Abstract
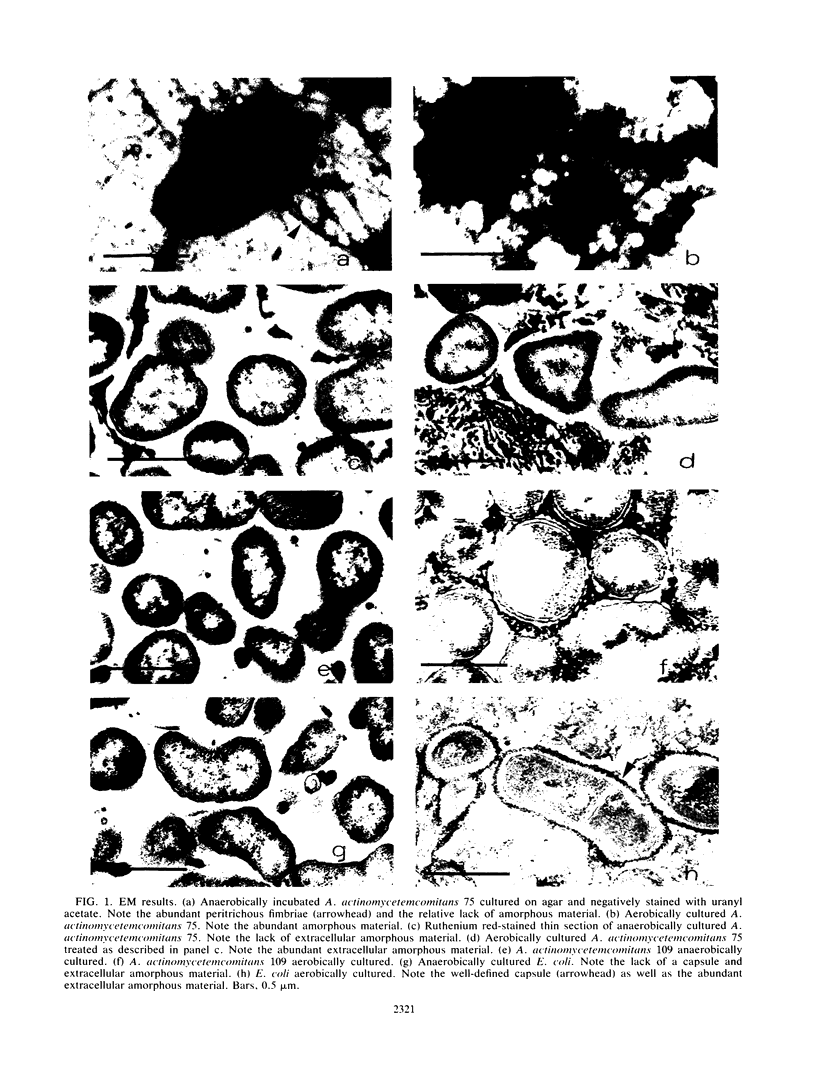
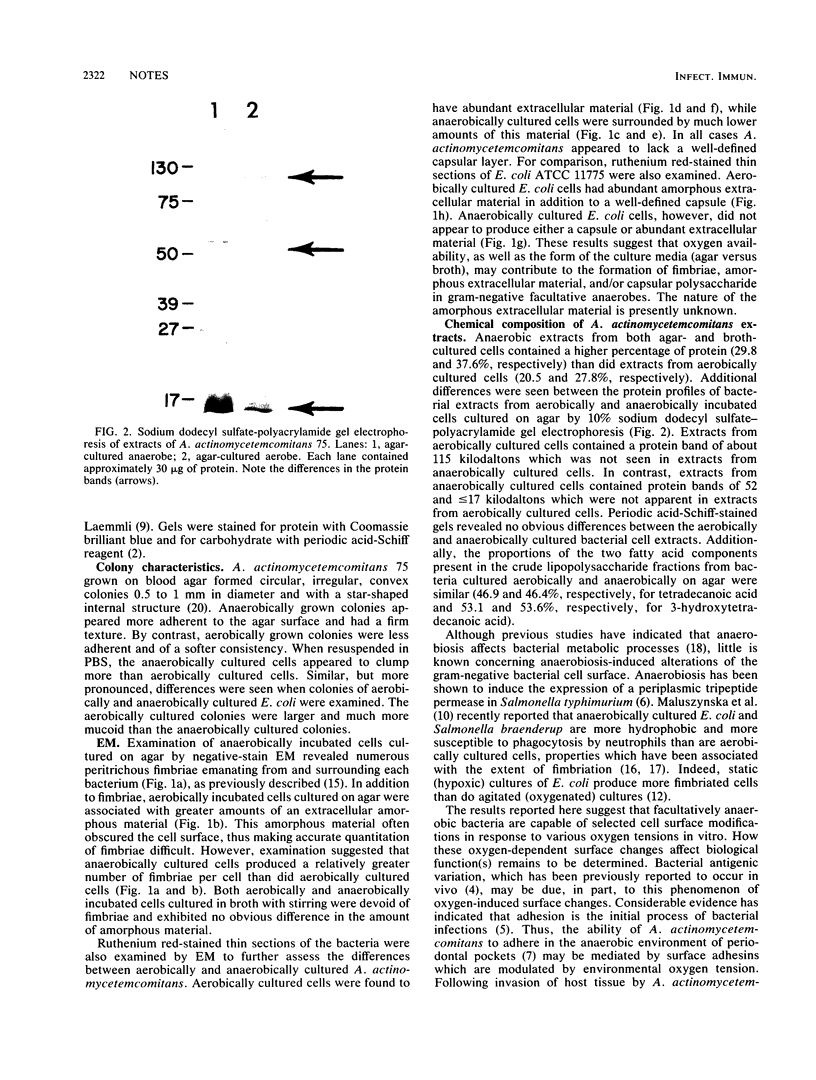
The ultrastructures and surface protein profiles of aerobically cultured Actinobacillus actinomycetemcomitans (Haemophilus actinomycetemcomitans) differed from those of cells cultured anaerobically. Similar ultrastructural differences were also observed when aerobic and anaerobic cultures of a strain of Escherichia coli were compared. These results suggest that oxygen-related variations in the bacterial cell surface may play a role in the adaptation of oral bacteria to different host environments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. R., Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson D. J., Higgins C. F. Anaerobic and leucine-dependent expression of a peptide transport gene in Salmonella typhimurium. J Bacteriol. 1984 Oct;160(1):131–136. doi: 10.1128/jb.160.1.131-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney E. B., Ash M. M., Jr Oxidation reduction potential of developing plaque, periodontal pockets and gingival sulci. J Periodontol. 1969 Nov;40(11):630–633. doi: 10.1902/jop.1969.40.11.630. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Hardy L. N., Markevics L. J., Evans J. D., Wicken A. J. Comparative studies on the effect of growth conditions on adhesion, hydrophobicity, and extracellular protein profile of Streptococcus sanguis G9B. Infect Immun. 1985 Nov;50(2):545–554. doi: 10.1128/iai.50.2.545-554.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maluszynska G. M., Stendahl O., Magnusson K. E. Interaction between human polymorphonuclear leukocytes (PMNL) and bacteria cultivated in aerobic and anaerobic conditions. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):139–143. doi: 10.1111/j.1699-0463.1985.tb02865.x. [DOI] [PubMed] [Google Scholar]
- Millar S. J., Goldstein E. G., Levine M. J., Hausmann E. Modulation of bone metabolism by two chemically distinct lipopolysaccharide fractions from Bacteroides gingivalis. Infect Immun. 1986 Jan;51(1):302–306. doi: 10.1128/iai.51.1.302-306.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H., Pilowsky K., Zilm P. S. The effect of growth rate on the adhesion of the oral bacteria Streptococcus mutans and Streptococcus milleri. Arch Oral Biol. 1984;29(2):147–150. doi: 10.1016/0003-9969(84)90119-5. [DOI] [PubMed] [Google Scholar]
- Scannapieco F. A., Kornman K. S., Coykendall A. L. Observation of fimbriae and flagella in dispersed subgingival dental plaque and fresh bacterial isolates from periodontal disease. J Periodontal Res. 1983 Nov;18(6):620–633. doi: 10.1111/j.1600-0765.1983.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensäter G., Takahashi-Abbe S., Abbe K., Birkhed D., Yamada T., Edwardsson S. Anaerobic and aerobic metabolism of sorbitol in Streptococcus sanguis and Streptococcus mitior. J Dent Res. 1985 Nov;64(11):1286–1289. doi: 10.1177/00220345850640110601. [DOI] [PubMed] [Google Scholar]
- Zambon J. J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985 Jan;12(1):1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Slots J., Genco R. J. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983 Jul;41(1):19–27. doi: 10.1128/iai.41.1.19-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]