Abstract
Background
Sphingosine-1-phosphate (S-1-P) is a bioactive sphingolipid that stimulates the migration of vascular smooth muscle cell (VSMC) through G-protein coupled receptors; it has been shown to activate reduced nicotinamide dinucleotide phosphate hydrogen (NAD[P]H) oxidase. The role of phospholipase C (PLC) in oxygen free radical generation, and the regulation of VSMC migration in response to S-1-P, are poorly understood.
Methods
Rat arterial VSMC were cultured in vitro. Oxygen free radical generation was measured by fluorescent redox indicator assays in response to S-1-P (0.1µM) in the presence and absence of the active PLC inhibitor (U73122; U7, 10nM) or its inactive analog U73343 (InactiveU7, 10nM). Activation of PLC was assessed by immunoprecipitation and Western blotting for the phosphorylated isozymes (β and γ). Small interfering (si) RNA to the G-proteins Gαi, Gαq, and Gα12/13 was used to downregulate specific proteins. Statistics were by one-way analysis of variance (n = 6).
Results
S-1-P induced time-dependent activation of PLC-β and PLC-γ; PLC-β but not PLC-γ activation was blocked by U7 but not by InactiveU7. PLC-β activation was Gαi-independent (not blocked by pertussis toxin, a Gαi inhibitor, or Gαi2 and Gαi3 siRNA) and Gαq-independent (not blocked by glycoprotein [GP] 2A, a Gαq inhibitor, or Gαq siRNA). PLC-β activation and cell migration was blocked by siRNA to Gα12/13. Oxygen free radical generation induced by S-1-P, as measured by dihydroethidium staining, was significantly inhibited by U7 but not by InactiveU7. Inhibition of oxygen free radicals with the inhibitor diphenyleneiodonium resulted in decreased cell migration to S-1-P. VSMC mitogen-activated protein kinase activation and VSMC migration in response to S-1-P was inhibited by PLC- inhibition.
Conclusion
S-1-P induces oxygen free radical generation through a Gα12/13, PLC-β-mediated mechanism that facilitates VSMC migration. To our knowledge, this is the first description of PLC-mediated oxygen free radical generation as a mediator of S-1-P VSMC migration and illustrates the need for the definition of cell signaling to allow targeted strategies in molecular therapeutics for restenosis.
Clinical Relevance
Activation of vascular smooth muscle cells by growth factors leads to cell proliferation and migration, which are integral features of the healing response in a vessel that leads to the development of intimal hyperplasia after bypass grafting, angioplasty, and stenting. Sphingosine-1-phosphate (S-1-P) is a common phospholipid, released from activated platelets at sites of vessel injury. It is a G-protein–coupled receptor agonist that induces smooth muscle cell migration, a key event in the development of intimal hyperplasia. Mechanisms of cell migration are not well defined, and understanding the mechanisms of signal transduction is important in defining potential targets for therapeutic intervention. The present study shows that S-1-P induces oxygen free radical generation through a Gα12/13, PLC-β–mediated mechanism that facilitates smooth muscle cell migration. Targeting choke points in cell signaling, such as membrane G-proteins, is an attractive molecular target in developing therapeutic strategies to moderate restenosis.
Activation of vascular smooth muscle cells (VSMC) by growth factors leads to cell proliferation and migration, which are integral features of the healing response in a vessel.1 Targeting key molecules at the level of the membrane, within the cell and within the nucleus, appears to be a novel way to achieve local control of the activated SMCs. Multiple pathways, including mammalian target of rapamycin, mitogen-activated protein kinase (MAPK), and cell-cycle proteins, have been successfully targeted by therapeutic agents. In human restenotic lesions, migration rather than proliferation may be a primary biologic event leading to intimal expansion, and the signaling leading to these events is not fully defined.1
Sphingosine-1-phosphate (S-1-P) is a bioactive sphin-golipid released in large amounts from activated platelets that has been shown to have numerous biologic effects in a large number of vascular cell types. It can act as a secondary mediator for cell responses mediated by platelet-derived growth factor (PDGF) and tissue plasminogen activator (tPA).2,3 S-1-P has been identified as a chemoattractant for VSMCs expressing G-protein–coupled receptors S-1-P1, S-1-P2 and S-1-P3 receptors.2,4–7 S-1-P1 couples exclusively to G-proteins of the Gαi type, while S-1-P2 and S-1-P3 couple to G-proteins of Gαi, Gαq, and Gα12/13 types.8,9
We have previously demonstrated that migration stimulated by S-1-P in VSMCs involves PI3-kinase, extracellular signal-regulated kinases 1/2 (ERK1/2), and p38MAPK.4–6 Others have shown that exogenous S-1-P will stimulate oxygen free radical generation in association with an increase in intracellular Ca2+ concentration and an increase in inositol phosphate production, reflecting activation of phospholipase C (PLC).
Very limited data are available on the role of PLC-β in arterial injury. One report has shown that PLC-β activities are not significantly elevated ≤24 hours of balloon injury in the rat aorta, but PLC-β activity has increased twofold by 14 days.10 Thus, it appears that PLC-β activity is not temporally associated with VSMC apoptosis or medial VSMC proliferation but occurs during the periods of VSMC migration and intimal expansion.1 S-1-P can activate PLC-β, but its role in S-1-P–mediated VSMC migration and oxygen free radical generation activation is not defined.
This study examined the role of the phospholipase C signaling during oxygen free radical generation and VSMC migration in response to S-1-P and tested the hypothesis that G-protein–induced PLC activation mediates VSMC migration through oxygen free radical–dependent mechanisms.
METHODS
Experimental design
Aortic arterial VSMCs from Sprague-Dawley rats (derived by the collagenase method), passages 3 to 6, were cultured in vitro. Activation of PLC was assessed by immunoprecipitation and Western blotting for the phosphorylated isozymes (β and γ). Small interfering (si) RNA to the G-proteins Gαi, Gαq, and Gα12/13 and adenoviral COOH-terminus β-adrenergic receptor kinase (β-ARKct) was developed commercially and cells transfected by a superlipofectamine method with appropriate downregulation of targets before examination of S-1-P–mediated PLC activation was confirmed.
Oxygen free radical generation was measured by fluorescent redox indicator assays in response to S-1-P (0.1µM) in the presence and absence of the active PLC inhibitor (U73122; U7, 0.1–1000nM) or its inactive analog U73343 (InactiveU7, 0.1–1000nM) and the nicotinamide dinucleotide phosphate hydrogen (NAD[P]H) oxidase inhibitor diphenyleneiodonium (DPI, 10µM). Cell migration in response to S-1-P was tested in a wound assay and a Boyden chamber assay under various inhibitory conditions.
Materials (available online only)
Western blotting
Cells were allowed to grow to 80% confluence and starved for 48 hours. These cells were stimulated with S-1-P alone (0.1µM) and in the presence of the inhibitors and harvested at time points from 0 to 60 minutes. At each time point, cells were washed once in ice-cold Dulbecco phosphate buffered saline (DPBS) and then mechanically removed in the presence of ice cold kinase buffer (200 µL; 25mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonate [HEPES, pH 7.5], 10% glycerol, 5mM ethylenediaminetetraacetic acid, 5mM ethyleneglycotetraacetic acid, 150mM sodium chloride, 100mM benzamidine, 0.1% 2-mercaptoethanol, 1% Triton X-100 [Union Carbide, Houston, Tex], 1mM pepstatin A, 2 mg/mL leupeptin, and 20 KIU/mL aprotinin).
Protein determinations were performed using a bicin-chonic acid assay (Biorad Laboratories, Richmond, Calif) with bovine serum albumen as the standard. Equal amounts of protein lysates (20 µg) were loaded onto a 10% polyacrylamide gel separated by electrophoresis and then transferred to a nitrocellulose membrane (Biorad).11 The membrane was blocked in 5% nonfat milk.
The blot was then immunostained with antibodies against either phosphorylated or total protein of interest. Protein bands were visualized using an antimouse immunoglobulin G horseradish peroxidase-conjugated antibody, followed by enhanced chemiluminescence (Amersham Bioscience, Piscataway, NJ) using the manufacturer’s protocols. Band intensity was measured using Gel-Imaging software (Kodak, Rochester, NY). All experiments were performed at least three times.
Immunoprecipitation
Time courses of S-1-P-induced PLC-β activation were performed in the presence and absence of U73122 (100nM) and the Inactive U7 (100nM), as previously described.12 Total PLC-β was immunoprecipitated from total cell extracts with a polyclonal antibody (Upstate Biotechnology, Lake Placid, NY) according to the manufacturer’s instructions. Immunocomplexes were then collected using protein A–Sepharose beads (Pharmacia Biotech, Piscataway, NJ), washed several times with Tween 20 (Union Carbide, Houston, Tex) in DPBS (Cellgro, Herndon, Va), fractionated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and probed with PY20 for tyrosine phosphorylation protein.
Small interfering RNA transfection
Pre-designed 2-hydroxyphytanoyl CoA lyase purified siRNA for gene knockdown for Gαi2 and Gαi3 proteins were purchased from Ambion Inc (Austin, Tex). VSMC of 50% confluence in 60-mm plates were starved overnight in 4 mL of Opti-MEM reduced serum medium (Gibco). Small interfering RNA is transfected using Lipofectamine 2000 (Invitrogen Inc, Carlsbad, Calif) following product protocol. Briefly, 22 µL of Lipofectamine 2000 was first incubated in a total volume of 250 µL of Opti-MEM for 5 minutes at room temperature. It was then added to 250 µL of Opti-MEM containing 440 pmol of siRNA. The solution was mixed gently and incubated for 20 minutes at room temperature, after which it was added to the starved plates of VSMC. The medium was changed after 4 to 6 hours of incubation. The cells were then used 72 hours after transfection for PLC-β assays. Scrambled siRNA served as a control.
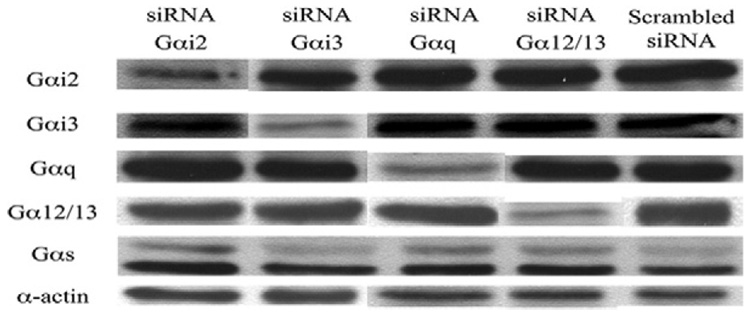
Using the methodologies described, we conducted concentration dependent experiments with siRNA to Gαi2, Gαi3, Gαq, and Gα12/13 and demonstrated a concentration dependent decrease in protein expression that was specific for the Gα protein targeted without altering the expression of the other Gα subunits (Fig E1, on-line only).
Fig E1 (online only). Western blots of small interfering RNA (si RNA) for G-proteins.
We examined the expression of the G-proteins in the presence of specific siRNA toGαi2,Gαi3,Gαq, and Gα12/13 after performing concentration-dependent suppression experiments. Western blots of Gαi2, Gαi3, Gαq, and Gα12/13, are shown in the presence of each siRNA used and the scrambled siRNA control. There is effective downregulation of each G-protein with the respective G-protein siRNA without affecting other G-proteins. We have also added Gαs and α-actin as loading controls.
Adenoviral infection
Adenoviral vectors were constructed by Welgen Inc (Worcester, MA) using purified plasmid encoding β-ARKCT, obtained from Guthrie cDNA Resource center. VSMC were plated at 70% confluence in 100-mm dishes and allowed to grow overnight. Recombinant adenovirus was then added at the appropriate concentrations (β-ARKCT; 100 MOI) in a reduced volume of media (1.5 to 2 mL). After 48 hours of incubation, the media was changed and cells were grown for an additional 24 hours. The cells were then used for PLC-β assays. Empty vector served as the control.
Dihydroethidium staining
Oxygen free radical generation was assayed using the oxygen free radical-sensitive fluorescent dye dihydroethidium (DHE; 10 µmol/L, Sigma). S-1-P (100nM) was added to growth-arrested VSMC in 24-well plates for variable times from 0 to 60 minutes. Dulbecco minimal essential medium (DMEM) and angiotensin II were used as positive and negative controls. Additional cells were examined in the presence of DPI and apocynin to verify the role of NAD(P)H oxidase activation by S-1-P. Plates were washed with PBS and then incubated in the dark for 5 minutes in DMEM lacking phenol red containing DHE (10 µmol/L). After the incubation, dishes were transferred to a fluorescent microscope for photography. Intracellular reactive oxygen species generation was quantitated spectrophotometrically by measurement of red fluorescent compound ethidium, which is produced when DHE specifically reacts with intracellular O2−.
Cell migration
VSMC migration in response to S-1-P (0.1µM) was performed in the presence and absence of U73122 (0.1 to 1000nM) and InactiveU7 (0.1 to 1000nM) using both the linear wound assay and the Boy-den microchemotaxis chamber assays, as previously described.5,6
Data and statistical analysis
All data are presented as the mean ± standard error of the mean. Statistical differences between groups were tested with a Kruskal-Wallis nonparametric test with post hoc Dunn multiple comparison correction, where appropriate, using Instat 3 software (Graph Pad Software Inc, San Diego, Calif). A value of P < .05 was regarded as significant. Nonsignificant P values are expressed as P = NS.
RESULTS
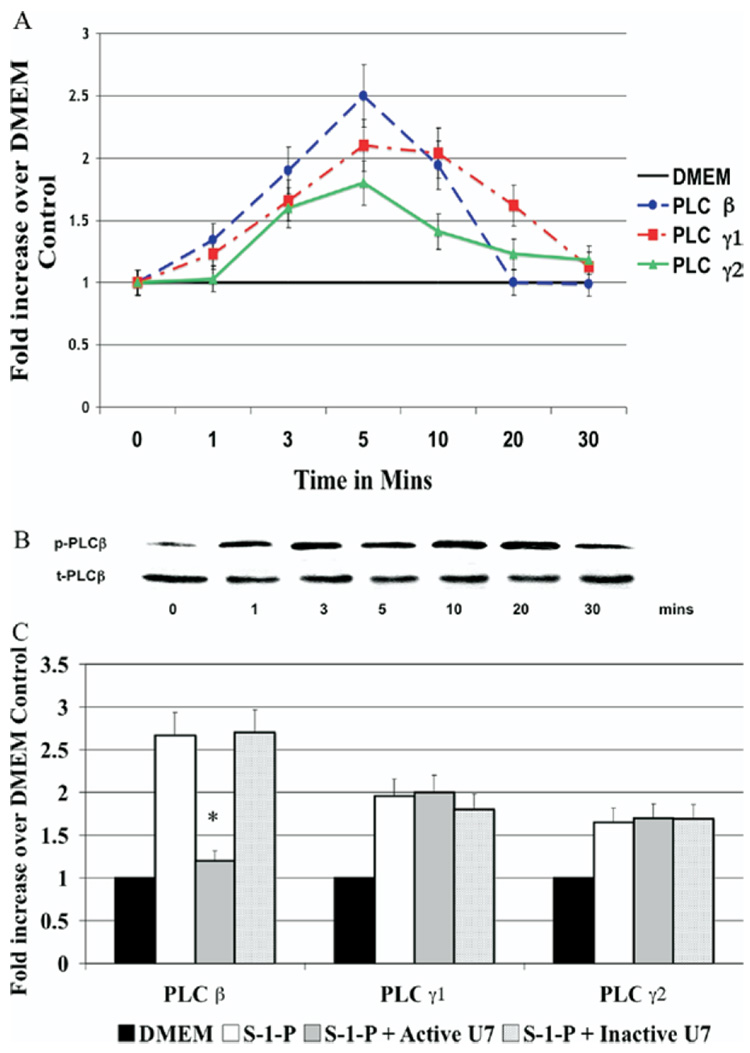
We examined the activation of PLC-β and PLC-γ isoforms in the VSMC by Western blotting in response to S-1-P (0.1µM). S-1-P–induced time-dependent activation of PLC-β, PLC-γ1, and PLC-γ2 was determined by phosphorylation of the respective protein relative to the total unphosphosylated protein; peak activation of PLC-β was at 10 minutes (Fig 1, A). PLC-β activation was blocked by the active PLC-β inhibitor (U73122; U7) but not by InactiveU7; Fig 1, B). Neither inhibitor effected PLC-γ activation in response to S-1-P, suggesting specificity of the inhibitor (Fig 1, B).
Fig. 1. Activation of phospholipase C-β (PLC-β).
A, Sphingosine-1-phosphate (S-1-P, 0.1µM)–induced time-dependent activation of PLC-β, PLC-γ1, or PLC-γ2, as measured by Western blotting. Maximal activation was at 5 minutes. PLC-β activation was blocked by U7 (10nM) but not by InactiveU7 (10nM). B, A representative Western blot for PLC-β is shown. C, Bar chart shows quantification of S-1-P–induced time-dependent activation of PLC-β, PLC-γ1, or PLC-γ2, in the presence and absence of U7 and InactiveU7. Values are the mean ± SEM of the ratio of the phosphorylation of the respective protein relative to the total unphosphorylated protein (n = 6). P < .05 compared with the control. DMEM, Dulbecco minimal essential medium.
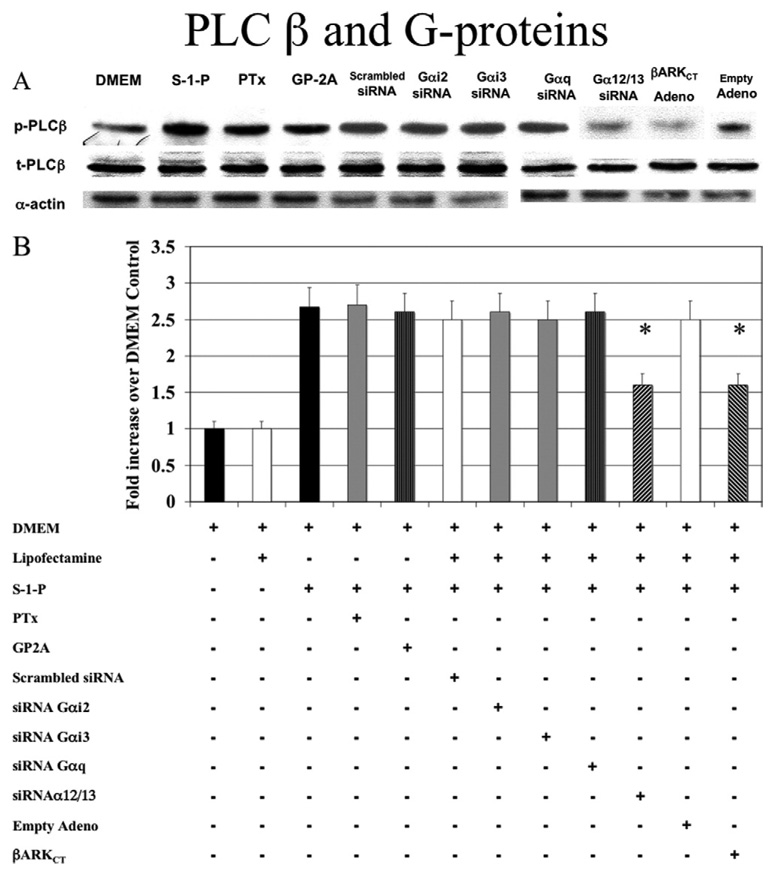
We have previously shown that S-1-P–mediated cell migration is dependent on G-protein coupled receptors. The VSMCs express Gαi2, Gαi3, Gαq, Gα12/13, and Gβγ. We, therefore, examined the role of G-proteins subunits Gα and Gβ in the activation of PLC-β by S-1-P using a strategy of both pharmacologic and molecular inhibitors. The activation of PLC-β by S-1-P was Gαi-independent (not blocked by preincubation with pertussis toxin, a Gαi inhibitor, or transfection with Gαi2 and Gαi3 siRNA) and Gαq-independent (not blocked by preincubation with glycoprotein-2A, a Gαq inhibitor, or transfection with Gαq siRNA; Fig 2). In contrast, siRNA to Gα12/13 blocked PLC-β activation (Fig 2). Scrambled siRNA for constructs against Gαi2, Gαi3, and Gα12/13 were used as controls and had no effect (data not shown). Adenoviral transfection with β-ARKCT, a Gβγ inhibitor, also blocked PLC-β activation in response to S-1-P. Empty adenovirus had no effect (Fig 2).
Fig. 2. G-proteins and phospholipase C-β (PLC-β).
A, Activation of PLC-β by sphingosine-1-phosphate (0.1µM) was Gαi-independent; that is, it was not blocked by pertussis toxin (PTx), a Gαi inhibitor, or Gαi2 and Gαi3 small interfering RNA (siRNA); and it was Gαq-independent (not blocked by GP-2A, a Gαq inhibitor, or Gαq siRNA). Small interfering RNA to Gα12/13 blocked PLC-β activation. Transfection with adenoviral COOH-terminus β-adrenergic receptor kinase (β-ARKCT) also blocked PLC-β activation. Representative Western blots are shown with α-actin as a loading control. B, The bar chart shows the quantification of the experiments representative in Fig 1, A and also shows additional controls. Scrambled siRNA and empty adenovirus did not significantly alter the response compared with control values. Values are the mean ± SEM of the ratio of the phosphorylation of PLC-β (p-PLC-β) relative to the total unphosphorylated PLC-β (t-PLC-β; n = 6). *P < .05 compared with control. DMEM, Dulbecco minimal essential medium; GP2A, glycoprotein 2A.
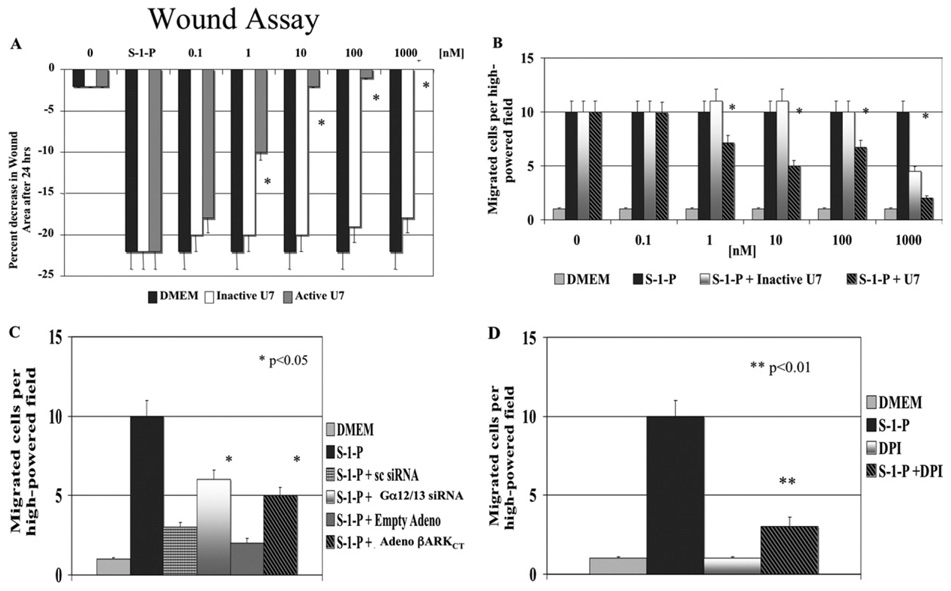
These data suggest that S-1-P mediates PLC-β activation through a Gα12/13 and Gβγ–mediated pathway. To test the biologic relevance of this assumption, we examined S-1-P–mediated cell migration. VSMC migration in response to S-1-P in the wound and the Boyden chamber assays of migration was inhibited by the PLC-β inhibitor U7 in a concentration-dependent manner and was unaffected by similar concentrations of Inactive U7 (Fig 3, A and B). Similarly when siRNA to Gα12/13 and transfection with β-ARKCT were used, S-1-P mediated migration was also significantly decreased (Fig 3, C).
Fig. 3. Phospholipase C-β (PLC-β) and cell migration.
A, Vascular smooth muscle cell migration in response to sphingosine-1-phosphate (S-1-P; 0.1µM) in both the wound assay and in (B) the Boyden chamber assays of migration was inhibited by increasing concentrations of the PLC-β inhibitor U7 (0.1 to 1000nM, preincubated with the cells for 1 hour before each assay) but not the inactive analogue, InactiveU7 (0.1 to 1000nM, preincubated with the cells for 1 hour before each assay). C, Migration in response to S-1-P (0.1µM) in the Boyden chamber assay was inhibited by small interfering RNA (siRNA) to Gα12/13 and adenoviral COOH-terminus β-adrenergic receptor kinase (adeno β-ARKCT) transfection. The transfection of scrambled siRNA or empty adenoviral had no effect. D, Cell migration in response to S-1-P (0.1µM) in the Boyden chamber was also inhibited by the nicotinamide dinucleotide phosphate hydrogen (NAD[P]H) oxidase inhibitor, diphenyleneiodonium chloride (DPI). Values are the mean ± SEM (n = 6). *P < .05; **P < .01 compared with control.
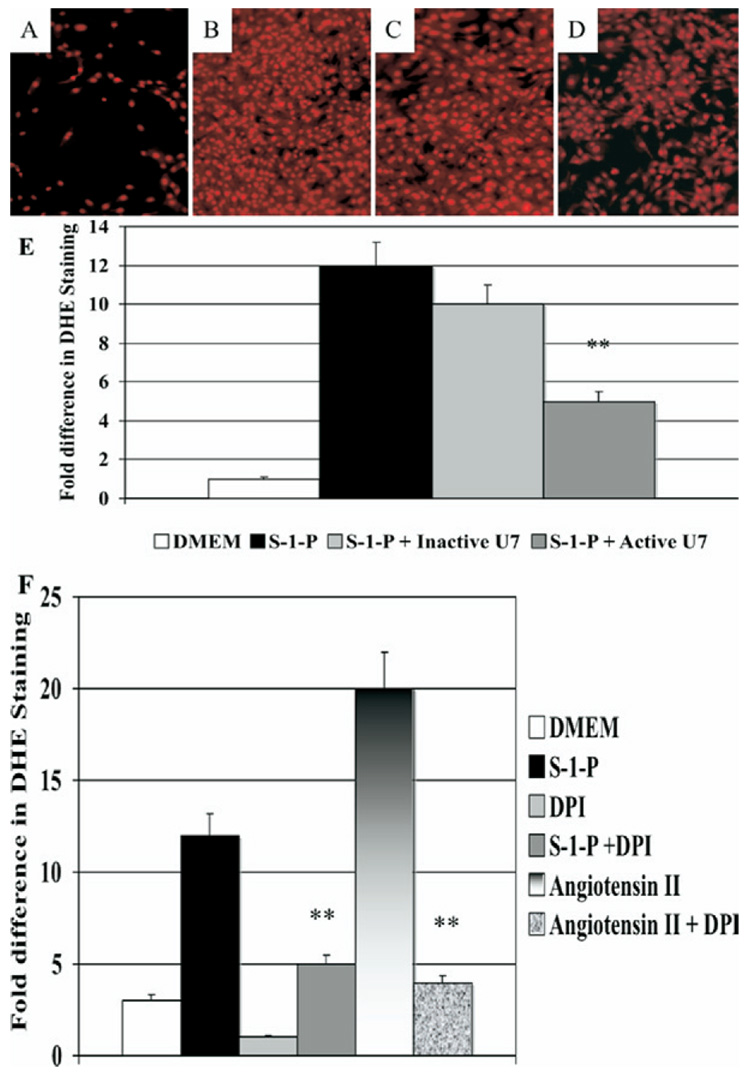
Phospholipases can control the generation of oxygen free radicals in cells, and oxygen free radicals are an important mediator of cell signaling and cell migration. S-1-P–induced oxygen free radical production as measured by DHE staining (Fig E2, A to D online only) and estimated by spectrophotometry was significantly inhibited by preincubation with U7 but not with InactiveU7 (Fig E2, E online only), suggesting that PLC-β is involved in S-1-P–mediated oxygen free radical activation. The increased DHE staining was not seen in the presence of DMEM alone and was significantly augmented in the presence of angiotensin II (Fig E2, F online only). S-1-P–mediated augmentation of DHE staining is inhibited by DPI, suggesting that NAD(P)H oxidase is the enzyme responsible for oxygen free radical generation in these VSMCs.
Fig E2 (online only). Oxygen free radical production: sphingosine-1-phosphate (S-1-P; 0.1µM)–induced oxygen free radical production as measured by quantitative dihydroethidium staining was significantly inhibited by U7 (10nM) but not by InactiveU7 (10nM). Representative photographs of the staining are shown.
A, Dulbecco minimal essential medium (DMEM); (B) S-1-P; (C) S-1-P with InactiveU7; and (D) S-1-P with U7). E, The bar chart shows the quantification of the experiment A to D. F, The bar chart shows oxygen free radical production in response to angiotensin II and S-1-P in the presence and absence of absence of the nicotinamide dinucleotide phosphate hydrogen oxidase inhibitor, diphenyleneiodonium chloride (DPI; 10µM), as measured by quantitative dihydroethidium staining. Values are the mean ± SEM (n = 6). *P < .05; **P < 0.01 compared with control.
When siRNA to Gα12/13 and transfection with β-ARKCT were used, S-1-P–mediated augmentation of DHE staining was also significantly decreased. VSMC migration in response to S-1-P in both the wound and the Boyden chamber assays of migration was inhibited by the NAD(P)H oxidase inhibitor DPI in a concentration-dependent manner and unaffected by similar concentrations of Inactive U7 (Fig 3, D).
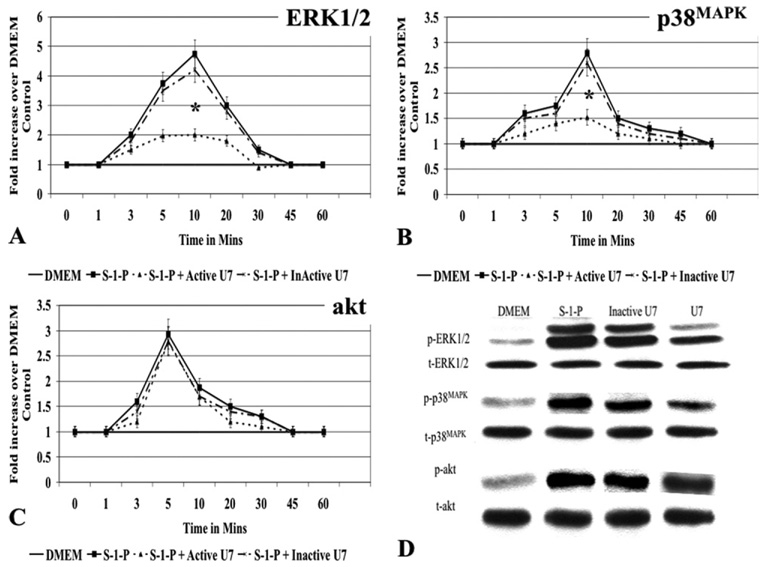
We have previously shown that S-1-P will activate the MAPKs ERK1/2 and p38MAPK and the kinase Akt. To determine if the MAPK pathways were downstream of PLC-β, we examined ERK1/2 and p38MAPK activation in the presence and absence of PLC-β inhibitor. S-1-P induced MEK1 and ERK1/2, and MKK3/6 and p38MAPK activation was significantly inhibited by U7 but not by InactiveU7 (Fig E3, A, B, and D, online only). Time-dependent Akt phosphorylation was partially but not significantly inhibited by U7; InactiveU7 had no effect (Fig E3, C and D, online only). These data suggest that PLC-β is upstream of the MAPK ERK1/2 and p38MAPK.
Fig E3 (online only). Mitogen-activated protein kinase (MAPK) and phospholipase C-β (PLC-β).
A, The presence of U7 (10nM) attenuated sphingosine-1-phosphate (S-1-P; 0.1µM)–mediated (ERK1/2) and (B) p38MAPK activation but (C) had no effect on akt activation as measured by Western blotting. The presence of InactiveU7 (10nM) had no effect, and the results could be superimposed on the control curves. D, A representative Western blot of the maximum response for each kinase is shown. Both activated and total protein expression is shown. Values are the mean ± SEM of the ratio of the phosphorylation of the respective protein relative to the total unphosphorylated protein (n = 6). *P < .05 compared with control. DMEM, Dulbecco minimal essential medium.
DISCUSSION
S-1-P is a G-protein–coupled receptor agonist that can stimulate VASMC migration. This migration is dependent mainly on S-1-P1 (Gαi mediated) and S-1-P3 (Gαi, Gαq, and Gα12/13 mediated) receptors. Exogenous S-1-P will stimulate oxygen free radical generation in association with an increase in intracellular Ca2+ concentration and an increase in inositol phosphate production, reflecting activation of PLC. These three S-1-P–induced events can be inhibited partially by pertussis toxin and markedly by U73122, a PLC inhibitor, and are mediated by S-1-P1 receptors suggesting a link between Gα proteins and PLC.13–15 The present study examines the G-protein pathways involved in PLC activation and oxygen free radical generation and demonstrates that S-1-P induces time dependent activation of PLC-β through a Gα12/13 Gβγ pathway, which in turn induces oxygen free radical generation. Inhibition of PLC-β will block oxygen free radical generation, MAPK activation, and vascular smooth muscle cell migration in response to S-1-P.
There are a multitude of pathways in the cell, and many are redundant. However, membrane events are a choke point in these processes, and both G-protein–coupled receptors and receptor-linked tyrosine kinases appear to be important therapeutic targets to moderate cell behavior. The receptor-regulated PLC-β pathway is an important component in signaling cascades that regulate many cell functions; it can be activated by both G-protein coupled receptor agonists (eg, S-1-P, angiotensin II, or bradykinin) and by receptor-linked tyrosine kinase agonists (eg, platelet-derived growth factor).16
PLC-β exists as a dimer within the cell and can interact with Gαq and Gα12/13 G-proteins.17 We have demonstrated that both are present in the VSMC. These G-proteins are part of a trimeric molecular complex with the Gβ and Gγ G-proteins. Gβ and Gγ G-proteins are tightly associated. PLC-β1 and PLC-γ are expressed in human and rat aortic vascular smooth muscle cells. PLC-β1 appears to be the isoform critical for angiotensin II–regulated PLC-β signaling in VSMC.18 S-1-P signaling shares many similarities with angiotensin II signaling. Our data show that PLC-β and PLC-γ can be activated by S-1-P in a time-dependent manner and that PLC-β activity in response to S-1-P is mediated by both Gα12/13 and Gβγ proteins and can modulate VSMC migration. Neither Gαi nor Gαq appear to be involved.
Consistent with these findings, S-1-P has previously been shown to activate Gα12/13 and stimulate PLC-β1 and PLC-β3 and that this activity was partially inhibited by Gβ antibodies.19 The receptor S-1-P2 has also been linked to activation of PLC-β via Gβγ subunits.20 We have previously shown that the VSMC express S-I-P1, S-1-P2, and S-1-P3 receptors.4 In this report, we show a similar effect on PLC-β activation using the Gβγ inhibitor β-ARKCT. Silencing of the receptor S-1-P2 but not receptor S-1-P1 receptors abolishes activation of Gαq, Gα12/13, and Gαi2, and suppresses S-1-P–stimulated PLC-β. The selective S-1-P1 receptor agonist, SEW2871, was found not to stimulate PLC-β activity, further supporting the hypothesis that S-1-P2 receptors may be the principal S-1-P receptor leading to activation of PLC-β.20
We have previously demonstrated that S-1-P can induce ERK1/2 and p38MAPK activation and that these events were Gαi-mediated and that PI3-K was involved.4,6 The present report suggests that PLC-β is also involved in the upstream mediation of ERK1/2 and p38MAPK. A similar finding has been seen with the G-protein–coupled receptor agonist angiotensin II.21 Angiotensin II–mediated MAPK activation is also regulated by the generation of reactive oxygen species derived from NAD(P)H oxidases, which is in part mediated by PLC.22 The present data set demonstrates that PLC-β plays a role in S-1-P–mediated oxygen free radical generation in VSMCs. Evidence of the importance of S-1-P and oxygen free radicals has been shown in physiologic preparations of VSMCs.
One part of the restenosis process is constrictive remodeling where the vessel appears to heal by constriction that reduces the effective luminal diameter. Keller et al23 examined myogenic vasoconstriction in SMCs and found that it required the activation of sphingosine kinase (the enzyme that produces S-1-P) and the generation of oxygen free radicals. The rapid and transient production of oxygen free radicals was enhanced by expression of wild-type sphingosine kinase and inhibited by a dominant-negative mutant. Exogenous S-1-P was also able to induce oxygen free radical production. Chemical (DPI), peptide (gp91ds-tat/gp91ds), and genetic (N17Rac) inhibition strategies against NAD(P)H oxidase indicated that NAD(P)H oxidase was the likely source of the oxygen free radicals detected in response to S-1-P.23 This data set is consistent with our data on S-1-P–mediated oxygen free radical generation likely from NAD(P)H oxidase and that we can inhibit DHE staining and S-1-P–mediated migration with DPI.
We have demonstrated that inhibition of PLC-β will attenuate oxygen free radical generation and downstream MAPK activation. Arterial injury will also induce an immediate profound vascular oxidative stress, likely accounted for by activation of vessel wall NAD(P)H oxidase.24,25 Superoxide production increases significantly ≤24 hours after balloon-induced injury. These changes appear NAD(P)H oxidase–dependent and are associated with augmented NAD(P)H oxidase activity and upregulation of p47(phox) and p67(phox).26 One can speculate that the platelet-rich thrombus present on the surface provides a rich source of S-1-P that could increase oxygen free radical production in a manner similar to that described by Keller et al.23
Diabetes mellitus is also a disease with an oxidative component. It has recently been reported that S-1-P prevents monocyte adhesion to the aortas of type 1 diabetic mice. Activation of the receptor S-1-P1 on endothelium by S-1-P decreases nuclear factor-κB nuclear translocation and p65 phosphorylation, resulting in reduced monocyte adhesion. 27 It thus appears that S-1-P and oxygen free radical production are important in a vessel’s response to injury. The present data set suggests that S-1-P can mediate these effects through G-proteins and PLC-β.
CONCLUSION
S-1-P can induce oxygen free radical generation through a Gα12/13–Gβγ, PLC-β–mediated mechanism, which facilitates VSMC MAPK activation and cell migration. To our knowledge, this is the first description of PLC-mediated oxygen free radical generation as a mediator of S-1-P VSMC migration and illustrates the need for the definition of cell signaling to allow targeted molecular strategies designed to modulate vessel remodeling and prevent restenosis.
Acknowledgments
This research was supported by grants to Dr Davies from NIH (HL086968) from the American College of Surgeons Junior Faculty Award and from the Mentored Clinical Scientist Development Award, sponsored by the NIH-NHLBI/Lifeline Foundation (K08 HL 67746). Dr Nicholl received grant support from the AHA through a Post Doctoral Fellowship (0225696T). Support also received by US Public Health Service HL67746 and the American Heart Association (NY) 0225696T.
APPENDIX (online only)
Materials
D-erythro-sphingosine-1-phosphate was purchased from Avanti Polar-Lipids (Alabaster, Ala). Pertussis toxin was purchased from Sigma Chemical Co (St Louis, Mo). GP-2A was purchased from Biomol (Plymouth Meeting, Penn). U73122 (U7), the inactive analog U73343, and PD98059 were purchased from Calbiochem (La Jolla, Calif). Small interfering RNA to Gαi2 and Gαi2, Gαq, and Gα12/13 were purchased from (Ambion, Inc Austin, Tex). Empty adenovirus and adenoviral COOH-terminus β-adrenergic receptor kinase were purchased from Weigen Inc. Peroxidase-conjugated antirabbit immunoglobulin G (IgG) antibody (raised in goat) and peroxidase-conjugated antimouse IgG antibody (raised in goat) were purchased from Jackson ImmunoResearch Laboratories (West Grove, Pa). Phospho-extracellular signal-regulated kinases 1/2 (ERK1/2) antibody was purchased from Promega, Inc (Madison, Wis). Total ERK 1/2 antibody was purchased from BD Transduction Laboratories (Lexington, Ky). Phospho-p38MAPK antibody was purchased from Biosource (Camarillo, Calif). Total p38MAPK mitogen-activated protein kinase, phospho-MEK1/2 (ser217/221), and phospho-MKK3/6 (ser189/207) antibodies were purchased from Cell Signaling (Beverley, Mass). Dulbecco minimal essential medium (DMEM) and Dulbecco phosphate buffered saline (DPBS) were purchased from Cellgro (Herndon, Va).
Footnotes
Competition of interest: none.
Presented at the Annual Meeting of the Association of Academic Surgery, 2nd Academic Surgical Congress, Phoenix, Ariz, Feb 9–12, 2007.
Additional material for this article may be found online at www.jvascsurg.org.
REFERENCES
- 1.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 2.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, et al. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 3.Maupas-Schwalm F, Auge N, Robinet C, Cambus JP, Parsons SJ, Salvayre R, et al. The sphingomyelin/ceramide pathway is involved in ERK1/2 phosphorylation, cell proliferation, and uPAR overexpression induced by tissue-type plasminogen activator. FASEB J. 2004;18:1398–1400. doi: 10.1096/fj.03-1123fje. [DOI] [PubMed] [Google Scholar]
- 4.Fegley AJ, Tanski WJ, Roztocil E, Davies MG. Sphingosine-1-phosphate stimulates smooth muscle cell migration through Gαi-and PI3-kinase-dependent p38MAPK activation. J Surg Res. 2003;113:32–41. doi: 10.1016/s0022-4804(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 5.Tanski WJ, Nicholl SM, Kim D, Fegley AJ, Roztocil E, Davies MG. Sphingosine-1-phosphate-induced smooth muscle cell migration involves the mammalian target of rapamycin. J Vasc Surg. 2005;39:91–98. doi: 10.1016/j.jvs.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 6.Tanski WJ, Roztocil E, Davies MG. Sphingosine-1-phosphate induces Gαi-coupled, PI3K/ras dependent smooth muscle cell migration. J Surg Res. 2002;108:98–106. doi: 10.1006/jsre.2002.6529. [DOI] [PubMed] [Google Scholar]
- 7.Kluk MJ, Colmont C, Wu MT, Hla T. Platelet-derived growth factor (PDGF)-induced chemotaxis does not require the G protein-coupled receptor S1P1 in murine embryonic fibroblasts and vascular smooth muscle cells. FEBS Lett. 2003;533:25–28. doi: 10.1016/s0014-5793(02)03742-0. [DOI] [PubMed] [Google Scholar]
- 8.Takuwa Y, Okamoto H, Takuwa N, Gonda K, Sugimoto N, Sakurada S. Subtype-specific, differential activities of the EDG family receptors for sphingosine-1-phosphate, a novel lysophospholipid mediator. Mol Cell Endocrinol. 2001;177:3–11. doi: 10.1016/s0303-7207(01)00441-5. [DOI] [PubMed] [Google Scholar]
- 9.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the Gαi), Gαq), and Gα12) families of heterotrimeric G proteins. J Biol Chem. 1999;274:27351–27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- 10.Wang XT, Wu LL, Sun YP, Bai H, Gao ZF, Xu JT. Roles of Gαq/11 mediated- and platelet-derived growth factor mediated-signal transduction pathways in rat aorta restenosis. Sheng Li Xue Bao. 2001;53:231–234. [PubMed] [Google Scholar]
- 11.Tanski WJ, Fegley AJ, Roztocil E, Davies MG. Domain dependent actions of urokinase on smooth muscle cell responses. J Vasc Surg. 2004;39:214–222. doi: 10.1016/s0741-5214(03)01031-0. [DOI] [PubMed] [Google Scholar]
- 12.Tanski WJ, Roztocil E, Hemady EA, Williams JA, Davies MG. Role of Gαq in smooth muscle cell proliferation. J Vasc Surg. 2004;39:639–644. doi: 10.1016/j.jvs.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto H, Takuwa N, Gonda K, Okazaki H, Chang K, Yatomi Y, et al. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem. 1998;273:27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- 14.Okajima F, Tomura H, Sho K, Kimura T, Sato K, Im D, et al. Sphingosine 1-phosphate stimulates hydrogen peroxide generation through activation of phospholipase C-Ca2+ system in FRTL-5 thyroid cells: possible involvement of guanosine triphosphate-binding proteins in the lipid signaling. Endocrinology. 1997;138:220–229. doi: 10.1210/endo.138.1.4883. [DOI] [PubMed] [Google Scholar]
- 15.Okajima F, Tomura H, Sho K, Nochi H, Tamoto K, Kondo Y. Involvement of pertussis toxin-sensitive GTP-binding proteins in sphingosine 1-phosphate-induced activation of phospholipase C-Ca2+ system in HL60 leukemia cells. FEBS Lett. 1996;379:260–264. doi: 10.1016/0014-5793(95)01526-4. [DOI] [PubMed] [Google Scholar]
- 16.Sternweis PC, Smercka AV. G-proteins in signal transduction: the regulation of phospholipase C. Ciba Found Symp. 1993;176:96–106. doi: 10.1002/9780470514450.ch7. [DOI] [PubMed] [Google Scholar]
- 17.Singer AU, Waldo GL, Harden TK, Sondek J. A unique fold of phospholipase C-b mediates dimerization and interaction with Gαq. Nat Struct Biol. 2002;9:32–36. doi: 10.1038/nsb731. [DOI] [PubMed] [Google Scholar]
- 18.Schelling JR, Nkemere N, Konieczkowski M, Martin KA, Dubyak GR. Angiotensin II activates the b1 isoform of PLC in vascular smooth muscle cells. Am J Physiol. 1997;272:C1558–C1566. doi: 10.1152/ajpcell.1997.272.5.C1558. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am J Physiol Cell Physiol. 2004;286:C1130–C1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]
- 20.Hu W, Mahavadi S, Huang J, Li F, Murthy KS. Characterization of S1P1 and S1P2 receptor function in smooth muscle by receptor silencing and receptor protection. Am J Physiol Gastrointest Liver Physiol. 2006;291:G605–G610. doi: 10.1152/ajpgi.00147.2006. [DOI] [PubMed] [Google Scholar]
- 21.Cetin A, Ozturk OH, Tokay A, Akcit F, Caglar S, Yesilkaya A. Angiotensin II-induced MAPK phosphorylation mediated by Ras and/or phospholipase C-dependent phosphorylations but not by protein kinase C phosphorylation in cultured rat vascular smooth muscle cells. Pharmacology. 2007;79:27–33. doi: 10.1159/000097539. [DOI] [PubMed] [Google Scholar]
- 22.Seshiah P, Weber D, Rocic P, Valppu L, Taniyama Y, Gaiendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 23.Keller M, Lidington D, Vogel L, Peter BF, Sohn HY, Pagano PJ, et al. Sphingosine kinase functionally links elevated transmural pressure and increased reactive oxygen species formation in resistance arteries. FASEB J. 2006;20:6. doi: 10.1096/fj.05-4075fje. [DOI] [PubMed] [Google Scholar]
- 24.Lassègue B, Clempus R. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 25.Souza HP, Souza LC, Anastacio VM, Pereira AC, Junqueira ML, Krieger JE, et al. Vascular oxidant stress early after balloon injury: evidence for increased NAD(P)H oxidoreductase activity. Free Radic Biol Med. 2000;28:1232–1242. doi: 10.1016/s0891-5849(00)00240-9. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, Niculescu R, Wang D, Patel S, Davenpeck KL, Zalewski A. Increased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injury. Arterioscler Thromb Vasc Biol. 2001;21:739–745. doi: 10.1161/01.atv.21.5.739. [DOI] [PubMed] [Google Scholar]
- 27.Morris M, Ley K, Hedrick C, Whetzel A, Bolick D, Srinivasan S, et al. Sphingosine-1 phosphate prevents monocyte/endothelial interactions in type 1 diabetic NOD mice through activation of the S1P1 receptor. Circ Res. 2006;99:731–739. doi: 10.1161/01.RES.0000244088.33375.52. [DOI] [PubMed] [Google Scholar]