Summary
Background
Malaria is a major cause of morbidity and mortality in Africa. International effort and funding for control has been stepped up, with substantial increases from 2003 in the delivery of malaria interventions to pregnant women and children younger than 5 years in The Gambia. We investigated the changes in malaria indices in this country, and the causes and public-health significance of these changes.
Methods
We undertook a retrospective analysis of original records to establish numbers and proportions of malaria inpatients, deaths, and blood-slide examinations at one hospital over 9 years (January, 1999–December, 2007), and at four health facilities in three different administrative regions over 7 years (January, 2001–December, 2007). We obtained additional data from single sites for haemoglobin concentrations in paediatric admissions and for age distribution of malaria admissions.
Findings
From 2003 to 2007, at four sites with complete slide examination records, the proportions of malaria-positive slides decreased by 82% (3397/10861 in 2003 to 337/6142 in 2007), 85% (137/1259 to 6/368), 73% (3664/16932 to 666/11333), and 50% (1206/3304 to 336/1853). At three sites with complete admission records, the proportions of malaria admissions fell by 74% (435/2530 to 69/1531), 69% (797/2824 to 89/1032), and 27% (2204/4056 to 496/1251). Proportions of deaths attributed to malaria in two hospitals decreased by 100% (seven of 115 in 2003 to none of 117 in 2007) and 90% (22/122 in 2003 to one of 58 in 2007). Since 2004, mean haemoglobin concentrations for all-cause admissions increased by 12 g/L (85 g/L in 2000–04 to 97 g/L in 2005–07), and mean age of paediatric malaria admissions increased from 3·9 years (95% CI 3·7–4·0) to 5·6 years (5·0–6·2).
Interpretation
A large proportion of the malaria burden has been alleviated in The Gambia. Our results encourage consideration of a policy to eliminate malaria as a public-health problem, while emphasising the importance of accurate and continuous surveillance.
Funding
UK Medical Research Council.
Introduction
Malaria is a major public-health problem in Africa, including The Gambia where it has been one of the main causes of mortality and morbidity in children younger than 5 years,1 pregnant women,2 and non-immune visitors.3 Investigations into ways to control malaria have been undertaken in The Gambia for more than 50 years.4 Early studies showed that chemoprophylaxis was highly effective in reduction of clinical attacks in children.5 A later trial of seasonal chemoprophylaxis with Maloprim (pyrimethamine and dapsone) administered by village health workers showed a reduction in morbidity from malaria, and a decrease in overall mortality in children younger than 5 years of more than 30%, emphasising the importance of malaria as a cause of death in children in The Gambia.6 These findings were confirmed by subsequent trials of insecticide-treated bednets, which showed substantial reductions in malaria-related morbidity7 and mortality8,9 in children.
Insecticide-treated bednets, prompt and effective treatment of clinical cases of malaria, intermittent preventive treatment in pregnant women and, in some populations, indoor residual spraying are now being deployed widely across Africa, with increasing amounts of coverage achieved.10 These initiatives follow more effective advocacy and support from the Bill & Melinda Gates Foundation, WHO, and public–private partnerships, and have been led by programmes such as the Global Fund to fight AIDS, Tuberculosis and Malaria and the Roll Back Malaria partnership, which have both contributed to substantially increased provision of insecticide-treated bednets in The Gambia since 2003. Furthermore, programmes such as the President's Malaria Initiative have supported control in other African countries.11
However, whether most affected communities12—who generally have inadequate surveillance of malaria—have benefited, is not yet clear.13 Published data are mostly from the fringes of endemic areas. Highly organised programmes for indoor residual spraying have substantially reduced malaria in South Africa and neighbouring areas of Mozambique and Swaziland,14 and separately on the island of Bioko in Equatorial Guinea.15 In Eritrea, a major fall in morbidity and mortality from malaria has been attributed to use of indoor residual spraying together with distribution of insecticide-treated bednets and strengthening of malaria case management in the community.16 A reduction in malaria on the island of Zanzibar occurred after highly effective artemisinin-based combination therapy was introduced, and was consolidated after increased distribution of insecticide-treated bednets.17 In Kenya, the number of malaria admissions has fallen in the coastal area,18 and reduced risk has been attributed to increasing use of such bednets as a result of social marketing and free distribution.19 Short survey visits suggest that malaria might be decreasing in other countries in which interventions have increased,20,21 but there are few data from West Africa, where a large proportion of global malaria cases arise.22
We undertook a retrospective analysis to investigate the changes that have occurred in The Gambia over the past 9 years, their potential causes, and public-health significance.
Methods
Study site and population
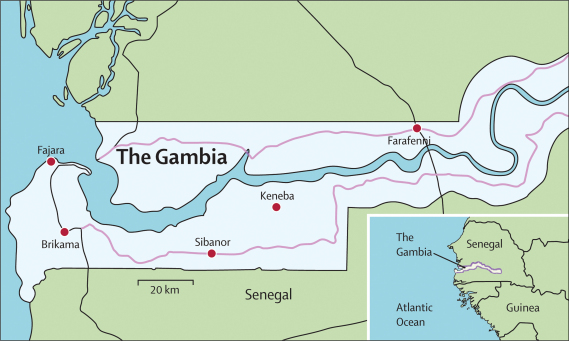
The Gambia is situated on the Atlantic coast of west Africa at the interface of the Sudan Savannah and northern Guinea Savannah zones. The country has one short rainy season from June to October, and most malaria transmission occurs between August and December.23 Almost all clinical cases of malaria are due to Plasmodium falciparum, which is transmitted by three species of the Anopheles gambiae complex.24 Patterns of malaria in three of the five different administrative regions of The Gambia have been surveyed at five health facilities, in which we identified high quality and continuous records (figure 1). In the Western Region, we obtained data for three facilities: Medical Research Council (MRC) hospital in Fajara (ward admissions, deaths, and outpatient haematology laboratory data); WEC (Worldwide Evangelisation for Christ) mission hospital in Sibanor (ward admissions, number of blood transfusions, haemoglobin concentration, and deaths); and the Government Major Health Centre in Brikama (haematology laboratory data). In the Lower River Region, we obtained laboratory haematology data from the MRC Keneba Clinic that serves the surrounding villages. In the North Bank Region, we gathered records for paediatric ward admissions and haematology laboratory data from the AFPRC (Armed Forces Provisional Ruling Council) District Hospital in Farafenni, which is the second largest hospital in the country.
Figure 1.
Location of the five sites studied for hospital admissions, inpatient deaths, and laboratory slide microscopy in The Gambia
The facilities at Fajara and Brikama serve mostly urban and peri-urban communities in the most densely populated part of the country near the coast; Farafenni is a small town with a large hospital serving a predominantly rural population; and facilities at Sibanor and Keneba are in large villages serving surrounding rural communities.
Data collection
We examined hospital and laboratory records for 9 years from January, 1999, to December, 2007. The MRC Scientific Co-ordinating Committee and the Joint MRC and Gambian Government Ethical Committee reviewed and approved the design of the data collection and analysis. The study was undertaken in two consecutive phases.
In phase 1, data were collected from the complete series of admission books at the MRC Fajara ward to enumerate the numbers of all admissions, admissions of malaria cases, all deaths, and deaths that were attributed to malaria. We also collected data from the complete series of laboratory books of the MRC outpatient haematology laboratory to enumerate the number of slides read and the number that were positive for malaria parasites. Throughout the study period, a thick blood film examination (with Field's staining) was done routinely for all patients attending the outpatient clinic for consultation with a physician, after screening by a nurse for fever and other signs and symptoms including headache and vomiting that are commonly, but not specifically, associated with clinical malaria. Routine examination was undertaken on 100 high-power fields (×1000 magnification) of every slide to identify parasites.
In phase 2, data were subsequently collected for 7 years from January, 2001, to December, 2007, from additional facilities (Brikama, Sibanor, Farafenni, and Keneba), which all have established links with the MRC for laboratory quality assurance and training. We designed a standard form to count the total number of overall admissions and number of admissions attributed to malaria every month, date of admission, and patient information such as age, sex, nationality, clinical diagnosis, and outcome. Clinical data for all patients admitted at the health facilities that we surveyed had been recorded in standard medical record books, which were kept at the wards of each facility by trained experienced field workers and nurses in charge of the health facilities. We designed a second form to count numbers of slides examined and malaria-positive slides every month.
Malaria was managed as described in the clinical protocols of the national guidelines for clinical management in The Gambia. The first-line treatment for mild malaria was chloroquine until the end of 2004, when a change was made to a combination of chloroquine and sulfadoxine plus pyrimethamine. Quinine is used as first-line treatment for severe malaria cases. In 2005, a policy decision was made for a future change of first-line malaria treatment to Coartem (artemether plus lumefantrine), which was subsequently supported by an award from the Global Fund Round 6. At the end of 2007, training and logistics were launched for introduction of Coartem at health facilities during 2008.
Monthly rainfall data for the study period were available from three meteorological stations in the Greater Banjul Area (Banjul, Serrekunda, and Yundum), and we averaged the results from these stations. Data for coverage of malaria-control interventions including insecticide-treated bednets were available from the multiple indicator cluster surveys (MICS),25,26 which were undertaken in all regions of The Gambia in 2000 (May–June) and 2006 (started in December, 2005, but mostly done between January and March, 2006). Both surveys coincided with the long dry season in the respective years when malaria would have been fairly infrequent, and net use by individuals might have been slightly less than in the rainy season when mosquitoes would be most abundant.
Serology
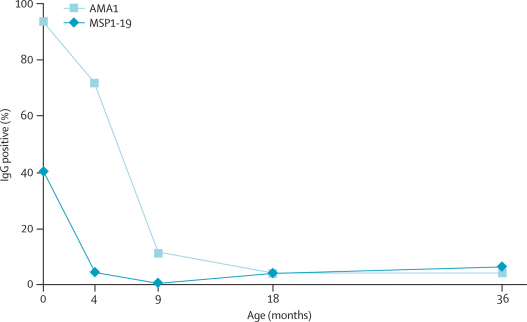
We assayed serum samples from a cohort study (not yet published) of infants born in Sukuta (Western Health Region) between July, 2003, and February, 2004, to investigate the acquisition of antibodies to blood stage P falciparum antigens during the first 3 years of life. This peri-urban community located between Fajara and Brikama was selected for study because it previously had an incidence of malaria that was representative of many Gambian communities in the Western Region27,28 and it is the only site in the country with a continuous birth cohort, which was recruited to study responses to infant vaccines. The samples that we used in this study were collected as part of a subcohort study of measles vaccine responses, and we undertook the assay of malaria antibodies after review and approval by the MRC Scientific Co-ordinating Committee and the Joint MRC and Gambian Government Ethical Committee. IgG reactivity to the whole ectodomain of Apical Membrane Antigen 1 (AMA1) and to the 19 kilodalton C-terminal part of merozoite surface protein 1 (MSP1-19) was measured by ELISA in sera that were obtained from 53 children at birth (cord blood) and at 4, 9, 18, and 36 months of age. The assay protocols have been previously described in studies of endemic populations.29,30
Statistical analysis
Clinical and laboratory data collected from record books from health facilities were given to the investigator who was responsible for data management (IA). Data were double entered and validated into a Microsoft Access database. A clean database was generated and converted into Stata (version 9) format with use of Stat Transfer (version 8). We analysed data using Stata (version 9). We calculated proportions and mean values with 95% CIs on the estimates as population samples, and risk ratios (RR) with 95% CIs to show differences in proportions between discrete time periods. We assessed the significance of differences in the proportions of malaria cases, malaria deaths, and positive slides over time by χ2 tests for trend. Linear regression was also applied to log transformed data from Fajara to test the significance of decreases over different periods.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
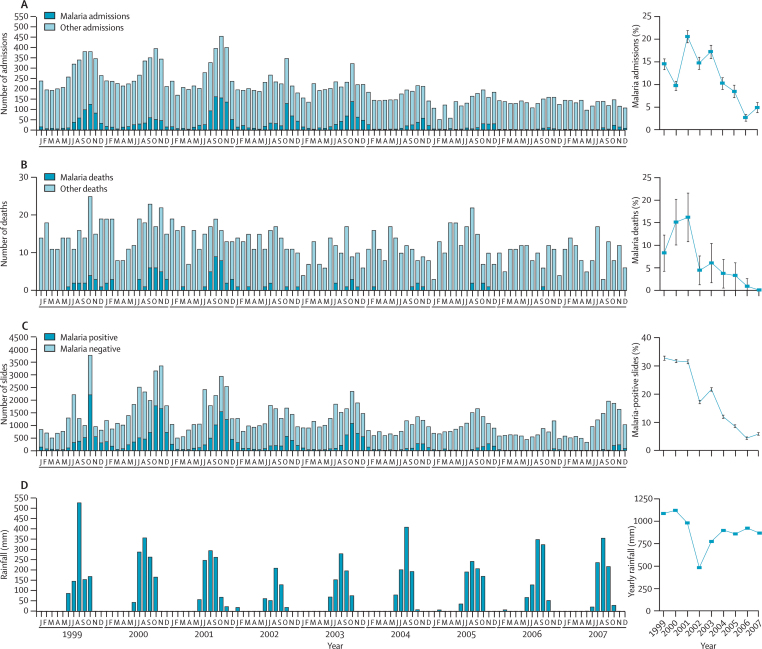
During the 9 years from January, 1999, to December, 2007, 21 820 patients were admitted to the MRC hospital in Fajara, 1341 inpatients died, and 129 397 slides were examined at the outpatient haematology laboratory. Every year, the number of admissions attributed to malaria, deaths attributed to malaria, and positive slides showed a highly seasonal distribution, with a peak from September to November at the end of the rainy season and with few cases during the driest months of the year (figure 2). During the study period, we detected a substantial decrease in the yearly number of admissions and deaths attributed to malaria and malaria-positive slides, particularly since 2003 (figure 2). 2002 had an unusually low yearly rainfall and a low number of cases of malaria, which is a normal pattern of inter-year variation, but the reduction since 2003 was not related to any decrease in yearly rainfall (figure 2).
Figure 2.
Monthly numbers (left panel) and yearly proportions (right panel) of malaria hospital admissions (A), deaths (B), and positive slides in outpatients (C) at the MRC in Fajara from January, 1999, to December, 2007
Monthly and yearly rainfall in the Greater Banjul Area over the period is also shown (D). Error bars indicate 95% CIs.
Compared with 2003, we recorded a 74% reduction in the proportion of malaria cases admitted in 2007 (435/2530 in 2003 vs 69/1531 in 2007; RR 0·26 [95% CI 0·20–0·34]), a 100% reduction in the proportions of deaths attributed to malaria (seven of 115 vs none of 117; p=0·006), and a 73% reduction in the proportion of slides positive for malaria (3664/16 932 vs 666/11 333; RR 0·27 [0·25–0·29]) at the MRC in Fajara (figure 2). Regression analysis for the whole 1999–2007 data series suggests that until 2003, there was no linear decrease in positive slides or malaria admissions, although the reduction in malaria deaths was significant, whereas all these indices fell substantially and significantly after 2003 (webfigure and webtable). The number of deaths attributed to malaria had already fallen sharply in 2002, increased only slightly in 2003, and then decreased to the lowest levels ever recorded (only one case of malaria with a fatal outcome was admitted in the last 2 years of the study period).
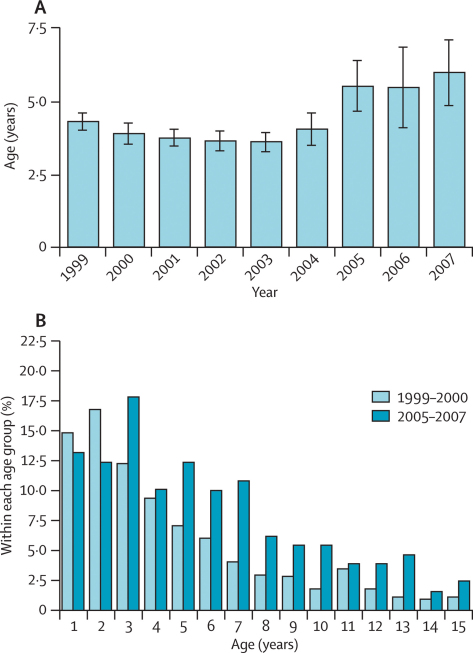
The age of malaria admissions in children (up to 15 years of age) at the MRC ward has significantly increased since 2004 (figure 3), suggesting that the reduction in malaria has been greatest in younger children. The mean age shifted from 3·9 years (95% CI 3·7–4·0) in 1999–2003 to 5·6 years (5·0–6·2) in 2005–07 (figure 3). The age distribution at the beginning of the study period (1999–2000) was strongly skewed towards very young children compared with the end of the period (2005–07) when the distribution was flatter and the proportions of children older than 5 years had significantly increased (figure 3).
Figure 3.
Age distribution of malaria cases admitted to the MRC ward from April, 1999, to December, 2007
(A) Mean age of paediatric cases (≤ 15 years) of malaria in every year from 1999 to 2007. Bars represent mean and error bars 95% CI. (B) Proportion of paediatric cases (≤ 15 years) of malaria in every age class in 1999–2000 and 2005–07.
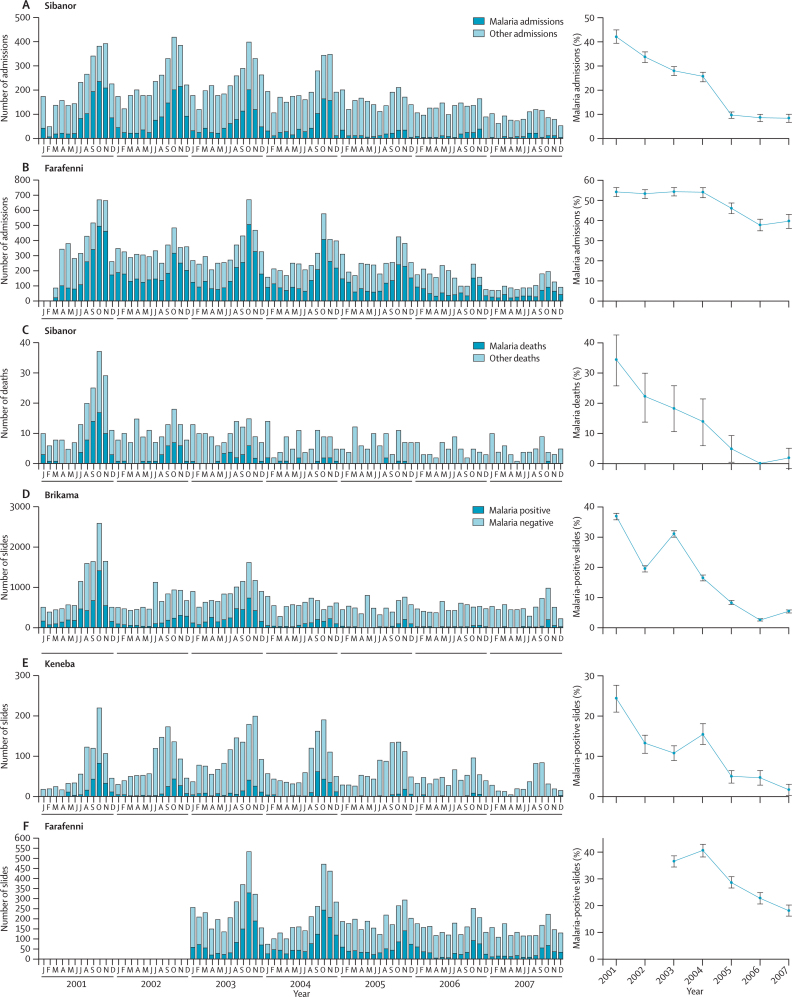
In four other health facilities that we investigated over the 7 years from January, 2001, to December, 2007, complete admissions data were available from Sibanor (14 877 all-cause admissions) and Farafenni (21 050 all-cause admissions); data for attributed cause of death were also available from Sibanor (716 all-cause inpatient deaths). Reliable and continuous records of slide examinations were available over the whole study period from Brikama (55 486 slides examined), Keneba (5768 slides examined), and for the last 5 years from Farafenni (12 513 slides examined) (figure 4).
Figure 4.
Monthly numbers (left panel) and yearly proportions (right panel) of malaria hospital admissions in Sibanor (A) and Farafenni (B); malaria mortality in Sibanor (C); and malaria-positive slides in Brikama (D) and Keneba (E) from January, 2001, to December, 2007, and in Farafenni (F) from January, 2003, to December, 2007
Error bars indicate 95% CIs.
All data sets show the same seasonal peaks that were present in Fajara, and show large and highly significant decreases in the numbers and proportions of malaria cases, deaths, and positive slides since 2003 (figure 4). The 69% reduction in proportions of admissions attributed to malaria in Sibanor (797/2824 in 2003 vs 89/1032 in 2007; RR 0·31 [95% CI 0·25–0·38]) was more pronounced than was the 27% decrease in Farafenni (2204/4056 vs 496/1251; RR 0·73 [0·68–0·79]) (figure 4). As we noted in Fajara, mortality from malaria in Sibanor has almost disappeared (only one death from malaria was recorded at Sibanor hospital in the last 2 years of the study period) (figure 4).
Since 2003, the 82% decrease in proportions of slides that were positive for malaria parasites in Brikama (3397/10861 in 2003 vs 337/6142 in 2007; RR 0·18 [95% CI 0·16–0·20]), and the 85% reduction in the proportions that were positive in Keneba (137/1259 vs six of 368; RR 0·15 [0·07–0·34]) (figure 4), were even greater than was the reduction noted at Fajara (figure 2). In Farafenni, the proportion of malaria-positive slides has been halved (1206/3304 vs 336/1853; RR 0·50 [0·45–0·55]).
Maternally derived antibodies to the P falciparum blood stage antigens AMA1 and MSP1-19 fell rapidly in the 53 children born in Sukuta in 2003–04, and almost all infants were antibody negative for MSP1-19 by the fourth month and for AMA1 by the ninth month of life (children had higher starting levels against AMA1) (figure 5). At 18 months of age, two (4%) children had antibodies to AMA1 and MSP1-19; by 36 months of age, only two (4%) and three (6%) children were positive to these antigens, respectively (figure 5).
Figure 5.
Proportion of maternally derived antibodies and frequency of new responses to malaria antigens in children born in 2003–04
The plot shows the percentage seropositive for IgG to the merozoite antigens AMA1 and MSP1-19 in 53 children at birth and at 4, 9, 18, and 36 months of age. The cut-off values for positivity were the mean plus 3 SD of ELISA OD (optical density) levels for 20 sera obtained from non-exposed individuals.
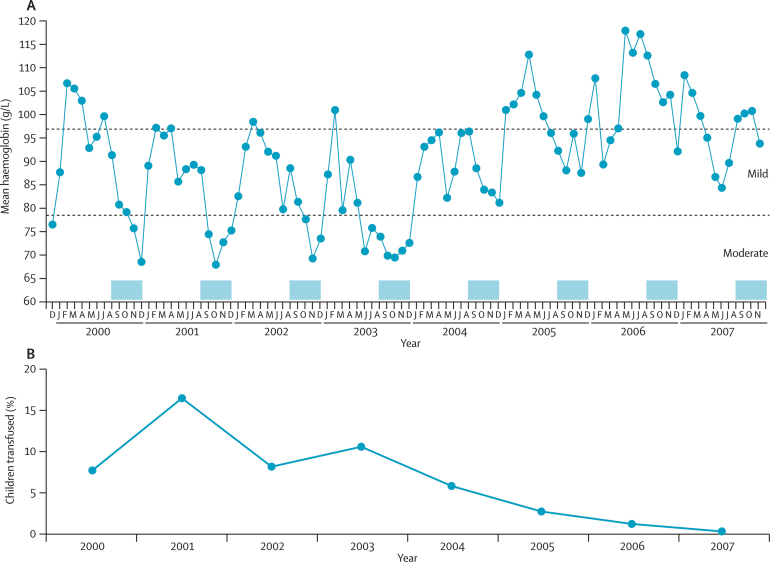
During the 8 years from December, 1999, to November, 2007, 18 906 patients were admitted to the hospital in Sibanor. 9763 (52%) of these patients were younger than 5 years, and haemoglobin values were available for 7408 cases. We noted that a major trough in haemoglobin concentrations coincided with the end of the rainy season (figure 6), which is attributable to a combination of seasonal malaria and nutritional deficiency during a so-called hungry season before most crops are harvested; however, we noted a substantial increase in haemoglobin values from 2004 onwards. We recorded a mean difference of 12·4 g/L (95% CI 10·6–13·8; p<0·0001) in haemoglobin concentration in patients who were admitted to the health facilities before 2004 (mean 84·9 [SD 11·0] g/L) and after 2004 (97·3 [9·3] g/L); from this point on, the magnitude and timing of the seasonal trough has become less clear. The effect on admission of severe anaemia and inpatient care is indicated by the yearly proportions of children younger than 5 years needing transfusion (figure 6). The decrease in the number of children needing transfusion (p<0·0001) has been so profound that during 2007, the hospital management decided to discontinue transfusion services and transfer the few patients with severe anaemia to other hospitals. The transfusion criteria did not change over the period studied, during which a typical threshold of haemoglobin concentration less than 45 g/L was applied and transfusion compliance was very high. Furthermore, we noted no change in concern about infection risk that might have modified transfusion rates over this time, during which community prevalence rates of HIV 1 and 2 were low (<2%).
Figure 6.
Rate of anaemia in children younger than 5 years who were admitted to Sibanor health facilities
Data are from 7408 children from 2000 to 2007. (A) Monthly mean haemoglobin concentrations of patients. The months of the yearly malaria seasons (August–December) are marked with blue shading. (B) Percentage of children receiving blood transfusion every year from 2000 to 2007.
The table shows data from the two most recent MICS surveys. Between 2000 and 2006, we recorded a more than three-fold increase in the proportion of children who slept under an insecticide-treated bednet, and an almost ten-fold increase in the proportion of pregnant women who received appropriate intermittent preventive treatment. The proportion of children reported to have had a fever in the 2 weeks before interview was almost halved between these two surveys (table).
Table.
Changes in coverage of malaria interventions and febrile illness between 2000 and 2006, shown in multiple indicator cluster surveys (MICS) in The Gambia
| 2000 | 2006 | Risk ratio (95% CI)* | p value | |
|---|---|---|---|---|
| Children younger than 5 years | ||||
| Had febrile illness during 2 weeks before the survey | 14·8% | 8·4% | 0·57 (0·51–0·63) | <0·001 |
| Slept under an ITN the night before the survey | 14·5% | 49·0% | 3·38 (3·11–3·67) | <0·001 |
| Slept under any net the night before the survey | 41·8% | 63·0% | 1·51 (1·44–1·57) | <0·001 |
| Women | ||||
| Received two doses of SP (IPT) during last pregnancy | (3·4%)† | 32·5% | 9·56 (undefined) | Undefined |
ITN=insecticide-treated bednet. SP=sulfadoxine plus pyrimethamine. IPT=intermittent preventive treatment.
95% CIs could be calculated for the data for children younger than 5 years because sample sizes were available (N=3632 in 2000; N=6543 in 2006),25,26 but not for the data for IPT in women.
IPT in pregnant women was not reported in the 2000 MICS survey (the figure in parentheses is from an unpublished survey in 2002).
Discussion
During the past few years, efforts to control malaria in Africa have expanded to a level that has not been seen since the time of the malaria eradication programme 50 years ago. In The Gambia, we recorded changes at all five facilities studied, particularly since 2003. We noted a progressive reduction in the proportions of ward admissions and deaths attributed to malaria, and of positivity rates of outpatient malaria slides. Actual numbers have decreased even more substantially, since admissions attributed to other causes and total numbers of slides examined have also slightly decreased. Previous trials of chemoprophylaxis and insecticide-treated bednets against malaria in The Gambia have shown reductions in all-cause morbidity and mortality greater than that directly attributable to malaria.8,31 Furthermore, evidence suggests that malaria can have a major indirect contribution to the comorbidities from other infectious diseases,32 although our study was not designed to test this hypothesis. The potentially complex relations between common infectious diseases in children need to be better understood now that malaria is substantially decreasing.
Data from health facilities do not always represent disease trends in the general population. For this retrospective study, we considered it important that data for malaria blood slides were obtained only from laboratories with trained staff and continuously monitored laboratory procedures in place. Similarly, data for hospital admissions were gathered from well established health facilities serving populations that might be able to access interventions to prevent malaria. Thus, we cannot assume that our findings are applicable to the whole country. Nevertheless, all the indices that we measured from all the sites are concordant and very encouraging, which is notable since the institutional facilities differed and included two government facilities, two MRC research sites, and a non-governmental organisation mission hospital, in urban and rural areas. Data were not available for analysis from private pharmacies, but discussions with retail managers suggested that sales of antimalarial drugs have fallen, which is consistent with the general perception in the community that the incidence of malaria has decreased. Recognition of the importance of prospectively collecting and ensuring quality of data from a systematic sample of facilities has led the National Malaria Control Programme to begin a surveillance system to detect future changes, incorporating one major government health centre in each administrative district in the country.
Other means of investigating changes in malaria in the population should also be considered, especially since high-quality surveillance is still almost universally absent from areas where malaria is most common. The nationally collected MICS data indicate a 43% reduction between 2000 and 2006 in episodes of fever reported by parents in children younger than 5 years, suggesting a substantially reduced malaria incidence in the community. More specific measurements should be incorporated as part of regular community surveillance if further changes in the malaria endemic are to be sensitively detected. Indices for the entomological inoculation rate (the product of the sporozoite prevalence in vector mosquitoes and the human biting rate) are relevant for estimating transmission rates, but decreases are difficult to measure accurately when sporozoite prevalence is already low (<1%) as in most previously published surveys from The Gambia33 and in surveys of villages in the Farafenni area in 2002–03 (unpublished data).
Community surveys of parasite infection and enlarged spleen rates23 are informative, and serological surveys of whole communities can provide a practical alternative to infer the force of infection in a community with stable endemicity;34 however, these initiatives have not been clearly applied to changes in malaria transmission over time. In the peri-urban area of Sukuta that previously had a moderate to high endemicity that is typical of many Gambian communities,27,28 few children born in 2003–04 seroconverted during the first 3 years of life, although maternally transferred antibody reactivity in most cord-blood samples was an indicator of substantial previous exposure to malaria. Serological studies of infants were previously used to document the local eradication of malaria,35 and surveys of infants and young children might now provide one of the more effective means to quantify recent local transmission.
We recorded a substantial shift in the mean age of children who were admitted to the MRC Fajara ward with malaria after 2004. The mean age of malaria admissions until 2004 was similar to that recorded 10 years previously,28 so our finding of a trend towards older ages of malaria cases is new. A more substantial decrease of malaria admissions in younger children might be mainly due to increased use of insecticide-treated bednets, since this intervention is targeted to children younger than 5 years; however, the reduction in malaria could cause some delay in the acquisition of immunity to P falciparum.28 A trial in Ghana showed that initial reduction in child mortality associated with the introduction of insecticide-treated bednets was not followed by an increase in child mortality in older children, 7 years after the intervention.36 A study in Burkina Faso reported similar findings 6 years after the introduction of insecticide-treated curtains.37 So far, all indices are towards a large improvement in public health. The large increase in mean haemoglobin concentration and major reduction in the number of children needing blood transfusions in Sibanor, coinciding with the malaria decrease from 2004 onwards, has had an effect on health-service delivery locally. The closure of a transfusion facility because of low demand emphasises that more investigation of the results of the malaria decrease is needed at all levels.
We have considered possible reasons for the decrease of malaria in The Gambia. Changes in rainfall cause some fluctuations in malaria from year to year, but could not account for the progressive reduction that we recorded since 2003. Socioeconomic changes and improvements in communications and access to education have probably had contributing effects, although these factors are gradual and not expected to produce such a rapid change as we reported here at diverse sites. A change in chemotherapy is likely to have had a substantial role, although it might not have initiated the decrease. Until the end of 2004, chloroquine alone was used as first-line therapy for uncomplicated malaria in most health facilities in The Gambia, even though parasite resistance to this drug had gradually increased to high levels since it was first detected in 1987.38 The decreasing therapeutic efficacy of chloroquine could have resulted in an increase in outpatient treatment failure of clinical malaria cases, and thus an increased risk of disease for which the patient needs to be admitted to hospital.39 At the beginning of 2005, sulfadoxine plus pyrimethamine combined with chloroquine was adopted for first-line treatment in government health facilities, but it had been prescribed in a few independent facilities previously. Although the efficacy of sulfadoxine plus pyrimethamine has fallen in east Africa because of resistance to this combination, it is still quite highly effective in The Gambia when combined with chloroquine.40 Its use in presumptive therapy together with the long plasma half-lives of its components means that it might also have some effect in prevention of infection.
The most substantial change in measures to prevent malaria has been the increase of coverage of insecticide-treated bednets, with MICS data showing a more than three-fold increase to 49% reported coverage of children younger than 5 years between 2000 and 2006. This initiative was possible because of support from the Global Fund—awarded in 2003 and implemented from 2004 onwards—for free distribution of insecticide-treated bednets to women who are pregnant and mothers of children younger than 5 years in the Western Health Region (which incorporates 55% of the population; three of the health facilities that we studied were in this area). Additionally, nationwide re-treatment of bednets was undertaken in 2003 and 2005 with support from UNICEF and WHO. The Gambia now has the highest reported coverage of insecticide-treated bednets for children younger than 5 years,10 and the intended beneficial effect is consistent with our findings.
These findings support the proposal that increased investment in malaria interventions in Africa can have a major effect on reducing morbidity and mortality from this disease. A large reduction might be more achievable in areas where transmission is highly seasonal as in The Gambia, than in areas that have more perennial transmission; however, this hypothesis has not been proven. Adequate data are needed from many other countries to identify whether this change is being achieved broadly throughout Africa, to allow monitoring and sustaining of reduced incidence where it has occurred, and appropriate targeting of resources to countries with the greatest need.11 A renewed call for eradication of malaria41 is being debated worldwide, but the epidemiological transition has encouraged the Gambian Government to launch a policy in February, 2008, that malaria should be eliminated as a public-health problem. The means to achieve this policy are still unknown in any continental African country, but intended efforts in The Gambia include introduction of free artemisinin-based combination therapy (artemether-lumefantrine) at health centres, and indoor residual spraying with dichlorodiphenyltrichloroethane in some areas. These and other interventions could further reduce malaria, and efforts should now tackle challenges to effective delivery and establishment of sustainable prospective monitoring.
Acknowledgments
Acknowledgments
Financial support for the research was provided by the UK Medical Research Council (MRC). We thank Jula Jaiteh, and Lamin Sanyang (Senior Nursing Officers at Government health facilities) for providing hospital records and helping to extract data; Gibril Bah, Lamin Manneh, Bernard Ndaye, Lamin Gibba, Fabakary Sanyang, Hawa Manneh, Demba B T Sambou, and Yorro Bah (MRC staff) for data extraction of the haematology laboratory and ward records; Aja Abbie Khan (Head of Regional Health Team for Western Division) for support in implementing the project at the Government health facilities; Andrew Prentice (Head of MRC Nutrition Research Programme) for supporting the Keneba laboratory record database management; Pamela Collier Njie and Muminatou Jallow for help with the provision of clinical data from the MRC ward in Fajara; and many other colleagues and partner organisations who have encouraged this work.
Contributors
SJC and CC-P contributed equally to this work. SJC coordinated the examination and collection of source data from laboratory and health facilities, examined datasets, and contributed to analysis and writing of the report. CC-P had a leading role in checking and analysing the data, and writing of the report. JE provided, checked, and analysed the data from Sibanor, and contributed to the report. SEA contributed to the analysis and writing of the report. NOD did the malaria antibody analyses. SSSS and OS guided and helped supervise the data collection from Farafenni. AJCF checked and provided the data from Keneba and contributed to the report. IA supervised the data entry. SD and KAB advised on data collection and epidemiology. TC was responsible for the clinical services and records at MRC Fajara and advised on policy implications. AP and MF advised on malaria control and policy implications. HCW provided serum samples from Sukuta, and contributed to the report. BMG advised and participated in many aspects of the study including preparation of the report. DJC initiated and supervised the study, and prepared the report on behalf of all authors.
Conflict of interest statement
We declare that we have no conflict of interest.
Web Extra Material
Linear regression on Fajara malaria admissions (A), malaria-positive slides (B), and malaria deaths (C) from 1999, to 2007, analysing distributions of monthly data across years, with log transformation of numbers for admissions and slide data
Linear regression on Fajara malaria admissions, malaria-positive slides, malaria deaths, non-malaria admissions, and malaria-negative slides from 1999 to 2007
References
- 1.Jaffar S, Leach A, Greenwood AM. Changes in the pattern of infant and childhood mortality in upper river division, The Gambia, from 1989 to 1993. Trop Med Int Health. 1997;2:28–37. doi: 10.1046/j.1365-3156.1997.d01-131.x. [DOI] [PubMed] [Google Scholar]
- 2.Anya SE. Seasonal variation in the risk and causes of maternal death in the Gambia: malaria appears to be an important factor. Am J Trop Med Hyg. 2004;70:510–513. [PubMed] [Google Scholar]
- 3.Williams CJ, Jones J, Chiodini P. High case-fatality from falciparum malaria in UK travellers returning from The Gambia: a case series. Travel Med Infect Dis. 2007;5:295–300. doi: 10.1016/j.tmaid.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood BM, Pickering H. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, west Africa. 1. A review of the epidemiology and control of malaria in The Gambia, west Africa. Trans R Soc Trop Med Hyg. 1993;87(suppl 2):3–11. doi: 10.1016/0035-9203(93)90169-q. [DOI] [PubMed] [Google Scholar]
- 5.McGregor IA, Gilles HM, Walters JH, Davies AH, Pearson FA. Effects of heavy and repeated malarial infections on Gambian infants and children; effects of erythrocytic parasitization. BMJ. 1956;2:686–692. doi: 10.1136/bmj.2.4994.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon A, Snow RW, Byass P, Greenwood BM, Hayes RJ, N'Jie AB. Sustained protection against mortality and morbidity from malaria in rural Gambian children by chemoprophylaxis given by village health workers. Trans R Soc Trop Med Hyg. 1990;84:768–772. doi: 10.1016/0035-9203(90)90071-l. [DOI] [PubMed] [Google Scholar]
- 7.Snow RW, Lindsay SW, Hayes RJ, Greenwood BM. Permethrin-treated bed nets (mosquito nets) prevent malaria in Gambian children. Trans R Soc Trop Med Hyg. 1988;82:838–842. doi: 10.1016/0035-9203(88)90011-9. [DOI] [PubMed] [Google Scholar]
- 8.Alonso PL, Lindsay SW, Armstrong JR. The effect of insecticide-treated bed nets on mortality of Gambian children. Lancet. 1991;337:1499–1502. doi: 10.1016/0140-6736(91)93194-e. [DOI] [PubMed] [Google Scholar]
- 9.D'Alessandro U, Olaleye BO, McGuire W. Mortality and morbidity from malaria in Gambian children after introduction of an impregnated bednet programme. Lancet. 1995;345:479–483. doi: 10.1016/s0140-6736(95)90582-0. [DOI] [PubMed] [Google Scholar]
- 10.UNICEF . Malaria and children: progress and intervention coverage. UNICEF; New York: 2007. pp. 1–70.http://www.unicef.org/health/files/Malaria0831.pdf (accessed May 2, 2008). [Google Scholar]
- 11.Snow RW, Guerra CA, Mutheu JJ, Hay SI. International funding for malaria control in relation to populations at risk of stable Plasmodium falciparum transmission. PLoS Med. 2008;5:e142. doi: 10.1371/journal.pmed.0050142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kouyate B, Sie A, Ye M, De Allegri M, Muller O. The great failure of malaria control in Africa: a district perspective from Burkina Faso. PLoS Med. 2007;4:e127. doi: 10.1371/journal.pmed.0040127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler D. Poor follow-up hampers malaria projects. Nature. 2007;450:144–145. doi: 10.1038/450144b. [DOI] [PubMed] [Google Scholar]
- 14.Sharp BL, Kleinschmidt I, Streat E. Seven years of regional malaria control collaboration-Mozambique, South Africa, and Swaziland. Am J Trop Med Hyg. 2007;76:42–47. [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp BL, Ridl FC, Govender D, Kuklinski J, Kleinschmidt I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar J. 2007;6:52. doi: 10.1186/1475-2875-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyarango PM, Gebremeskel T, Mebrahtu G. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar J. 2006;5:33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattarai A, Ali SS, Kachur SP. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okiro EA, Hay SI, Gikandi PW. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:e151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fegan GW, Noor AM, Akhwale WS, Cousens S, Snow RW. Effect of expanded insecticide-treated bednet coverage on child survival in rural Kenya: a longitudinal study. Lancet. 2007;370:1035–1039. doi: 10.1016/S0140-6736(07)61477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambers RG, Gupta RK, Ghebreyesus TA. Responding to the challenge to end malaria deaths in Africa. Lancet. 2008;371:1399–1401. doi: 10.1016/S0140-6736(08)60609-1. [DOI] [PubMed] [Google Scholar]
- 21.WHO . Impact of long-lasting insecticidal nets (LLINs) and artemisinin-based combination therapies (ACTs) measured using surveillance data, in four African countries. Preliminary Report. World Health Organization; Geneva: 2008. http://www.who.int/malaria/docs/ReportGFImpactMalaria.pdf (accessed April 29, 2008). [Google Scholar]
- 22.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenwood BM, Bradley AK, Greenwood AM. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81:478–485. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- 24.Caputo B, Nwakanma D, Jawara M. Anopheles gambiae complex along The Gambia river, with particular reference to the molecular forms of An. gambiae s.s. Malar J. 2008;7:182. doi: 10.1186/1475-2875-7-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MICS . The Gambia Multiple Indicator Cluster Survey 2005/6. The Gambia Bureau of Statistics; Banjul: 2007. pp. 1–243. [Google Scholar]
- 26.MICS . The Gambia Multiple Indicator Cluster Survey 2000. The Gambia Bureau of Statistics; Banjul: 2002. pp. 1–116. [Google Scholar]
- 27.Marsden PD. The Sukuta Project. A longitudinal study of health in Gambian children from birth to 18 months of age. Trans R Soc Trop Med Hyg. 1964;58:455–489. doi: 10.1016/0035-9203(64)90101-4. [DOI] [PubMed] [Google Scholar]
- 28.Snow RW, Omumbo JA, Lowe B. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 29.Cavanagh DR, Elhassan IM, Roper C. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- 30.Polley SD, Mwangi T, Kocken CH. Human antibodies to recombinant protein constructs of Plasmodium falciparum Apical Membrane Antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004;23:718–728. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Greenwood BM, Bradley AK, Byass P. Comparison of two strategies for control of malaria within a primary health care programme in the Gambia. Lancet. 1988;1:1121–1127. doi: 10.1016/s0140-6736(88)91949-6. [DOI] [PubMed] [Google Scholar]
- 32.Shanks GD, Hay SI, Bradley DJ. Malaria's indirect contribution to all-cause mortality in the Andaman Islands during the colonial era. Lancet Infect Dis. 2008;8:564–570. doi: 10.1016/S1473-3099(08)70130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates across Africa: literature survey, internet access and review. Trans R Soc Trop Med Hyg. 2000;94:113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drakeley CJ, Corran PH, Coleman PG. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruce-Chwatt LJ, Draper CC, Konfortion P. Seroepidemiological evidence of eradication of malaria from Mauritius. Lancet. 1973;2:547–551. doi: 10.1016/s0140-6736(73)92361-1. [DOI] [PubMed] [Google Scholar]
- 36.Binka FN, Hodgson A, Adjuik M, Smith T. Mortality in a seven-and-a-half-year follow-up of a trial of insecticide-treated mosquito nets in Ghana. Trans R Soc Trop Med Hyg. 2002;96:597–599. doi: 10.1016/s0035-9203(02)90321-4. [DOI] [PubMed] [Google Scholar]
- 37.Diallo DA, Cousens SN, Cuzin-Ouattara N, Nebie I, Ilboudo-Sanogo E, Esposito F. Child mortality in a West African population protected with insecticide-treated curtains for a period of up to 6 years. Bull World Health Organ. 2004;82:85–91. [PMC free article] [PubMed] [Google Scholar]
- 38.Menon A, Otoo LN, Herbage EA, Greenwood BM. A national survey of the prevalence of chloroquine resistant Plasmodium falciparum malaria in The Gambia. Trans R Soc Trop Med Hyg. 1990;84:638–640. doi: 10.1016/0035-9203(90)90130-7. [DOI] [PubMed] [Google Scholar]
- 39.Meerman L, Ord R, Bousema JT. Carriage of chloroquine-resistant parasites and delay of effective treatment increase the risk of severe malaria in Gambian children. J Infect Dis. 2005;192:1651–1657. doi: 10.1086/496887. [DOI] [PubMed] [Google Scholar]
- 40.Dunyo S, Ord R, Hallett R. Randomised trial of chloroquine/sulphadoxine-pyrimethamine in Gambian children with malaria: impact against multidrug-resistant P falciparum. PLoS Clin Trials. 2006;1:e14. doi: 10.1371/journal.pctr.0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Lancet Is malaria eradication possible? Lancet. 2007;370:1459. doi: 10.1016/S0140-6736(07)61609-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linear regression on Fajara malaria admissions (A), malaria-positive slides (B), and malaria deaths (C) from 1999, to 2007, analysing distributions of monthly data across years, with log transformation of numbers for admissions and slide data
Linear regression on Fajara malaria admissions, malaria-positive slides, malaria deaths, non-malaria admissions, and malaria-negative slides from 1999 to 2007