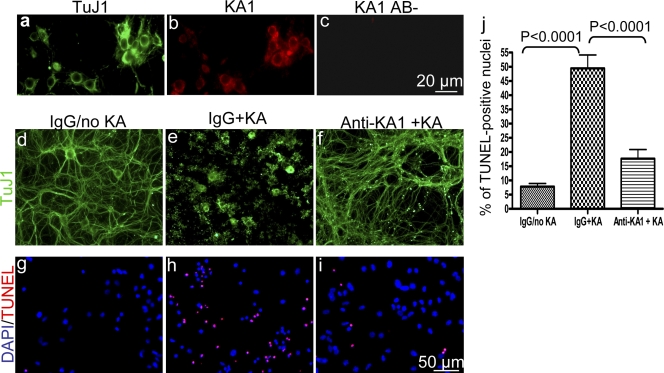
Antibody blockage of the KA1 subunit protects neurons from KA-induced death in vitro. (a and b) Primary cortical neuronal cultures were double stained with anti-TuJ1 (a) and anti-KA1 antibody (b). KA1 is expressed in the TuJ1-positive neurons in vitro. (c) In the absence of the primary antibody there was no staining. 14-d in vitro cortical neuronal cultures were incubated with rabbit IgG or anti-KA1 antibody for 3 h, and 50 μM KA was added to the culture media. Fresh rabbit IgG or anti-KA1 antibody was readded to the cultures 24 h later. 48 h after KA treatment, the cultures were stained for TuJ1 (d–f) or TUNEL (g–i). KA induced dramatic neuronal morphological changes (e) and apoptosis (h) compared with IgG treatment alone (d and g). In the anti-KA1 antibody–treated cultures, neuronal morphology was preserved (f), and there were many fewer apoptotic cells (i) than in IgG-treated cultures after KA (e and h). (j) Quantitative analyses showed that the numbers of KA-induced TUNEL-positive nuclei in the anti-KA1 antibody–treated cultures were significantly fewer than in the IgG-treated cultures (n = 45 for each group). Error bars indicate SEM.
Western blot analysis using this antibody revealed that it is specific and stained a single band corresponding to the size of the KA1 subunit (Fig. S3 B). Immunocytochemistry analysis revealed that the KA1 subunit is expressed in neurons in vitro (Fig. 8, a and b). Therefore, neuronal cultures were treated with control IgG or anti-KA1 antibody 3 h before KA treatment, and 24 h later, fresh IgG or anti-KA1 antibody was readded. 2 d after KA treatment, neuronal morphological changes were analyzed by neuronal class III β-tubulin (TuJ1) immunocytochemistry, and cell death was analyzed by TUNEL assay. In IgG-treated cultures, KA induced the degradation of TuJ1-positive cells as revealed by immunocytochemistry (Fig. 8, compare d with e). However, in anti-KA1 antibody–treated neuronal cultures, KA caused only slight neuronal morphological changes, and most neurites maintained their integrity (Fig. 8, compare d–f). To quantify the neuronal protective effect of the anti-KA1 antibody, we compared the percentage of TUNEL-positive nuclei with control IgG and anti-KA1 antibody–treated cultures after KA. In anti-KA1 antibody–treated cultures, KA induced dramatically less neuronal death than control IgG–treated neurons (Fig. 8, g–j). These experiments demonstrate that interfering with KA1 function protects against KA-induced neuronal death in vitro.
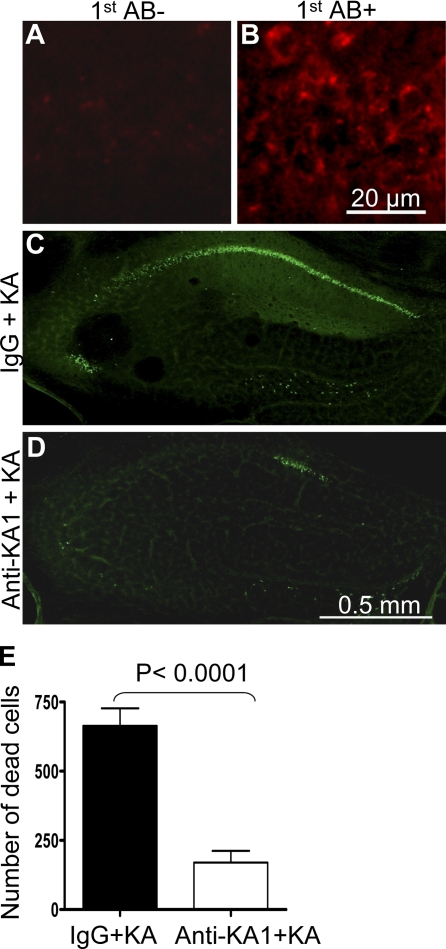
To investigate the in vivo neuronal protective effect of the anti-KA1 antibody that showed neuronal protective effect in vitro, we infused the antibody into the hippocampus of control mice and injected KA. As shown in Fig. 9, this antibody binds to fresh brain sections (Fig. 9, A and B). The mice infused with the anti-KA1 antibody showed significantly less neuronal cell death after KA injection compared with control IgG–infused mice (Fig. 9, C–E). This experiment demonstrates that the KA1 subunit of the KA receptor plays a critical role in excitotoxic neuronal death in vivo.
Figure 9.
KA1 antibodies bind to hippocampal neurons and inhibit KA-induced neuronal degeneration. (A) A control without primary antibody showed no staining. (B) The anti-KA1 antibody stained fresh frozen brain sections. (C and D) Mice were infused with control IgG or anti-KA1 antibody for 5 d, injected with KA, and killed 2 d later. FJB staining showed that infusion of anti-KA1 antibodies dramatically inhibited KA-induced neuronal death. (E) Quantitative results of FJB-positive cells are shown as bar graphs (n = 7 per group). Error bars indicate SEM.
Discussion
Excitotoxicity is a primary cause of neuronal loss in many neurological disorders. Many facets of this cell death pathway are understood. For example, in conditions such as ischemic stroke, loss of blood flow leads to energy depletion in neurons and accumulation of excitatory amino acids like glutamate. The excess glutamate can depolarize the neurons, leading to an increased release of glutamate and the establishment of a deleterious cycle. This massive depolarization causes Ca2+ influx and activation of cell death pathways (Kemp and McKernan, 2002; Hara and Snyder, 2007).
Although a general understanding of the process is known, many molecular details are unclear. In the work reported in this study, we shed light on two fundamental features of the cell death pathway: the critical role of the KA1 receptor and the role of proteases and the ECM.
KA receptors are one of the three subtypes of ionotropic receptors for the excitatory transmitter glutamate (Dingledine et al., 1999). The other two subtypes are N-methyl-d-aspartate and AMPA receptors (Mayer and Westbrook, 1987). KA receptors are tetrameric combinations of five subunits: KA1, KA2, GluR5, GluR6, and GluR7 (Hollmann and Heinemann, 1994; Lerma, 2006). Among these subunits, GluR5, GluR6, and GluR7 have low affinity KA-binding sites, whereas KA1 and KA2 have high affinity binding sites (London and Coyle, 1979; Unnerstall and Wamsley, 1983; Hampson et al., 1987). Therefore, high affinity receptors are heteromeric assemblies that include the KA1 or KA2 subunits, whereas receptors lacking KA1 or KA2 have lower affinity (Contractor et al., 2003). GluR5–7 subunits can form functional homomeric or heteromeric receptors, whereas KA1 and KA2 subunits only participate in heteromeric receptors and can partner with any of the GluR5–7 subunits (Egebjerg et al., 1991; Werner et al., 1991; Herb et al., 1992; Sommer et al., 1992; Schiffer et al., 1997; Huettner, 2003; Lerma, 2003, 2006). However, the exact combinations of subunits of the KA receptor in different cell types and brain regions are not clear (Huettner, 2003; Lerma, 2003, 2006).
In the hippocampus, KA1 mRNA localization is detected only in the CA3 region and DG (Bahn et al., 1994; Kask et al., 2000). However, studies using immunohistochemistry showed that the protein is present in the CA1 pyramidal neuronal cell layers, stratum lucidum of CA3, and the polymorphic layer of DG under basal conditions (Fogarty et al., 2000; Darstein et al., 2003). Although studies using gene KO techniques have shed light on functions of KA2, GluR5, GluR6, and GluR7 subunits (Mulle et al., 1998, 2000; Contractor et al., 2003; Pinheiro et al., 2007), the exact physiological and pathological functions of the KA1 subunit are not known (Huettner, 2003; Lerma, 2003, 2006).
Our results demonstrated that KA1 and all other KA subunits are expressed in adult mouse hippocampi as determined by Western blotting. However, after KA injection, only KA1 was significantly increased in wild-type mice. Further analysis using immunohistochemistry revealed that KA1 was mainly up-regulated in the CA1 pyramidal neuronal cell layers. Because the KA1 subunit contains a high affinity agonist-binding site, increased expression may increase the binding of the receptors to KA and therefore sensitize neurons to excitotoxic neuronal death. In keeping with this idea, KA1 was not up-regulated in mice resistant to neuronal loss (see below). These experiments established a correlation between KA1 up-regulation and neuronal death after KA injection.
Mice deficient in tPA or plasminogen are resistant to excitotoxic neuronal death (Tsirka et al., 1995, 1997). Analysis of these mice implicated the degradation of laminin as a component of the death pathway. A likely explanation was that loss of laminin resulted in loss of matrix interaction and cell death termed anoikis (Chen and Strickland, 1997; Gilmore, 2005; Reddig and Juliano, 2005). If this was the mechanism, mice lacking laminin in the hippocampus should be sensitized to neuronal degeneration.
However, mice deficient in laminin in the hippocampus were resistant to neuronal loss. In tPA or lamγ1 KO mice, the KA1 subunit was not significantly increased after KA injection. One mechanism that would explain the previous results with tPA/plasmin-deficient mice and the new results with hippocampal lamγ1 KO mice was that laminin degradation released products that contributed to neuronal death (Chen and Strickland, 1997). Our results indicate that laminin degradation by plasmin generates biologically active fragments that promote neuronal death. In this model, loss of tPA or plasmin would protect neurons because laminin would not be degraded. Laminin deficiency would also be protective because even in the presence of proteases, laminin degradation products would not be generated. Our results support this hypothesis. Infusion of laminin degradation products restores neuronal sensitivity to either lamγ1 or tPA KO mice.
Various studies have shown that tPA is rapidly released (within 30 min) upon membrane depolarization (Gualandris et al., 1996; Parmer et al., 1997; Baranes et al., 1998). Laminin is degraded in 1–2 h after KA injection (Chen and Strickland, 1997). The KA1 subunit of the KA receptor is up-regulated during the same time frame (1–2 h) in control mice (Fig. S5 A) but is not increased in either tPA or lamγ1 KO mice (Figs. 4 and 5). Because KA1 is predominantly up-regulated in the CA1 region of control mice after KA injection (Fig. 4), the lack of KA1 up-regulation in the CA1 region of lamγ1 KO mice impaired c-fos induction in CA1 4 h after KA injection and the spread and maintenance of c-fos expression in other regions of the hippocampus at later time points (Fig. 2 A). The timing of these molecular changes supports our hypothesis that plasmin-mediated degradation of laminin up-regulates the KA1 subunit, which is necessary for c-fos induction and neuronal cell death in the CA1 region of the hippocampus.
Infusion of plasmin-digested laminin into the hippocampus of either tPA or lamγ1 KO mice restored KA1 up-regulation after KA injection. However, infusion of intact laminin only restored KA1 up-regulation in lamγ1 KO mice but not in tPA KO mice (Fig. 6). Lamγ1 KO mice would still generate plasmin activity after KA, which could degrade the infused intact laminin. In contrast, the infused intact laminin could not be degraded in tPA KO mice because there is no tPA to activate plasminogen, and thus KA1 was not up-regulated. Therefore, the degradation products of laminin generated by plasmin play an important role in KA1 up-regulation. These experiments showed that proteolytic fragments of laminin can regulate KA1 subunit expression and therefore modulate excitotoxic neuronal degeneration. However, the mechanism by which laminin fragments regulate KA1 subunit expression is currently unclear. For example, laminin fragments could bind to as yet unidentified receptors and activate intracellular signaling pathway to regulate KA1 subunit expression. Laminin fragments could regulate KA1 expression at transcriptional, translational, or posttranslational levels.
Based on previous work (Tsirka et al., 1995, 1997; Chen and Strickland, 1997; Nagai et al., 1999; Chen et al., 2003) and our current results, we propose a new model of excitotoxic neuronal death. In the presence of excess excitatory amino acid, tPA is released from neurons and converts plasminogen to plasmin. Plasmin catalyzes the degradation of laminin, generating laminin fragments that up-regulate the KA1 subunit of KA receptors. Up-regulation of KA1 could bias the subunit composition of KA receptors so that more KA1 subunits participate in channel formation. Alternatively, increased expression of KA1 subunits could increase the number of KA receptors, which would be composed of one KA1 subunit with other subunits. Either one of these changes would lead to an increased ratio of KA1 subunits. Because the KA1 subunit is a high affinity site for KA, this occurrence would increase the affinity of the receptors to KA, therefore promoting excitotoxic neuronal death.
The up-regulation of the KA1 subunit mainly occurred in the CA1 region of the hippocampus, which normally exhibits low KA1 expression. Therefore, KA-induced excitotoxic neuronal death in CA1 may represent an effect of KA that is secondary to the up-regulation of KA1 subunits. In support of this idea, interfering with KA1 function by anti-KA1 antibodies dramatically decreased neuronal loss after KA injection. Given the location of KA1 up-regulation, it is possible that our model is most relevant to those pathologies in which neuronal death occurs mostly in the CA1 region.
Our results have implications for the mechanisms by which the ECM controls cell viability. It has been shown that the loss of the matrix attachment can lead to cell death via anoikis (Meredith et al., 1993; Frisch and Ruoslahti, 1997; Frisch and Screaton, 2001; Grossmann, 2002). It is possible that in some cases, it is not simply the loss of attachment but the generation of toxic degradation products from the matrix that contribute to cell death. This idea is similar in concept to a recent study showing that fragments of collagen can have antiangiogenesis effects (Marneros and Olsen, 2005).
Finally, there is no effective therapy for the neuronal death that occurs in ischemia. General glutamate receptor antagonists can protect against excitotoxic neuronal death, but such antagonists have dramatically deleterious effects on brain activity (e.g., impaired memory, decreased alertness, and coma). These side effects prevent their use in patients (Kemp and McKernan, 2002; Lipton, 2006). Demonstration that the single subunit KA1 is important for neuronal death suggests a more specific therapeutic approach that might protect neurons while leaving most brain activity unaffected.
Materials and methods
Animals
Adult male mice (8–10 wk of age) backcrossed to C57BL6 for 10 generations were used. Lamγ1 KO mice were homozygous for the floxed lamγ1 allele and also carried the Cre recombinase transgene under the control of the CaMKII promoter (Chen and Strickland, 2003). Littermates homozygous for the floxed lamγ1 allele without the Cre recombinase transgene were used as controls. tPA KO mice on a C57BL6 background were obtained from The Jackson Laboratory.
Intrahippocampal infusion and KA injection
Intrahippocampal reagent infusions were performed as described previously (Chen and Strickland, 1997; Chen et al., 2003). In brief, the mice were injected intraperitoneally with atropine (0.6 mg/kilogram of body weight) and were anesthetized with 2.5% avertin (0.02 ml/gram of body weight). They were placed on a stereotaxic apparatus (Stoelting). A microosmotic pump (Alza) containing 100 μl purified mouse laminin-1 (0.5 mg/ml; Sigma-Aldrich), 100 μl plasmin-digested laminin-1 (0.5 mg/ml; proteases were heat inactivated after digestion), 100 μl anti-KA1 antibody (80 μg/ml; Santa Cruz Biotechnology, Inc.), 100 μl rabbit IgG (80 μg/ml; control for KA1 antibody; Sigma-Aldrich), 100 μl PBS (control for laminin infusion), or 100 μl of control buffers used for laminin digestion was placed subcutaneously in the back of the animals. A brain infusion cannula was inserted in the brain (at coordinates bregma, −2.5 mm; medial lateral, 0.5 mm; and dorsoventral, 1.6 mm), and was connected to a pump to deliver the compound into the hippocampus. The infusion rate was 0.5 μl/h. The pumps were allowed to infuse the designated solution for 7 d.
For KA or PBS injection into the CA1 region, a total volume of 300 nl PBS or 0.5 mM KA (Tocris Cookson) for a dose of 0.15 nmol was delivered unilaterally using a microinjection apparatus (Stoelting) via a 2.5-μl Hamilton syringe equipped with a 33-gauge needle over the course of 60 s. After retracting the needle 0.1 mm, the needle was kept in place for 2 min to allow diffusion of KA, after which it was completely removed. The coordinates for the injections were bregma, −2.5 mm; medial lateral, 1.7 mm; and dorsoventral, 1.6 mm. The mice were perfused through the heart with ice-cold saline (for Western blotting) or further perfused with 4% PFA in 0.1 M phosphate buffer, pH 7.4 (for immunohistochemistry), for either 2 h , 4 h, 6 h, 2 d, or 6 d after experimental injections. For Western blot analyses, the hippocampi were collected in dry ice. For immunohistochemistry, the brains were removed, postfixed overnight at 4°C, and incubated in 30% sucrose in PBS for 48 h at 4°C. 30-μm coronal brain sections were cut on a microtome, collected in PBS, and processed for further analysis.
Immunohistochemistry
Immunohistochemistry was performed as previously described (Chen et al., 1999). In brief, brain sections were washed in PBS and blocked in 0.3% Triton X-100 and 5% proper normal serum in PBS. The primary antibodies used were rat anti-lamγ1 (1:50; Millipore), rabbit anti–c-fos (1:500; EMD), and goat anti-KA1 receptor (1:100; Santa Cruz Biotechnology, Inc.). The sections were incubated overnight with primary antibody in 0.3% Triton X-100 and 3% normal serum in PBS at 4°C. After rinsing in PBS, the sections were incubated with appropriate secondary antibodies for 2 h and examined under a fluorescence microscope.
To screen the potential binding capacity of five different anti-KA1 antibodies (obtained from Millipore, Abcam, and Santa Cruz Biotechnology, Inc.), we used fresh frozen brain sections for immunohistochemistry. Brain sections were briefly fixed in 4% PFA, washed, and incubated with anti-KA1 antibodies. The binding of anti-KA1 antibodies to brain sections was visualized using appropriate fluorescent dye–conjugated secondary antibodies. Three anti-KA1 antibodies (Santa Cruz Biotechnology, Inc.) showed positive staining. These three antibodies were used for in vitro analyses for their functional blocking effects.
FJB and silver staining
FJB staining was performed as described previously (Schmued and Hopkins, 2000; Chen et al., 2003). Fixed free-floating brain sections were mounted onto slides, dried at 55°C for 2 h, and stained with 0.0004% FJB (Histo-Chem) for 20 min. Staining was visualized using a fluorescein isothiocyanate filter on a microscope (Axiovert 200; Carl Zeiss, Inc.).
The NeuroSilver kit I (FD NeuroTechnologies, Inc.) was used for silver staining according to the manufacturer's instructions. After staining, the sections were mounted onto slides, dehydrated, coverslipped with DPX (1,3-diethyl-8-phenylxanthine; Sigma-Aldrich), and examined under a microscope (Axiovert 200; Carl Zeiss, Inc.). For quantitative analyses, silver-stained or FJB-positive cells were counted using a profile counting method under a 100× Plan Apochromat oil lens (Carl Zeiss, Inc.; Guillery and Herrup, 1997). Five matched sections containing the hippocampus from each animal were counted (each section was 180 μm apart, so dead cells were only counted once). The total numbers of dead cells from all five sections were counted for each animal, and statistical analyses were based on seven animals in each group. The boundary between CA1 and CA2/3 is according to the shape of the hippocampus that is distinguishable under a microscope. The differences between control and mutant mice were analyzed by Student's t test. For GFP visualization of the mice carrying the reporter transgene, PFA-fixed brain sections were directly examined under a fluorescence microscope (Axiovert 200; Carl Zeiss, Inc.).
Western blot analysis
After treatment, the hippocampi were dissected and processed for Western blot analysis as described previously (Yu et al., 2005). In brief, hippocampi were homogenized in lysis buffer containing 25 mM Tris, pH 7.4, 95 mM NaCl, 2% SDS, 10 mM EDTA, phosphatase inhibitor cocktail I and II, and protease inhibitor cocktail (Sigma-Aldrich). Protein concentrations were determined by the Lowry method (Bio-Rad Laboratories). 30–50 μg of proteins was fractionated on 10% SDS-PAGE, blotted onto polyvinylidene difluoride membrane (Millipore), and probed with primary antibodies. Primary antibodies were KA1 (1:2,000) and KA2 (1:1,000) obtained from Millipore, KA1 (1:1,000) and c-fos (1:1,500) obtained from Santa Cruz Biotechnology, Inc., and GluR5 (1:2,000), GluR6, GluR7 (1:1,000), phospho–c-Jun (1:1,000), phospho-JNK (1:2,000), and phospho-SEK1/MKK4 (1:1,000) obtained from Cell Signaling Technology. After incubation with appropriate secondary antibodies (GE Healthcare), proteins were visualized by chemiluminescence according to the manufacturer's instructions (Thermo Fisher Scientific). Seven control and mutant hippocampi were used for each Western blot, and samples were blotted three times. For loading controls, each membrane was reprobed with anti–β-actin antibody (1:8,000; Sigma-Aldrich). The signal intensity of the Western blot film was quantified by ImageJ (National Institutes of Health). All signal intensities were normalized to actin. The differences in signal intensity between control and mutant samples or between differently treated mice for each Western blot were analyzed by either Student's t test or one-way analysis of variance (ANOVA) as indicated in the figure legends.
Cortical neuronal culture and KA treatment
Embryonic day 18.5 wild-type embryos were collected from pregnant female mice and decapitated, and their brains were dissected in Hank's balanced salt solution using sterile techniques. Cortical and hippocampal tissues from each embryo were combined and dissociated in 0.25% trypsin, triturated using a burn polished pipette, spun briefly, resuspended in plating medium (2 mM glutamine, 1× B27, 10% FBS, and NeuroBasal media), and seeded in poly-d-lysine–coated coverslips placed in12-well plates in a density of 640 cells/mm2. 4 h later, the plating medium was replaced with growth medium (plating medium without FBS). Cultures were maintained in a humidified incubator at 37°C with a 5% CO2 atmosphere. The medium was changed every 3–4 d by half volume. Using this protocol, the cultured cells were 99% neuronal (Brewer et al., 1993; unpublished data).
To determine the effect of anti-KA1 antibodies on KA-induced neuronal death in vitro, 14-d in vitro neuronal cultures were washed with NeuroBasal media three times to remove dead cells. The neuronal cultures were incubated in fresh growth media overnight, and control IgG or anti-KA1 antibodies were added to the media and incubated for 3 h. 50 μM KA was added to the media and incubated for 48 h. Additional control IgG or anti-KA1 antibodies were added to the cultures 24 h after the first addition. Neuronal cultures were fixed in 4% PFA and processed for TuJ1 immunocytochemistry and terminal deoxynucleotidyl transferase–mediated biotinylated UTP nick-end labeling assay (TUNEL staining). TUNEL staining was performed using the In Situ Cell Death Detection kit (Roche) according to the instructions of the manufacturer. Neuronal cultures were permeabilized in 0.1% Triton X-100/0.1% sodium citrate on ice for 2 min and incubated with the TUNEL reaction mixture at 37°C for 1 h in a humidified chamber. After TUNEL staining, the cultures were incubated with mouse anti-TuJ1 antibody (1:1,000; Covance), and the staining was visualized using fluorescent dye–conjugated donkey anti–mouse IgG. Nuclei were counterstained with DAPI. TUNEL and DAPI double-labeled nuclei were determined. Five random fields were analyzed in each culture well, and each experiment group was composed of three wells. The statistical analyses were based on results from three different batches of cultures. The differences in percentage of TUNEL-positive nuclei in different groups were analyzed by one-way ANOVA.
Image analysis
All images were acquired by using an AxioVision System (Carl Zeiss, Inc.) under a microscope (Axiovert 200; Carl Zeiss, Inc.) using a camera (Axiocam; Carl Zeiss, Inc.), and images were processed with Photoshop (Adobe).
Online supplemental material
Fig. S1 shows KA-induced neuronal death in the CA2/3 region of lamγ1 KO mice and the long-term neuronal protective effect of lamγ1 deficiency. Fig. S2 shows that AMPA receptor subunits are not changed in either control or lamγ1 KO mice after KA injection. Fig. S3 shows specificity of the anti-KA1 antibodies and the effect of plasmin-digested laminin on KA1 up-regulation. Fig. S4 shows that infusion of plasmin-digested laminin into tPA KO mice restores neuronal sensitivity to excitotoxicity. Fig. S5 shows that KA1 up-regulation precedes neuronal death after KA injection. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200803107/DC1.
Supplementary Material
Acknowledgments
We would like to thank Dr. Erin H. Norris and Ms. Emily R. Lowry for reading the manuscript and members of the Strickland laboratory for useful discussions.
This work was supported by grants from the National Institutes of Health (NS035704, NS050537, and AA014630 to S. Strickland), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (grant to S. Strickland), the Muscular Dystrophy Association (grant MDA4066 to Z.-L. Chen), the Marie Curie Excellence program of the European Commission (grant MEXT-CT-2006-042265 to R. Pawlak), and the Medical Research Council (grant RC G0500231/73852 to R. Pawlak).
Z.-L. Chen and H. Yu contributed equally to this paper.
Abbreviations used in this paper: ANOVA, analysis of variance; CaMKII, calcium/calmodulin-dependent protein kinase II α; DG, dentate gyrus; FJB, Fluoro Jade B; KA, kainate; KO, knockout; lamγ1, laminin γ1; MKK4, MAPK kinase 4; tPA, tissue plasminogen activator.
References
- Andersson, P.-B., V.H. Perry, and S. Gordon. 1991. The kinetics and morphological characteristics of the macrophage-microglial response to kainic acid-induced neuronal degeneration. Neuroscience. 42:201–214. [DOI] [PubMed] [Google Scholar]
- Arundine, M., and M. Tymianski. 2003. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 34:325–337. [DOI] [PubMed] [Google Scholar]
- Bahn, S., B. Volk, and W. Wisden. 1994. Kainate receptor gene expression in the developing rat brain. J. Neurosci. 14:5525–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranes, D., D. Lederfein, Y. Huan, M. Chen, C.H. Bailey, and E.R. Kandel. 1998. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 21:813–825. [DOI] [PubMed] [Google Scholar]
- Behrens, A., M. Sibilia, and E.F. Wagner. 1999. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 21:326–329. [DOI] [PubMed] [Google Scholar]
- Borsello, T., P.G. Clarke, L. Hirt, A. Vercelli, M. Repici, D.F. Schorderet, J. Bogousslavsky, and C. Bonny. 2003. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat. Med. 9:1180–1186. [DOI] [PubMed] [Google Scholar]
- Brewer, G.J., J.R. Torricelli, E.K. Evege, and P.J. Price. 1993. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35:567–576. [DOI] [PubMed] [Google Scholar]
- Chen, Z.-L., and S. Strickland. 1997. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 91:917–925. [DOI] [PubMed] [Google Scholar]
- Chen, Z.L., and S. Strickland. 2003. Laminin γ1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 163:889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z.-L., J.A. Indyk, T.H. Bugge, K.W. Kombrinck, J.L. Degen, and S. Strickland. 1999. Neuronal death and blood-brain barrier breakdown after excitotoxic injury are independent processes. J. Neurosci. 19:9813–9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z.L., J.A. Indyk, and S. Strickland. 2003. The hippocampal laminin matrix is dynamic and critical for neuronal survival. Mol. Biol. Cell. 14:2665–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor, A., A.W. Sailer, M. Darstein, C. Maron, J. Xu, G.T. Swanson, and S.F. Heinemann. 2003. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J. Neurosci. 23:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle, J.T., M.E. Molliver, and M.J. Kuhar. 1978. In situ injection of kainic acid: a new method for selectively lesioning neural cell bodies while sparing axons of passage. J. Comp. Neurol. 180:301–323. [DOI] [PubMed] [Google Scholar]
- Cuenda, A. 2000. Mitogen-activated protein kinase kinase 4 (MKK4). Int. J. Biochem. Cell Biol. 32:581–587. [DOI] [PubMed] [Google Scholar]
- Darstein, M., R.S. Petralia, G.T. Swanson, R.J. Wenthold, and S.F. Heinemann. 2003. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J. Neurosci. 23:8013–8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine, R., K. Borges, D. Bowie, and S.F. Traynelis. 1999. The glutamate receptor ion channels. Pharmacol. Rev. 51:7–61. [PubMed] [Google Scholar]
- Dragatsis, I., and S. Zeitlin. 2000. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 26:133–135. [DOI] [PubMed] [Google Scholar]
- Egebjerg, J., B. Bettler, I. Hermans-Borgmeyer, and S. Heinemann. 1991. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 351:745–748. [DOI] [PubMed] [Google Scholar]
- Fogarty, D.J., F. Perez-Cerda, and C. Matute. 2000. KA1-like kainate receptor subunit immunoreactivity in neurons and glia using a novel anti-peptide antibody. Brain Res. Mol. Brain Res. 81:164–176. [DOI] [PubMed] [Google Scholar]
- Frisch, S.M., and E. Ruoslahti. 1997. Integrins and anoikis. Curr. Opin. Cell Biol. 9:701–706. [DOI] [PubMed] [Google Scholar]
- Frisch, S.M., and R.A. Screaton. 2001. Anoikis mechanisms. Curr. Opin. Cell Biol. 13:555–562. [DOI] [PubMed] [Google Scholar]
- Gilmore, A.P. 2005. Anoikis. Cell Death Differ. 12(Suppl 2):1473–1477. [DOI] [PubMed] [Google Scholar]
- Grossmann, J. 2002. Molecular mechanisms of “detachment-induced apoptosis–Anoikis”. Apoptosis. 7:247–260. [DOI] [PubMed] [Google Scholar]
- Gualandris, A., T.E. Jones, S. Strickland, and S.E. Tsirka. 1996. Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J. Neurosci. 16:2220–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery, R.W., and K. Herrup. 1997. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J. Comp. Neurol. 386:2–7. [DOI] [PubMed] [Google Scholar]
- Hagg, T., D. Muir, E. Engvall, S. Varon, and M. Manthorpe. 1989. Laminin-like antigen in rat CNS neurons: distribution and changes upon brain injury and nerve growth factor treatment. Neuron. 3:721–732. [DOI] [PubMed] [Google Scholar]
- Hagg, T., C. Portera-Cailliau, M. Jucker, and E. Engvall. 1997. Laminins of the adult mammalian CNS; laminin-alpha2 (merosin M-) chain immunoreactivity is associated with neuronal processes. Brain Res. 764:17–27. [DOI] [PubMed] [Google Scholar]
- Hampson, D.R., D. Huie, and R.J. Wenthold. 1987. Solubilization of kainic acid binding sites from rat brain. J. Neurochem. 49:1209–1215. [DOI] [PubMed] [Google Scholar]
- Hara, M.R., and S.H. Snyder. 2007. Cell signaling and neuronal death. Annu. Rev. Pharmacol. Toxicol. 47:117–141. [DOI] [PubMed] [Google Scholar]
- Herb, A., N. Burnashev, P. Werner, B. Sakmann, W. Wisden, and P.H. Seeburg. 1992. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 8:775–785. [DOI] [PubMed] [Google Scholar]
- Hollmann, M., and S. Heinemann. 1994. Cloned glutamate receptors. Annu. Rev. Neurosci. 17:31–108. [DOI] [PubMed] [Google Scholar]
- Huettner, J.E. 2003. Kainate receptors and synaptic transmission. Prog. Neurobiol. 70:387–407. [DOI] [PubMed] [Google Scholar]
- Indyk, J.A., Z.-L. Chen, S.E. Tsirka, and S. Strickland. 2003. Laminin chain expression suggests that laminin-10 is a major isoform in the mouse hippocampus and is degraded by the tissue plasminogen activator/plasmin protease cascade during excitotoxic injury. Neurosci. 116:359–371. [DOI] [PubMed] [Google Scholar]
- Jucker, M., M. Tian, and D.K. Ingram. 1996. Laminins in the adult and aged brain. Mol. Chem. Neuropathol. 28:209–218. [DOI] [PubMed] [Google Scholar]
- Kask, K., J. Jerecic, D. Zamanillo, J. Wilbertz, R. Sprengel, and P.H. Seeburg. 2000. Developmental profile of kainate receptor subunit KA1 revealed by Cre expression in YAC transgenic mice. Brain Res. 876:55–61. [DOI] [PubMed] [Google Scholar]
- Kasof, G.M., A. Mandelzys, S.D. Maika, R.E. Hammer, T. Curran, and J.I. Morgan. 1995. Kainic acid-induced neuronal death is associated with DNA damage and a unique immediate-early gene response in c-fos-lacZ transgenic rats. J. Neurosci. 15:4238–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, J.A., and R.M. McKernan. 2002. NMDA receptor pathways as drug targets. Nat. Neurosci. 5(Suppl):1039–1042. [DOI] [PubMed] [Google Scholar]
- Kuan, C.Y., A.J. Whitmarsh, D.D. Yang, G. Liao, A.J. Schloemer, C. Dong, J. Bao, K.J. Banasiak, G.G. Haddad, R.A. Flavell, et al. 2003. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl. Acad. Sci. USA. 100:15184–15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal La Salle, G. 1988. Long-lasting and sequential increase of c-fos oncoprotein expression in kainic acid-induced status epilepticus. Neurosci. Lett. 88:127–130. [DOI] [PubMed] [Google Scholar]
- Lerea, L.S., and J.O. McNamara. 1993. Ionotropic glutamate receptor subtypes activate c-fos transcription by distinct calcium-requiring intracellular signaling pathways. Neuron. 10:31–41. [DOI] [PubMed] [Google Scholar]
- Lerma, J. 2003. Roles and rules of kainate receptors in synaptic transmission. Nat. Rev. Neurosci. 4:481–495. [DOI] [PubMed] [Google Scholar]
- Lerma, J. 2006. Kainate receptor physiology. Curr. Opin. Pharmacol. 6:89–97. [DOI] [PubMed] [Google Scholar]
- Lerma, J., A.V. Paternain, A. Rodriguez-Moreno, and J.C. Lopez-Garcia. 2001. Molecular physiology of kainate receptors. Physiol. Rev. 81:971–998. [DOI] [PubMed] [Google Scholar]
- Lipton, S.A. 2006. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat. Rev. Drug Discov. 5:160–170. [DOI] [PubMed] [Google Scholar]
- London, E.D., and J.T. Coyle. 1979. Specific binding of [3H]kainic acid to receptor sites in rat brain. Mol. Pharmacol. 15:492–505. [PubMed] [Google Scholar]
- Manning, A.M., and R.J. Davis. 2003. Targeting JNK for therapeutic benefit: from junk to gold? Nat. Rev. Drug Discov. 2:554–565. [DOI] [PubMed] [Google Scholar]
- Marneros, A.G., and B.R. Olsen. 2005. Physiological role of collagen XVIII and endostatin. FASEB J. 19:716–728. [DOI] [PubMed] [Google Scholar]
- Mayer, M.L., and G.L. Westbrook. 1987. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog. Neurobiol. 28:197–276. [DOI] [PubMed] [Google Scholar]
- Meredith, J.E. Jr., B. Fazeli, and M.A. Schwartz. 1993. The extracellular matrix as a cell survival factor. Mol. Biol. Cell. 4:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle, C., A. Sailer, I. Perez-Otano, H. Dickinson-Anson, P.E. Castillo, I. Bureau, C. Maron, F.H. Gage, J.R. Mann, B. Bettler, and S.F. Heinemann. 1998. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 392:601–605. [DOI] [PubMed] [Google Scholar]
- Mulle, C., A. Sailer, G.T. Swanson, C. Brana, S. O'Gorman, B. Bettler, and S.F. Heinemann. 2000. Subunit composition of kainate receptors in hippocampal interneurons. Neuron. 28:475–484. [DOI] [PubMed] [Google Scholar]
- Nagai, N., T. Urano, A. Endo, H. Takahashi, Y. Takada, and A. Takada. 1999. Neuronal degeneration and a decrease in laminin-like immunoreactivity is associated with elevated tissue-type plasminogen activator in the rat hippocampus after kainic acid injection. Neurosci. Res. 33:147–154. [DOI] [PubMed] [Google Scholar]
- Novak, A., C. Guo, W. Yang, A. Nagy, and C.G. Lobe. 2000. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 28:147–155. [PubMed] [Google Scholar]
- Parmer, R.J., M. Mahata, S. Mahata, M.T. Sebald, D.T. O'Connor, and L.A. Miles. 1997. Tissue plasminogen activator (t-PA) is targeted to the regulated secretory pathway. Catecholamine storage vesicles as a reservoir for the rapid release of t-PA. J. Biol. Chem. 272:1976–1982. [DOI] [PubMed] [Google Scholar]
- Pinheiro, P.S., D. Perrais, F. Coussen, J. Barhanin, B. Bettler, J.R. Mann, J.O. Malva, S.F. Heinemann, and C. Mulle. 2007. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. USA. 104:12181–12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddig, P.J., and R.L. Juliano. 2005. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 24:425–439. [DOI] [PubMed] [Google Scholar]
- Salles, F.J., and S. Strickland. 2002. Localization and regulation of the tissue plasminogen activator-plasmin system in the hippocampus. J. Neurosci. 22:2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito, M.S., E.R. Brown, S. Carswell, A.M. DiCamillo, M.S. Miller, C. Murakata, N.T. Neff, J.L. Vaught, and F.A. Haun. 1998. Preservation of cholinergic activity and prevention of neuron death by CEP-1347/KT-7515 following excitotoxic injury of the nucleus basalis magnocellularis. Neuroscience. 86:461–472. [DOI] [PubMed] [Google Scholar]
- Savinainen, A., E.P. Garcia, D. Dorow, J. Marshall, and Y.F. Liu. 2001. Kainate receptor activation induces mixed lineage kinase-mediated cellular signaling cascades via post-synaptic density protein 95. J. Biol. Chem. 276:11382–11386. [DOI] [PubMed] [Google Scholar]
- Schiffer, H.H., G.T. Swanson, and S.F. Heinemann. 1997. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 19:1141–1146. [DOI] [PubMed] [Google Scholar]
- Schmued, L.C., and K.J. Hopkins. 2000. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 874:123–130. [DOI] [PubMed] [Google Scholar]
- Sommer, B., N. Burnashev, T.A. Verdoorn, K. Keinanen, B. Sakmann, and P.H. Seeburg. 1992. A glutamate receptor channel with high affinity for domoate and kainate. EMBO J. 11:1651–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, M., T. Hagg, N. Denisova, B. Knusel, E. Engvall, and M. Jucker. 1997. Laminin-alpha2 chain-like antigens in CNS dendritic spines. Brain Res. 764:28–38. [DOI] [PubMed] [Google Scholar]
- Tsirka, S.E., A. Gualandris, D.G. Amaral, and S. Strickland. 1995. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 377:340–344. [DOI] [PubMed] [Google Scholar]
- Tsirka, S.E., A.D. Rogove, T.H. Bugge, J.L. Degen, and S. Strickland. 1997. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J. Neurosci. 17:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnerstall, J.R., and J.K. Wamsley. 1983. Autoradiographic localization of high-affinity [3H]kainic acid binding sites in the rat forebrain. Eur. J. Pharmacol. 86:361–371. [DOI] [PubMed] [Google Scholar]
- Werner, P., M. Voigt, K. Keinanen, W. Wisden, and P.H. Seeburg. 1991. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 351:742–744. [DOI] [PubMed] [Google Scholar]
- Wu, D.C., W. Ye, X.M. Che, and G.Y. Yang. 2000. Activation of mitogen-activated protein kinases after permanent cerebral artery occlusion in mouse brain. J. Cereb. Blood Flow Metab. 20:1320–1330. [DOI] [PubMed] [Google Scholar]
- Yang, D.D., C.Y. Kuan, A.J. Whitmarsh, M. Rincon, T.S. Zheng, R.J. Davis, P. Rakic, and R.A. Flavell. 1997. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 389:865–870. [DOI] [PubMed] [Google Scholar]
- Yin, Y., Y. Kikkawa, J.L. Mudd, W.C. Skarnes, J.R. Sanes, and J.H. Miner. 2003. Expression of laminin chains by central neurons: analysis with gene and protein trapping techniques. Genesis. 36:114–127. [DOI] [PubMed] [Google Scholar]
- Yu, W.M., M.L. Feltri, L. Wrabetz, S. Strickland, and Z.L. Chen. 2005. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J. Neurosci. 25:4463–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q.X., D.S. Pei, Q.H. Guan, Y.F. Sun, X.M. Liu, and G.Y. Zhang. 2006. Blockade of the translocation and activation of mitogen-activated protein kinase kinase 4 (MKK4) signaling attenuates neuronal damage during later ischemia-reperfusion. J. Neurochem. 98:170–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.