Summary
A transient state of tolerance to microbial molecules accompanies many infectious diseases. Thought to minimize inflammation-induced injury, it may also alter host defenses. Here we report that recovery from the tolerant state induced by Gram-negative bacteria is greatly delayed in mice that lack acyloxyacyl hydrolase (AOAH), a lipase that partially deacylates the bacterial cell wall lipopolysaccharide (LPS). Whereas wild-type mice regained normal responsiveness within 14 days after they received an intraperitoneal injection of LPS or Gram-negative bacteria, AOAH-deficient mice had greatly reduced pro-inflammatory responses to a second LPS injection for at least 3 weeks. In contrast, their plasma IL-10 response was maintained. LPS-primed Aoah−/− mice experiencing prolonged tolerance were highly susceptible to virulent E. coli challenge. To our knowledge, this is the first evidence that inactivating a stimulatory microbial agonist is important for restoring effective host defenses following an infectious exposure in animals.
Introduction
As key sensors and effectors of innate immunity, phagocytes sense conserved microbial molecules and mount inflammatory responses that destroy invading microorganisms. For hours to a few days after such an encounter, however, the cells’ pro-inflammatory responses to the same and other microbial molecules may be greatly reduced. This period of tolerance (also called reprogramming, since some anti-inflammatory responses are usually preserved (Henricson et al., 1993; Shnyra et al., 1998)), is thought to minimize inflammation-induced damage during recovery from infectious diseases. Although tolerance induced by Gram-negative bacteria or their LPS (endotoxin) has been studied most intensively, a similar phenomenon has also been noted in response to other bacterial agonists (Cross, 2002; Medvedev et al., 2006; Wang et al., 2003) and during some viral infections (Didierlaurent et al., 2008).
Tolerance to LPS has been documented in several clinical settings: in adults with pyelonephritis (McCabe, 1963), in the alveolar macrophages of individuals who have smoked endotoxin-containing tobacco (Chen et al., 2007), and in the blood leukocytes of children with bacterial meningitis (Helminen and Vesikari, 1990a), adults with alcoholic cirrhosis (Von Baehr et al., 2000) and adults with sepsis (McCall et al., 1993; Faist et al., 1988; Munoz et al., 1991). Perhaps most significantly, tolerance in blood leukocytes has been one of the most consistently-observed correlates of infection-induced immunosuppression in humans (West and Heagy, 2002). Tolerance has also been noted in the blood cells of uninfected patients following major trauma (West and Heagy, 2002), with greater tolerance noted in those who had elevated plasma endotoxin levels (Kelly et al., 1997). In an investigation of patients with severe sepsis, the blood monocytes of patients with Gram-negative bacterial infections showed greater tolerance than did those from patients with Gram-positive infections (Munoz et al., 1991; McCabe, 1963).
Whereas much is now known about how LPS initiates reprogramming in both monocyte-macrophages and neutrophils (Medvedev et al., 2006; Wysocka et al., 2001; Cross, 2002; Cavaillon et al., 2003; McCall and Yoza, 2007; Foster et al., 2007), the mechanisms that terminate the reprogrammed (tolerant) state and restore host defenses are poorly understood (McCall and Yoza, 2007; Wysocka et al., 2005). Indeed, the duration of tolerance may vary greatly. In children with Haemophilus influenzae type b meningitis, leukocyte hyporesponsiveness to LPS resolved as the patients recovered over a few days (Helminen and Vesikari, 1990b). In contrast, adult cancer patients who received a bolus intravenous injection of LPS remained tolerant for almost two weeks (Engelhardt et al., 1991) and the peripheral blood leukocytes of septic adults have often been reprogrammed for many days or weeks after apparently successful treatment of the inciting infection (Weighardt et al., 2000). In septic patients, recovery from tolerance has been associated with survival (Munoz et al., 1991; Weighardt et al., 2000). Understanding how tolerance is terminated in vivo thus might suggest ways to decrease the duration of post-infection immunosuppression and improve outcome.
Here we show that recovery from tolerance elicited by LPS or Gram-negative bacteria in vivo requires acyloxyacyl hydrolase (AOAH), a macrophage, dendritic cell and neutrophil lipase that inactivates LPSs by removing secondary fatty acyl chains from the lipid A moiety (Munford and Hall, 1986). The duration and degree of tolerance in vivo correlated with the presence of fully acylated LPS in peritoneal macrophages. Moreover, mice experiencing prolonged tolerance were highly susceptible to E. coli challenge. These results suggest that killing Gram-negative bacteria does not allow full restoration of homeostasis and effective host defenses: to prevent prolonged immune suppression, the dominant bacterial ‘signal’ molecule, LPS, must also be inactivated.
Results
AOAH allows recovery from tolerance induced by LPS or Gram-negative bacteria in vivo
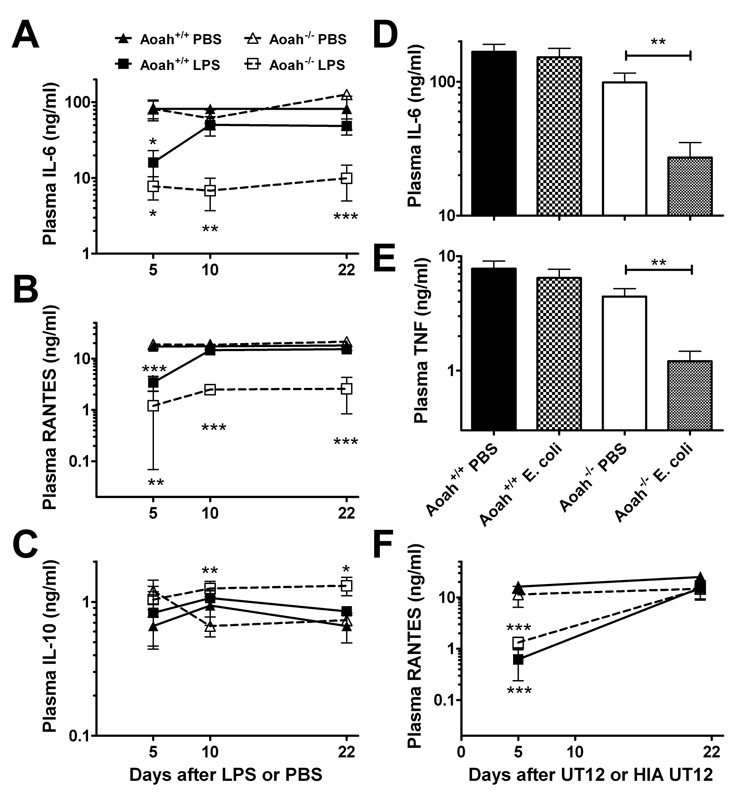
To investigate a possible role for AOAH in recovery from tolerance, we gave a small intraperitoneal dose of LPS (10 µg) or PBS to wild-type (Aoah+/+) and AOAH-deficient (Aoah−/−) mice and measured their responses to a second LPS challenge (20 µg) given 5, 10, or 22 days later. Aoah−/− and Aoah+/+ mice did not differ in their acute cytokine responses to the first LPS injection (see data for PBS-primed mice in Fig. 1A–C), and the mice in both groups were tolerant on day 5 after the initial LPS dose. In contrast, whereas wild-type mice regained their ability to respond normally to a second LPS dose within 5 to 10 days, LPS-primed Aoah−/− mice had greatly reduced plasma IL-6, TNF and RANTES responses to a second dose for the entire 22 day period after the initial LPS exposure (Fig. 1A, B; Fig. S1). In keeping with previous reports that LPS-induced macrophage reprogramming alters responses along both the MyD88-dependent and - independent arms of the intracellular signaling pathway downstream of TLR4 (Bagchi et al., 2007; Sato et al., 2002), in vivo responses to LPS challenge that were MyD88-dependent (TNF [Figure S1], IL-6) or MyD88-independent (RANTES (Bjorkbacka et al., 2004), MCP-1 (Mata-Haro et al., 2007) [Figure S1]) were impaired. AOAH is thus required for mice to recover their ability to mount several normal pro-inflammatory responses to LPS. In contrast, LPS-primed Aoah−/− mice produced slightly higher plasma concentrations of IL-10, a major anti-inflammatory cytokine, than did the other groups (Fig. 1C). Retention of a normal IL-10 response to LPS despite greatly reduced pro-inflammatory responses suggests that the tolerant animal’s reactions to LPS are reprogrammed, not globally depressed (Henricson et al., 1993; Shnyra et al., 1998; Medvedev et al., 2006).
Fig. 1. Aoah−/− mice experience prolonged LPS-induced tolerance in vivo.
(A-C) Aoah−/− and Aoah+/+ mice received i.p. injections of PBS or LPS (10 µg) in PBS on day 0. On days 5, 10, or 22, separate groups of mice (n = 4 – 11 mice/group) were challenged i.p. with 20 µg LPS. Cytokine or chemokine levels were measured in plasma samples collected 4 hours later. Combined data from 4 experiments. (D, E) Intact Gram-negative bacteria also induce prolonged tolerance in Aoah−/− mice. Mice were primed i.p. with 1.5 × 107 colony-forming units of an avirulent strain of E. coli K12. Fourteen days later they were challenged i.p. with LPS and plasma samples were collected 1hr (TNF) and 4 hrs (IL-6) later for cytokine analysis. n = 8–10, data combined from 2 experiments. (F) Aoah−/− mice recover from tolerance induced by an agonistic antibody to MD-2—TLR4. Mice were injected i.p. with 1 µg UT12 IgG or heat inactivated (HIA) UT12 on day 0. On days 5 and 21, separate groups of mice were challenged i.p. with 20 µg LPS. RANTES levels were measured in plasma 4 hours after LPS challenge. n = 4–5. Closed symbols, Aoah+/+; open symbols, Aoah−/− ; squares, UT12 priming; triangles, HIA UT12 priming. Similar results were observed using TNF and IL-6 as response read-outs (not shown) and in a second experiment (n = 4–5). Differences from PBS control group (or HIA UT12 group) of same genotype: * p <0.05; **p <0.01; *** p <0.001. Error bars: ± 1 SEM. Note log scales.
Since Gram-negative bacteria also contain non-LPS activators of innate immune responses (peptidoglycan, lipoproteins, others), it was important to know whether AOAH is also required to terminate tolerance induced by infection with intact Gram-negative bacteria. We gave a sub-lethal infectious dose (1.5 × 107 colony forming units [cfu]) of an avirulent E. coli strain to Aoah+/+ and Aoah−/− mice and challenged them 14 days later with an intraperitoneal injection of either a virulent E. coli strain (O18K1, 3 × 107 cfu) or 20 µg LPS. As shown in Fig. 1D and EM, Aoah−/− mice previously exposed to E. coli had significantly lower plasma IL-6 and TNF responses to the LPS challenge than did Aoah+/+ mice; similar results were seen following virulent E. coli challenge (Fig. S2). These findings confirm the ability of AOAH to terminate tolerance induced by whole Gram-negative bacteria and support the conclusion that LPS is the dominant Gram-negative “molecular pattern” recognized by animal cells (Somerville, Jr. et al., 1996; Munford, 2008).
MD-2—TLR4 activation by an agonistic monoclonal antibody does not induce prolonged tolerance in AOAH-deficient animals
It is possible that AOAH prevents prolonged tolerance through a mechanism that does not involve its ability to deacylate LPS. To test this hypothesis, we gave Aoah+/+ and Aoah−/− mice an i.p. injection of a monoclonal antibody that stimulates cells via MD-2—TLR4 in the absence of LPS (Ohta et al., 2006) and tested their responses to an intraperitoneal dose of LPS given 5 days or 21 days later. We found identical responses for the two genotypes: in both Aoah+/+ and Aoah−/− mice, tolerance on day 5 was followed by recovery by day 21 (Fig. 1F [compare with the responses to a priming dose of LPS in Fig. 1B]). AOAH is thus important for recovery from tolerance induced by its substrate, LPS, but not from tolerance induced by a non-LPS agonist that activates the same signaling pathway.
LPS-induced antibodies are not required to prolong tolerance in AOAH-deficient mice
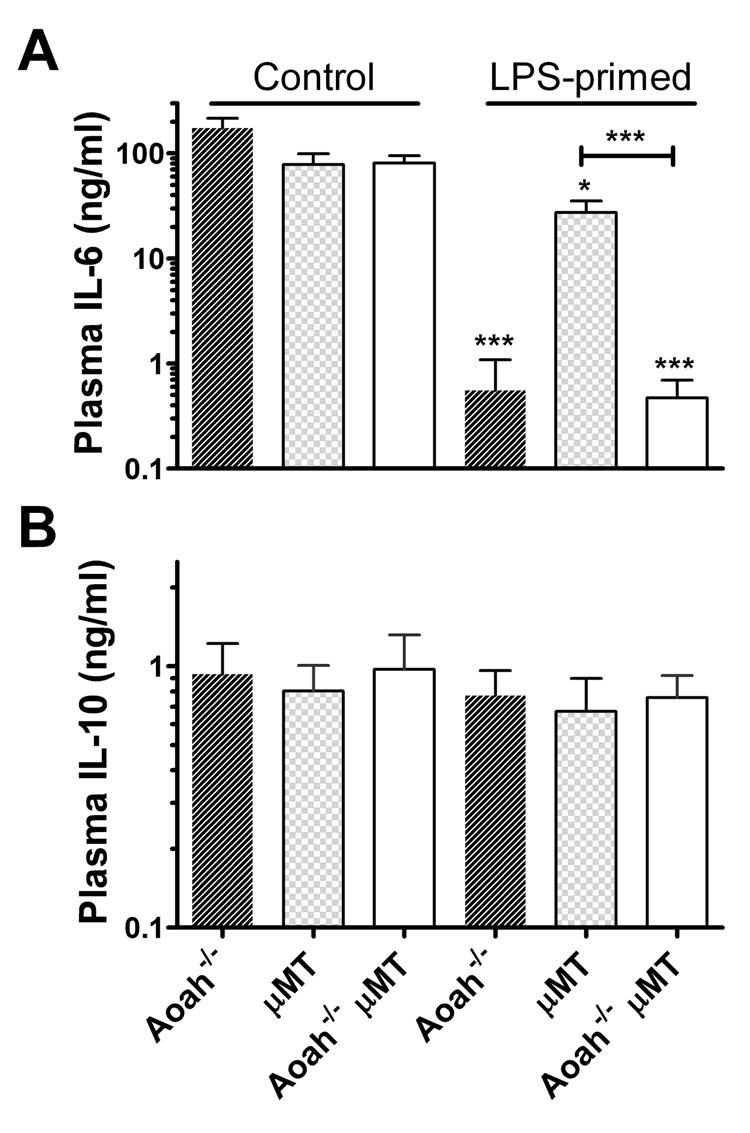
Since Aoah−/− mice have greatly exaggerated polyclonal IgM and IgG3 antibody responses to LPS (Lu et al., 2005), we tested the hypothesis that antibody-mediated LPS neutralization prevents responses to the second LPS challenge in primed animals (Greisman et al., 1969). Mice that lack both B cells (Kitamura et al., 1991) and AOAH (µMT, AOAH double knockouts) exhibited prolonged LPS tolerance in the absence of circulating IgM and IgG (Fig. 2). B cells or antibody responses to the priming LPS thus do not account for persistent LPS tolerance in Aoah−/− mice.
Fig. 2. Prolonged tolerance is not antibody-mediated.
Aoah−/−, µMT, and Aoah−/− µMT mice were primed with LPS i.p. on day 0 and challenged with LPS on day 14. Plasma samples were collected 4 hrs later and cytokine levels were measured by ELISA. Combined data from 3 experiments, n = 5 – 15 mice/group. Error bars: ± 1 SEM. Differences from PBS control group of same genotype: *** p <0.001.
Peritoneal macrophages also exhibit reprogrammed responses to microbial ligands
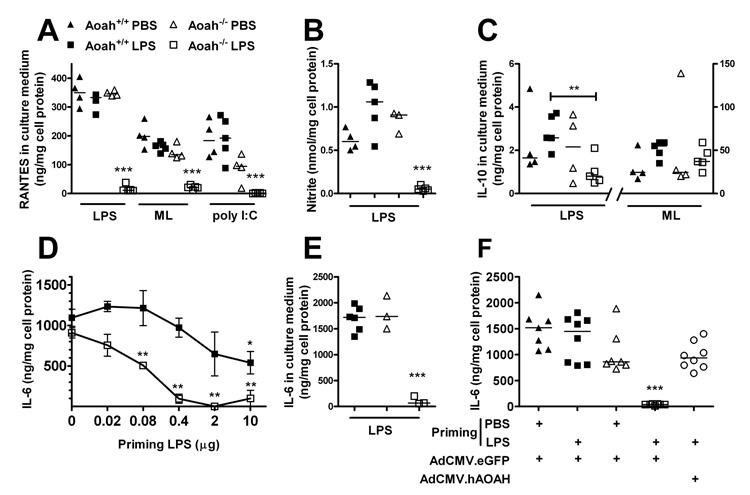
Macrophages harvested from the peritoneal cavities of LPS-primed Aoah−/− mice were also reprogrammed. Re-exposure to LPS ex vivo was associated with greatly diminished LPS-induced MyD88-dependent (TNF, IL-6, IL-1β [not shown]) and MyD88-independent (RANTES, nitric oxide production) responses (Fig. 3A, B). In addition, the production of TNF, IL-6 (not shown) and RANTES was reduced in response to Micrococcus luteus, a TLR2- dependent stimulus, and there was significantly impaired RANTES production after stimulation with a MyD88-independent TLR3 agonist, poly I:C (Alexopoulou et al., 2001) (Fig. 3A). LPS thus induced prolonged cross-tolerance (or “heterotolerance” (Dobrovolskaia et al., 2003)) to other microbial agonists in Aoah−/− animals. In contrast, whereas LPS-primed Aoah−/− macrophages produced wildtype levels of IL-10 when incubated with Micrococcus luteus, their IL-10 response to LPS was approximately one-third that of macrophages from primed Aoah+/+ mice (Fig. 3C). A more extensive survey of peritoneal macrophage responses to LPS is presented in Supplemental Table 1.
Fig. 3. Reprogramming persists in explanted peritoneal macrophages from LPS-primed Aoah−/− mice.
A–C, Aoah−/− and Aoah+/+ mice were given LPS i.p. (primed mice) or PBS (control mice) on day 0 and on day 10. Ten days after the second injection, peritoneal macrophages were isolated and treated ex vivo with LPS, Micrococcus luteus (ML) or poly I:C for 6 (A,C), or 24 (B) hours (A) RANTES; (B) nitrite; (C) IL- 10. (D) LPS induction of tolerance in vivo is dose-dependent. Mice (n=3–6) received various doses of E. coli O14 LPS or PBS i.p. on day 0. On day 14, isolated peritoneal macrophages were exposed to 1 µg/ml LPS for 6 hr and medium IL-6 was measured. Error bars: ± 1 SEM. (E) Responses to LPS of macrophages explanted from mice primed with 10 µg LPS 8 weeks previously. In A – E, significant differences from PBS-primed mice of same genotype are indicated by * p <0.05; **p <0.01; *** p <0.001. (F) Recombinant AOAH prevents prolonged tolerance in Aoah−/− mice. Mice that had received an i.v. injection of 1 × 108 transgene equivalents of Ad.CMV-hAOAH or Ad.CMV-eGFP 14 days previously were primed i.p. with PBS or 1 µg LPS. Peritoneal macrophages were isolated 14 days later and stimulated with LPS ex vivo. *** denotes p < 0.001 vs. other groups. Data were combined from two independent experiments. The symbol designations in panel A are used in the other panels. In F, the open circles represent LPS-primed Aoah−/− mice that had received Ad.CMV-hAOAH.
In Aoah−/− animals, tolerance can be induced by very low LPS doses and last for two months
LPS-induced tolerance was dose-dependent (Fig. 3D); as little as 0.1 µg intraperitoneal LPS induced tolerance for 2 weeks in Aoah−/− peritoneal macrophages whereas 10 µg or more was required in Aoah+/+ animals. Moreover, tolerance lasted for at least two months in the absence of AOAH (Fig. 3E). Aoah−/− mice of another genetic background (C3H/HeN) also were tolerant for 14 days or more following exposure to 10 µg LPS i.p., excluding a strain-specific effect of the Aoah−/− mutation (data not shown).
Recombinant AOAH prevents prolonged tolerance in Aoah−/− animals
To confirm that AOAH deficiency allows prolonged LPS-induced tolerance, we expressed recombinant human AOAH in AOAH-deficient mice using a gutted adenoviral vector, Ad.CMV-hAOAH. Fourteen days after viral infection the mice were given either 1 µg LPS or PBS i.p. When they were harvested 2 weeks later and stimulated with LPS ex vivo, macrophages from LPS-primed Aoah−/− mice that had received Ad.CMV-hAOAH produced 20-fold more IL-6 and TNF than did macrophages from Aoah−/− mice that had received a control virus, Ad.CMV-eGFP (Fig. 3F), and their ability to produce several cytokines and chemokines in response to LPS was also restored (Supplement Table 1). Providing recombinant AOAH thus prevented prolonged tolerance in Aoah−/− animals.
Retention of acylated LPS by macrophages is associated with prolonged tolerance in vivo
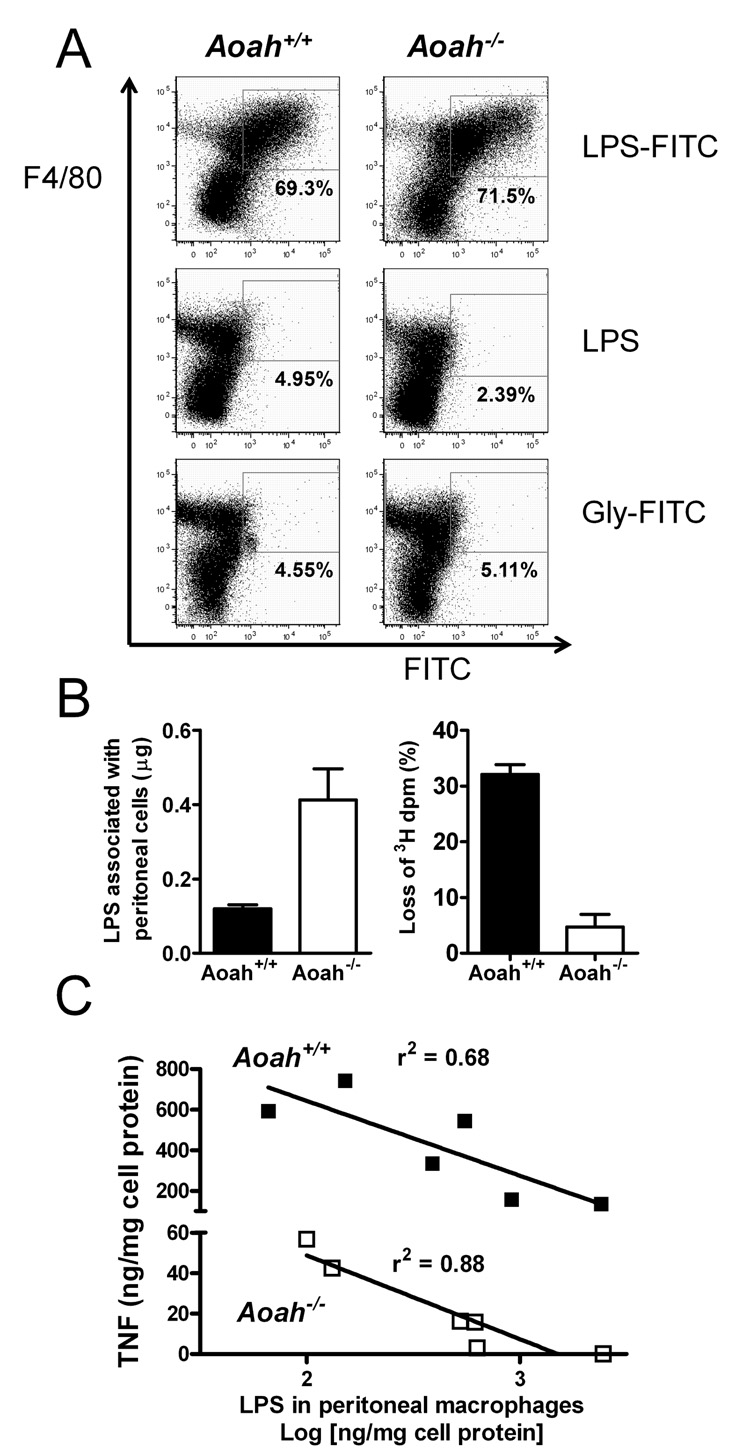
Ten days after intraperitoneal priming there were more macrophages, neutrophils, and B cells in the peritoneal washes of Aoah−/− mice that had been primed with LPS than in the other groups (Supplement Table 2). Flow cytometric examination of peritoneal cells 10 days after i.p. injection of a fluorescent LPS preparation (FITC-LPS) revealed that the FITC-LPS was associated almost exclusively with F4/80+ cells (macrophages) (Fig. 4A). Approximately 60–70 per cent of the recovered macrophages was FITC+ in both Aoah−/− and Aoah+/+ mice. The mean fluorescence intensity was similar for macrophages of both genotypes. To measure the uptake and deacylation of LPS by peritoneal cells in vivo, we used [3H/14C]LPS. Ten days after i.p. injection of 10 µg radiolabeled LPS, more of the LPS remained associated with peritoneal cells in Aoah−/− mice, which had more macrophages (Fig. 4B, left); very little of this LPS had been deacylated (Fig. 4B, right). In contrast, the [3H/14C]LPS found in Aoah+/+ cells lacked one-third of its 3H-acyl chains, consistent with loss of the two secondary acyl chains that are released by AOAH (Shao et al., 2007). Collectively, these findings suggest that the macrophages from LPS-primed Aoah−/− and Aoah+/+ mice contained similar amounts of LPS per cell when they were studied 10 days later; in Aoah+/+ macrophages the LPS had lost one-third of its acyl chains whereas in Aoah−/− macrophages it remained fully acylated. Importantly, production of TNF following LPS exposure ex vivo was inversely related to the amount of cell-associated 14C-LPS (Fig. 4C). Prolonged tolerance thus correlated directly with the retention of fully acylated LPS by peritoneal macrophages.
Fig. 4. Fully acylated LPS remains macrophage-associated in primed Aoah−/− mice and induces prolonged reprogramming.
(A) Flow cytometric analysis of peritoneal monocyte-macrophages harvested 10 days after i.p. injection of FITC-LPS, LPS, or FITC-glycine. (B) Peritoneal cells were harvested from Aoah+/+ and Aoah−/− mice that had received i.p. injections of 10 µg [3H/14C]LPS 10 days earlier. Recovery of LPS (left) and its deacylation (% loss of 3H dpm, right). AOAH removes 2 of the 6 acyl chains. Error bar = 1 SEM. n = 7–8 mice/group. (C) Ten days after LPS injection i.p., there was an inverse correlation between the amount of TNF produced following LPS stimulation of explanted macrophages ex vivo and the amount of radiolabeled LPS associated with the explanted cells.
Prolonged tolerance is immunosuppressive
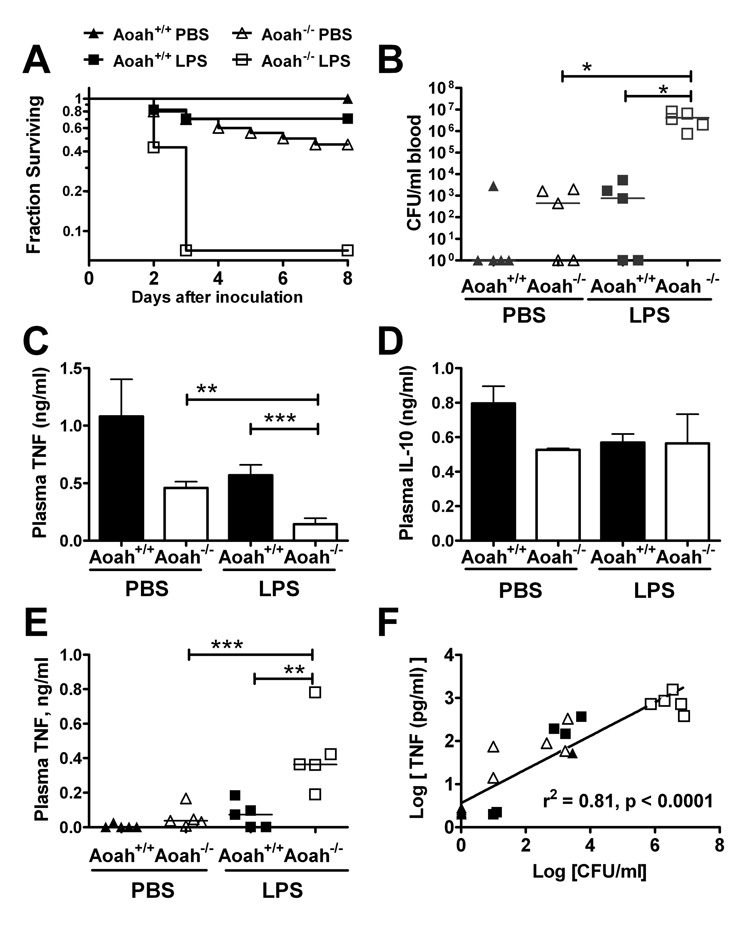
To find out how prolonged in vivo tolerance influences host defense, we compared the ability of Aoah−/− and Aoah+/+ mice to resist a virulent E. coli O18 strain (Cross et al., 1995) two weeks after a priming dose of LPS. Whereas no PBS-primed Aoah+/+ mice succumbed to the bacterial challenge, 90% of the LPS-primed Aoah−/− mice did not survive (Fig. 5A). When we repeated the bacterial challenge and measured colony forming units (cfu) in various organs 24 hrs later, we recovered 102- to 103-fold more cfu from the blood of LPS-primed Aoah−/− mice than from mice in the other experimental groups (Fig. 5B). A similar pattern was observed in the liver, spleen, lung, kidney and peritoneal fluid (data not shown). In the absence of AOAH, LPS priming thus severely impairs the ability to control E. coli infection.
Fig. 5. LPS-primed Aoah−/− mice are more susceptible to E. coli challenge.
(A) Groups of mice received a priming intraperitoneal dose of 10 µg E. coli O14 LPS or PBS. Ten to 14 days later, they were challenged intraperitoneally with 800 colony-forming units (cfu) E. coli O18K1. Data combined from 3 independent experiments (n = 15 – 19). Each survival curve is different from the others at p < 0.01 (Mantel-Cox). In another experiment, cultures were performed 24 hrs after i.p. inoculation of 800 cfu E. coli. Panel (B) shows the cfu recovered from cultures of blood. In a third experimental design, 5 × 107 cfu E. coli were injected i.p. and plasma cytokine levels were measured one hour later. At this time point, plasma TNF levels (C) were significantly lower in primed Aoah−/− mice than in the other groups, whereas the plasma IL-10 was more variable (D)(data combined from 2 experiments, each with n=4). (F) Twenty-four hours post-challenge (800 cfu), in contrast, the concentrations of TNF in the blood (see panel (E)) correlated with the number of recovered bacteria shown in (B). Similar correlations were observed between IL-6 and cfu, and for both peritoneal fluid and blood (not shown). * p <0.05; **p <0.01; *** p <0.001.
The impact of prolonged tolerance was evident shortly following the infectious challenge. One hour after challenge with 3 × 107 E. coli O18K1, LPS-primed Aoah−/− mice had lower plasma TNF (Fig. 5C) and IL-6 (not shown) than did animals in the other groups; the IL-10 response was more variable (Fig. 5D). Similar results were found when mice had been primed with live E. coli and then challenged with the virulent O18K1 strain (Fig. S2). Animals that retained fully acylated LPS in peritoneal macrophages thus mounted sluggish pro-inflammatory cytokine responses when challenged with live bacteria. Twenty-four hours after infection, the plasma and peritoneal levels of TNF and IL-6 (not shown) were directly related to the numbers of bacteria found in these fluids (Fig. 5 E, F). Uncontrolled infection in LPS-primed Aoah−/− mice thus can induce inflammatory responses that likely contribute to lethality.
Discussion
We studied the duration of endotoxin tolerance in vivo and found strong evidence that terminating tolerance to Gram-negative bacteria requires enzymatic inactivation of their major bacterial TLR ligand, LPS. The inactivating enzyme, acyloxyacyl hydrolase, is produced by monocyte-macrophages, neutrophils, dendritic cells (Lu et al., 2003) and renal cortical epithelial cells (Feulner et al., 2004). It removes the secondary acyl chains that are required for LPS recognition by MD-2—TLR4, the LPS signaling receptor (Munford and Varley, 2006). LPS deacylation occurs over many hours in vivo and does not appreciably diminish the acute inflammatory response to LPS or Gram-negative bacteria. On the other hand, persistently acylated LPS can induce prolonged, exaggerated antibody responses (Lu et al., 2005), hepatomegaly (Shao et al., 2007), and low-grade elevations in circulating levels of certain cytokines (IL-10) (B. Shao, unpublished). Here we found that AOAH deficiency has a profound impact on the intensity and duration of endotoxin tolerance. The LPS dose required to induce prolonged reprogramming in animals that could not deacylate LPS was at least 20-fold lower than that needed in wild-type mice (Fig. 3D) and the tolerant state lasted for at least 2 months (Fig. 3E). The intensity of the tolerant state, as assessed by the production of TNF by explanted macrophages, was directly related to the amount of macrophage—associated acylated LPS (Fig. 4C). Prolonged tolerance did not occur in Aoah−/− animals when MD-2—TLR4 was activated by an agonistic monoclonal antibody (Fig. 1F), providing strong evidence that the enzyme’s ability to inactivate LPS is essential to its ability to limit tolerance.
In the categorization developed by Greisman (Greisman et al., 1969), early endotoxin tolerance is mediated by changes in cellular responsiveness to LPS (this phase was later attributed to macrophages (Freudenberg and Galanos, 1988)), whereas (late) tolerance that lasts more than a few days is caused by neutralization of the challenge LPS dose by antibodies raised in response to the initial LPS exposure. The latter phenomenon seemed a likely explanation for prolonged tolerance in Aoah−/− mice, which react to low doses of LPS by mounting robust polyclonal IgM and IgG3 responses that include antibodies to many different LPSs (Lu et al., 2005). Indeed, we also found increased numbers of B cells in the peritoneal washes of LPS-primed Aoah−/− mice (Supplemental Table 1). We were thus surprised to find that prolonged LPS reprogramming occurred in Aoah−/− mice that lack B cells (Fig. 2) and was also present in macrophages that had been removed from the antibody-rich in vivo environment (Fig. 3). In animals that cannot deacylate LPS, prolonged endotoxin tolerance is not mediated by antibodies or B cells.
Many previous investigators have found that early endotoxin tolerance (lasting hours or a few days) can boost host defenses (Lehner et al., 2001; Freudenberg et al., 2001; Cross, 2002; Cavaillon et al., 2003; Echtenacher and Mannel, 2002). Enhanced resistance to infectious challenge during early tolerance might have several mechanisms: the ability of TNF, produced in response to the priming dose of LPS, to boost host defenses (Echtenacher and Mannel, 2002; Cavaillon et al., 2003), a greater influx of neutrophils into the site of bacterial inoculation (Lehner et al., 2001), diminished neutrophil apoptosis (Feterowski et al., 2001), interferon-mediated hypersensitivity to LPS (Freudenberg et al., 2001), or increased production of antimicrobial proteins (Foster et al., 2007). In contrast, other groups have found that early tolerance can diminish host defenses. In particular, tolerance induced by intraperitoneal infection or intraportal injection of LPS can diminish antibacterial resistance in the lungs of mice (Mason et al., 1997; Deng et al., 2006), pointing to complex interactions between the systemic and local compartments (Fitting et al., 2004). In all of these studies, microbial challenge was administered within a few hours or days of the priming exposure.
The interval between primary LPS exposure and bacterial challenge was much longer in our studies than in those previously reported. Remarkably, for at least two weeks after LPS priming, AOAH-deficient animals had increased numbers of intraperitoneal B cells and macrophages (Supplemental Table 2), suggesting that the fully acylated LPS remains stimulatory in vivo. Nonetheless, they mounted sluggish pro-inflammatory (TNF, IL-6) responses when challenged with live bacteria (Fig. 4C and Fig. S2), and were more likely to die following intraperitoneal bacterial challenge (Fig. 4A). Moreover, macrophages explanted from LPS-primed Aoah−/− mice produced significantly lower levels of many chemokines and cytokines when they were stimulated ex vivo with LPS (Fig. 3 and Supplemental Table 1). The immunodeficiency observed here seems quite similar to that described by Cross et al., who found that TLR4-dependent inflammatory responses were indispensible for host defense vs. virulent E. coli challenge (Cross et al., 1995). In the present case, it seems likely that a dampened, sluggish initial response allowed bacteria to proliferate to levels that then induced sustained, harmful levels of pro-inflammatory mediators.
Several previous observations have suggested that persistence of MD-2—TLR4 agonists can prolong tolerance in vivo. Whereas a single i.v. dose of LPS could induce tolerance in humans for less than 14 days (Engelhardt et al., 1991), daily administration of LPS maintained tolerance for as long as 30 days (Greisman et al., 1963) and weekly injections for as long as 42 days (Engelhardt et al., 1991). In mice, which generally recover from LPS-induced tolerance within 5 to 7 days (Wysocka et al., 2001), the agonistic UT12 monoclonal antibody to MD-2—TLR4 induced tolerance that lasted at least 9 days (Ohta et al., 2006). These reports and the inverse correlation found here between ex vivo TNF production and macrophage-associated LPS (Fig. 4C) suggest that persistent TLR4 activation is sufficient to perpetuate, at least in part, mechanisms that reprogram cellular responses to LPS and several other microbial agonists. Remarkably, LPS-primed Aoah−/− mice may not regain normal responsiveness to LPS. For convenience we terminated almost all experiments 10 to 14 days after priming, a time at which significant differences between Aoah−/− and Aoah+/+ mice were consistently found. LPS-primed Aoah−/− mice remained tolerant in vivo for at least 22 days (Fig. 1), however, and explanted macrophages were reprogrammed when they were studied 8 weeks after the priming LPS dose (Fig. 3E). In other studies we have found that LPS-exposed Aoah−/− mice develop lymph node enlargement (Lu et al., 2005) and hepatomegaly (Shao et al., 2007) that lasted at least 6 and 4 weeks, respectively. Enzymatic deacylation may thus be required to terminate many different responses to LPS in vivo.
We found that providing AOAH in an adenoviral vector could prevent prolonged LPS-induced tolerance in Aoah−/− animals (Fig. 2F). This result raises the possibility that increasing AOAH activity in vivo might hasten the recovery of host defenses following tissue-invasive Gram-negative bacterial diseases and help prevent secondary infections. Enhancing AOAH activity might also improve pulmonary defenses in individuals whose alveolar macrophages have been tolerized by the endotoxin in tobacco smoke (Chen et al., 2007), enhance responses to infection in patients with hepatic cirrhosis, whose circulating monocytes may be tolerized by gut-derived endotoxin (Von Baehr et al., 2000), or diminish systemic immune activation in patients with human immunodeficiency virus disease (Brenchley et al., 2006). It should also be worthwhile to look for enhanced infection susceptibility related to differences in AOAH expression among such individuals (Gao et al., 2007).
Although exogenously-administered alkaline phosphatase can evidently inactivate LPS in vivo in rodents (Beumer et al., 2003; Koyama et al., 2002; Poelstra et al., 1997; van Veen et al., 2005), until recently there was only indirect evidence that endogenous phosphatases play this role. Inactivation of LPS by an intestinal alkaline phosphatase has now been reported to prevent gut mucosal inflammation in zebrafish (Bates et al., 2007), which do not produce AOAH, suggesting that LPS dephosphorylation may be an important mechanism for inactivating the large amounts of LPS found in the intestine. Our findings indicate, in contrast, that enzymatic deacylation plays a distinctive role in inactivating LPS in extraintestinal tissues, even in animals that produce endogenous phosphatases (Peterson and Munford, 1987; Lu et al., 2005; Shao et al., 2007; Vaishnava and Hooper, 2007). Most importantly, we have provided evidence that disabling this potent microbial agonist is required to restore effective antibacterial host defenses following Gram-negative bacterial infection in vivo. It is possible that host mechanisms for inactivating other microbial molecules, such as bacterial lipopeptides and peptidoglycan fragments (Hedl et al., 2007) or viral TLR agonists (Didierlaurent et al., 2008), may also play a role in terminating infection-induced immunosuppression in animals.
Experimental Procedures
Mice
Targeted disruption of mouse Aoah was accomplished as described (Lu et al., 2003). The mutation was back-crossed 8 generations into the C57Bl/6 and C3H/HeN backgrounds. All mice were kept in a specific pathogen−free barrier facility. µMT Aoah−/− double knockouts, obtained by crossing µMT with Aoah−/− mice, lacked serum IgM and IgG. Mouse protocols were approved by the Institutional Animal Care and Utilization Committee of The University of Texas Southwestern Medical Center (Dallas, Texas).
Reagents
E. coli O14 LPS was prepared by the phenol-chloroform-petroleum ether method (Galanos et al., 1969). Rc S. typhimurium LPS (3H-labeled fatty acyl chains and 14C-labeled glucosamine backbone) was prepared as described previously (Munford and Erwin, 1992); 1 µg had approximately 150,000 dpm 3H and 10,000 dpm 14C. We measured the ratio of 3H dpm to 14C dpm in cell lysates to estimate per cent deacylation (Shao et al., 2007). FITC-LPS was prepared as described by Tobias et al. (Tobias et al., 1995). LPS from E. coli O14 was resuspended (2 mg/ml) in 0.1 M borate, pH 10.5. Five µg radiolabeled LPS was added so that the concentration of the final product could be calculated. 10 mg of solid FITC was then added to 2.5 ml suspension and incubated for 3 hrs at 37°C. A 10-fold excess of glycine was added to stop the reaction. The suspension was dialyzed (1000 MW cut off) against PBS until the dialysis buffer was no longer yellow. The FITC-LPS was then precipitated by adding a two-fold excess of ethanol. The pellet was washed three times with 70% ethanol and resuspended in PBS. The degree of labeling was 0.17 FITC/LPS (mol/mol). Glycine-FITC was made by mixing glycine with FITC in PBS. The solution was diluted so that its OD494 was the same as that of the FITC-LPS. The FITC mean fluorescence intensity (MFI) of peritoneal cells did not change after adding trypan blue, which quenches extracellular FITC. To enhance intracellular FITC, cells were permeabilized with 0.5% saponin (Sigma) and then stained with Qdot® 565 goat anti-fluorescein conjugate (Invitrogen).
Murine monoclonal anti-MD-2—TLR4 antibody, UT12 (Ohta et al., 2006), was prepared from mouse ascites using a T-GEL MacroPAC™ kit from SCIPAC (Sittingbourne, Kent, UK). Purity was ascertained by SDS-PAGE with Coomassie blue staining. The purified mAb was approximately 500-fold more potent than was heat-denatured mAb (95°C, 5 min) at eliciting TNF release from C56Bl/6 (Tlr4+/+) murine macrophages, and it did not stimulate Tlr4−/− macrophages to release TNF (data not shown).
Gutted adenoviral vectors
A vector that expresses human AOAH (gAd.CMV-hAOAH) was prepared by cloning the 1.7 kb AOAH cDNA into a gutted-Ad shuttle plasmid, pACParentCmF.CMVp-hGHTerm, as an Eco RI fragment. The product was linearized with Pme I and co-transformed with undigested gutted-Ad packaging plasmid, pAd4, into E. coli strain BJ5183 (gift of B. Vogelstein, Johns Hopkins School of Medicine) to allow homologous recombination (He et al., 1998), which results in the transfer of the expression cassette and Kan marker from the shuttle plasmid into the packaging plasmid. Kanamycin-resistant clones were screened by digestion with Pac I, and clones showing the expected pattern were verified by transformation into 293 cells followed by assaying for AOAH activity. The finished packaging plasmid was then digested with Pac I to liberate the complete gutted-Ad genome, which was transformed into 116cre cells (Parks et al., 1996). High titer, purified gAd.CMV-hAOAH was then prepared by the cre-lox system of Graham and co-workers (Parks et al., 1996)(6) as modified by Palmer and Ng (Palmer and Ng, 2003). A control virus that expresses eGFP (cDNA from Clontech, Palo Alto, CA) was produced in the same way. The viral preps were assayed by quantitative PCR to determine the number of transgene equivalents per ml. In preliminary experiments we found that sustained production of AOAH in vivo occurred from 2 to 4 weeks after i.v. injection of Ad.CMV-hAOAH.
In vivo and ex vivo experiments
Mice were injected i.p. with 10 µg E. coli 014 LPS in 300 ul PBS, 1 µg UT12 mAb in PBS, 1.5 × 107 E. coli LCD25 (Munford et al., 1992) or PBS only. This E. coli inoculum contains approximately 150 ng LPS (Ingraham et al., 1983). They were challenged on later dates with 20 µg E. coli O111 LPS in 300 ul PBS i.p. Tail vein blood was obtained 1 and 4 hours after challenge. EDTA was used as anticoagulant. Live bacterial challenge was performed using E. coli O18K1, a virulent strain provided by A. Cross (U. Maryland School of Medicine). For ex vivo experiments, mice were primed with E. coli 014 LPS or PBS i.p.; on some occasions, two priming doses were given. Ten or 14 days after the last priming dose, mice were euthanized with CO2 and their peritoneal cells were flushed with 5 ml PBS containing 3 mM EDTA. After adherence for 16 h and washing with complete RPMI (cRPMI) medium (Lu et al., 2005), the cells were treated with 1 µg/ml of E. coli O111 LPS (Sigma), 40 µg/ml of killed Micrococcus luteus cells (Sigma), or 10 µg/ml of poly I:C (Sigma) for 6 hours. The media were harvested for cytokine assays and the cells were washed prior to lysis in PBS 0.1% Triton X-100 to measure cell protein (Biorad). In results not shown, the TNF response to polyI:C was very low in all of the study groups, indicating that the preparation used here was endotoxin-free, and Micrococcus luteus cells induced similar production of TNF by Tlr4+/+ and Tlr4−/− murine macrophages, as expected for a TLR2 agonist.
Assays
The RANTES, TGFβ, and MIP-1α ELISA kits were from R&D systems, the IL-1β ELISA kit from eBioscience and the Griess Reagent Kit from Invitrogen. Other cytokines and chemokines were measured using ELISA kits from Becton-Dickenson or a Searchlight array (Thermo Scientific Life Science Research Products, Rockford, IL). Flow cytometry and data analysis were performed as described (Lu et al., 2005). Fc binding was blocked with 0.5 mg/ml of normal mouse IgG (Caltag). Cells were then incubated with antibodies for 1 hr on ice, washed, and analyzed using FACScan™ (Becton-Dickenson) with flow Jo software (TreeStar, Inc.). Anti-mouse F4/80-FITC (Clone CI:A3-1) was from Serotec; Anti-mouse F4/80 (Biotin and PE) (Clone BM8) was from Caltag. CD11b Clone M1/70; Gr-1 Clone RB6-8C5; B220 Clone RA3-6B2; CD5 Clone 53-7.3; CD3 Clone 145-2C11; NK1.1 Clone PK136; and CD11c Clone HL3 were all from Becton-Dickenson.
Statistics
Unpaired Student’s t test (2-tailed) was used for comparisons between groups. Log-rank (Mantel-Cox) test was used for survival curve analysis.
Supplementary Material
Acknowledgments
Supported by grant AI18188 from the National Institute of Allergy and Infectious Diseases and the Jan and Henri Bromberg Chair in Internal Medicine, UT-Southwestern Medical Center. We thank F. L. Graham and B. Vogelstein for essential reagents, David Farrar, Beth Levine and Lora Hooper for helpful advice, and Baomei Shao for titrating the adenovirus preps.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll- like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J. MyD88-Dependent and MyD88-Independent Pathways in Synergy, Priming, and Tolerance between TLR Agonists. The Journal of Immunology. 2007;178:1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host. Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer C, Wulferink M, Raaben W, Fiechter D, Brands R, Seinen W. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J. Pharmacol. Exp. Ther. 2003;307:737–744. doi: 10.1124/jpet.103.056606. [DOI] [PubMed] [Google Scholar]
- Bjorkbacka H, Fitzgerald KA, Huet F, Li X, Gregory JA, Lee MA, Ordija CM, Dowley NE, Golenbock DT, Freeman MW. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol Genomics. 2004;19:319–330. doi: 10.1152/physiolgenomics.00128.2004. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Endotoxin tolerance: is there a clinical relevance? J Endotoxin Res. 2003;9:101–107. doi: 10.1179/096805103125001487. [DOI] [PubMed] [Google Scholar]
- Chen H, Cowan MJ, Hasday JD, Vogel SN, Medvedev AE. Tobacco Smoking Inhibits Expression of Proinflammatory Cytokines and Activation of IL-1R-Associated Kinase, p38, and NF-{kappa}B in Alveolar Macrophages Stimulated with TLR2 and TLR4 Agonists. The Journal of Immunology. 2007;179:6097–6106. doi: 10.4049/jimmunol.179.9.6097. [DOI] [PubMed] [Google Scholar]
- Cross A, Asher L, Seguin M, Yuan L, Kelly N, Hammack C, Sadoff J, Gemski P., Jr The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J Clin. Invest. 1995;96:676–686. doi: 10.1172/JCI118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AS. Endotoxin tolerance -- current concepts in historical perspective. J. Endotoxin Res. 2002;8:83–98. doi: 10.1179/096805102125000227. [DOI] [PubMed] [Google Scholar]
- Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J. Clin. Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp. Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Medvedev AE, Thomas KE, Cuesta N, Toshchakov V, Ren T, Cody MJ, Michalek SM, Rice NR, Vogel SN. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR "homotolerance" versus "heterotolerance" on NF-kappa B signaling pathway components. J Immunol. 2003;170:508–519. doi: 10.4049/jimmunol.170.1.508. [DOI] [PubMed] [Google Scholar]
- Echtenacher B, Mannel DN. Requirement of TNF and TNF receptor type 2 for LPS-induced protection from lethal septic peritonitis. J Endotoxin. Res. 2002;8:365–369. doi: 10.1179/096805102125000696. [DOI] [PubMed] [Google Scholar]
- Engelhardt R, Mackensen A, Galanos C. Phase I Trial of Intravenously Administered Endotoxin (Salmonella abortus equi) in Cancer Patients. Cancer Res. 1991;51:2524–2530. [PubMed] [Google Scholar]
- Faist E, Mewes A, Strasser T, Walz A, Alkan S, Baker C, Ertel W, Heberer G. Alteration of monocyte function following major injury. Arch. Surg. 1988;123:287–292. doi: 10.1001/archsurg.1988.01400270021002. [DOI] [PubMed] [Google Scholar]
- Feterowski C, Weighardt H, Emmanuilidis K, Hartung T, Holzmann B. Immune protection against septic peritonitis in endotoxin-primed mice is related to reduced neutrophil apoptosis. Eur. J Immunol. 2001;31:1268–1277. doi: 10.1002/1521-4141(200104)31:4<1268::aid-immu1268>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Feulner JA, Lu M, Shelton JM, Zhang M, Richardson JA, Munford RS. Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract. Infect. Immun. 2004;72:3171–3178. doi: 10.1128/IAI.72.6.3171-3178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting C, Dhawan S, Cavaillon J. Compartmentalization of Tolerance to Endotoxin. The Journal of Infectious Diseases. 2004;189:1295–1303. doi: 10.1086/382657. [DOI] [PubMed] [Google Scholar]
- Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- Freudenberg MA, Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect. Immun. 1988;56:1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg MA, Merlin T, Gumenscheimer M, Kalis C, Landmann R, Galanos C. Role of lipopolysaccharide susceptibility in the innate immune response to Salmonella typhimurium infection: LPS, a primary target for recognition of Gram-negative bacteria. Microbes and Infection. 2001;3:1213–1222. doi: 10.1016/s1286-4579(01)01481-2. [DOI] [PubMed] [Google Scholar]
- Galanos C, Luderitz O, Westphal O. A new method for the extraction of R lipopolysaccharides. Eur. J. Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gao L, Tsai YJ, Grigoryev DN, Barnes KC. Host defense genes in asthma and sepsis and the role of the environment. Curr. Opin. Allergy Clin. Immunol. 2007;7:459–467. doi: 10.1097/ACI.0b013e3282f1fb9a. [DOI] [PubMed] [Google Scholar]
- Greisman SE, Hornick RB, Carozza FA, Jr, Woodward TE. The role of endotoxin during typhoid fever and tularemia in man. I. Acquisition of tolerance to endotoxin. J. Clin. Invest. 1963;42:1064–1075. doi: 10.1172/JCI104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisman SE, Young EJ, Carozza FA., Jr Mechanisms of Endotoxin Tolerance: V. Specificity of the early and late phases of pyrogenic tolerance. The Journal of Immunology. 1969;103:1223–1236. [PubMed] [Google Scholar]
- He T-C, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proceedings of the National Academy of Sciences. 2007;104:19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helminen M, Vesikari T. Increased interleukin-1 (IL-1) production from LPS-stimulated peripheral blood monocytes in children with febrile convulsions. Acta Paediatr. Scand. 1990a;79:810–816. doi: 10.1111/j.1651-2227.1990.tb11559.x. [DOI] [PubMed] [Google Scholar]
- Helminen M, Vesikari T. Interleukin-1 production in bacterial meningitis. Scand. J Infect. Dis. 1990b;22:105–108. doi: 10.3109/00365549009023128. [DOI] [PubMed] [Google Scholar]
- Henricson BE, Manthey CL, Yu Perera P, Hamilton TA, Vogel SN. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect. Immun. 1993;61:2325–2333. doi: 10.1128/iai.61.6.2325-2333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham JL, Maaloe O, Neidhardt FC. Growth of the Bacterial Cell. Sunderland, MA: Sinauers Associates, Inc.; 1983. [Google Scholar]
- Kelly JL, O'Sullivan C, O'Riordain M, O'Riordain D, Lyons A, Doherty J, Mannick JA, Rodrick ML. Is circulating endotoxin the trigger for the systemic inflammatory response syndrome seen after injury. Ann. Surg. 1997;225:530–541. doi: 10.1097/00000658-199705000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Koyama I, Matsunaga T, Harada T, Hokari S, Komoda T. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin. Biochem. 2002;35:455–461. doi: 10.1016/s0009-9120(02)00330-2. [DOI] [PubMed] [Google Scholar]
- Lehner MD, Ittner J, Bundschuh DS, Van Rooijen N, Wendel A, Hartung T. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar typhimurium infection despite attenuated cytokine response. Infect Immun. 2001;69:463–471. doi: 10.1128/IAI.69.1.463-471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Zhang M, Kitchens RL, Fosmire S, Takashima A, Munford RS. Stimulus-dependent deacylation of bacterial lipopolysaccharide by dendritic cells. J. Exp. Med. 2003;197:1745–1754. doi: 10.1084/jem.20030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Zhang M, Takashima A, Weiss J, Apicella MA, Li XH, Yuan D, Munford RS. Lipopolysaccharide deacylation by an endogenous lipase controls innate antibody responses to Gram-negative bacteria. Nat. Immunol. 2005;6:989–994. doi: 10.1038/ni1246. [DOI] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Summer WR, Nelson S. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J Infect Dis. 1997;176:1293–1302. doi: 10.1086/514125. [DOI] [PubMed] [Google Scholar]
- Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The Vaccine Adjuvant Monophosphoryl Lipid A as a TRIF-Biased Agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- McCabe WR. Endotoxin tolerance. II. Its occurrence in patients with pyelonephritis. J Clin. Invest. 1963;42:618–625. doi: 10.1172/JCI104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall CE, Grosso-Wilmoth LM, LaRue K, Guzman RN, Cousart SL. Tolerance to endotoxin-induced expression of the interleukin- 1β gene in blood neutrophils of humans with the sepsis syndrome. J. Clin. Invest. 1993;91:853–861. doi: 10.1172/JCI116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall CE, Yoza BK. Gene silencing in severe systemic inflammation. Am J Respir. Crit Care Med. 2007;175:763–767. doi: 10.1164/rccm.200610-1436CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- Munford RS. Sensing gram-negative bacterial lipopolysaccharides: a human disease determinant? Infect. Immun. 2008;76:454–465. doi: 10.1128/IAI.00939-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford RS, DeVeaux LC, Cronan JE, Jr, Rick PD. Biosynthetic radiolabeling of bacterial lipopolysaccharide to high specific activity. J. Immunol. Methods. 1992;148:115–120. doi: 10.1016/0022-1759(92)90164-o. [DOI] [PubMed] [Google Scholar]
- Munford RS, Erwin AL. Eucaryotic lipopolysaccharide deacylating enzyme. Meth. Enzymol. 1992;209:485–492. doi: 10.1016/0076-6879(92)09059-c. [DOI] [PubMed] [Google Scholar]
- Munford RS, Hall CL. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234:203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- Munford RS, Varley AW. Shield as signal: lipopolysaccharides and the evolution of immunity to Gram-negative bacteria. PLoS Pathog. 2006;2:e67. doi: 10.1371/journal.ppat.0020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz C, Carlet J, Fitting C, Misset B, Bieriot J-P, Cavaillon J-M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J. Clin. Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S, Bahrun U, Shimazu R, Matsushita H, Fukudome K, Kimoto M. Induction of Long-Term Lipopolysaccharide Tolerance by an Agonistic Monoclonal Antibody to the Toll-Like Receptor 4/MD-2 Complex. Clin. Vaccine Immunol. 2006;13:1131–1136. doi: 10.1128/CVI.00173-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D, Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AA, Munford RS. Dephosphorylation of the lipid A moiety of Escherichia coli lipopolysaccharide by mouse macrophages. Infect. Immun. 1987;55:974–978. doi: 10.1128/iai.55.4.974-978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelstra K, Bakker WW, Klok PA, Hardonk MJ, Meijer DKF. A physiologic function for alkaline phosphatase: Endotoxin detoxification. Lab. Invest. 1997;76:319–327. [PubMed] [Google Scholar]
- Sato S, Takeuchi O, Fujita T, Tomizawa H, Takeda K, Akira S. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and - independent pathways. Int. Immunol. 2002;14:783–791. doi: 10.1093/intimm/dxf046. [DOI] [PubMed] [Google Scholar]
- Shao B, Lu M, Katz SC, Varley AW, Hardwick J, Rogers TE, Ojogun N, Rockey DC, DeMatteo RP, Munford RS. A Host Lipase Detoxifies Bacterial Lipopolysaccharides in the Liver and Spleen. J. Biol. Chem. 2007;282:13726–13735. doi: 10.1074/jbc.M609462200. [DOI] [PubMed] [Google Scholar]
- Shnyra A, Brewington R, Alipio A, Amura C, Morrison DC. Reprogramming of lipopolysaccharide-primed macrophages is controlled by a counterbalanced production of IL-10 and IL-12. J. Immunol. 1998;160:3729–3736. [PubMed] [Google Scholar]
- Somerville JE, Jr, Cassiano L, Bainbridge B, Cunningham MD, Darveau RP. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J. Clin. Invest. 1996;97:359–365. doi: 10.1172/JCI118423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J. Biol. Chem. 1995;270:10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Hooper LV. Alkaline phosphatase: keeping the peace at the gut epithelial surface. Cell Host. Microbe. 2007;2:365–367. doi: 10.1016/j.chom.2007.11.004. [DOI] [PubMed] [Google Scholar]
- van Veen SQ, van Vliet AK, Wulferink M, Brands R, Boermeester MA, van Gulik TM. Bovine Intestinal Alkaline Phosphatase Attenuates the Inflammatory Response in Secondary Peritonitis in Mice. Infect. Immun. 2005;73:4309–4314. doi: 10.1128/IAI.73.7.4309-4314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Baehr V, Döcke WD, Plauth M, Liebenthal C, Küpferling S, Lochs H, Baumgarten R, Volk HD. Mechanisms of endotoxin tolerance in patients with alcoholic liver cirrhosis: role of interleukin 10, interleukin 1 receptor antagonist, and soluble tumour necrosis factor receptors as well as effector cell desensitisation. Gut. 2000;47:281–287. doi: 10.1136/gut.47.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Doyle M, Manning BJ, Blankson S, Wu QD, Power C, Cahill R, Redmond HP. Bacterial lipoprotein induces endotoxin-independent tolerance to septic shock. J. Immunol. 2003;170:14–18. doi: 10.4049/jimmunol.170.1.14. [DOI] [PubMed] [Google Scholar]
- Weighardt H, Heidecke CD, Emmanuilidis K, Maier S, Bartels H, Siewert JR, Holzmann B. Sepsis after major visceral surgery is associated with sustained and interferon-gamma-resistant defects of monocyte cytokine production. Surgery. 2000;127:309–315. doi: 10.1067/msy.2000.104118. [DOI] [PubMed] [Google Scholar]
- West MA, Heagy W. Endotoxin tolerance: A review. Crit Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]
- Wysocka M, Montaner LJ, Karp CL. Flt3 ligand treatment reverses endotoxin tolerance-related immunoparalysis. J Immunol. 2005;174:7398–7402. doi: 10.4049/jimmunol.174.11.7398. [DOI] [PubMed] [Google Scholar]
- Wysocka M, Robertson S, Riemann H, Caamano J, Hunter C, Mackiewicz A, Montaner LJ, Trinchieri G, Karp CL. IL-12 suppression during experimental endotoxin tolerance: Dendritic cell loss and macrophage hyporesponsiveness. J. Immunol. 2001;166:7504–7513. doi: 10.4049/jimmunol.166.12.7504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.