Abstract
Background
Leafy vegetables are good sources of folates and food shops nowadays offer an increasing number of lettuce varieties.
Objective
To obtain data on the folate content and forms in common lettuce varieties and spinach sold in the Nordic countries, and to investigate effects of different storage conditions and preparations in the consumer's home or at lunchtime restaurants.
Design
Folate was analysed in eight different lettuce varieties and spinach using a validated high-performance liquid chromatographic method and the detected forms of folates were confirmed by a mass spectrometric detector [liquid chromatography–mass spectrometry (LC-MS)] following heat extraction, deconjugation with rat serum and purification by solid-phase extraction.
Results
Folate content, expressed in folic acid equivalents, in the lettuce samples varied six-fold, from 30 to 198 µg 100 g−1 on a fresh weight basis. The folate content was decreased by 14% after storage at 4°C for 8 days and by 2–40% after storage at 22°C for 2–4 h, depending on whether samples were stored as whole leaves, or small torn or cut pieces. LC-MS confirmed the identity of the folate forms: H4folate, 5-CH3-H4folate, 5-HCO-H4folate and 10-HCO-H4folate.
Conclusion
The considerable variation in folate content between varieties of lettuce in this pilot study, with one variety reaching the level found in spinach, indicates the potential to increase folate intake considerably by choosing folate-rich varieties of lettuce and storing at low temperatures.
Keywords: folate analysis; folate retention; HPLC, LC-MS; leafy vegetables; storage
Introduction
Leafy vegetables are common food items in a well-balanced diet, and increased consumption of fruit and vegetables is a general goal in public health work in Western countries. In a recent survey green salad was the third most popular vegetable in Sweden and Åland, fourth most popular in Finland and Denmark, and sixth most popular in Norway 1. Most lettuce varieties offered in Scandinavian food shops today derive from the four genera Lactuca (e.g. iceberg lettuce, oakleaf lettuce), Chicorium (e.g. frillice lettuce), Eruca (e.g. rocket) and Valerianella (e.g. manche).
Adequate intake of folate is an important factor in the prevention of neural tube defects and may be a factor in decreasing the risk of chronic diseases 2–5. The average daily intake of folate in the Nordic population, especially among fertile woman, is lower (∼200 µg folate day−1) than recommended (400 µg folate day−1) 3. In a review 6, plant foods (vegetables, fruit and potatoes) were stated to be predominant contributors to folate intake in Europe. Even in countries from Northern Europe, where plant food consumption is lower than in the various Mediterranean diets, plant foods are estimated to contribute approximately 40% of the total folate intake among adults 6. Despite the importance of folate in human nutrition, data on folate content in different vegetables, including lettuce varieties, are limited.
Multiple forms of folates with different susceptibility to degradation during storage exist in plant foods, and the precise distribution of these different forms depends not only on the species, but also on growth, harvest and postharvest conditions. The effect of different storage conditions on the folate concentrations in spinach was studied by Chen et al. 7, who found that the content of folate was reduced by 7% when stored for 10 h at room temperature and by 26% when stored for 7 days in the refrigerator (4°C). Pandrangi and LaBorde 8 studied the effect of storage temperature on retention of folate in commercially packaged fresh spinach and found that only 53% of folate was retained after 8 days at 4°C. In contrast, Mullin et al. 9 reported that folate levels in fresh spinach remained unchanged after storage at 4°C for 14 days. To the authors’ knowledge, only one study has been performed on other green leafy vegetables: Gami and Chen 10 kept Swiss chard at several temperatures and reported folate losses of 30% after 24 h at 21°C and 12% after 10 days at 4°C. However, data on folate content in lettuce stored in regular light for several hours, common in lunchtime restaurants, are not available.
Analysis of folate has been a challenge for a long time because of the large number of structural analogues, their instability and low levels in natural samples. There is no official method for measuring natural folates in foods. The traditionally used microbiological assay does not distinguish between individual forms of folate. High-performance liquid chromatography (HPLC) has to be used. However, a mass detector [i.e. liquid chromatography–mass spectrometry (LC-MS)] may be needed to quantify low levels of different forms of folates in food samples 11.
The aims of this pilot study were to obtain data on the main forms of folate (tetrahydrofolate, 5-methyl-tetrahydrofolate and 5-formyl-tetrahydrofolate) in common lettuce varieties and spinach sold in the Nordic countries, to investigate the effect of different storage conditions and preparations in the consumer's home or in lunchtime restaurants, and to detect other forms of folate with an LC-MS method.
Materials and methods
Sampling of lettuce and spinach
Fresh whole head lettuce was bought in three different local shops (ICA, Willys and Coop) on one or two occasions. Samples of butterhead lettuce (Lactuca sativa. var. capitata) (130 g), oakleaf lettuce (Lactuca sativa var. crispa) (175 g), frillice lettuce (Cichorium endivia) (130 g + 200 g), iceberg (Lactuca sativa var. capitata) (130 g + 360 g) and lollo rosso (Lactuca sativa var. crispa) (175 g + 200 g) sold as whole lettuce heads, stored in a plastic carton (PET), plastic wrapping [polypropylene (PP) or polyethylene (PE)] or without any wrapping were collected. Random leaves (∼12 g each) were ripped off the heads and immediately put in three plastic tubes (4 g each).
Frillice lettuce (105 g) and romaine lettuce (Lactuca sativa var. longifolia) (100 g) were bought as plants in a pot. Random leaves (12 g) were ripped off the plants and immediately put in three plastic tubes (4 g each).
Frillice lettuce (60 g), lollo rosso (40 g), mânche (Valerianella locusta) (60 g), rocket (Eruca sativa) (60 g) and spinach (Spinacia oleracea) (60 g) were bought as self-service picked salad. Random leaves were picked from each lettuce sample and immediately put in three plastic tubes (4 g each).
Rocket (175 g) and spinach (150 g) were bought packaged in a modified atmosphere (PET), already rinsed. Random leaves were picked from the bags and immediately put in three plastic tubes (4 g each). Rocket (Eruca sativa) (500 g + 500 g) was also bought as leaves in a plastic carton (PET). Random leaves were picked from the carton and immediately put in three plastic tubes (4 g each).
All tubes were flushed with nitrogen and immediately stored at −80°C.
Two samples of iceberg lettuce (samples no. 1 and 2) were placed on plates at room temperature (22°C) under regular light for 2 and 4 h. The leaves were either torn into pieces or cut with a smooth-edged knife. Samples of spinach, rocket and frillice lettuce were also placed on plates at room temperature for 2 and 4 h. Random leaves were picked from the plates and immediately put in three plastic tubes (4 g each).
Certified reference material, CRM 485 (lyophilized mixed vegetable), with certified folate content, was obtained from the Institute for Reference Materials and Measurements (Geel, Belgium) and stored as vacuum-packed subsamples (2 g) at −80°C until analysis.
Reagents and standards
All chemicals were purchased from Merck (Darmstadt, Germany) unless not otherwise stated. Acetonitrile was of isocratic grade for HPLC. Other chemicals were of analytical quality. Water was purified using a Milli-Q system (Millipore, USA). Thermostable α-amylase solution (E-BLAAM, 3000 U ml−1) was obtained from Megazyme International (Cork, Republic of Ireland) and used for sample pretreatment without additional preparation. Rat serum (RS) (Scanbur, Sollentuna, Sweden) was used as folate conjugase (γ-glutamyl hydrolase) source. RS was dialysed to remove endogenous folates as earlier described 12. Protease was purchased from Sigma Chemical Co. (St Louis, MO, USA). Protease solution (5 mg ml−1) was prepared in 10 mmol l−1 sodium acetate buffer and dialysed at 4°C with stirring in three steps using 800 ml of the 50 mmol l−1 phosphate buffer (pH 6.1) containing 0.1% (v/v) 2-mercaptoethanol (MCE) in each 40 min step. The dialysed protease solution was stored at −20°C until use within 2 weeks and the dialysed RS was stored in small portions (0.5 ml) at −80°C and used within 3 months.
Folic acid, (6S)-5-HCO-H4folate, sodium salt, (6S)-5-CH3-H4folate, sodium salt (6R)-5,10-CH+-H4folate and (6S)-H4folate, sodium salt, were donated by Eprova AG (Schaffhausen, Switzerland) and 10-HCO-folic acid was obtained from Dr Schirck's Laboratories (Jona, Switzerland). Standard stock solutions of 10-HCO-H2folate and 5,10-CH+-H4folate were prepared according to Pfeiffer et al. (1997) 13, while solutions of folic acid, 5-HCO-H4folate, 10-HCO-folic acid and 5-CH3-H4folate were prepared as described previously 14. Stock solution of H4folate (200 µg ml−1, purity corrected) was prepared in elution buffer [0.1 M sodium acetate containing 10% (w/v) NaCl, 1% (w/v) ascorbic acid (AA) and 0.1% (v/v) 2.3-dimercapto-1-propanol (BAL) (Sigma-Aldrich, St Louis, MO, USA)]. Aliquots of standard stock solutions were placed in separate tubes, flushed with nitrogen and stored at −80°C for a maximum of 90 days. Calibration solutions were prepared immediately before use by dilution of stock solutions with elution buffer.
Dry matter
Dry matter of lettuce samples was determined in duplicate according to AOAC method 950.46B 15 at 105–109°C for 16.5 h.
Sample preparation
All samples were extracted in triplicate. Samples were protected against folate oxidation throughout the preparation process by nitrogen, subdued light and cooling on ice after heating. The sample tubes (4 g) were taken from the freezer (−80°C) and immediately put in an igloo containing liquid nitrogen. After 1 min the cap was opened and the lettuce sample was crushed with a cooled spatula. Freshly prepared extraction buffer [15 ml of 0.1 M phosphate buffer (pH 6.1) containing 1% (w/v) AA and 0.1% (v/v) BAL] was added to the sample tube. For CRM 485, 0.3 g lyophilized powder was added to 15 ml in extraction buffer. Samples were extracted, conjugated and centrifuged as described previously 14. For deconjugation of polyglutamate forms of folate, 75 µl RS was added to 3.2 ml supernatant. To evaluate the effect of dienzyme and trienzyme treatment, rocket samples, in triplicate, were also prepared with 40 µl α-amylase during the extraction step and incubation with 1 ml protease solution for 1.5 h at 37°C followed by 5 min deactivation by boiling before the deconjugation step.
All prepared extracts were frozen below −20°C before sample clean-up. Blank samples containing the enzyme suspensions were prepared and treated in the same way as the lettuce samples for corrections of endogenous folate. Before quantification, extracts were purified by solid-phase extraction (SPE) on strong anion exchange (SAX) Isolute cartridges (500 mg; International Sorbent Technology, Mid-Glamorgan, UK). Aliquots (2.5 ml) of extract were applied to the preconditioned cartridges and eluted with freshly prepared elution buffer (prepared as described in Reagents and standards). The first portion (0.5 ml) of eluate was discarded and the second portion (3.5 ml) was weighed and collected for HPLC analysis. To eliminate interference more effectively, a combination with PH EC cartridges prior to the SAX sorbents was also investigated, as described previously 16.
Recovery tests were performed by adding known amounts of H4folate and 5-CH3-H4folate at two levels to a spinach sample in triplicate before extraction. The repeatability of the analytical procedure was checked by always including a certified reference material CRM 485 sample on each extraction day.
High-performance liquid chromatography
Quantification by HPLC was performed on an Agilent 1100 system as described previously 12. The folates were separated on a Thermo Electron-Corporation Aquasil C18 column (3 µm, 150×4.6 mm) with a C18 (1 mm) guard column (Optimize Technologies). The chromatographic conditions for gradient elution were as follows: column temperature, 23°C; autosampler temperature, 8°C, flow rate 0.4 ml min−1; volume injected, 20 µl; fluorescence detection (FD), 290 nm excitation and 360 nm emission; ultraviolet (UV) detection, 280, 290 and 300 nm. The mobile phase consisted of 30 mM potassium phosphate buffer (pH 2.3), using a gradient with acetonitrile starting at 6% (v/v) which was maintained isocratically for the first 5 min; thereafter, the acetonitrile concentration was increased linearly to 25% within 20 min and the total run time was 42 min. Peaks were identified by retention times and identity was confirmed by comparing ratios of fluorescence and UV peak heights (h) at different wavelengths; in addition, fluorescence and diode array spectra were used to verify individual folates. Quantification was based on an external standard method. The peak area was plotted against concentration and least-square regression analysis was used to fit lines to the data. A multilevel calibration curve was used (n=7) and the amount of each form of folate was calculated in its free acid form. Total folate was calculated as the sum of H4folate, 5-CH3-H4folate and 5-HCO-H4folate, expressed as folic acid equivalents (MW, 441.4) considering different molecular weights for the three native folate forms.
Liquid chromatography–mass spectrometry
To investigate further forms of folates in the lettuce samples, seven of the sample extracts were analysed on an LC-MS single quadrupole system (Agilent 1100 series) using an in-house optimized and validated method 17. The column (Ace C18, 150 mm×4.6 mm, 3 µm; Advanced Chromatography Technologies, Aberdeen, UK) was used for separation. The flow rate was 0.3 ml min−1, the injection volume was 20 µl and the mobile phase used was acetonitrile–methanol (6%) and 0.01 M acetic acid, under linear gradient elution conditions. Electrospray ionization was operated in positive ion mode. Fragmentor potential was set to 100 V for all forms of folates. For quantification of folates selected ion monitoring of protonated molecular ions [M + H]+ was used at m/z 460 for 5-CH3-H4folate, m/z 442 for folic acid, m/z 472 for 10-HCO-H2folate, m/z 474 for 5-HCO-H4folate, m/z 470 for 10-HCO-folic acid, m/z 446 for H4folate and m/z 457 for 5,10-CH+-H4folate. Mass spectral data as well as retention times of compounds were used for peak identification and quantification was based on an external standard method (n=8).
Statistical analysis and calculation of nutrient retention
The results are presented as mean values from triplicates, except for the results of the LC-MS analysis, where single values are presented. Statistical analysis were performed with Tukey's pairwise comparison (α = 0.05) using Minitab software, release 14 (Minitab, Coventry, UK) and significant differences were considered from p<0.05.
Results and discussion
Folates in leafy vegetables
The folate content and forms of eight different lettuce varieties and spinach based on LC-FD analyses are presented in Table 1. The lettuce varieties were purchased in three local shops, originated from six European countries and were sold with different kinds of wrapping as described in Materials and methods and Table 1. The total folate concentration expressed in folic acid equivalents and calculated from the sum of H4folate, 5-CH3-H4folate and 5-HCO-H4folate varied from 30 to 198 µg 100 g−1 on a fresh weight (FW) basis. Spinach contained in total 172–177 µg 100 g−1 FW. The three rocket samples showed a large variation in the total folate concentrations (108, 181 and 198 µg 100 g−1 FW). Lollo rosso could also be considered as a good folate source since all three samples contained total folate concentrations above 90 µg 100 g−1 FW (90–125 µg 100 g−1 FW). In contrast, the popular iceberg lettuce contained only 30, 38 and 43 µg total folate 100 g−1 FW in the three samples analysed. Frillice lettuce (48–112 µg 100 g−1 FW), romaine lettuce (56 µg 100 g−1 FW), butterhead lettuce (71 µg 100 g−1 FW) and oakleaf (74 µg 100 g−1 FW) were shown to be good to moderate folate sources.
Three different forms of folate were separated and quantified by LC-FD. The major form of folate was 5-CH3-H4folate, followed by 5-HCO-H4folate and H4folate, but their relative proportions varied between cultivars and within varieties. The most labile form, H4folate, for self-service picked spinach was only half that of spinach packaged in a modified atmosphere, which could be expected since H4folate is susceptible to oxidative degradation. The degradation of H4folate could also be observed when comparing self-service picked rocket with rocket packed in a modified atmosphere. However, it is difficult to draw any conclusions on the preferred packing method. A reasonable explanation is that not only did the method of packaging differ between samples belonging to the same leafy vegetable cultivar, but also that they originated from batches that differed in cultivation conditions, harvest and postharvest conditions. More experiments with several lettuce samples from each packing method are needed to be able to draw significant conclusions.
Folate retention during storage
One sample (in triplicate) each of self-service picked frillice lettuce and of self-service picked spinach was selected to investigate the retention of folates when stored at room temperature (22°C) and in regular light on a plate for 2 or 4 h to simulate the condition in lunchtime restaurants. One sample of rocket sold in a plastic carton was also chosen for this study and lettuce from that sample was also placed in the refrigerator (4°C) for 8 days, stored in its original plastic carton.
The total folate content (folic acid equivalents) was reduced by 14–40% on a dry matter basis in all the stored lettuce samples. As shown in Fig. 1, frillice lettuce showed a significant (p<0.05) reduction of 40% in total folates after 4 h. The rocket purchased in a plastic carton also lost significant (p<0.05) amounts (22%) of total folates after 4 h at 22°C. In the refrigerator, 14% of the total folates in rocket were lost. This reduction, however, was not significant (p>0.05). Spinach already showed a significant (p<0.05) reduction of 30% in total folate content after 2 h in room temperature. Given the minor changes in moisture content (< 1% in all processes), folate losses in the stored samples were due to degradation of folate.
Fig. 1.
(%) of total folates in rocket, frillice lettuce and spinach on a dry matter basis when stored on a plate in regular light for 2 or 4 h at 22°C. Leaves from frillice were also stored in the refrigerator (4°C) for 8 days. Values are means±SD (for three replicates) as shown by vertical bars. Different letters show statistically significant (p<0.05) differences in folate content after different storage conditions compared with the product directly after purchase.
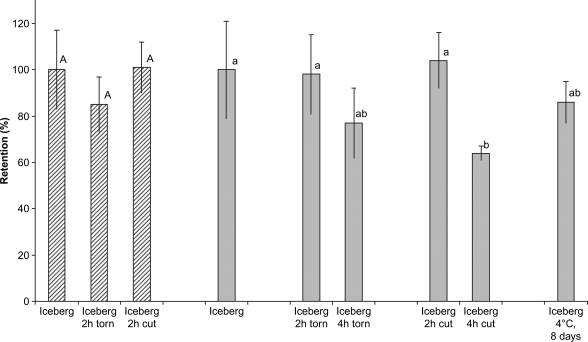
As shown in Fig. 2, the content of total folates in two different iceberg lettuce heads (nos 1 and 2) was well retained in iceberg lettuce pieces torn or cut from the heads and stored for 2 h. After 4 h, 23% of the total folate content was lost in the pieces that were torn from the lettuce head (no. 2) and 36% of the total folates in the pieces cut from the head (no. 2). However, only the reduction in the lettuce pieces cut with a knife from the iceberg head was significant (p<0.05). In the refrigerator 14% of total folate was lost after 8 days’ storage, a non-significant change, which is similar to the 12% losses of total folates reported in Swiss chard after 10 days 10.
Fig. 2.
(%) of total folates (calculated on a dry matter basis) in two different samples (no. 1, striped and no. 2, shaded) of torn or cut iceberg lettuce stored on a plate in regular light for 2 and 4 h at 22°C, respectively, compared with whole heads. Leaves from one lettuce head (no. 2) were stored in the refrigerator (4°C) for 8 days. Values are means±SD (for three replicates) as shown by vertical bars. Different letters show statistically significant (p<0.05) differences in folate content after different storage conditions compared with the product directly after purchase.
Methodological comparisons and quality assurance of folate analysis
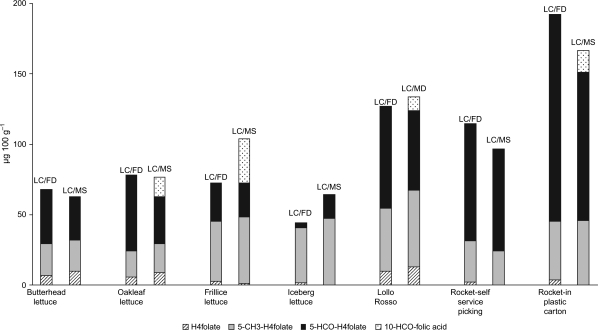
In the present study, LC-MS was used as a quantification method to confirm the forms of folate found in the leafy vegetables analysed. 5-CH3-H4folate and 5-HCO-H4folate were detected in all leafy vegetables with both LC-FD and LC-MS. H4folate was found in all samples using LC-FD, but only in small amounts in iceberg lettuce (<1.5 µg 100 g−1). When LC-MS was used as a quantification method no H4folate was detected in iceberg lettuce or rocket, but data were similar for the three forms of folate (H4folate, 5-CH3-H4folate and 5-HCO-H4folate) when analysed by either LC-FD or LC-MS (Fig. 3). The LC-MS method also detected 10-HCO-folic acid in four out of the seven lettuce samples analysed, whereas 10-HCO-H2folate and 5,10-CH+-H4folate were not detected in any of the samples.
Fig. 3.
Comparisons between the distribution of different forms of folate in seven different varieties of lettuce when quantified with liquid chromatography with fluorescence detector (LC-FD) (n=3) and liquid chromatography with mass spectrometer detector (LC-MS) (n=1).
The LC-MS data indicated that formyl forms of folate are the dominant form of folate in all lettuce varieties except for iceberg lettuce. This was not as consistent when using LC-FD as the quantification method (Table 1). Formyl folates in leafy vegetables analysed by LC-FD or LC-MS-MS have been reported previously 11, 18 19, but not as the dominant form as seen in this study. Therefore, further analyses with LC-MS or LC-MS-MS are needed to confirm these results.
Table 1.
Mean content of individual and total folate (µg 100 g−1) in several lettuce varieties and spinach on a fresh weight basisa
| Name | Description | Country of origin | H4folateb (µg 100 g−1) | 5-CH3-H4folatec (µg 100 g−1) | 5-HCO-H4folated (µg 100 g−1) | Total folatee (folic acid equivalents) (µg 100 g−1) |
|---|---|---|---|---|---|---|
|
| ||||||
| Butterhead lettucef | Whole head, not packed | Sweden | 7.0±3.7 | 22.3±2.5 | 38.7±13 | 70.8±7.5 |
| Frillice lettuceg | As a plant | Sweden | 6.3±3.2 | 32.3±9.1 | 11.2±5.7 | 47.8±3.4 |
| Frillice lettucef | Whole head in plastic carton | Sweden | 2.5±2.1 | 42.7±6.1 | 27.5±2.3 | 69.4±2.3 |
| Frillice lettuceh | In plastic bags | Denmark | 3.6±3.5 | 68.5±3.8 | 7.3±2.7 | 72.8±3.1 |
| Frillice lettucef | Self-service picked salad | Sweden | 9.3±2.0 | 65.5±1.0 | 42.2±20 | 112±21 |
| Icebergf | Whole head in plastic bag | Germany | 1.2±0.0 | 26.8±1.9 | 2.9±1.2 | 29.7±0.6 |
| Icebergf | Whole head in plastic wrapping | Spain | 0.2±0.1 | 34.7±3.2 | 7.5±0.4 | 38.2±7.1 |
| Icebergh | Whole head in plastic wrapping | Germany | 1.5±0.4 | 39.1±7.6 | 3.8±3.3 | 42.7±9.0 |
| Lollo rossof | In plastic carton | France | 5.3±1.1 | 38.8±1.1 | 51±1.5 | 90.5±1.5 |
| Lollo rossof | Self-service picked salad | Sweden | 9.6±1.4 | 54.2±3.6 | 42.0±12 | 101±8.2 |
| Lollo rossoh | In plastic bags | Denmark | 9.7±3.7 | 44.9±17 | 72.2±17 | 125±22 |
| Manchef | Self-service picked salad | France | 6.4±1.7 | 41.9±1.9 | 72.0±13 | 114±9.5 |
| Oakleaf lettucef | In plastic carton | Sweden | 5.6±2.7 | 18.7±4.1 | 54.0±19 | 74.2±13 |
| Rocketg | Self-service picked salad | Italy | 2.0±0.3 | 29.5±0.7 | 83.0±12 | 108±15 |
| Rocketf | In plastic carton | Italy | 3.5±2.8 | 41.7±6.7 | 147±1.4 | 181±9.7 |
| Rocketg | In protective atmosphere; rinsed | Unknown | 2.8±3.8 | 54.0±8.1 | 152±7.5 | 198±17 |
| Romaine lettucef | As a plant | Sweden | 2.9±1.3 | 39.4±8.8 | 21.9±9.9 | 56.6±13 |
| Spinachg | In protective atmosphere; rinsed | Unknown | 28.1±3.8 | 101±34 | 51.1±1.7 | 172±29 |
| Spinachf | Free-choice delicatessen salad | Italy | 14.5±6.5 | 95.1±24 | 76.1±22 | 177±34 |
Folates were analysed by liquid chromatography–fluorescence detection (LC-FD).
a All folate results are means of triplicates±SD; b H4folate = tetrahydrofolate; c 5-CH3-H4folate = 5-methyl-tetrahydrofolate; d 5-HCO-H4folate = 5-formyl-tetrahydrofolate; e total folate = measured as the sum of H4folate, 5-CH3-H4folate, 5-HCO-H4folate, expressed as folic acid equivalents; f, g, h samples purchased at three different shops: ICA, Coop and Willys, respectively.
Previously published data on folate content based on microbiological assay are of the same magnitude as the present total folate data obtained with LC-FD or LC-MS. The few comparative data found on folate concentrations in the literature based on LC-FD or LC-MS-MS are also in similar ranges. Thus, Konings et al. 19 reported a total folate content of 42 µg 100 g−1 in iceberg lettuce using an LC-FD method. In the Nordic food tables the total folate values in iceberg lettuce are reported to be 56 µg 100 g−1 20, 53 µg 100 g−1 21 and 89 µg 100 g−1 21, respectively, all analysed with the traditional microbiological assay.
One of the critical steps in folate analyses is sample preparation, e.g. heat extraction, combined with different enzyme treatments to release efficiently all folates from the food matrix. In the present study, folates were extracted from the food matrix using 0.1 M phosphate buffer with added antioxidants (ascorbic acid and BAL). BAL was chosen as an antioxidant since it has been shown to be the best stabilizer of the most labile form of folate, H4folate, in phosphate buffer 23. To improve folate extraction from the food matrix and simultaneously stabilize the folates, liquid nitrogen was used to homogenize the lettuce samples efficiently and gently before heat extraction and enzyme treatments, as previously reported 11. Monoenzyme treatment with rat serum conjugase was chosen since lettuce and spinach are known to contain negligible amounts of starch and protein and have previously been shown not to require amylase and protease treatment 19, 24.
Conclusions
Data on the concentration of individual forms of folates (H4folate, 5-CH3-H4folate and 5-HCO-H4folate) were obtained for eight different lettuce varieties and spinach sold in different wrappings and as whole lettuce heads or loose leaves. The forms of folates were, in decreasing proportions: formylated reduced folates > methylated reduced folates > tetrahydrofolate. These results are in contrast to previous reported results, where methylated reduced folates were dominant 11, 18 19. This study clearly indicated the need for a mass spectrometry detector to quantify and characterize all forms of folate. Further studies on folate quantification in foods should use optimized and validated sample preparation combined with LC-MS or LC-MS-MS analysis.
Storage of torn or cut fresh lettuce for up to 2 h in room temperature or for 8 days in a refrigerator caused no significant folate losses. After 4 h at room temperature folate was significantly decreased in spinach, rocket and frillice lettuce. Spinach lost significant amounts of folate after 2 h at room temperature.
The total folate content in the lettuce samples varied from 30 to 198 µg 100 g−1 on a fresh weight basis; rocket contained total folate concentrations above 100 µg 100 g−1, while iceberg contained only 30–43 µg total folate 100 g−1. These figures are within the ranges reported previously 19, 20 25. The six-fold variation in total folate content between various lettuce varieties in this pilot study argues for a replacement of the most popular iceberg lettuce with varieties higher in folate, e.g. lollo rosso and rocket. Further analyses with optimized and validated LC-MS or LC-MS-MS analyses are needed to obtain more representative data on total folate content and forms in lettuce and other leafy vegetables.
Acknowledgments
The gift of folate standards from Eprova AG Schaffhausen is gratefully acknowledged. Special thanks to Christina Nilsson, Division of Meat Science, Department of Food Science, SLU, for assistance with the liquid nitrogen. We also thank the folate group, especially Veronica Öhrvik and Johan Patring at the Division of Food Chemistry, Department of Food Science, SLU, for valuable support with the methodology.
References
- 1.Similä M, Fagt S, Vaask S, Thorgeirsdottir H, Padule I, Petkeviciene J, et al. The NORBAGREEN 2002 study. Copenhagen: Nordic Council of Ministers; 2003. [Google Scholar]
- 2.Anon. WHO TRS 916. Diet, nutrition and the prevention of chronic diseases. Report from a joint FAO/WHO Expert Consultation; Geneva: World Health Organization; 2003. [Google Scholar]
- 3.NNR, Nordic Nutrition Recommendations 2004; Copenhagen: Nordic Council of Ministers; 2004. Nord 2004:13. [Google Scholar]
- 4.Bailey LB, Rampersaud GC, Kauwell GPA. Folic acid supplements and fortification affect the risk for neural tube defects, vascular disease and cancer: evolving science. J Nutr. 2003;133:1961–8S. doi: 10.1093/jn/133.6.1961S. [DOI] [PubMed] [Google Scholar]
- 5.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA. 1995;274:1049–57. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 6.de Bree A, van Dusseldorp M, Brouwer IA, van het Hof KH, Steegers-Theunissen RPM. Folate intake in Europe: recommended, actual and desired intake. Eur J Clin Nutr. 1997;51:643–60. doi: 10.1038/sj.ejcn.1600467. [DOI] [PubMed] [Google Scholar]
- 7.Chen T-S, Song Y-O, Kirsch AJ. Effects of blanching, freezing and storage on folacin contents of spinach. Nutr Rep Int. 1983;28:317–23. [Google Scholar]
- 8.Pandrangi S, LaBorde LF. Retention of folate, carotenoids, and other quality characteristics in commercially packaged fresh spinach. J Food Sci. 2004;69:C702–7. [Google Scholar]
- 9.Mullin WJ, Wood DF, Howsam SG. Some factors affecting folacin content of spinach, Swiss chard, broccoli and brussels sprouts. Nutr Rep Int. 1982;26:7–16. [Google Scholar]
- 10.Gami DB, Chen T-S. Kinetics of folacin destruction in Swiss chard during storage. J Food Sci. 1985;50:447–9. [Google Scholar]
- 11.Zhang GF, Storozhenko S, van der Straeten D, Lambert WE. Investigation of the extraction behaviour of the main monoglutamate folates from spinach by liquid chromatography–electrospray ionization tandem mass spectrometry. J Chromatogr A. 2005;1078:59–66. doi: 10.1016/j.chroma.2005.04.085. [DOI] [PubMed] [Google Scholar]
- 12.Patring JDM, Jastrebova JA, Hjortmo SB, Andlid T, Jägerstad MI. Development of a simplified method for simultaneous determination of folates in bakers’ yeast by liquid chromatography with ultraviolet and fluorescence detection. J Agric Food Chem. 2005;53:2406–11. doi: 10.1021/jf048083g. [DOI] [PubMed] [Google Scholar]
- 13.Pfeiffer CM, Rogers LM, Bailey LB, Gregory JF. Absorption of folate from fortified cereal-grain products and of supplemental folate consumed with or without food determined using a dual-label stable-isotope protocol. Am J Clin Nutr. 1997;66:1388–97. doi: 10.1093/ajcn/66.6.1388. [DOI] [PubMed] [Google Scholar]
- 14.Holte Stea T, Johansson M, Jägerstad M, Frølich W. Retention of folates in cooked, stored and reheated peas, broccoli and potatoes for use in modern large-scale service systems. Food Chem. 2006;101:1095–107. [Google Scholar]
- 15.AOAC. Official methods of analysis. In: Horwitz W, editor. AOAC official method 992.05 folic acid (pteroylglutamic acid) in infant formula; Gaithersburg, MD: AOAC International; 2000. 50.024–50.6. [Google Scholar]
- 16.Nilsson C, Johansson M, Yazynina E, Strålsjö L, Jastrebova J. Solid-phase extraction for HPLC analysis of dietary folates. Eur Food Res Technol. 2004;219:199–204. [Google Scholar]
- 17.Patring JDH, Jastrebova JA. Application of liquid chromatography – electrospray ionisation mass spectrometry for determination of dietary folates: Effects of buffer nature and mobile phase composition on sensitivity and selectivity. J Chromatogr A. 2007;1143:72–82. doi: 10.1016/j.chroma.2006.12.079. [DOI] [PubMed] [Google Scholar]
- 18.Freisleben A, Schieberle P, Rychlik M. Specific and sensitive quantification of folate vitamers in foods by stable isotope dilution assays using high-performance liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem. 2003;376:149–56. doi: 10.1007/s00216-003-1844-y. [DOI] [PubMed] [Google Scholar]
- 19.Konings EJ, Roomans HH, Dorant E, Goldbohm RA, Saris WH, van den Brandt PA. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am J Clin Nutr. 2001;73:765–76. doi: 10.1093/ajcn/73.4.765. [DOI] [PubMed] [Google Scholar]
- 20.SLV. Food composition table – energy and nutrients. Uppsala: SLV; 2002. [Google Scholar]
- 21.Fineli. Finnish food composition database. Helsinki: Finnish Public Health Institute, Nutrition Unit; 2004. [Google Scholar]
- 22.Møller A, Saxholt E, Christensen AT, Hartkopp HB, Hess Ygil K. Danish food composition databank, revision 6.0; 2005. Department of Nutrition, Danish Institute for Food and Veterinary Research. http://www.foodcomp.dk/:Food Informatics. [Google Scholar]
- 23.Patring JDM, Johansson MS, Yazynina E, Jastrebova JA. Evaluation of impact of different antioxidants on stability of dietary folates during food sample preparation and storage of extracts prior to analysis. Anal Chim Acta. 2005;553:36–42. [Google Scholar]
- 24.Shrestha AK, Arcot J, Paterson J. Folate assay of food by traditional and tri-enzyme treatments using cryoprotected Lactobacillus casei . Food Chem. 2000;71:545–52. [Google Scholar]
- 25.Simonne A, Simonne E, Eitenmiller R, Coker CH. Bitterness and composition of lettuce varieties grown in the southeastern United States. Horttechnology. 2002;12:721–6. [Google Scholar]