Abstract
The mitochondrial inner membrane contains different translocator systems for the import of presequence-carrying proteins and carrier proteins. The translocator assembly and maintenance protein 41 (Tam41/mitochondrial matrix protein 37) was identified as a new member of the mitochondrial protein translocator systems by its role in maintaining the integrity and activity of the presequence translocase of the inner membrane (TIM23 complex). Here we demonstrate that the assembly of proteins imported by the carrier translocase, TIM22 complex, is even more strongly affected by the lack of Tam41. Moreover, respiratory chain supercomplexes and the inner membrane potential are impaired by lack of Tam41. The phenotype of Tam41-deficient mitochondria thus resembles that of mitochondria lacking cardiolipin. Indeed, we found that Tam41 is required for the biosynthesis of the dimeric phospholipid cardiolipin. The pleiotropic effects of the translocator maintenance protein on preprotein import and respiratory chain can be attributed to its role in biosynthesis of mitochondrial cardiolipin.
Introduction
The mitochondrial inner membrane contains the large complexes of the respiratory chain, numerous carrier proteins for shuttling metabolites, and specific machineries for translocation and assembly of precursor proteins. About 1,000 different proteins are imported into mitochondria. The translocase of the outer membrane (TOM complex) functions as general import gate for precursor proteins. Subsequently, the import pathways diverge. Most proteins are transported into or across the inner membrane by using either the presequence pathway or the carrier pathway (Jensen and Johnson, 2001; Endo et al., 2003; Koehler, 2004; Oka and Mihara, 2005; Dolezal et al., 2006; Kutik et al., 2007; Neupert and Herrmann, 2007). The carrier translocase of the inner membrane (TIM22 complex) directs polytopic proteins with internal targeting signals into the inner membrane, using the membrane potential Δψ as driving force.
The presequence translocase (TIM23 complex) recognizes the preproteins with cleavable N-terminal presequences and inserts them into the Tim23 import channel in a Δψ-dependent manner. Preproteins carrying a hydrophobic sorting signal are laterally released into the inner membrane, whereas the majority of cleavable preproteins are completely translocated into the matrix. Reconstitution experiments with proteoliposomes revealed that the TIM23 complex and an energized cardiolipin-rich membrane represented a minimal system for membrane integration of cleavable preproteins (van der Laan et al., 2007). In organello, however, the TIM23 complex dynamically interacts with several further protein machineries: the translocase of the outer membrane for preprotein transfer from the outer to the inner membrane (Chacinska et al., 2005; Mokranjac et al., 2005), complexes III and IV of the respiratory chain to stimulate the Δψ-driven membrane insertion of preproteins with sorting signal (van der Laan et al., 2006; Wiedemann et al., 2007; Saddar et al., 2008), and the presequence translocase-associated motor (PAM) with the heat shock protein 70 that drives protein transport into the matrix at the expense of ATP (Kutik et al., 2007; Neupert and Herrmann, 2007; D'Silva et al., 2008).
The TIM23 complex is a multistep machine and its assembly and mode of cooperation with the motor PAM are only partially understood. Two recent studies identified a mitochondrial protein, which is peripherally attached to the inner membrane from the matrix side and involved in the assembly and maintenance of the activity of the TIM23 complex (Gallas et al., 2006; Tamura et al., 2006). The protein was termed translocator assembly and maintenance protein 41 (Tam41) or mitochondrial matrix protein 37 (Mmp37). Yeast cells lacking Tam41/Mmp37 show a temperature-sensitive growth defect, and the import of presequence-carrying mitochondrial preproteins is impaired at elevated temperature in vivo and in organello. Tam41 does not stably bind to the TIM23 complex, yet its absence influences the integrity of the TIM23 complex and the cooperation with the motor PAM. It was thus concluded that Tam41 is not a structural subunit of the TIM23 complex but a new member of the mitochondrial translocator systems required to maintain the proper assembly state and activity of the TIM23 complex (Gallas et al., 2006; Tamura et al., 2006). Although both studies agreed well on the effects of Tam41/Mmp37 on the presequence pathway, different findings were reported on the second protein import pathway to the inner membrane, the carrier pathway. Gallas et al. (2006) reported that tam41Δ mitochondria were impaired in the import of a noncleavable carrier precursor, whereas Tamura et al. (2006) did not observe a defect in the translocation of carrier precursors to a protease-protected location. The molecular function of Tam41 remained open.
We used a native assembly assay to characterize the biogenesis of noncleavable carrier proteins in mitochondria lacking Tam41. Surprisingly, we observed a strong defect in carrier assembly even at low temperature, suggesting that the defect of tam41Δ mitochondria may be more pronounced in the biogenesis of carrier proteins than in the presequence pathway. The subsequent analysis revealed pleiotropic effects of Tam41 on the mitochondrial membrane potential and the assembly state of respiratory chain supercomplexes. We report that these seemingly nonrelated effects are caused by the involvement of Tam41 in the biosynthesis of cardiolipin.
Results and discussion
Mitochondria lacking Tam41 are blocked in the assembly of carrier proteins
We generated a yeast strain lacking the TAM41 gene. As tam41Δ cells are temperature sensitive for growth (Gallas et al., 2006; Tamura et al., 2006), the cells were grown at low temperature to minimize indirect effects. Mitochondria were isolated and subjected to a short heat shock. The steady-state levels of proteins from the four mitochondrial compartments were comparable for tam41Δ and wild-type mitochondria, whereas the import of presequence-carrying preproteins was impaired in the mutant mitochondria (unpublished data), in agreement with the reported defect of tam41Δ mitochondria in the presequence pathway (Gallas et al., 2006; Tamura et al., 2006). These two studies reported different observations for the import of noncleavable carrier proteins as analyzed by protease protection assays. Carrier proteins are imported in a multistep process (Fig. 1 A) and thus precursors accumulated at stage III on the intermembrane space side can be protected against externally added protease when low to moderate concentrations of protease are used (Pfanner and Neupert, 1987; Ryan et al., 1999). Importantly, mitochondria with a defect of Δψ or the TIM22 complex can efficiently accumulate carrier proteins at stage III and, thus, depending on the strength of the protease treatment and the stability of the outer membrane, protease protection of carrier precursors can be observed despite an inner membrane import defect. To directly monitor the assembly stages of mitochondrial carrier proteins, we used a blue native gel assay, which separates stage III (intermediate in intermembrane space) from stage V (mature assembled carrier) upon lysis of mitochondria with a mild detergent such as digitonin (Ryan et al., 1999; Dyall et al., 2003; Rehling et al., 2003). Surprisingly, the Δψ-dependent formation of stage V carrier was blocked in tam41Δ mitochondria, as analyzed by importing the 35S-labeled precursors of the ADP/ATP carrier (AAC) and dicarboxylate carrier (DIC; Fig. 1 B). Instead a smaller form (AAC* and DIC*) was observed, migrating above the stage III intermediate. The formation of AAC* and DIC* was inhibited by dissipation of Δψ in contrast to the stage III intermediate (Fig. 1 B), demonstrating that the generation of AAC* and DIC* depended on the activity of the inner membrane. Even without heat shock treatment, carrier assembly to the oligomeric form (stage V) was completely blocked into tam41Δ mitochondria (Fig. 1 C). AAC imported into tam41Δ mitochondria was resistant to extraction at pH 11.5 (Fig. 1 D). Together with the Δψ dependence of import, this indicates that the carrier was inserted into the lipid phase of the inner membrane. Thus tam41Δ mitochondria are defective in carrier assembly, suggesting that the function of Tam41 extends beyond the maintenance of the TIM23 complex.
Figure 1.
Protein import and assembly defects in tam41Δ mitochondria. (A) Biogenesis stages I–V of mitochondrial carrier proteins (Ryan et al., 1999). (B) Isolated mitochondria from wild-type (WT) or tam41Δ yeast cells grown at 21°C were heat shocked in vitro and incubated with radiolabeled AAC or DIC precursor proteins at 25°C. The mitochondria were lysed with digitonin and analyzed by blue native electrophoresis and digital autoradiography. *, carrier form in tam41Δ mitochondria. (C) The assembly of AAC was analyzed as for B without in vitro heat shock. (D) After incubation with AAC, mitochondria were treated at pH 11.5. Total (T), pellet (P), and supernatant (S) were analyzed by SDS-PAGE followed by autoradiography and immunodecoration. (E) Isolated mitochondria (in vitro heat shock) were incubated with Fe/S protein. (F) Quantification of Fe/S protein maturation in the absence or presence of an in vitro heat shock in tam41Δ mitochondria. 20-min import into wild-type mitochondria was set to 100%. Error bars represent SEM (n = 3).
Interestingly, we observed that preprotein import via the presequence pathway was temperature sensitive in tam41Δ mitochondria, shown here with the 35S-labeled cleavable preprotein of the Rieske Fe/S-protein. Although the import into the mutant mitochondria was impaired after the heat shock, it was only moderately affected at low temperature (Fig. 1, E and F). We assessed the Δψ of tam41Δ mitochondria by fluorescence quenching. When the yeast cells were grown at low temperature, the fluorescence decrease of tam41Δ mitochondria was close to that of wild-type mitochondria (Fig. 2 A), indicating no major alteration of Δψ. When the cells were grown at elevated temperature, however, a strong decrease of the Δψ of tam41Δ mitochondria was observed (Fig. 2 A). For in vitro protein import experiments, the mitochondria were isolated from cells grown at low temperature and subjected to a short heat shock. This in vitro heat shock led to a reduction of the fluorescence quenching, i.e., a reduction of Δψ (Fig. 2 B). Thus, both protein import via the presequence pathway and the inner membrane potential are impaired in tam41Δ mitochondria in a temperature-sensitive manner. In contrast, the assembly of carrier proteins is also blocked at low temperature. Because under these conditions the Δψ of the mutant mitochondria is close to that of wild-type mitochondria, Δψ cannot be the critical determinant for the lack of carrier assembly in tam41Δ mitochondria.
Figure 2.
Temperature sensitivity of Δψ generation in tam41Δ mitochondria. (A) Yeast cells were grown in YPLac at low temperature (21°C) and shifted to 30 or 37°C for 8 h in vivo before isolation of mitochondria. Δψ was assessed at 25°C by fluorescence quenching. (B) Yeast cells were grown at 21°C. Mitochondria were isolated and Δψ was assessed.
Collectively, the assembly of carrier proteins is more strongly affected in tam41Δ mitochondria than the biogenesis of presequence-containing proteins. Neither maintenance of TIM23 function nor Δψ generation seems to be a primary target of the functional defect of tam41Δ mitochondria.
Alteration of inner membrane protein complexes in mitochondria lacking Tam41 or cardiolipin biosynthetic enzymes
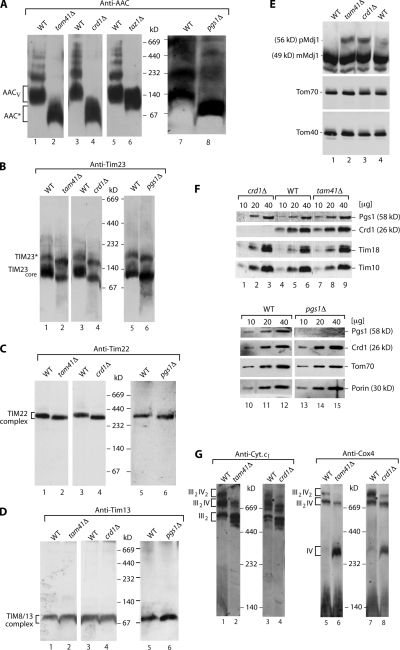
AAC binds cardiolipin, a unique phospholipid with dimeric structure, and it was reported that the lack of cardiolipin synthase (Crd1) alters the oligomeric state of AAC (Hoffmann et al., 1994; Jiang et al., 2000; Nury et al., 2005; Claypool et al., 2008). A direct comparison of tam41Δ and crd1Δ mitochondria by Western blot analysis of blue native gels revealed a remarkable similarity of the pattern of AAC in both mutant mitochondria (Fig. 3 A). Oligomeric forms, including the dominant AACV, were virtually absent in both mutants, and the carrier molecules migrated in the low molecular mass range (Fig. 3 A). Mitochondria lacking phosphatidylglycerophosphate (PGP) synthase (Pgs1), which functions upstream of Crd1 in cardiolipin biosynthesis (Chang et al., 1998; Mileykovskaya et al., 2005; Li et al., 2007), showed a similar alteration of the AAC pattern (Fig. 3 A). We also studied mitochondria lacking Taz1, the orthologue of human tafazzin, which is affected in the Barth syndrome (Vreken et al., 2000; Brandner et al., 2005; Claypool et al., 2006; McKenzie et al., 2006). The putative acyltransferase or transacylase Taz1 is involved in the last step of cardiolipin maturation, the remodeling of fatty acid side chains (Gu et al., 2004; Valianpour et al., 2005; Xu et al., 2006; Li et al., 2007). In taz1Δ mitochondria, the higher oligomers of AAC were absent, whereas AACV was still present and only slightly altered in its mobility (Fig. 3 A; Brandner et al., 2005). Thus, the blue native pattern of AAC in tam41Δ mitochondria was different from that of taz1Δ mitochondria but similar to the pattern of crd1Δ and pgs1Δ mitochondria.
Figure 3.
Inner membrane complexes are altered in tam41Δ, crd1Δ, and pgs1Δ mitochondria. (A–D and G) Mitochondria isolated from yeast cells grown at low temperature were analyzed by blue native electrophoresis. Whole cell protein extracts (E) and mitochondria (F) were analyzed by SDS-PAGE and immunodecoration.
We compared the effect of Tam41 versus Crd1 and Pgs1 deletion on the TIM complexes by Western blot analysis of blue native gels. The TIM23 complex migrates in several forms on blue native gels (Chacinska et al., 2005; van der Laan et al., 2007). Gallas et al. (2006) and Tamura et al. (2006) showed that the blue native pattern of TIM23 was substantially altered in tam41Δ mitochondria. Indeed, tam41Δ, crd1Δ, and pgs1Δ mitochondria showed a similar alteration of the mobility of TIM23 complexes (Fig. 3 B). A slight mobility shift was observed for the TIM22 complex in the mutant mitochondria (Fig. 3 C), whereas the soluble Tim8–Tim13 complex of the intermembrane space was not altered (Fig. 3 D). Cells lacking Tam41 were shown to accumulate mitochondrial precursor proteins in vivo (Gallas et al., 2006; Tamura et al., 2006). We observed that not only tam41Δ cells but also crd1Δ cells accumulated the precursor of the cleavable protein Mdj1 (Fig. 3 E). Thus the lack of cardiolipin leads to similar effects on TIM complexes and preprotein accumulation as the lack of Tam41. To exclude that the lack of Tam41 led to a loss of Crd1 or Pgs1, we determined the steady-state levels of the proteins and did not observe a difference between wild-type and tam41Δ mitochondria (Fig. 3 F).
Cardiolipin stabilizes supercomplexes of the mitochondrial respiratory chain, in particular complexes up to 1 Md formed by complexes III and IV (Schägger, 2002; Zhang et al., 2002, 2005; Pfeiffer et al., 2003; Zhong et al., 2004). Assuming that Tam41 plays a role in the biosynthesis of cardiolipin, the III and IV supercomplexes should be altered in the deletion mutant. We analyzed digitonin-lysed mitochondria by blue native electrophoresis and immunodecoration for cytochrome c1 (complex III) and Cox4 (complex IV). Indeed, the supercomplexes were strongly altered in both tam41Δ and crd1Δ mitochondria (Fig. 3 G).
Collectively, the characterization of tam41Δ mitochondria reveals pleiotropic effects on complexes of the mitochondrial inner membrane and a remarkable resemblance of the defects observed in mitochondria lacking cardiolipin.
tam41Δ mitochondria are deficient in cardiolipin
To directly address if the lack of Tam41 affected the levels of mitochondrial cardiolipin, we extracted lipids from isolated wild-type and tam41Δ mitochondria and subjected them to mass spectrometry. Fig. 4 A shows the liquid chromatography/mass spectrometry (LCMS) profiles of wild-type and tam41Δ mitochondrial lipid extracts. The major ions in the spectra correspond to phosphatidylethanolamine and phosphatidylinositol, whereas cardiolipins appear as minor ions in the lipid profiles (Fig. 4 A, top). Using an unbiased profiling approach (Guan et al., 2006; Shui et al., 2007), we compared the lipid profile of tam41Δ mitochondria relative to the wild-type profile and expressed this as a differential profile. The main difference observed was a down-regulation of several ions (mass-to-charge ratio [m/z] of 685.5, 699.5, and 713.5 and 1371.9, 1399.9, and 1427.9) in tam41Δ, corresponding to the doubly and singly charged species of cardiolipin molecules found in Saccharomyces cerevisiae (Fig. 4 A, middle; ions 1117.8 and 1145.8 were identified as fragments of cardiolipin). For comparison, we also analyzed lipid extracts of crd1Δ and taz1Δ mitochondria. As expected, monolysocardiolipin accumulated in taz1Δ mitochondria (m/z 1055.8, 1137.8, and 1165.8) and phosphatidylglycerol (PG) accumulated in crd1Δ mitochondria (m/z 719.5, 747.5, and 775.5; Fig. 4 A, bottom; Tuller et al., 1998; Jiang et al., 2000; Zhang et al., 2003; Gu et al., 2004; Valianpour et al., 2005; Claypool et al., 2006).
Figure 4.
tam41Δ mitochondria are deficient in cardiolipin. (A) Lipids were extracted from isolated mitochondria and subjected to LCMS. Averaged normalized spectra from wild-type and tam41Δ mitochondria. Differential lipid profiles (log10 ratio) of tam41Δ/wild-type, crd1Δ/wild-type, and taz1Δ/wild-type mitochondria. IS, internal standard (tetramyristoyl cardiolipin); PE, phosphatidylethanolamine; PI, phosphatidylinositol. (B) Analysis of cardiolipin levels in wild-type and tam41Δ mitochondria. (C) Wild-type and tam41Δ cells harboring the indicated plasmids were subjected to fivefold serial dilutions and grown at 37°C on YPD. (D) Cardiolipin levels of mitochondria from tam41Δ yeast cells harboring the indicated plasmids. Bars represent the range of two MS analyses.
The levels of cardiolipin were assessed using cardiolipin internal standards based on the integration of the peak area of the LCMS chromatograms. Most species of cardiolipin were virtually absent in tam41Δ mitochondria (Fig. 4 B). To directly determine if the lack of Tam41 was responsible for the observed defects, Tam41 was reexpressed from a plasmid in tam41Δ cells. Thereby, the growth of the cells at elevated temperature was restored (Fig. 4 C) and cardiolipin was synthesized (Fig. 4 D). Thus, the expression of Tam41 in tam41Δ cells is sufficient for cardiolipin synthesis and cell viability at elevated temperature.
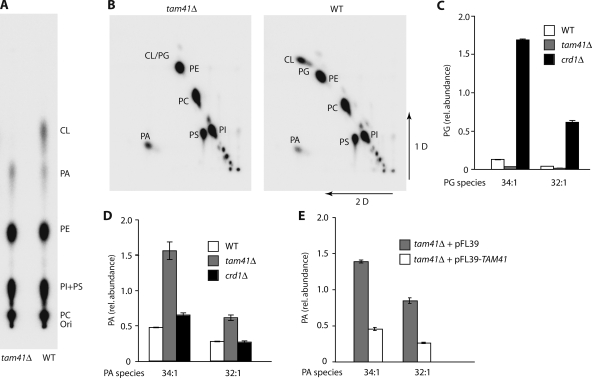
Upon in vivo labeling with [32PO4], the lipids of enriched mitochondria were analyzed by one- and two-dimensional separation (Fig. 5, A and B). Cardiolipin and PG were not detected in tam41Δ mitochondria. Tandem mass spectrometry–based quantification of PG levels demonstrated that the levels of PG were significantly reduced in tam41Δ mitochondria in contrast to the strong increase in crd1Δ mitochondria (Fig. 5 C; Tuller et al., 1998; Jiang et al., 2000; Zhang et al., 2003). These results demonstrate that Tam41 does not function at the stage of Crd1, but the reduced levels of PG in tam41Δ indicate that Tam41 acts upstream of Crd1. There was no evidence that tam41Δ mitochondria accumulated large amounts of PGP (the immediate precursor of PG), which would run very close to the origin in the two-dimensional system (Fig. 5 B). Collectively, the results obtained so far raise the possibility that Tam41 functions at an early step of cardiolipin biosynthesis. To address this, we determined the levels of phosphatidic acid (PA), the first metabolite in the biosynthesis of cardiolipin, and indeed found a considerable increase in tam41Δ mitochondria but not in crd1Δ mitochondria (Fig. 5 D). The increased levels of PA were also observed by one- and two-dimensional separation of [32PO4] lipids (Fig. 5, A and B). Reexpression of Tam41 in tam41Δ cells decreased the levels of PA to wild-type levels (Fig. 5 E), demonstrating that the increase of PA was caused by the lack of Tam41.
Figure 5.
tam41Δ mitochondria are deficient in PG but accumulate PA. (A) Cellular lipids in wild type and tam41Δ were labeled with [32PO4], extracted, subjected to one-dimensional thin layer chromatography, and imaged. CL, cardiolipin; Ori, origin; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine. (B) Cellular lipids labeled and extracted as in A were subjected to two-dimensional thin layer chromatography. (C and D) Analysis of PG and PA levels in wild-type, tam41Δ, and crd1Δ mitochondria. (E) PA levels of tam41Δ mitochondria from a yeast strain reexpressing Tam41. Bars represent the range of two MS analyses.
Conclusions
We report an unexpected role of the peripheral mitochondrial inner membrane protein Tam41. Mitochondria lacking Tam41 revealed various, at first glance pleiotropic, defects, and the analysis of the mutant mitochondria led to several surprising twists. First, we confirmed the findings of Gallas et al. (2006) and Tamura et al. (2006), who identified Tam41 based on its influence on the assembly and maintenance of the presequence pathway (TIM23 complex). Second, tam41Δ mitochondria showed a reduced Δψ and a defect in the assembly of carrier proteins, which are imported via the TIM22 complex. Importantly, at low temperature, tam41Δ mitochondria were still strongly impaired in carrier assembly, whereas Δψ and presequence pathway were not or only moderately affected, suggesting that the presequence pathway was not the primary site of action of Tam41.
Third, various inner membrane protein complexes, including supercomplexes of the respiratory chain, carrier complexes, and TIM translocases, were altered in tam41Δ mitochondria, resembling the broad spectrum of defects observed in mitochondria lacking cardiolipin (Jiang et al., 2000; Schägger, 2002; Zhang et al., 2002, 2005; Pfeiffer et al., 2003; Zhong et al., 2004; Nury et al., 2005; van der Laan et al., 2007; Claypool et al., 2008). Finally, a detailed analysis by mass spectrometry and in vivo radiolabeling of lipids proved that the levels of cardiolipin, as well as of PG, were significantly reduced in tam41Δ mitochondria. The sequence of cardiolipin synthesis in yeast is PA to CDP-diacylglycerol to PGP to PG to cardiolipin (Mileykovskaya et al., 2005; Li et al., 2007). We did not observe an accumulation of PGP, but a significant accumulation of PA in tam41Δ mitochondria. In contrast, mitochondria lacking Crd1 accumulate large amounts of PG but not PA (Tuller et al., 1998; Jiang et al., 2000; Zhang et al., 2003; this study), demonstrating that Tam41 acts upstream of Crd1. Moreover, tam41Δ mitochondria contain wild-type levels of Crd1 and Pgs1. The accumulation of the early metabolite PA thus suggests that Tam41 is required at an early stage of cardiolipin biosynthesis.
In summary, the available results indicate that Tam41 is required for the biosynthetic pathway of cardiolipin or the regulation of the pathway. This dimeric mitochondrial phospholipid exerts a broad influence on numerous functions of the inner membrane, including the assembly states of the respiratory chain, metabolite carriers, and the TIM23 complex. The apparently pleiotropic effects of Tam41 can thus be explained by its involvement in lipid biosynthesis.
Materials and Methods
Yeast strains
YPH499 and BY4741 were used as wild-type S. cerevisiae strains. tam41Δ (fomp7Δ-A; YBG-0800-A) was generated by plasmid shuffling (Chacinska et al., 2005). pgs1Δ, taz1Δ, and crd1Δ strains have been described previously (Chang et al., 1998; Brandner et al., 2005; Euroscarf). The region encoding Tam41, plus 500 bp upstream/downstream, was cloned into pFL39 using SacI and BamHI restriction sites, yielding pFL39-TAM41.
Growth conditions and mitochondrial protein import
Cells were grown in liquid YP medium (1% [wt/vol] yeast extract; 2% [wt/vol] Bacto Peptone; and either 3% [vol/vol] glycerol (YPG), 2% [wt/vol] sucrose [YPS], or 2.2% [vol/vol] lactic acid; 0.05% [wt/vol] glucose; 0.04% [wt/vol] CaCl2; 0.03% [wt/vol] KH2PO4; 0.1% [wt/vol] NH4Cl; 0.05% [wt/vol] NaCl, pH 4.8 [YPLac]) or on 2% (wt/vol) glucose (YPD) agar plates. Mitochondria were isolated by differential centrifugation and stored at −80°C in SEM (250 mM sucrose, 1 mM EDTA, and 10 mM MOPS/KOH, pH 7.2). Proteins were synthesized in rabbit reticulocyte lysate in the presence of [35S]methionine and incubated with isolated mitochondria in import buffer (3% [wt/vol] BSA, 250 mM sucrose, 80 mM KCl, 5 mM methionine, 5 mM MgCl2, 2 mM KH2PO4, 10 mM MOPS-KOH, pH 7.2, 2 mM NADH, 4 mM ATP, 20 mM creatine phosphate, and 0.1 mg/ml creatine kinase; Ryan et al., 1999). Where indicated, mitochondria were subjected to a 15-min heat shock (37°C). Δψ was dissipated by addition of 8 μM antimycin A, 20 μM oligomycin, and 1 μM valinomycin before the import experiment. After import, mitochondria were treated with 50 μg/ml of proteinase K for 15 min on ice. Analysis was performed by digital autoradiography (Storm imaging system; GE Healthcare) and ImageQuant software (GE Healthcare). For alkaline extraction, mitochondria were resuspended in 0.1 M Na2CO3 and incubated for 15 min on ice. Pellet and supernatant fractions of mitochondrial proteins were separated by 60-min centrifugation at 100,000 g. Fractions were precipitated by trichloroacetic acid precipitation (12% vol/vol).
Lipid analysis
Mitochondrial lipids were extracted using chloroform/methanol/water (10:10:3 vol/vol) and desalted using butanol extraction. Tetramyristoyl cardiolipin (Avanti Polar Lipids, Inc.) was added as internal standard. Profiles of polar lipids were obtained using a micromass spectrometer (Q-Tof micro; Waters) in the negative electrospray ionization ion mode with upfront LCMS separation as described previously (Shui et al., 2007). Mass spectra were recorded from m/z of 600 to 1500. Lipid chromatograms combined to generate averaged spectra and profiles of wild-type and mutant conditions were compared in a nontargeted fashion based on a chemometric method (Guan et al., 2006). Ions corresponding to cardiolipin were selected and their chromatograms were extracted using MassLynx 4.0 software (Waters). Integrated peak areas and their normalized values (using tetramyristoyl cardiolipin as internal standards) were used for semiquantitative analysis (Shui et al., 2007). Quantification of individual molecular species of PA and PG was performed using multiple reaction monitoring with a 4000 Q-Trap mass spectrometer (Applied Biosystems) as described by Fei et al. (2008).
For in vivo labeling, cells were grown at least six generations to late log phase at 25°C in YPG medium supplemented with 10 μCi/ml of [32PO4]. A mitochondrial enriched fraction was prepared as previously described (Chang et al., 1998). In brief, cells were disrupted using silica/zirconium beads in a Mini-Bead-Beater 8 (BioSpec Products) and unbroken cells were removed by centrifugation. Enriched mitochondria were isolated by a final centrifugation at 27,000 g. Enriched mitochondria were suspended in 0.5 M NaCl and 0.1 N HCl/methanol/chloroform (1:2:1 vol/vol) and, after vortexing, a two-phase system was generated by adding an additional part of the NaCl solution. The chloroform phase was applied to either 10 × 10- or 10 × 20-cm HPTLC Silica 60 plates (EMD) for two- or one-dimensional separation of lipids, respectively. For two-dimensional separation, chloroform/methanol/25% NH3 (65:35:5 vol/vol) was used in the first dimension and chloroform/methanol/acetone/acetic acid/water (50:20:10:10:5 vol/vol) was used in the second dimension. For one-dimensional separation, chloroform/methanol/acetic acid (65:25:8) was used. Radiolabel was imaged using a Molecular Imager FX (Bio-Rad Laboratories). Lipids were identified based on mobilities of known standards.
Miscellaneous
Δψ of isolated mitochondria was assessed by fluorescence quenching using the potential-sensitive fluorescent dye 3,3′-dipropylthiadicarbocyanine iodide in membrane potential buffer (0.6 M sorbitol, 0.1% [wt/vol] BSA, 10 mM MgCl2, 0.5 mM EDTA, and 20 mM KPi, pH 7.2) as described previously (Brandner et al., 2005). In some figures, nonrelevant gel lanes were removed. For Western blot analysis, mitochondrial proteins or protein complexes were separated by SDS-PAGE or blue native electrophoresis (Ryan et al., 1999; Schägger, 2002) and blotted on polyvinylidene fluoride membranes.
Acknowledgments
We thank Drs. T. Endo, M. Greenberg, and R. Stuart for critical comments on the manuscript. We are grateful to Drs. C. Meisinger, T. Major, M. van der Laan, and W. Voos for experimental advice and discussion.
This work was supported by the Deutsche Forschungsgemeinschaft (P. Rehling and N. Pfanner), Sonderforschungsbereich 746, Excellence Initiative of the German Federal and State Governments (EXC 294), Gottfried Wilhelm Leibniz Program, Fonds der Chemischen Industrie (N. Pfanner), Singapore National Research Foundation, National University of Singapore via the Life Science Institute and the Academic Research Fund, Biomedical Research Council of Singapore (M.R. Wenk), National Institutes of Health (grant GM56389 to W. Dowhan), and Boehringer Ingelheim Fonds (predoctoral fellowship to S. Kutik).
S. Kutik and M. Rissler contributed equally to this paper.
Abbreviations used in this paper: AAC, ADP/ATP carrier; Crd1, cardiolipin synthase; DIC, dicarboxylate carrier; LCMS, liquid chromatography/mass spectrometry; Mmp37, mitochondrial matrix protein 37; PA, phosphatidic acid; PAM, presequence translocase-associated motor; PG, phosphatidylglycerol; PGP, phosphatidylglycerophosphate; Pgs1, PGP synthase; Tam41, translocator assembly and maintenance protein 41; TIM, translocase of the inner membrane.
References
- Brandner, K., D.U. Mick, A.E. Frazier, R.D. Taylor, C. Meisinger, and P. Rehling. 2005. Taz1, an outer mitochondrial membrane protein, affects stability and assembly of inner membrane protein complexes: implications for Barth Syndrome. Mol. Biol. Cell. 16:5202–5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacinska, A., M. Lind, A.E. Frazier, J. Dudek, C. Meisinger, A. Geissler, A. Sickmann, H.E. Meyer, K.N. Truscott, B. Guiard, et al. 2005. Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell. 120:817–829. [DOI] [PubMed] [Google Scholar]
- Chang, S.-C., P.N. Heacock, C.J. Clancey, and W. Dowhan. 1998. The PEL1 gene (renamed PGS1) encodes the phosphatidylglycero-phosphate synthase of Saccharomyces cerevisiae. J. Biol. Chem. 273:9829–9836. [DOI] [PubMed] [Google Scholar]
- Claypool, S.M., J.M. McCaffery, and C.M. Koehler. 2006. Mitochondrial mislocalization and altered assembly of a cluster of Barth syndrome mutant tafazzins. J. Cell Biol. 174:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool, S.M., Y. Oktay, P. Boontheung, J.A. Loo, and C.M. Koehler. 2008. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 182:937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Silva, P.R., B. Schilke, M. Hayashi, and E.A. Craig. 2008. Interaction of the J-protein heterodimer Pam18/Pam16 of the mitochondrial import motor with the translocon of the inner membrane. Mol. Biol. Cell. 19:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezal, P., V. Likic, J. Tachezy, and T. Lithgow. 2006. Evolution of the molecular machines for protein import into mitochondria. Science. 313:314–318. [DOI] [PubMed] [Google Scholar]
- Dyall, S.D., S.C. Agius, C. De Marcos Lousa, V. Trezeguet, and K. Tokatlidis. 2003. The dynamic dimerization of the yeast ADP/ATP carrier in the inner mitochondrial membrane is affected by conserved cysteine residues. J. Biol. Chem. 278:26757–26764. [DOI] [PubMed] [Google Scholar]
- Endo, T., H. Yamamoto, and M. Esaki. 2003. Functional cooperation and separation of translocators in protein import into mitochondria, the double-membrane bounded organelles. J. Cell Sci. 116:3259–3267. [DOI] [PubMed] [Google Scholar]
- Fei, W., G. Shui, B. Gaeta, X. Du, L. Kuerschner, P. Li, A.J. Brown, M.R. Wenk, R.G. Parton, and H. Yang. 2008. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 180:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallas, M.R., M.K. Dienhart, R.A. Stuart, and R.M. Long. 2006. Characterization of Mmp37p, a Saccharomyces cerevisiae mitochondrial matrix protein with a role in mitochondrial protein import. Mol. Biol. Cell. 17:4051–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, Z., F. Valianpour, S. Chen, F.M. Vaz, G.A. Hakkaart, R.J. Wanders, and M.L. Greenberg. 2004. Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol. Microbiol. 51:149–158. [DOI] [PubMed] [Google Scholar]
- Guan, X.L., X. He, W.-Y. Ong, W. Kiang Yeo, G. Shui, and M.R. Wenk. 2006. Non-targeted profiling of lipids during kainate-induced neuronal injury. FASEB J. 20:1152–1161. [DOI] [PubMed] [Google Scholar]
- Hoffmann, B., A. Stockl, M. Schlame, K. Beyer, and M. Klingenberg. 1994. The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. J. Biol. Chem. 269:1940–1944. [PubMed] [Google Scholar]
- Jensen, R.E., and A.E. Johnson. 2001. Opening the door to mitochondrial protein import. Nat. Struct. Biol. 8:1008–1010. [DOI] [PubMed] [Google Scholar]
- Jiang, F., M.T. Ryan, M. Schlame, M. Zhao, Z. Gu, M. Klingenberg, N. Pfanner, and M.L. Greenberg. 2000. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J. Biol. Chem. 275:22387–22394. [DOI] [PubMed] [Google Scholar]
- Koehler, C.M. 2004. New developments in mitochondrial assembly. Annu. Rev. Cell Dev. Biol. 20:309–335. [DOI] [PubMed] [Google Scholar]
- Kutik, S., B. Guiard, H.E. Meyer, N. Wiedemann, and N. Pfanner. 2007. Cooperation of translocase complexes in mitochondrial protein import. J. Cell Biol. 179:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G., S. Chen, M.N. Thompson, and M.L. Greenberg. 2007. New insights into the regulation of cardiolipin biosynthesis in yeast: implications for Barth syndrome. Biochim. Biophys. Acta. 1771:432–441. [DOI] [PubMed] [Google Scholar]
- McKenzie, M., M. Lazarou, D.R. Thorburn, and M.T. Ryan. 2006. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J. Mol. Biol. 361:462–469. [DOI] [PubMed] [Google Scholar]
- Mileykovskaya, E., M. Zhang, and W. Dowhan. 2005. Cardiolipin in energy transducing membranes. Biochemistry (Mosc.). 70:154–158. [DOI] [PubMed] [Google Scholar]
- Mokranjac, D., D. Popov-Celeketic, K. Hell, and W. Neupert. 2005. Role of Tim21 in mitochondrial translocation contact sites. J. Biol. Chem. 280:23437–23440. [DOI] [PubMed] [Google Scholar]
- Neupert, W., and J.M. Herrmann. 2007. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76:723–749. [DOI] [PubMed] [Google Scholar]
- Nury, H., C. Dahout-Gonzalez, V. Trezeguet, G. Lauquin, G. Brandolin, and E. Pebay-Peyroula. 2005. Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett. 579:6031–6036. [DOI] [PubMed] [Google Scholar]
- Oka, T., and K. Mihara. 2005. A railroad switch in mitochondrial protein import. Mol. Cell. 18:145–146. [DOI] [PubMed] [Google Scholar]
- Pfanner, N., and W. Neupert. 1987. Distinct steps in the import of ADP/ATP carrier into mitochondria. J. Biol. Chem. 262:7528–7536. [PubMed] [Google Scholar]
- Pfeiffer, K., V. Gohil, R.A. Stuart, C. Hunte, U. Brandt, M.L. Greenberg, and H. Schägger. 2003. Cardiolipin stabilizes respiratory chain supercomplexes. J. Biol. Chem. 278:52873–52880. [DOI] [PubMed] [Google Scholar]
- Rehling, P., K. Model, K. Brandner, P. Kovermann, A. Sickmann, H.E. Meyer, W. Kühlbrandt, R. Wagner, K.N. Truscott, and N. Pfanner. 2003. Protein insertion into the mitochondrial inner membrane by a twin-pore translocase. Science. 299:1747–1751. [DOI] [PubMed] [Google Scholar]
- Ryan, M.T., H. Müller, and N. Pfanner. 1999. Functional staging of ADP/ATP carrier translocation across the outer mitochondrial membrane. J. Biol. Chem. 274:20619–20627. [DOI] [PubMed] [Google Scholar]
- Saddar, S., M.K. Dienhart, and R.A. Stuart. 2008. The F1Fo-ATP synthase complex influences the assembly state of the cytochrome bc1-cytochrome oxidase supercomplex and its association with the TIM23 machinery. J. Biol. Chem. 283:6677–6686. [DOI] [PubMed] [Google Scholar]
- Schägger, H. 2002. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim. Biophys. Acta. 1555:154–159. [DOI] [PubMed] [Google Scholar]
- Shui, G., A.K. Bendt, K. Pethe, T. Dick, and M.R. Wenk. 2007. Sensitive profiling of chemically diverse bioactive lipids. J. Lipid Res. 48:1976–1984. [DOI] [PubMed] [Google Scholar]
- Tamura, Y., Y. Harada, K. Yamano, K. Watanabe, D. Ishikawa, C. Ohshima, S. Nishikawa, H. Yamamoto, and T. Endo. 2006. Identification of Tam41 maintaining integrity of the TIM23 protein translocator complex in mitochondria. J. Cell Biol. 174:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuller, G., C. Hrastnik, G. Achleitner, U. Schiefthaler, F. Klein, and G. Daum. 1998. YDL142c encodes cardiolipin synthase (Cls1p) and is non-essential for aerobic growth of Saccharomyces cerevisiae. FEBS Lett. 421:15–18. [DOI] [PubMed] [Google Scholar]
- Valianpour, F., V. Mitsakos, D. Schlemmer, J.A. Towbin, J.M. Taylor, P.G. Ekert, D.R. Thorburn, A. Munnich, R.J. Wanders, P.G. Barth, and F.M. Vaz. 2005. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J. Lipid Res. 46:1182–1195. [DOI] [PubMed] [Google Scholar]
- van der Laan, M., N. Wiedemann, D.U. Mick, B. Guiard, P. Rehling, and N. Pfanner. 2006. A role for Tim21 in membrane-potential-dependent preprotein sorting in mitochondria. Curr. Biol. 16:2271–2276. [DOI] [PubMed] [Google Scholar]
- van der Laan, M., M. Meinecke, J. Dudek, D.P. Hutu, M. Lind, I. Perschil, B. Guiard, R. Wagner, N. Pfanner, and P. Rehling. 2007. Motor-free mitochondrial presequence translocase drives membrane integration of preproteins. Nat. Cell Biol. 9:1152–1159. [DOI] [PubMed] [Google Scholar]
- Vreken, P., F. Valianpour, L.G. Nijtmans, L.A. Grivell, B. Plecko, R.J. Wanders, and P.G. Barth. 2000. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem. Biophys. Res. Commun. 279:378–382. [DOI] [PubMed] [Google Scholar]
- Wiedemann, N., M. van der Laan, D.P. Hutu, P. Rehling, and N. Pfanner. 2007. Sorting switch of mitochondrial presequence translocase involves coupling of motor module to respiratory chain. J. Cell Biol. 179:1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., A. Malhotra, M. Ren, and M. Schlame. 2006. The enzymatic function of tafazzin. J. Biol. Chem. 281:39217–39224. [DOI] [PubMed] [Google Scholar]
- Zhang, M., E. Mileykovskaya, and W. Dowhan. 2002. Gluing the respiratory chain together: cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 277:43553–43556. [DOI] [PubMed] [Google Scholar]
- Zhang, M., X. Su, E. Mileykovskaya, A.A. Amoscato, and W. Dowhan. 2003. Cardiolipin is not required to maintain mitochondrial DNA stability or cell viability for Saccharomyces cerevisiae grown at elevated temperatures. J. Biol. Chem. 278:35204–35210. [DOI] [PubMed] [Google Scholar]
- Zhang, M., E. Mileykovskaya, and W. Dowhan. 2005. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 280:29403–29408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, Q., V.M. Gohil, L. Ma, and M.L. Greenberg. 2004. Absence of cardiolipin results in temperature sensitivity, respiratory defects, and mitochondrial DNA instability independent of pet56. J. Biol. Chem. 279:32294–32300. [DOI] [PubMed] [Google Scholar]