Abstract
Sunlight (UVB) triggers cutaneous (CLE) and systemic lupus through an unknown mechanism. We tested the hypothesis that UVB triggers CLE through a CSF-1-dependent, macrophage (Mø) -mediated mechanism in MRL-Faslpr mice. By constructing mutant MRL-Faslpr strains expressing varying levels of CSF-1 (high, intermediate, none), and use of an ex-vivo gene transfer to deliver CSF-1 intra-dermally, we determined that CSF-1 induces CLE in lupus-susceptible, MRL-Faslpr mice, but not in lupus-resistant, BALB/c mice. Notably, UVB incites an increase in Mø, apoptosis in the skin and CLE in MRL-Faslpr, but not in CSF-1-deficient MRL-Faslpr mice. Furthermore, UVB did not induce CLE in BALB/c mice. Probing further, UVB stimulates CSF-1 expression by keratinocytes leading to recruitment and activation of Mø that, in turn, release mediators, which induce apoptosis in keratinocytes. Thus, sunlight triggers a CSF-1-dependent, Mø-mediated destructive inflammation in the skin leading to CLE in lupus-susceptible MRL-Faslpr, but not lupus-resistant BALB/c mice. Taken together, we envision CSF-1 as the “match” and lupus-susceptibility as the “tinder” leading to CLE.
Keywords: Rodent, Macrophages, Autoimmunity, Skin, Transgenic mice
Introduction
Cutaneous involvement, cutaneous lupus erythematosus (CLE), is common in patients with lupus and is often the first manifestation (1). Sunlight exposure (UVB) to the skin triggers CLE and systemic lupus (1). Despite the pivotal position of the skin in lupus, the pathogenesis of CLE and the mechanisms responsible for UVB incited CLE are poorly understood. Progress in pinpointing the precise mechanisms responsible for CLE and the role of UVB has been hindered by lack of a well-studied animal model that shares features with the human illness.
While several mouse models for lupus nephritis are available, MRL-Faslpr is the only strain that develops a reliable multi-organ lupus that is similar to human lupus including skin, lung, salivary/lacrimal gland pathology and arthritis (2). These mice may prove valuable for dissecting the pathogenesis of CLE for several reasons. Similar to human CLE, skin lesions in MRL-Faslpr mice are common (80% incidence) and involve a chronic inflammatory process that is leukocyte-dependent (3–6). Furthermore, since CLE in MRL-Faslpr mice is evident early in life (3 mo of age), and the incidence and severity progressively increases until death (5–6 mo of age, 50% mortality), the tempo is conducive for dissecting the pathogenesis of CLE. By comparison, MRL mice that do not have a mutation in Fas (MRL-++) develop a far more indolent and milder cutaneous disease (7). As lupus susceptibility is related to MRL background genes, MRL mice may provide a means to decipher molecular pathways driving the development of CLE. Despite the overwhelming evidence supporting a role for T lymphocytes in CLE, innate immunity and inflammation are central to the pathogenesis of autoimmune skin disease (8, 9). The skin, the first line of defense from the environment, is rich in sentinel cells (Mø, dendritic cells [DC] and Langerhans cells [LC]), key leukocytes in innate immunity and inflammation (10). Mø and DC are the primary leukocytes in the dermis of a healthy mouse, with Mø far out numbering DC (6:1 ratio) (11). Moreover, larger numbers of Mø traffic to the dermis during inflammation (12–15). Upon activation, Mø release mediators that induce apoptosis of parenchymal cells, and therefore are central to tissue injury (16). Thus, we hypothesize that Mø are pivotal in CLE in MRL-Faslpr mice.
The major regulator of Mø development is CSF-1 (17). CSF-1 binds to a single receptor, encoded by the proto-oncogene c-fms, which is expressed on skin Mø, DC, and LC that are derived from monocytes (18, 19). While the contribution of Mø and CSF-1 to CLE has not been explored, we have identified multiple links between CSF-1, Mø, and inflammation in the development of systemic lupus in MRL-Faslpr mice: 1) CSF-1 expression increases in the serum and kidney prior to nephritis and rises with advancing disease (20); 2) Mø and T cells localize within intra-renal sites rich in CSF-1 (21); 3) systemic disease (kidney, salivary/lacrimal gland and lung pathology) is suppressed and skin lesions are not visible in CSF-1-deficient (Csf1op/op) MRL-Faslpr (Csf1op/op;MRL-Faslpr) mice (22); and 4) CSF-1 mediates Mø recruitment, activation, and in turn, Mø-dependent tubular epithelial cell (TEC) apoptosis during nephritis (23). Thus, we singled out CSF-1 receptor (CSF-1R)-bearing Mø for further study. Given the importance of sunlight in triggering CLE in human lupus and the central role of Mø in host defense from environmental stimuli, we tested the hypothesis that sunlight triggers CLE through the induction of a CSF-1-dependent, Mø-mediated mechanism in MRL-Faslpr mice.
Materials and Methods
Mice
Mice heterozygous for the osteopetrotic mutation (Csf1op) on the C57BL/6JxC3Heb/FeJ-a/a background, BALB/c, C57BL/6 (B6), MRL/MpJ-+/+ (MRL-++) and MRL/MpJ-Faslpr/Faslpr (MRL-Faslpr) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The Csf1op mutation was backcrossed onto the MRL-Faslpr background for 10 generations. Transgenic mice (C57BL/6 × CBAF1) expressing EGFP under the control of the CSF-1R (c-fms) promoter and first intron (Tgfms-EGFP), referred to as MacGreen mice, were provided by Dr. D.A. Hume, University of Queensland (Brisbane, Australia) (24) and this transgene, together with the TgN(FLCsf1)Ers (TgC) transgene (expressing the full-length CSF-1 gene driven by the CSF-1 promoter/first intron) from TgN(FLCsf1)9Ers/+ mice (25) were backcrossed onto the MRL-Faslpr background for 7 generations and were referred to as MacGreen;MRL-Faslpr and TgC/+;MRL-Faslpr, respectively. Mice were bred and housed at Harvard Medical School. Only female mice were used. The use of mice in this study was reviewed and approved by the Standing Committee on Animals in the Harvard Medical School in adherence to the NIH Guide for the Care and Use of Laboratory Animals.
CSF-1 ELISA
To quantify the levels of CSF-1 in serum, supernatants, and tissue homogenates, we evaluated samples using a previously published ELISA method (26). Briefly, we homogenized tissue samples using a MixMill 300 (Qiagen, Valencia, CA). We determined the protein concentration of each homogenate using the BCA Protein Assay Kit (PIERCE, Rockford, IL) and evaluated 200μg of protein per tissue sample. We did not dilute the serum and supernatants. The Abs and reagents in this assay were purchased from BD Bioscience (San Jose, CA).
Anti-SSA/Anti-SSB ELISA
To determine the level of anti-SSA (Ro) and anti-SSB (La) Abs in the circulation we used the ELISA kit (AESKULISA ANA-8Pro; AESKU. Diagnostics, Wendelsheim, Germany) and anti-mouse IgG (Sigma, St. Louis, MO) according to the manufactures instructions.
Gross skin lesions
We scored the severity of visible skin lesions as follows: 0: none; 1: mild (snout & ears), 2: moderate, less than 2 cm (snout, ears, intrascapular); 3: severe, more than 2 cm (snout, ears, intrascapular).
Skin histopathology
We fixed sections of the skin (5mm × 15mm) in 10% neutral buffered formalin for 24h, and stained paraffin sections with H&E as described previously (27). We assessed skin sections using the following categories: 0: normal unaffected skin; 1 (Mild): few focal dermal infiltrates (superficial and/or deep), normal thickness of epidermis (3–5 cell layer); 2 (Intermediate): disseminated dermal infiltrates (superficial and/or deep), increased epidermal thickness (acantosis, >5 cell layer) and hypergranulosis/hyperkeratosis, interface vacuolar changes with or without rare apoptotic keratinocytes (Civatte bodies); 3 (Advanced): disseminated dermal infiltrates (superficial and deep), increased epidermal thickness (acanthosis, >10cell layer) with hypergranulosis/hyperkeratosis and follicular plugging, interface vacuolar changes with scattered apoptotic keratinocytes (Civatte bodies) with/without basement membrane thickening, and scarring alopecia. Histopathology was graded using coded slides.
Immunofluorescence (Lupus band)
We fixed cryostat sectioned frozen tissue in 100% acetone, followed by incubation with fluorescein-conjugated Abs specific mouse IgG, IgG1, IgM, or C3 (ICN Biomedicals, Aurora, OH) for 30 min. Igs/C3 were detected using Nikon Eclipse E1000 upright fluorescence microscope.
Immunohistochemistry
We stained frozen skin sections for the presence of Mø, T cells, and apoptotic cells (cleaved caspase-3 positive cells) using anti-CD68 Ab (Serotec, Raleigh, NC), anti-CD4 Ab, anti-CD8 Ab (eBioscience, San Diego, CA) and anti-cleaved caspase-3 Ab (Asp175) (Cell-signalling, Danvers, MA), as previously described (28). The number of cells bearing CD4, CD8, CD68 determinants and apoptotic cells was assessed in 10 randomly selected high-power fields (hpf). To determine the number of CD68+CD11c− and CD68+CD11c+ cells in the skin, we fixed and incubated frozen skin sections with purified rat anti-CD68 Ab (Serotec), biotinylated rabbit anti-rat Ab, Streptavidin Texas Red (Vector) and CD11c-FITC Ab (eBioscience).
Keratinocyte cell line
The mouse keratinocyte line, PAM212 (provided by Dr. J.G. Rheinwald, Brigham and Women’s Hospital, Boston, MA), was cultured as previously described (29).
Isolating primary keratinocytes and dermal fibroblasts
We isolated keratinocytes and dermal fibroblasts from mouse ears (30, 31). Briefly, we divided ears into dorsal and ventral halves, treated them with 1% trypsin (Invitrogen, Carlsbad, CA), and then separated epidermis and dermis. To isolate keratinocytes, we filtered (100sm) epidermal cells, then seeded the cell suspension onto collagen Type I (BD Bioscience) coated plates in Keratinocyte SFM-Media (Invitrogen). To isolate dermal fibroblasts, we minced and digested the dermis with collagenase (Sigma, St. Louis, MO). The remaining undigested tissue was filtered (100sm). We cultured these cells in complete DMEM (Invitrogen) containing 10% FCS (Atlanta Biologicals, Lawrenceville, GA).
Constructing CSF-1 transduced cells (“CSF-1 carrier cells”)
We isolated and cultured primary dermal fibroblasts (see above) and renal TEC derived from MRL-Faslpr, CSF-1op/op;MRL-Faslpr and BALB/c mice, as previously described (32). CRIP packaging cell lines producing helper-free recombinant retroviruses carrying the CSF-1 gene were generated (33). Briefly, DNA sequences encoding CSF-1 (bp 160–1874) (provided by R.C. Mulligan, Boston, MA) were subcloned into the moloney murine leukaemia virus (mo-MuLV)-based MFG-vector. The MFG vector carrying the CSF-1 gene was introduced into a mammalian packaging cell line (CRIP). Using the virus-containing cell culture supernatants, we infected primary cultured TEC and dermal fibroblasts, referred to as “CSF-1 carrier cells”. For controls, TEC or dermal fibroblasts were infected with the supernatant of CRIP cells transfected with the MFG-vector alone, referred to as “empty vector carrier cells”. We verified successful transfection of TEC and dermal fibroblasts by quantifying CSF-1 in the supernatant.
Intra-dermal delivery of “carrier cells”
To deliver CSF-1 into the skin, we infused “CSF-1 carrier cells” or “empty vector carrier cells” (5×106) in HBSS (100 sl), into the intra-scapular area intra-dermally. The cell viability (>90%) was determined (trypan blue exclusion) immediately prior to infusion of these “carrier cells”. We excised and snap froze skin containing the area that was infused at day 3 and 28 post infusion. We compared the maximal lesions in each mouse, selected by evaluating serial sections (4sm) every 20μm.
UVB-exposure
In vivo
To expose the skin (2 mo of age) to UVB, we anesthetized mice and removed the hair on their backs with a shaver. We irradiated these mice with a set of four FS-20 UVB lamps (National Biological, Westinghouse), filtered by a Kodacel membrane to eliminate residual UVC. The energy output of the UVB-lamps was measured with an UV-radiometer at a distance of 17 cm. Mice were exposed daily with 500J/m2.
In vitro
The PAM 212 cell line and primary cultured mouse keratinocytes and dermal fibroblasts were grown until confluent and then exposed to UVB (500J/m2) either once or twice (24 h apart). CSF-1 levels in the supernatant were analysed (ELISA) 24 h after the last dose of UVB.
Adoptive transfer
We isolated bone marrow (BM) from MacGreen;MRL-Faslpr mice (34) and i.v. injected these cells (2×107) in the tail. We sacrificed these mice 48 h later and prepared skin (ear) and blood samples to detect the adoptively transferred EGFP+ cells using flow cytometry as previously described (35).
BM derived Mø (BMMø)
We isolated BMMø as previously described (34). When the cultured cells were confluent, we stimulated them with CSF-1 (10μg/ml) (Preprotech, Rocky Hill, NJ), LPS (5μg/ml) (Sigma-Aldrich) and IFN-γ (500 U/ml) (Sigma-Aldrich).
BMMø induced apoptosis
We collected supernatant from unstimulated and stimulated BMMø (CSF-1 or LPS/IFN-γ for 24 h). These supernatants were added to cultured mouse keratinocytes or dermal fibroblasts (cell line, PAM212). After 48 h, we assessed early (annexin) and late (annexin/PI) apoptosis by flow cytometry using the Annexin-V-FITC-PI kit (BD Bioscience) according to the manufacturer’s instructions (36).
Flow Cytometry
We prepared and stained single cell suspensions from skin as previously described (35). We used the following Abs for FACS analysis: PE-conjugated anti-CD86; PE-Cy5 conjugated anti-CD8 (53–6.7); anti-CD11c (N418); and allophycocyanin-conjugated anti-CD45.1 (A20), anti-CD4 (RM4–5), and anti-F4/80 (BM8) from eBioscience. We used FITC-conjugated CD11b (M1/70) from Pharmingen and FITC- and allophycocyanin- conjugated anti-CD68 (FA11) from Serotec. We collected 0.25 – 0.5 × 106 skin cells using a FACS Calibur (Becton Dickinson) and analyzed data using Flowjo software (Tree Star, Palo Alto, CA).
Statistical Analysis
The data represent the mean ± SEM and were prepared using GraphPad PRISM version 4.0. We used the non-parametric Mann-Whitney U test and the One-way ANOVA test to evaluate p values.
Results
MRL-Faslpr cutaneous lesions resemble human discoid lupus
Cutaneous lesions in MRL-Faslpr mice, identifiable within the intra-scapular area, on the ears, and face (snout), develop in the majority of MRL-Faslpr mice. Moreover, cutaneous lesions in MRL-Faslpr mice, presenting as erythematous follicular plaques with/without ulceration and scarring alopecia, share features with human lupus (Fig. 1A) (2, 5). Histopathologically, these cutaneous lesions are similar to the verrucoid variant of human discoid lupus (Fig. 1A). Cutaneous lesions in MRL-Faslpr mice consist of superficial and deep peri-vascular and peri-adnexal infiltrates with associated interface vacuolar degeneration of basal keratinocytes, apoptotic keratinocytes, papillomatosis and hyperkeratosis with follicular plugging, and scarring alopecia. Interestingly, a lupus band (detecting deposits of immune complexes at the dermal-epidermal junction), pathognomic for human CLE (37), is detected in skin with and without CLE lesions (Fig. 1B). In accordance with the photosensitivity in human lupus, UVB-irradiation of MRL-Faslpr mice, but not of lupus-resistant BALB/c mice (data not shown), induces a lupus band in advance of CLE (Fig. 1C). In another striking parallel to human CLE, Ro, and La auto-Abs are detected in MRL-Faslpr at 6 mo of age mice with CLE (Ro 3.17 ± 0.34; La 3.17 ± 0.41; n=6), but not in MRL-Faslpr at 2 mo of age mice before CLE (Ro 0.20 ± 0.06; La 0.15 ± 0.06; n=6; p<0.01), or in lupus-resistant control BALB/c mice (Ro 0.05 ± 0.01; La 0.04 ± 0.01; n=6; p<0.01). Taken together, the cutaneous pathology, photosensitivity, and accompanying formation of lupus bands and distinctive auto-Abs in MRL-Faslpr mice closely resemble manifestations of human CLE.
Figure 1.
Cutaneous lesions in MRL-Faslpr mice share features with human discoid CLE. A. Gross cutaneous lesions in the intra-scapular area and ears in MRL-Faslpr mice. Histopathologically cutaneous lesions in MRL-Faslpr mice resemble the verrucoid variant of discoid lupus characterized by interface vacuolar changes (black arrowheads) with apoptotic keratinocytes (white open arrow), acanthotic epidermis (double headed arrow) with hyperkeratosis (V), hypergranulosis (#) and follicular plugging (*), superficial and deep, as well as perifollicular inflammatory infiltrates (arrows) and associated scarring alopecia with markedly reduced hair follicles (H) and dermal fibrosis (@). Representative photomicrographs from MRL-Faslpr mice (5 mo of age), H&E stained sections (n=18). Magnification: 20x, inset 40x. Inset in right panel: apoptotic keratinocytes, 60x. B. Positive lupus band (IgG and C3 deposits) at the dermal-epidermal junction in lesional and non-lesional skin of MRL-Faslpr mice (3 mo of age) determined by immunofluorescence staining. Representative of n=5–7. The pattern of IgM and IgG1 staining at the dermal-epidermal junction (data not shown) was more focal than IgG and C3. We did not detect a lupus band in BALB/c skin (data not shown). Stars indicate dermal-epidermal junction; dotted line the border of the epidermis to the environment. C. MRL-Faslpr skin is photosensitive. UVB ‚irradiation (6 consecutive days, 500J/m2) induces the lupus band (IgG, C3) as well as IgM and IgG1 (data not shown) at the dermal-epidermal junctions in MRL-Faslpr mice (2 mo of age), but not in BALB/c mice (data not shown). Representative of n = 4–6, experiment repeated twice.
CSF-1 promoter/first intron drives disease-related tissue expression of CSF-1 in MRL-Faslpr mice
To test the hypothesis that robust expression of CSF-1 exacerbates CLE, we analyzed MRL-Faslpr CSF-1 transgenic mice. We have previously reported that CSF-1-deficient MRL-Faslpr (Csf1op/op;MRL-Faslpr) mice do not develop visible skin lesions, and systemic pathology characteristic of this strain (23). To verify that the CSF-1 promoter/first intron-driven full-length CSF-1 transgene (25) used to over-express CSF-1 restored disease related tissue expression of CSF-1, we created TgC/+;Csf1op/op;MRL-Faslpr mice, in which the only source of CSF-1 was encoded by the TgC transgene and compared circulating and tissue CSF-1 production and CLE development in this transgenic mouse with MRL-Faslpr mice. In the TgC/+;Csf1op/op;MRL-Faslpr mouse, CSF-1 expression in the serum, skin and other tissues (kidney, salivary gland, lung, spleen and LNs) as well as cutaneous and the systemic pathology (kidney, lung, spleen, and LNs) were restored to the values in age matched MRL-Faslpr mice (data not shown). CSF-1-deficient MRL-Faslpr mice were used as negative controls. Thus, the TgC transgene drives disease-related tissue expression of CSF-1 in MRL-Faslpr mice.
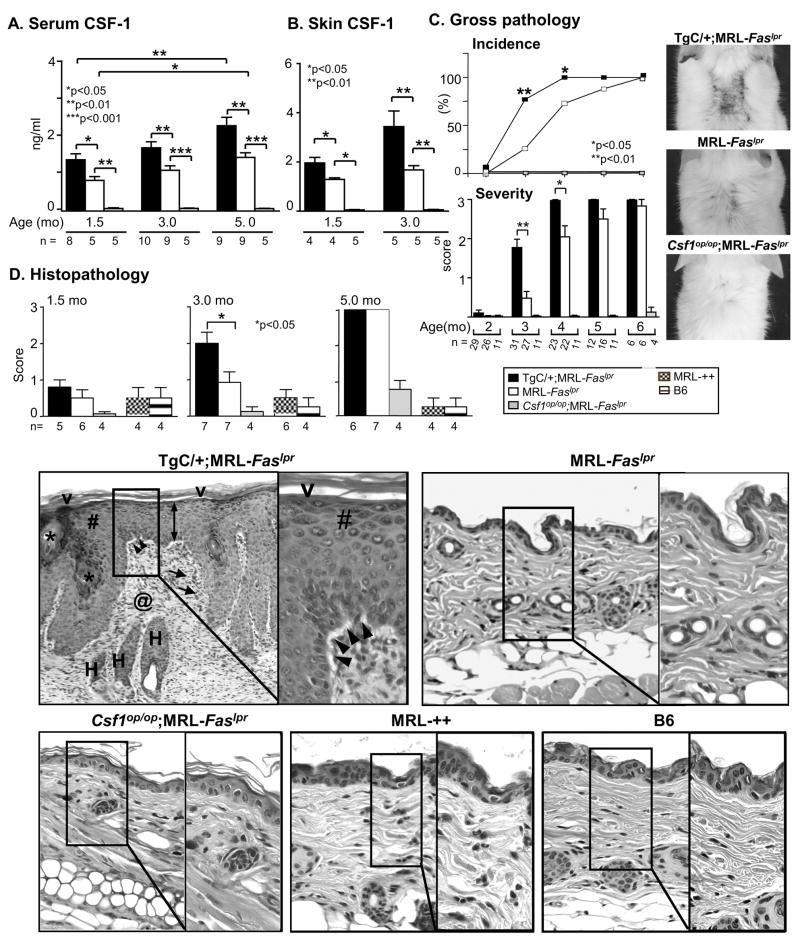
The CSF-1 transgene increases CSF-1 in the serum and skin in MRL-Faslpr mice
To over-express CSF-1 in MRL-Faslpr mice, we constructed TgC/+;MRL-Faslpr mice in which CSF-1 is expressed from both the wild-type CSF-1 gene and TgC transgene. To determine whether this strategy amplified CSF-1 expression, we compared CSF-1 levels in the serum and skin of TgC/+;MRL-Faslpr and MRL-Faslpr mice, using CSF-1-deficient MRL-Faslpr mice as a negative control. CSF-1 expression in MRL-Faslpr mice progressively rose as disease advanced (1.5, 3.0 and 5.0 mo of age) (Fig. 2 A, B). However, the increase in CSF-1 in the TgC/+;MRL-Faslpr was, as expected, greater than in MRL-Faslpr mice. By comparison, serum CSF-1 levels in age-matched MRL-++ and B6, lupus-resistant mice remained unchanged. Thus, we have constructed mutant MRL-Faslpr strains that allow analysis of disease expression in mice with varying levels of CSF-1 in serum and skin: TgC/+;MRL-Faslpr (high), MRL-Faslpr (intermediate) and Csf1op/op;MRL-Faslpr (none).
Figure 2.
Increasing CSF-1 expression in MRL-Faslpr mice accelerates the tempo of CLE. A. Serum CSF-1 levels in TgC/+;MRL-Faslpr, MRL-Faslpr and Csf1op/op;MRL-Faslpr mice at 1.5, 3.0, 5.0 mo of age and B. Skin CSF-1 levels in homogenates determined by ELISA. C. Time-related incidence and severity of skin lesions in TgC/+;MRL-Faslpr, MRL-Faslpr and Csf1op/op;MRL-Faslpr mice from 2–6 mo of age. Values are mean ± SEM. Representative photomicrographs from MRL-Faslpr at 3 mo of age. D. Skin (intrascapular) histopathology comparing TgC/+;MRL-Faslpr, MRL-Faslpr and Csf1op/op;MRL-Faslpr mice. MRL-++ and B6 mice with normal skin are controls. The cutaneous lesions in TgC/+;MRL-Faslpr mice include: interface vacuolar changes (black arrowheads), acanthotic epidermis (double arrow) with hyperkeratosis (V), hypergranulosis (#) and follicular plugging (*), superficial and deep, as well as perifollicular inflammatory infiltrates (arrows) and dermal scarring (@); Representative skin H&E stained sections (3 mo of age). Magnification: 20x, Inset 40x.
Increasing CSF-1 in MRL-Faslpr mice exacerbates leukocyte rich CLE
To determine whether an increase in CSF-1 influences the course of CLE, we compared the skin lesion in TgC/+;MRL-Faslpr, MRL-Faslpr and Csf1op/op;MRL-Faslpr mice. The development of gross and histopathologic skin lesions in TgC/+;MRL-Faslpr mice that have amplified CSF-1 expression, increased more rapidly as compared to MRL-Faslpr mice (Fig. 2C). The gross cutaneous pathology peaked in the TgC/+;MRL-Faslpr mice at 4 mo of age as compared with 6 mo of age in MRL-Faslpr mice. Furthermore, cutaneous histopathology was substantially greater in TgC/+;MRL-Faslpr as compared with the MRL-Faslpr strain at 3 mo of age (Fig. 2D). MRL-++ and B6 skin remained normal between 1.5–5.0 mo of age. Thus, the tempo of cutaneous pathology is accelerated by up-regulating CSF-1 in MRL-Faslpr mice.
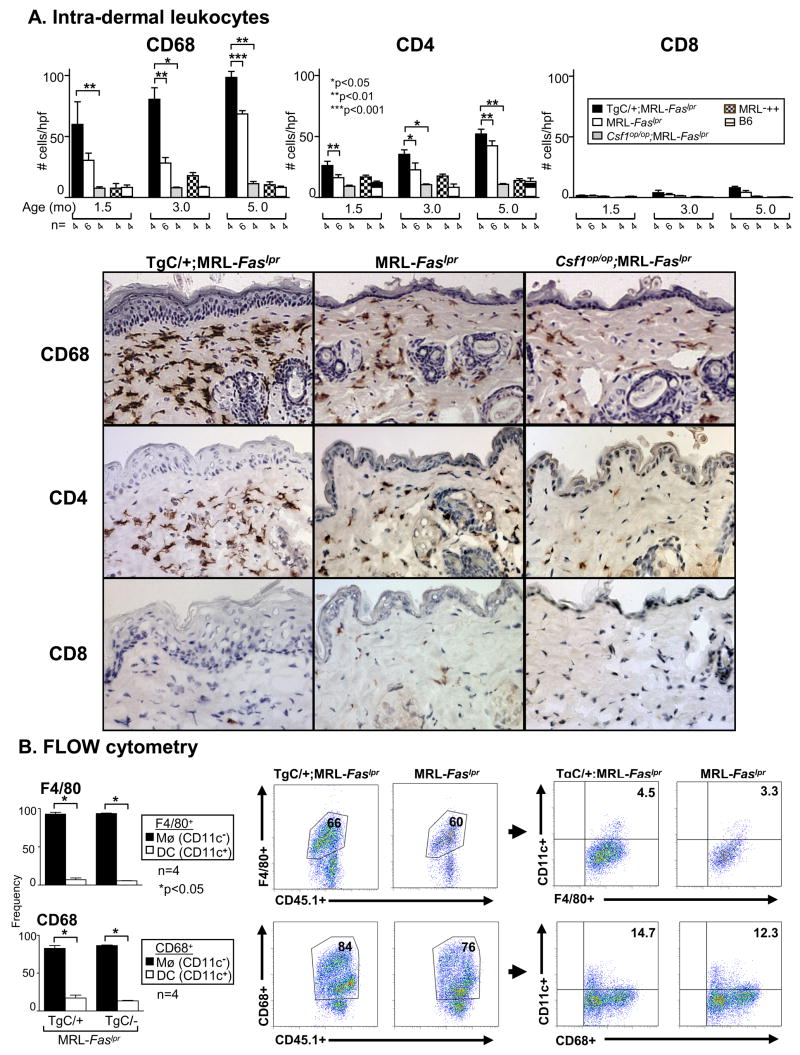
We detected a robust increase in CSF-1R-bearing Mø, and dermal DC (expressing CD68) in the TgC/+;MRL-Faslpr as compared to MRL-Faslpr skin (Fig. 3A). Notably, the proportion of CD68+ leukocytes in the skin that are Mø (~90%, F4/80+, CD68+CD11c−) grossly exceeds DC (~10%, CD68+CD11c+) as determined by flow cytometry and immunofluorescence (Fig. 3B). Infiltrating CD68+ leukocytes were more abundant than CD4+ T cells at all time points (Fig. 3A), while few CD8+ leukocytes were detected in the TgC/+; MRL-Faslpr or MRL-Faslpr mice. In contrast, leukocytes in the skin were much less abundant in mice without CLE (Csf1op/op;MRL-Faslpr, MRL-++ and B6 mice) (Fig. 3). Taken together, an increase in CSF-1 in the skin is associated with a rise in the infiltration of CD68+, most notably Mø, and CD4+ leukocytes in the skin and CLE expression in MRL-Faslpr mice.
Figure 3.
Intra-dermal leukocytes (CD68, CD4) increase in TgC/+;MRL-Faslpr mice compared with MRL-Faslpr mice. A. We evaluated the number of intra-dermal CD68+ cells (Mø, DC) and T cells (CD4+ and CD8+) from TgC/+;MRL-Faslpr, MRL-Faslpr, Csf1op/op;MRL-Faslpr mice, MRL-++ and B6 mice at 1.5, 3.0 and 5.0 mo of age. Representative photomicrographs are from mice at 3 mo of age. In addition, intra-epidermal leukocytes (CD68+ and CD4+) were increased (n= 4–7, data not shown). B. Intra-dermal CD68+ leukocytes in MRL-Faslpr mice are predominantly Mø. Flow cytometric analysis of intra-dermal Mø (F4/80+CD11c− and CD68+CD11c−) and DC (F4/80+CD11c+ and CD68+CD11c+) in TgC/+;MRL-Faslpr compared to MRL-Faslpr mice. Graphs depict the average frequency of CD11c− and CD11c+ cells in the CD45.1+ F4/80+ and CD68+ populations. Values are mean ± SEM.
Increasing CSF-1 in the skin incites CLE in lupus-susceptible MRL-Faslpr mice
To test the hypothesis that expression of CSF-1 in the skin incites CLE, we injected syngeneic dermal fibroblasts genetically modified to constitutively express CSF-1 (“CSF-1 carrier cells”) into the skin of lupus-susceptible, MRL-Faslpr mice before the advent of cutaneous lesions (2 mo of age), and lupus-resistant BALB/c mice (age-matched). MRL-Faslpr and BALB/c “CSF-1 carrier cells” generated similar levels of CSF-1 “in vitro” and these levels were substantially higher (~2X) than dermal fibroblasts transfected with an empty vector (“empty vector carrier cells”) (Fig. 4A.1). Infusing syngeneic “CSF-1 carrier cells”, but not “empty vector carrier cells”, into the skin similarly increased intra-dermal CSF-1 (area adjacent to infusion site) that spills over into the serum in MRL-Faslpr and BALB/c mice (3 days post-infusion) (Fig. 4A.2). The increase in intra-dermal CSF-1 using “CSF-1 carrier cells”, but not the “empty vector carrier cells”, incited CLE in MRL-Faslpr mice, but not in BALB/c mice, in the area adjacent (Fig. 4B), but not distal (data not shown) to the infusion site. Intra-dermal CSF-1 expression resulted in the infiltration of far more CD68+ (mainly Mø, F4/80+CD11b+CD11c−, data not shown) and CD4+ with few CD8+ leukocytes in the MRL-Faslpr skin than in BALB/c mice, a pattern similar to the spontaneous CLE (Fig. 4C). To eliminate the possibility that dermal fibroblasts uniquely contributed to these lesions, we repeated these studies using TEC instead of dermal fibroblasts. The results were similar (data not shown). Taken together, an intra-dermal increase in CSF-1 fosters the accumulation of mainly Mø and incites CLE in lupus-susceptible, but not in lupus-resistant mice.
Figure 4.
Increasing intra-dermal CSF-1 incites CLE in MRL-Faslpr mice. A.1) We verified that dermal fibroblasts from MRL-Faslpr and BALB/c mice genetically modified with a retroviral vector encoding CSF-1 constitutively express similar levels of CSF-1 (“CSF-1 carrier cells”),“empty vector carrier cells” serve as controls. A.2) CSF-1 was measured in the skin (area adjacent to infusion site) and serum of MRL-Faslpr and BALB/c mice following intra-dermal delivery of “carrier cells” at 3 days post-infusion. B. CLE is incited in the area of infusion and adjacent to infusion site 28 days post-infusion in MRL-Faslpr, but not BALB/c mice infused intra-dermally with “CSF-1 carrier cells” and characterized by interface vaculolar changes (black arrowheads) with hypergranulosis (#), and superficial, deep and peri-follicular inflammatory infiltrates (arrows) with scarring alopecia (@). Representative photomicrographs. Magnification: 20x, Inset 40x. C. Leukocyte-rich CLE incited by “CSF-1 carrier cells” in MRL-Faslpr, but not BALB/c mice. We evaluated intra-dermal Mø/DC (CD68+) and CD4+ and CD8+ T cells in the area adjacent to the “carrier cell” infusion. Data is representative of two separate experiments using dermal fibroblast and TEC “carrier cells” and an additional experiment infusing more (1.0 × 107) “carrier cells”. Values are mean ± SEM.
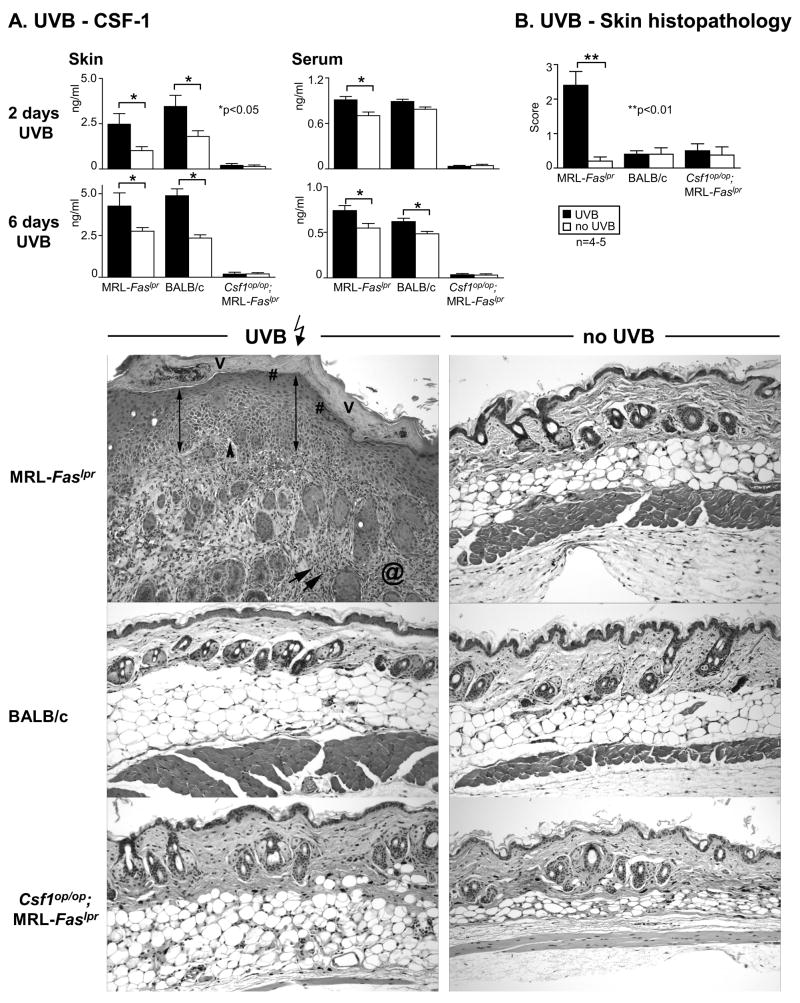
UVB induces CSF-1-dependent CLE in MRL-Faslpr mice
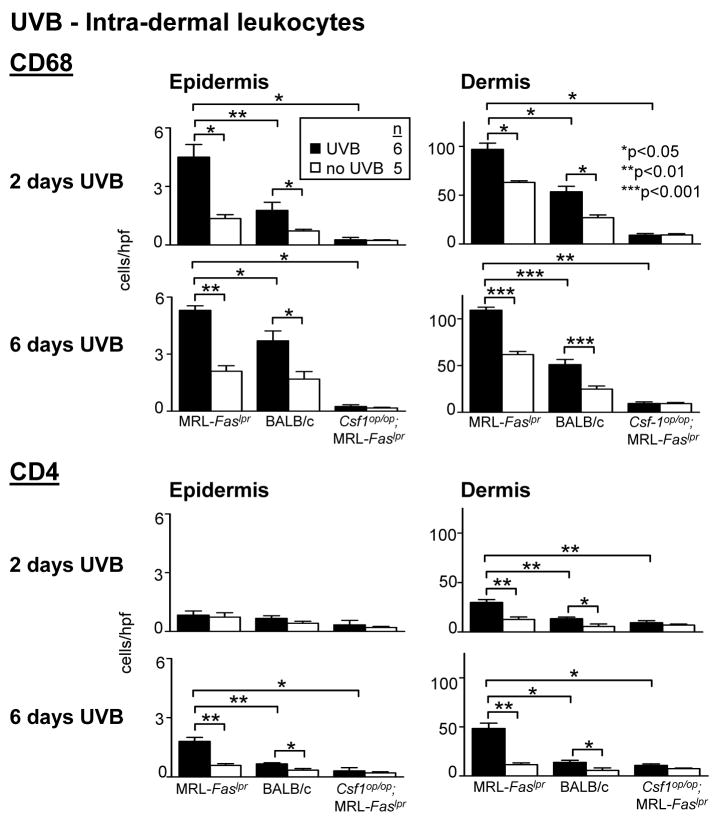
Sunlight triggers CLE in humans (1). Does UVB xinduce CSF-1 expression in the skin and CLE in MRL-Faslpr mice? Following UVB-exposure for 2 consecutive days, we detected a dramatic rise in CSF-1 expression in skin and serum in MRL-Faslpr and BALB/c mice (Fig. 5A). The CSF-1 level in the skin and serum in both strains remained similarly elevated after 6 consecutive days of UVB (Fig. 5A). At this point MRL-Faslpr mice, but not BALB/c mice, developed gross skin lesions (data not shown) with histopathologic features of CLE (Figure 5B). Of note, MRL-Faslpr and BALB/c are albino mice. UVB-exposure to other lupus-resistant albino strains including MRL-++ (Fas intact that do not develop skin lesions until the second year of life(38)), BALB/c-gld (mutation in Fas ligand), and non-obese diabetic mice (autoimmune diabetes) does not incite CLE (data not shown). Moreover, UVB did not incite CLE in Csf1op/op;MRL-Faslpr mice (Fig. 5B), indicating that expression of CLE requires CSF-1. While infiltration of CD68+ leukocytes increased in the epidermis and dermis in MRL-Faslpr and BALB/c mice following UVB-exposure (2 and 6 days) as compared to sham-treated controls, far more CD68+ leukocytes (~2x) accumulated in MRL-Faslpr than BALB/c mice (Fig. 6, top panel). The majority of CD68+ leukocytes were Mø (CD68+CD11c−) rather than DC (CD68+CD11c+) (~5:1 ratio). Of note, the proportion of intra-dermal CD68+ leukocytes that are Mø in comparison to DC did not change in MRL-Faslpr (UVB 79.88 ± 1.40; 15.02 ± 0.80 versus no UVB 78.47± 1.08; 16.87 ± 1.52 and BALB/c (UVB 80.05 ± 0.39; 14.71 ± 0.79 versus no UVB 73.50 ± 3.04; 15.23 ± 0.47) mice following UVB determined by flow cytometry. Notably, we detected few CD68+ leukocytes in Csf1op/op;MRL-Faslpr skin and this number did not rise following UVB-exposure (Fig. 6, top panel). Moreover, while intra-dermal CD4+ T cells increase in response to UVB, CD68+ leukocytes are more abundant. Interestingly, the initial response to UVB (2 days) resulted in a rise in Mø and not T cells in the epidermis (Fig. 6, bottom panel), suggesting that the initial impact on the epidermis is Mø-dependent. Taken together, UVB induces CSF-1-dependent CLE in lupus-susceptible MRL-Faslpr, but not in lupus-resistant BALB/c mice.
Fig 5.
UVB-irradiation increases CSF-1 expression and incites CLE in MRL-Faslp mice. A. We irradiated MRL-Faslpr, Csf1op/op;MRL-Faslpr and BALB/c mice daily (500J/m2 UVB) until we detected visible lesions (6 days). CSF-1 levels were measured (ELISA) in the skin and serum of MRL-Faslpr and BALB/c following 2 and 6 days of UVB-irradiation. UVB irradiated Csf1op/op;MRL-Faslpr mice served as negative controls. Values are mean ± SEM. B. Histopathologically, UVB incited CLE in MRL-Faslpr, but not BALB/c mice. The UVB incited features of CLE consisted of vacuolar interface changes (black arrowheads), apoptotic keratinocytes, acanthotic epidermis (double arrow) with hyperkeratosis (V), hypergranulosis (#), and superficial and deep, as well as perifollicular inflammatory infiltrates (arrows) with dermal fibrosis (@). Notably, UVB irradiated Csf1op/op;MRL-Faslpr mice did not develop CLE. Representative photomicrographs; Magnification: 20x. Data is representative of 2 separate experiments.
Figure 6.
More CD68+ leukocytes are in the skin of MRL-Faslpr, Csf1op/op;MRL-Faslpr and BALB/c following UVB exposure. MRL-Faslpr, Csf1op/op;MRL-Faslpr and BALB/c mice (2 mo of age) exposed to UVB for 2 and 6 days. We analyzed the epidermis and dermis for CD68+ leukocytes and CD4+ T cells. Values are mean ± SEM.
UVB induces CSF-1 mediated self-destruction in the skin
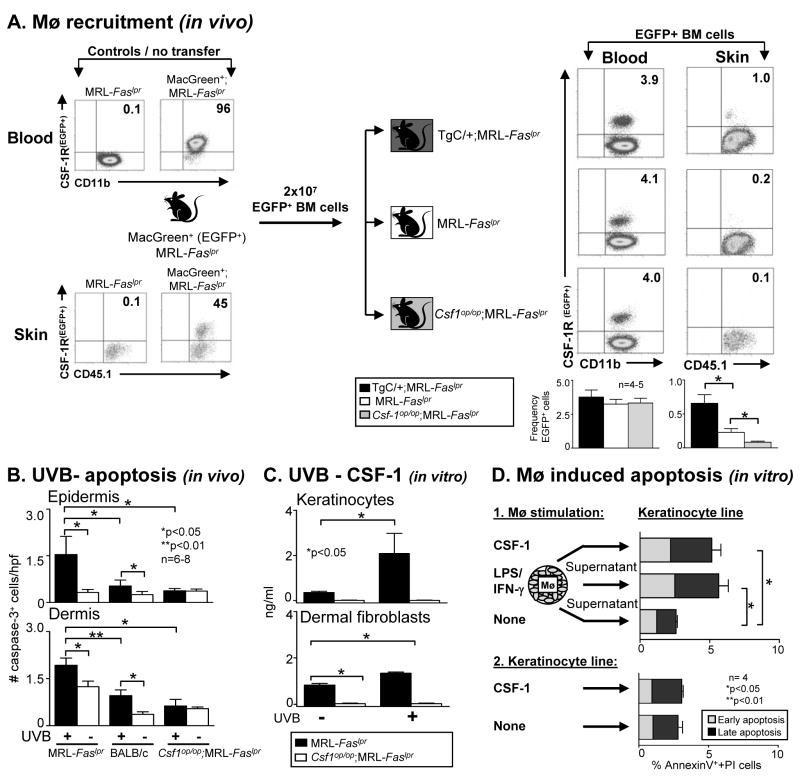
To determine the mechanism responsible for UVB-incited CSF-1-dependent CLE, we tested the hypothesis that CSF-1 expression in the skin recruits Mø from the circulation. For this purpose, we utilized BM cells from MacGreen;MRL-Faslpr mice that express EGFP under control of the c-fms promoter and in which CSF-1R+ leukocytes are readily detected in the blood (96% of CD11b+ leukocytes are EGFP+) and skin (45% of CD45.1+ leukocytes are EGFP+) (Fig. 7A). We adoptively transferred BM cells from MacGreen;MRL-Faslpr mice into MRL-Faslpr mice with varying levels of CSF-1 (TgC/+;MRL-Faslpr > MRL-Faslpr > Csf1op/op;MRL-Faslpr). First, we verified that equivalent numbers of EGFP+ cells were delivered into the blood (Fig. 7A). The number of EGFP+ Mø recruited into the skin paralleled the level of CSF-1 levels in the skin (TgC/+;MRL-Faslpr > MRL-Faslpr > Csf1op/op;MRL-Faslpr) (Fig. 7A). Thus, CSF-1 recruits c-fms+ BM-derived monocytes into the skin in MRL-Faslpr mice.
Figure 7.
CSF-1, generated by keratinocytes/dermal fibroblasts, recruits and activates Mø in the skin, which in turn, induce apoptosis in keratinocytes. A. CSF-1 recruits EGFP+ BM cells into the skin. We transferred BM cells from MacGreen (EGFP+);MRL-Faslpr mice into TgC/+;MRL-Faslpr, MRL-Faslpr and Csf1op/op;MRL-Faslpr mice and evaluated the recruitment of EGFP+ cells into the skin (flow cytometry). Data is representative of 3 separate experiments. B. UVB-induced apoptosis in epidermis and dermis is far more robust in MRL-Faslpr mice than in Csf1op/op;MRL-Faslpr and BALB/c mice. We evaluated the epidermis and dermis for apoptotic (cleaved-caspase-3+) cells following UVB-exposure (6 days). Sham-treated mice served as controls. Values are mean ± SEM. C. CSF-1 is up-regulated in mouse primary keratinocytes and dermal fibroblast (derived from MRL-Faslpr mice) irradiated with UVB one time with a dose of 500J/m2 in vitro. CSF-1 levels in the supernatant were analyzed 24 h after UVB-exposure (ELISA). Keratinocytes and dermal fibroblasts isolated from Csf1op/op;MRL-Faslpr mice served as negative controls. Data is representative of 2 separate experiments. D. Mø release mediators that induce apoptosis in keratinocytes. Supernatants from BMMø stimulated with CSF-1 (100μg/ml) released mediators that induced apoptosis in keratinocytes that was more robust than the apoptosis induced by supernatants from unstimulated BMMø (Annexin-V-FITC and PI using flow cytometry). Data is representative of 3 experiments. Values are mean ± SEM.
In lupus nephritis, activated Mø accumulate adjacent to cells (TEC) rich in CSF-1 and release mediators that induce TEC apoptosis (16). Thus, we hypothesized that CSF-1 attracted and activated Mø that induce apoptosis of skin parenchymal cells. Following UVB-irradiation (6 days) apoptotic cells increase in the epidermis and dermis of MRL-Faslpr mice more robustly than in UVB-irradiated BALB/c mice (Fig. 7B). In contrast, the number of apoptotic cells in UVB-irradiated Csf1op/op;MRL-Faslpr skin did not rise (Fig. 7B). Taken together, this indicates that UVB triggered CSF-1 expression in the skin is linked to the heightened infiltration by Mø and apoptosis.
To further examine the link between UVB, CSF-1 expression and apoptosis, we determined whether keratinocytes and dermal fibroblasts, cell types known to generate CSF-1 (39), are stimulated by UVB. As compared to unstimulated cells, CSF-1 expression was up-regulated in primary keratinocytes and dermal fibroblasts derived from MRL-Faslpr mice following UVB (Fig 7C). Keratinocytes and dermal fibroblasts derived from Csf1op/op;MRL-Faslpr mice served as negative controls. Moreover, up-regulation of CSF-1 in keratinocytes following UVB was as robust as other well-documented stimulants, IFN-γ and TNF-α/LPS (40) (data not shown). We detected similar findings using real-time-PCR and in human primary keratinocytes and dermal fibroblasts (data not shown). Since CSF-1 recruits Mø to the skin, we tested the hypothesis that the recruited, activated BMMø destroy the cellular source of CSF-1 in the epidermis, keratinocytes. For this purpose, we incubated keratinocytes and dermal fibroblasts with supernatant from BMMø (95% pure F480+CD11b+) following treatment with or without CSF-1. Incubation with IFN-γ/LPS, a conventional and potent Mø stimulant served as a positive control. CSF-1 directly activated BMMø as determined by up-regulation of CD86 (flow cytometry, data not shown). These activated BMMø released mediators that induced apoptosis of keratinocytes and dermal fibroblasts (Fig. 7D). In the context of these experiments, it is notable that CSF-1 stimulated BMMø (derived from MRL-Faslpr mice) released mediators that induced apoptosis in a greater number of keratinocytes (PAM 212 line) than unstimulated BMMø. Importantly, apoptosis is not induced in keratinocytes directly stimulated with CSF-1 alone (Fig. 7D). We detected similar findings when we repeated this experiment using BMMø derived from B6 mice (data not shown), indicating that the mediators inducing apoptosis were not unique to MRL-Faslpr mice. Taken together, in MRL-Faslpr mice, UVB induces CSF-1 expression in keratinocytes. CSF-1 attracts Mø to migrate from the circulation towards intra-dermal cells generating CSF-1. CSF-1 fosters Mø activation and these leukocytes release mediators that destroy CSF-1 generating cells (Fig. 8). Thus, in lupus-susceptible, but not lupus-resistant, mice, UVB-exposure induces CSF-1 that, in turn, activates a CSF-1-dependent pathway leading to CLE.
Figure 8.
Scheme of UVB triggered CSF-1 and Mø mediated apoptosis during cutaneous lupus in MRL-Faslpr mice. 1) UVB induces CSF-1 expression in keratinocytes. 2) BMMø are recruited by CSF-1 to sites rich in CSF-1 in the skin (eg, keratinocytes). 3) CSF-1 activates Mø that release mediators and 4) induce apoptosis of adjacent cells (eg, keratinocytes).
Discussion
The pathology of cutaneous lesions in MRL-Faslpr mice and human CLE are similar (41). Sunlight (UVB-exposure) triggers the expression of human CLE and human lupus (1). Since CSF-1 is required for nephritis in MRL-Faslpr mice, we analyzed the impact of CSF-1 in the pathogenesis of CLE in MRL-Faslpr mice (23). By constructing unique mutant MRL-Faslpr strains expressing varying levels of CSF-1 (high, intermediate, none), and using an ex-vivo gene transfer system to increase intra-dermal CSF-1, we determined that CSF-1 is necessary for and incites CLE in MRL-Faslpr mice. Hypothesizing that UVB exposure worsens CLE through a CSF-1-dependent mechanism, we showed that UVB-exposure increased Mø, apoptosis in the skin, and incited CLE in MRL-Faslpr, but not in CSF-1-deficient MRL-Faslpr mice. Probing deeper, we determined a CSF-1-dependent, Mø-mediated mechanism is responsible for CLE.
Lupus susceptibility genes are essential for CSF-1 mediated CLE. Using either “CSF-1 carrier cells” or UVB-exposure, CLE was induced in the lupus-susceptible MRL-Faslpr mice, but not in the lupus-resistant BALB/c mice. This finding is consistent with sunlight (UVB) triggering CLE in lupus patients, but not in normal individuals (42). Moreover, UVB-exposure incited a far greater accumulation of Mø within the skin of MRL-Faslpr as compared to lupus-resistant BALB/c mice, despite inducing similar levels of CSF-1 in the skin. This finding is consistent with hyper-proliferation of Mø to CSF-1 in MRL-Faslpr, but not in non-autoimmune strains (43). Thus, Mø responsiveness to CSF-1 may be a critical feature of lupus susceptibility leading to CLE.
Our findings highlight a central role for Mø in CLE. These are consistent with the essential contribution of Mø to psoriasis (an inflammatory skin condition traditionally thought to be triggered by T lymphocytes) in two distinct models, a T cell dependent and T cell independent (14, 15). In this autoimmune skin disorder, the recruitment and activation of Mø in the psoriatic skin is a key pathogenic event in the development and maintenance of psoriatic lesions. Thus, it is tempting to suggest that the underlying events that lead to Mø recruitment, activation and the “misbehavior” of these Mø in psoriasis are linked to CSF-1. Of note, we are aware that Mø are somewhat less prevalent in human CLE than in the MRL-Faslpr mice. We view the greater number of Mø in MRL-Faslpr CLE as an advantage to more clearly dissect the role of these CSF-1R-bearing leukocytes in the disease process.
Our findings indicate that a rise in CSF-1 alone is not sufficient for CLE, but rather require the MRL-Faslpr background genes. While our data suggests that Mø are central to UVB incited CLE, the increase in Mø is followed by a substantial rise in CD4 T cells. Thus, distinct populations of T cell may regulate CLE. In this regard, gross skin lesions are suppressed in MRL-Faslpr mice lacking CD4, but not CD8 T cells (44). On the other hand, expanding natural killer T cells (NKT) by provision of α-Galactosylceramide (45) suppresses dermitis, and eliminating CD1d, an antigen-presenting molecule know to activate NKT cells exacerbates dermitis (46). In contrast, CLE is accelerated in beta(2)-microglobulin deficient MRL-Faslpr mice in which normal development of CD1-dependent NKT cells is prevented (47). Thus, it is not clear whether NKT cells are central to CLE. With this in mind, we plan to pinpoint the distinct T cell populations in lupus-susceptible MRL-Faslpr mice, but not lupus-resistant strains required for UVB incited CLE.
We report the novel finding that UVB induces CSF-1 in keratinocytes, and dermal fibroblasts. We detected a rise in CSF-1 mRNA (real-time PCR) and/or protein (ELISA) following UVB-exposure in mouse keratinocytes (primary cells and PAM 212 line), primary human keratinocytes, primary mouse dermal fibroblasts and human dermal fibroblasts. In contrast, another report, using Northern analysis and proliferation assays, indicated that UVB decreases CSF-1 expression by PAM 212 mouse keratinocytes and fibroblasts of a mouse dermal line (39). The discrepancy between these findings and ours maybe related to the differences between primary cells and cell lines and/or the assay techniques used. We are confident of our findings since our in vitro studies are consistent with our and other’s in vivo evidence that UVB increases intra-dermal CSF-1 (48), and the reproducibility of our findings in two primary cell types, keratinocytes and dermal fibroblasts, derived from two species, mouse and humans.
UVB-exposure induces apoptosis in the skin and apoptotic cells have been implicated in the pathogenesis of lupus (49–51). There are several mechanisms that may be instrumental in inducing apoptosis in skin parenchymal cells. For example, UVB induces direct, ligand-independent activation of membrane death receptors such as Fas/Fas-ligand up-regulation (52). However, since MRL-Faslpr mice lack a functional Fas antigen, we can dismiss this mechanism. Of note, the release of TNF-α and subsequent ligation of the TNF receptor (p55) mediates keratinocyte apoptosis (53, 54). In this regard, TNF-a, along with nitric oxide and reactive oxygen species, are mediators released by activated Mø that induce apoptosis of renal parenchymal cells, TEC (16, 23). In the present study, the UVB induction of CSF-1 in the skin (keratinocytes and dermal fibroblasts) recruits and activates Mø. These Mø home to sites rich in CSF-1 and release mediators that induce apoptosis in adjacent cells. This suggests that the CSF-1-dependent influx of Mø is the culprit responsible for UVB-incited apoptosis in the skin. This is consistent with the enhanced numbers of intra-dermal apoptotic cells and Mø following UVB exposure in MRL-Faslpr, as compared to BALB/c and CSF-1-deficient MRL-Faslpr mice. We wish to point out that MRL-Faslpr mice are defective in phagocytosis of apoptotic cells, and that this inefficient clearance, which is also discussed in human lupus (55) may contribute, in part, to the accumulation of intra-dermal apoptotic cells (56, 57). Finally, evidence indicates that apoptotic cells may be instrumental in inducing auto-Abs that promote systemic lupus (3, 58) Thus, it is intriguing to speculate that UVB induction of CSF-1 in the skin may initiate systemic lupus by increasing apoptotic cells in the skin, and/or causing a rise in systemic CSF-1 and/or boosting the activated Mø in the skin that may egress and home to other tissues. Future experiments will test these possibilities.
In conclusion, our studies identify a UVB-incited, CSF-1-dependent, Mø-mediated sequence leading to CLE in lupus-susceptible strains. We envision CSF-1 as the “match” and lupus susceptibility genes as the “tinder” required for CLE.
Acknowledgments
We wish to thank Dr. A. Schwarting and M. Blanfeld for running the SS-A/SS-B ELISA assay and Dr. Terry B. Strom for editorial suggestions.
This work was supported by the National Institutes of Health grants DK 52369 (VRK), DK 36149 (VRK). J. Menke is supported by the Deutsche Forschungsgemeinschaft (ME-3194/1-1), J. Lucas by a Ruth L. Kirschstein National Research Service Award (F32 DK078416-01)
Footnotes
Abbreviations used in this paper: B6, C57BL/6; MRL-++, MRL/MpJ-+/+; MRL-Faslpr, MRL/MpJ-Faslpr/Faslpr; Mø, macrophages; WT, wild type; BM, bone marrow; CLE, cutaneous lupus erythematosus; LC, langerhans cells; DC, dendritic cell; hpf, high power field; TEC, tubular epithelial cell.
References
- 1.Lin JH, Dutz JP, Sontheimer RD, Werth VP. Pathophysiology of cutaneous lupus erythematosus. Clin Rev Allergy Immunol. 2007;33:85–106. doi: 10.1007/s12016-007-0031-x. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa F, Yoshimasu T. Animal models of spontaneous and drug-induced cutaneous lupus erythematosus. Autoimmun Rev. 2005;4:345–350. doi: 10.1016/j.autrev.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Werth VP. Cutaneous lupus: insights into pathogenesis and disease classification. Bull NYU Hosp Jt Dis. 2007;65:200–204. [PubMed] [Google Scholar]
- 4.Synkowski DR, Provost TT. Characterization of the inflammatory infiltrate in lupus erythematosus lesions using monoclonal antibodies. J Rheumatol. 1983;10:920–924. [PubMed] [Google Scholar]
- 5.Kanauchi H, Furukawa F, Imamura S. Characterization of cutaneous infiltrates in MRL/lpr mice monitored from onset to the full development of lupus erythematosus-like skin lesions. J Invest Dermatol. 1991;96:478–483. doi: 10.1111/1523-1747.ep12470176. [DOI] [PubMed] [Google Scholar]
- 6.David-Bajar KM, Davis BM. Pathology, immunopathology, and immunohistochemistry in cutaneous lupus erythematosus. Lupus. 1997;6:145–157. doi: 10.1177/096120339700600210. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa F, Kanauchi H, Wakita H, Tokura Y, Tachibana T, Horiguchi Y, Imamura S, Ozaki S, Takigawa M. Spontaneous autoimmune skin lesions of MRL/n mice: autoimmune disease-prone genetic background in relation to Fas-defect MRL/1pr mice. J Invest Dermatol. 1996;107:95–100. doi: 10.1111/1523-1747.ep12298305. [DOI] [PubMed] [Google Scholar]
- 8.Alaibac M, Berti E, Chizzolini C, Fineschi S, Marzano AV, Pigozzi B, Riboldi E, Sozzani S, Kuhn A. Role of cellular immunity in the pathogenesis of autoimmune skin diseases. Clin Exp Rheumatol. 2006;24:S14–19. [PubMed] [Google Scholar]
- 9.Clark RA, Kupper TS. Misbehaving macrophages in the pathogenesis of psoriasis. J Clin Invest. 2006;116:2084–2087. doi: 10.1172/JCI29441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark R, Kupper T. Old meets new: the interaction between innate and adaptive immunity. J Invest Dermatol. 2005;125:629–637. doi: 10.1111/j.0022-202X.2005.23856.x. [DOI] [PubMed] [Google Scholar]
- 11.Dupasquier M, Stoitzner P, van Oudenaren A, Romani N, Leenen PJ. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J Invest Dermatol. 2004;123:876–879. doi: 10.1111/j.0022-202X.2004.23427.x. [DOI] [PubMed] [Google Scholar]
- 12.Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Kiekens RC, Thepen T, Oosting AJ, Bihari IC, Van De Winkel JG, Bruijnzeel-Koomen CA, Knol EF. Heterogeneity within tissue-specific macrophage and dendritic cell populations during cutaneous inflammation in atopic dermatitis. Br J Dermatol. 2001;145:957–965. doi: 10.1046/j.1365-2133.2001.04508.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Peters T, Kess D, Sindrilaru A, Oreshkova T, Van Rooijen N, Stratis A, Renkl AC, Sunderkotter C, Wlaschek M, Haase I, Scharffetter-Kochanek K. Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation. J Clin Invest. 2006;116:2105–2114. doi: 10.1172/JCI27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratis A, Pasparakis M, Rupec RA, Markur D, Hartmann K, Scharffetter-Kochanek K, Peters T, van Rooijen N, Krieg T, Haase I. Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation. J Clin Invest. 2006;116:2094–2104. doi: 10.1172/JCI27179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest. 1999;103:73–80. doi: 10.1172/JCI4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 18.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yui MA, Brissette WH, Brennan DC, Wuthrich RP, Rubin-Kelley VE. Increased macrophage colony-stimulating factor in neonatal and adult autoimmune MRL-lpr mice. Am J Pathol. 1991;139:255–261. [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom RD, Florquin S, Singer GG, Brennan DC, Kelley VR. Colony stimulating factor-1 in the induction of lupus nephritis. Kidney Int. 1993;43:1000–1009. doi: 10.1038/ki.1993.141. [DOI] [PubMed] [Google Scholar]
- 22.Lenda DM, Stanley ER, Kelley VR. Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice. J Immunol. 2004;173:4744–4754. doi: 10.4049/jimmunol.173.7.4744. [DOI] [PubMed] [Google Scholar]
- 23.Lenda DM, Kikawada E, Stanley ER, Kelley VR. Reduced macrophage recruitment, proliferation, and activation in colony-stimulating factor-1-deficient mice results in decreased tubular apoptosis during renal inflammation. J Immunol. 2003;170:3254–3262. doi: 10.4049/jimmunol.170.6.3254. [DOI] [PubMed] [Google Scholar]
- 24.Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- 25.Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, Pollard JW, Stanley ER. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- 26.Faust J, Menke J, Kriegsmann J, Kelley VR, Mayet WJ, Galle PR, Schwarting A. Correlation of renal tubular epithelial cell-derived interleukin-18 up-regulation with disease activity in MRL-Faslpr mice with autoimmune lupus nephritis. Arthritis Rheum. 2002;46:3083–3095. doi: 10.1002/art.10563. [DOI] [PubMed] [Google Scholar]
- 27.Menke J, Lucas JA, Zeller GC, Keir ME, Huang XR, Tsuboi N, Mayadas TN, Lan HY, Sharpe AH, Kelley VR. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J Immunol. 2007;179:7466–7477. doi: 10.4049/jimmunol.179.11.7466. [DOI] [PubMed] [Google Scholar]
- 28.Moore KJ, Yeh K, Naito T, Kelley VR. TNF-alpha enhances colony-stimulating factor-1-induced macrophage accumulation in autoimmune renal disease. J Immunol. 1996;157:427–432. [PubMed] [Google Scholar]
- 29.Mee JB, Antonopoulos C, Poole S, Kupper TS, Groves RW. Counter-regulation of interleukin-1alpha (IL-1alpha) and IL-1 receptor antagonist in murine keratinocytes. J Invest Dermatol. 2005;124:1267–1274. doi: 10.1111/j.0022-202X.2005.23684.x. [DOI] [PubMed] [Google Scholar]
- 30.Dlugosz AA, Glick AB, Tennenbaum T, Weinberg WC, Yuspa SH. Isolation and utilization of epidermal keratinocytes for oncogene research. Methods Enzymol. 1995;254:3–20. doi: 10.1016/0076-6879(95)54003-2. [DOI] [PubMed] [Google Scholar]
- 31.Rogers KM, Black DH, Eberle R. Primary mouse dermal fibroblast cell cultures as an in vitro model system for the differential pathogenicity of cross-species herpesvirus papio 2 infections. Arch Virol. 2007;152:543–552. doi: 10.1007/s00705-006-0865-1. [DOI] [PubMed] [Google Scholar]
- 32.Wuthrich RP, Glimcher LH, Yui MA, Jevnikar AM, Dumas SE, Kelly VE. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 1990;37:783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]
- 33.Danos O, Mulligan RC. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988;85:6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 37.Blaszczyk M, Jablonska S, Chorzelski TP, Jarzabek-Chorzelska M, Beutner EH, Kumar V. Clinical relevance of immunologic findings in cutaneous lupus erythematosus. Clin Dermatol. 1992;10:399–406. doi: 10.1016/0738-081x(92)90086-e. [DOI] [PubMed] [Google Scholar]
- 38.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 39.Schuhmachers G, Ariizumi K, Kitajima T, Edelbaum D, Xu S, Shadduck RK, Gilmore GL, Taylor RS, Bergstresser PR, Takashima A. UVB radiation interrupts cytokine-mediated support of an epidermal-derived dendritic cell line (XS52) by a dual mechanism. J Invest Dermatol. 1996;106:1023–1029. doi: 10.1111/1523-1747.ep12338592. [DOI] [PubMed] [Google Scholar]
- 40.Chodakewitz JA, Lacy J, Edwards SE, Birchall N, Coleman DL. Macrophage colony-stimulating factor production by murine and human keratinocytes. Enhancement by bacterial lipopolysaccharide. J Immunol. 1990;144:2190–2196. [PubMed] [Google Scholar]
- 41.Furukawa F, Tanaka H, Sekita K, Nakamura T, Horiguchi Y, Hamashima Y. Dermatopathological studies on skin lesions of MRL mice. Arch Dermatol Res. 1984;276:186–194. doi: 10.1007/BF00414018. [DOI] [PubMed] [Google Scholar]
- 42.Hasan T, Nyberg F, Stephansson E, Puska P, Hakkinen M, Sarna S, Ros AM, Ranki A. Photosensitivity in lupus erythematosus, UV photoprovocation results compared with history of photosensitivity and clinical findings. Br J Dermatol. 1997;136:699–705. [PubMed] [Google Scholar]
- 43.Moore KJ, Naito T, Martin C, Kelley VR. Enhanced response of macrophages to CSF-1 in autoimmune mice: a gene transfer strategy. J Immunol. 1996;157:433–440. [PubMed] [Google Scholar]
- 44.Koh DR, Ho A, Rahemtulla A, Fung-Leung WP, Griesser H, Mak TW. Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells. Eur J Immunol. 1995;25:2558–2562. doi: 10.1002/eji.1830250923. [DOI] [PubMed] [Google Scholar]
- 45.Yang J-Q, Saxena V, Xu H, Van Kaer L, Wang C-R, Singh RR. Repeated {alpha}-Galactosylceramide Administration Results in Expansion of NK T Cells and Alleviates Inflammatory Dermatitis in MRL-lpr/lpr Mice. J Immunol. 2003;171:4439–4446. doi: 10.4049/jimmunol.171.8.4439. [DOI] [PubMed] [Google Scholar]
- 46.Jun-Qi Yang TC, Liu Hongzhu, Hong Seokmann, Bui Hai, Kaer Luc Van, Wang Chyung-Ru, Singh Ram Raj. CD1d deficiency exacerbates inflammatory dermatitis in MRL- European Journal of Immunology. 2004;34(lprlprI mice):1723–1732. doi: 10.1002/eji.200324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan OTM, Paliwal V, McNiff JM, Park S-H, Bendelac A, Shlomchik MJ. Deficiency in {beta}2-Microglobulin, But Not CD1, Accelerates Spontaneous Lupus Skin Disease While Inhibiting Nephritis in MRL-Faslpr Mice: An Example of Disease Regulation at the Organ Level. J Immunol. 2001;167:2985–2990. doi: 10.4049/jimmunol.167.5.2985. [DOI] [PubMed] [Google Scholar]
- 48.Scordi IA, Vincek V. Timecourse study of UVB-induced cytokine induction in whole mouse skin. Photodermatol Photoimmunol Photomed. 2000;16:67–73. doi: 10.1034/j.1600-0781.2000.d01-6.x. [DOI] [PubMed] [Google Scholar]
- 49.Van Laethem A, Claerhout S, Garmyn M, Agostinis P. The sunburn cell: regulation of death and survival of the keratinocyte. Int J Biochem Cell Biol. 2005;37:1547–1553. doi: 10.1016/j.biocel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Bijl M, Reefman E, Limburg PC, Kallenberg CG. Inflammatory clearance of apoptotic cells after UVB challenge. Autoimmunity. 2007;40:244–248. doi: 10.1080/08916930701357125. [DOI] [PubMed] [Google Scholar]
- 51.Reefman E, de Jong MC, Kuiper H, Jonkman MF, Limburg PC, Kallenberg CG, Bijl M. Is disturbed clearance of apoptotic keratinocytes responsible for UVB-induced inflammatory skin lesions in systemic lupus erythematosus? Arthritis Res Ther. 2006;8:R156. doi: 10.1186/ar2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takahashi H, Ishida-Yamamoto A, Iizuka H. Ultraviolet B irradiation induces apoptosis of keratinocytes by direct activation of Fas antigen. J Investig Dermatol Symp Proc. 2001;6:64–68. doi: 10.1046/j.0022-202x.2001.00020.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhuang L, Wang B, Sauder DN. Molecular mechanism of ultraviolet-induced keratinocyte apoptosis. J Interferon Cytokine Res. 2000;20:445–454. doi: 10.1089/10799900050023852. [DOI] [PubMed] [Google Scholar]
- 54.Zhuang L, Wang B, Shinder GA, Shivji GM, Mak TW, Sauder DN. TNF receptor p55 plays a pivotal role in murine keratinocyte apoptosis induced by ultraviolet B irradiation. J Immunol. 1999;162:1440–1447. [PubMed] [Google Scholar]
- 55.Gaipl US, Kuhn A, Sheriff A, Munoz LE, Franz S, Voll RE, Kalden JR, Herrmann M. Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun. 2006;9:173–187. doi: 10.1159/000090781. [DOI] [PubMed] [Google Scholar]
- 56.Licht R, Dieker JW, Jacobs CW, Tax WJ, Berden JH. Decreased phagocytosis of apoptotic cells in diseased SLE mice. J Autoimmun. 2004;22:139–145. doi: 10.1016/j.jaut.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol. 2003;170:3223–3232. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- 58.Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, Voll RE, Winkler T, Kuhn A, Kalden J, Kern P, Herrmann M. Clearance deficiency and systemic lupus erythematosus (SLE) J Autoimmun. 2007;28:114–121. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]