Abstract
We were unable to demonstrate the presence of the classic enterobacterium-type lipopolysaccharide in the cells of the Lyme disease spirochete, Borrelia burgdorferi B31. This finding was primarily based on chemical analysis and the absence of free lipid A upon mild acid hydrolysis of the appropriate cell extracts. These results do not preclude the possible existence of an unusual lipopolysaccharide-like compound(s) in B. burgdorferi.
Full text
PDF
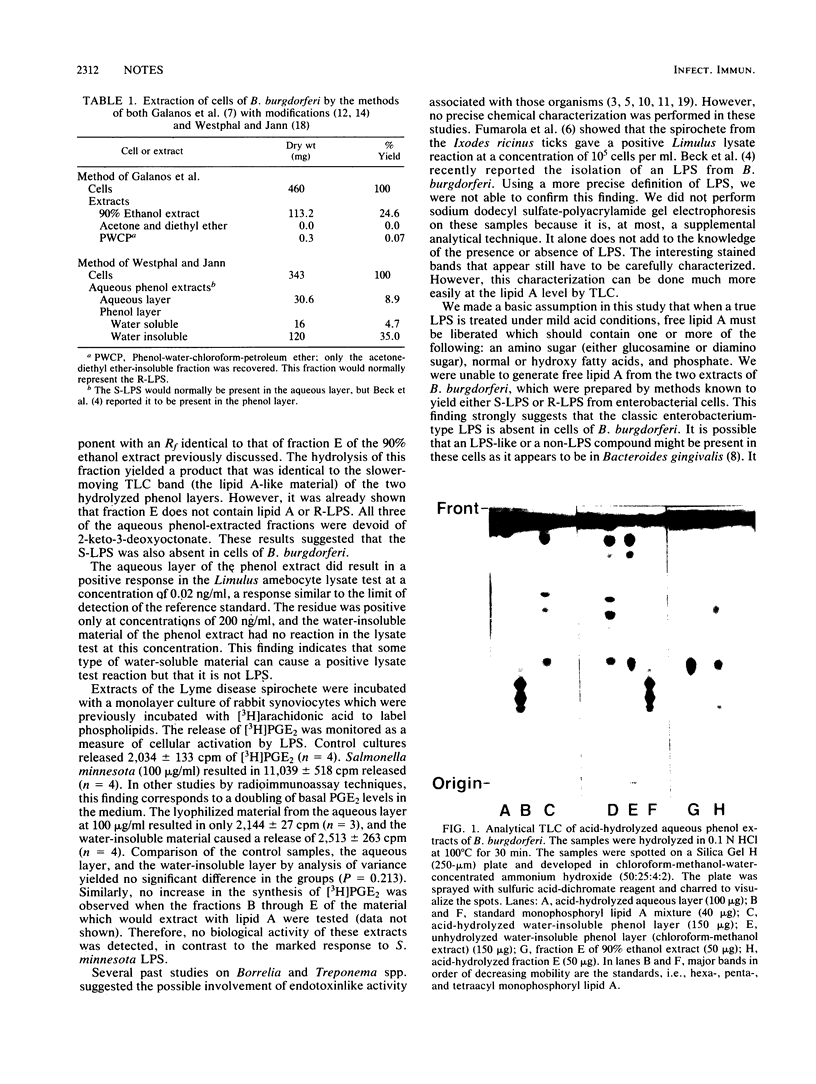

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum D. H., Joens L. A. Serotypes of beta-hemolytic Treponema hyodysenteriae. Infect Immun. 1979 Sep;25(3):792–796. doi: 10.1128/iai.25.3.792-796.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck G., Habicht G. S., Benach J. L., Coleman J. L. Chemical and biologic characterization of a lipopolysaccharide extracted from the Lyme disease spirochete (Borrelia burgdorferi). J Infect Dis. 1985 Jul;152(1):108–117. doi: 10.1093/infdis/152.1.108. [DOI] [PubMed] [Google Scholar]
- D'ALESSANDRO G., DEL CARPIO C. A lipopolysaccharide antigen of the Treponema. Nature. 1958 Apr 5;181(4614):991–992. doi: 10.1038/181991b0. [DOI] [PubMed] [Google Scholar]
- Fumarola D., Munno I., Miragliotta G. "Endotoxicity" of the Lyme disease spirochete. Infection. 1983 Nov-Dec;11(6):345–345. doi: 10.1007/BF01641362. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Koga T., Nishihara T., Fujiwara T., Nisizawa T., Okahashi N., Noguchi T., Hamada S. Biochemical and immunobiological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun. 1985 Mar;47(3):638–647. doi: 10.1128/iai.47.3.638-647.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuessen M. E., Birmingham J. R., Joens L. A. Biological activity of a lipopolysaccharide extracted from Treponema hyodysenteriae. Infect Immun. 1982 Jul;37(1):138–142. doi: 10.1128/iai.37.1.138-142.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuessen M. E., Joens L. A., Glock R. D. Involvement of lipopolysaccharide in the pathogenicity of Treponema hyodysenteriae. J Immunol. 1983 Aug;131(2):997–999. [PubMed] [Google Scholar]
- Qureshi N., Mascagni P., Ribi E., Takayama K. Monophosphoryl lipid A obtained from lipopolysaccharides of Salmonella minnesota R595. Purification of the dimethyl derivative by high performance liquid chromatography and complete structural determination. J Biol Chem. 1985 May 10;260(9):5271–5278. [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Heller D., Fenselau C. Position of ester groups in the lipid A backbone of lipopolysaccharides obtained from Salmonella typhimurium. J Biol Chem. 1983 Nov 10;258(21):12947–12951. [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P., Anderson L., Raetz C. R. Glucosamine-derived phospholipids in Escherichia coli. Structure and chemical modification of a triacyl glucosamine 1-phosphate found in a phosphatidylglycerol-deficient mutant. J Biol Chem. 1983 Dec 10;258(23):14245–14252. [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P., Nashed M. A., Anderson L., Raetz C. R. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A. Complete structure of A diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J Biol Chem. 1983 Jun 25;258(12):7379–7385. [PubMed] [Google Scholar]
- Weckesser J., Drews G., Fromme I., Mayer H. Isolation and chemical composition of the lipopolysaccharides of Rhodopseudomonas palustris strains. Arch Mikrobiol. 1973;92(2):123–138. doi: 10.1007/BF00425010. [DOI] [PubMed] [Google Scholar]
- Wright D. J. Reaction following treatment of murine borreliosis and Shwartzman type reacion with borrelial sonicates. Parasite Immunol. 1980 Autumn;2(3):201–221. doi: 10.1111/j.1365-3024.1980.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Yaron M., Yaron I., Smetana O., Eylan E., Zor U. Stimulation of prostaglandin E production by bacterial endotoxins in cultured human synovial fibroblasts. Arthritis Rheum. 1980 Aug;23(8):921–925. doi: 10.1002/art.1780230807. [DOI] [PubMed] [Google Scholar]




