Abstract
This study tested the hypothesis that implicit power motivation (nPower), in interaction with power incentives, influences activation of brain systems mediating motivation. Twelve individuals low (lowest quartile) and 12 individuals high (highest quartile) in nPower, as assessed per content coding of picture stories, were selected from a larger initial participant pool and participated in a functional magnetic resonance imaging study during which they viewed high-dominance (angry faces), low-dominance (surprised faces) and control stimuli (neutral faces, gray squares) under oddball-task conditions. Consistent with hypotheses, high-power participants showed stronger activation in response to emotional faces in brain structures involved in emotion and motivation (insula, dorsal striatum, orbitofrontal cortex) than low-power participants.
Keywords: implicit motives, facial expressions of emotion, motivation, power, dominance, personality, brain, fMRI
McClelland et al. (1989) distinguished between two fundamentally different motivational systems. The explicit motivational system represents individuals’ self-attributed (or explicit) motives, that is, motivational orientations and goals that people ascribe to themselves, that they can verbally report on and that give rise to controlled forms of behavior. The implicit motivational system represents individuals’ implicit motives, that is, motivational dispositions that operate outside of people's conscious awareness and that orient, select and energize spontaneous forms of behavior.
McClelland et al. (1989) furthermore argued that implicit motives are mediated by brain areas subserving motivation and the autonomic nervous system. Given the growing body of evidence for an involvement of implicit motives in hormone release (e.g. McClelland, 1989; Wirth et al., 2006; Schultheiss, in press), which is under control of motivational brain structures such as the amygdala and the hypothalamus, and the increasing evidence from biopsychology and the neurosciences for separate brain systems for automatic motivational processes and effortful control of action (e.g. Rolls, 1999; LeDoux, 2002; Berridge and Robinson, 2003; Lieberman et al., 2004), these claims appear to have merit. What is missing until this day, though, is a direct linking of implicit motives to brain structures critically involved in motivation. In the present paper, we aim to fill this gap by exploring how individual differences in the implicit need for power (or nPower) influence activation in motivational brain structures in response to social dominance cues.
THE NEED FOR POWER
nPower represents a capacity to derive pleasure from having physical, mental or emotional impact on other individuals or groups of individuals and, conversely, to experience the impact of others on oneself as aversive (Veroff and Veroff, 1972; Winter, 1973; Schultheiss et al., 2005b). nPower is usually assessed with the Picture Story Exercise (PSE). On the PSE, research participants write imaginative stories about ambiguous picture cues. Stories are later coded for power-related themes using coding systems derived from experimental studies. Coding systems for the assessment of nPower, such as Winter's (1973) revised system and Winter's (1994) running text system, have adequate retest reliability (Winter and Stewart, 1977; Winter, 1991) and predict a host of power-related phenomena, such as leadership behavior (e.g. McClelland and Boyatzis, 1982), persuasion (e.g. Schultheiss and Brunstein, 2002), partner abuse (e.g. Mason and Blankenship, 1987), frequency of sexual intercourse (e.g. Schultheiss et al., 2003), Pavlovian conditioning of dominance cues (Stanton et al., 2006), instrumental learning in the context of dominance contests (Schultheiss et al., 2005b) and changes in gonadal steroid and cortisol levels in response to dominance successes and defeats (Schultheiss and Rohde, 2002; Wirth et al., 2006; Stanton and Schultheiss, 2007). Notably, scores obtained with these power motive scoring systems generally do not substantially correlate with self-report measures of dominance—a finding that is consistent with the implicit nature of nPower (for a review, see Schultheiss, in press).
Power-motivated individuals seek to dominate others and avoid being dominated by others. As a consequence, they are particularly sensitive to social cues signaling others’ high or low dominance (e.g. Fodor et al., 2006). Schultheiss and colleagues have recently tried to pinpoint the social cues that carry particular meaning for power-motivated perceivers by examining the role of facial expressions of emotion as motivational incentives (Schultheiss et al., 2005a; Schultheiss and Hale, 2007). They have argued that for power-motivated perceivers, anger and surprise faces both hold high incentive value, whereas neutral faces do not.
Anger faces are perceived as a sign of the sender's dominance and high status (e.g. Knutson, 1996; Tiedens, 2001) and should therefore be aversive and challenging to individuals who seek power themselves (i.e. people high in nPower). In contrast, surprise is displayed when the surprisee's expectations have been violated (cf. Camras et al., 2002). In the case of a social interaction, such a violation is likely to have been committed, either verbally or nonverbally, by the person to whom the sender directs the surprised expression. Thus, surprise can reflect a power differential between sender's and perceiver's control over the interaction, with the surprisor having power over the surprisee (cf. Conway et al., 1999), and should therefore represent a salient reward for power-motivated individuals. Finally, faces that show a neutral expression and therefore signal neither the sender's dominance nor a lack thereof are assumed to hold no particular incentive value for high-power individuals.
In support of these predictions, Schultheiss and colleagues have shown that high-power individuals, but not low-power individuals, respond to the high-dominance anger expression with attentional avoidance and impaired instrumental learning, whereas they orient their attention toward the low-dominance expression of surprise and are reinforced by surprise faces on an instrumental learning task (Schultheiss et al., 2005a; Schultheiss and Hale, 2007). In contrast, individuals’ nPower did not predict systematic reinforcement effects of neutral faces (Schultheiss et al., 2005a). Taken together, these findings indicate that both anger faces and surprise faces represent potent motivational incentives for individuals with a strong power motive. In the present research, we built on this body of work by using these facial stimuli as a vantage point from which to explore the effects of nPower on brain activation patterns.
THE MOTIVATIONAL BRAIN
Research in the affective neurosciences has identified a network of core structures involved in motivation that are dedicated to invigorating the organism's response to incentive cues and guiding the organism toward rewards or away from punishers (for reviews, see Rolls, 1999; Cardinal et al., 2002; LeDoux, 2002; O’Doherty, 2004). These include, most prominently, the amygdala, which responds to, and directs learning about, emotionally salient stimuli (LeDoux, 1996); the striatum, which plays a critical role in the acquisition, execution and invigoration of behavior that is instrumental for incentive attainment (e.g. Rolls, 1999; Schultz et al., 2000); and the orbitofrontal cortex (OFC), which evaluates the hedonic impact of the manifest reinforcers encountered by the individual (Rolls, 2000; Kringelbach, 2005). These three structures receive highly processed and integrated information through association cortices such as the insula, which represents a critical interface between autonomic bodily responses to incentives and core structures of the motivational brain (Damasio, 1994; Craig, 2002) and plays a role in memory for incentive value (Balleine and Dickinson, 2000). Amygdala, striatum and OFC interact with each other and send their output to the motor cortex for the regulation of behavior and to the brain stem and hypothalamus for the regulation of autonomic responses, including hormone release. Amygdala, striatum and OFC as well as the insula are involved in identifying and responding to positive and negative incentives (e.g. LeDoux, 1996; Rolls, 1999; Baxter and Murray, 2002; Phan et al., 2002; Delgado et al., 2004; Kringelbach, 2005), and all of these structures are also involved in processing facial expressions of emotion (for a review, see Adolphs, 2002).
LeDoux (2002, pp. 255–258) has speculated that implicit motives such as nPower are closely tied to activity in motivational brain structures. This speculation appears plausible in light of the previously reviewed effects of nPower on hormone release (Schultheiss et al., 2005b; Stanton and Schultheiss, 2007) and Pavlovian and instrumental learning (Schultheiss et al., 2005a; Stanton et al., 2006). In the present research, we, therefore, proceeded on the assumption that effects of nPower on brain activation responses to facial expressions of emotion will be particularly likely to be observed in the amygdala, the striatum (caudate and nucleus accumbens), the OFC and the insula.
OVERVIEW OF THE PRESENT RESEARCH
We conducted a functional magnetic resonance imaging (fMRI) study to directly test the idea that individual differences in nPower affect activity in motivational brain structures in response to facial expressions signaling high or low dominance (anger and surprise; high incentive value), compared to responses to neutral faces and gray squares (low incentive value). To maximize our ability to detect a moderating effect of nPower on brain responses to facial expressions of emotion, we selected from an initial pool of 112 participants those who were either very high (highest quartile) or very low (lowest quartile) in nPower as assessed with a PSE. We expected high-power individuals to show greater activation than low-power individuals in response to emotional expressions (relative to neutral faces) in motivational brain areas. Based on our contention that neutral faces should hold no particular incentive value, we did not expect high-power participants to show increased activation in motivational brain areas in response to neutral faces, relative to a no-face control stimulus (gray squares).
METHOD
Participants and procedure
From mid-May to mid-July 2005, 112 individuals (63 women; mean age = 22.33 years, s.d. = 5.33) who had passed a prescreening for mental and physical health problems that would have excluded them from fMRI testing participated in screening sessions in which their nPower was assessed with a PSE. Twelve low-power and 12 high-power participants were then selected from this initial pool. This final sample consisted of 14 women and 10 men, with a mean age of 20.96 years (s.d. = 2.18). They participated in fMRI scanner sessions scheduled between July and September 2005.
Design
The study had a 2 (nPower: low, high) × 3 (facial emotion: anger, surprise, neutral) design, with the first factor varying between subjects and the other factor blocked within subjects. Dependent variables were changes in blood oxygenation level dependent contrasts in a priori regions of interest (ROIs: amygdala, caudate, nucleus accumbens, OFC, insula).
nPower assessment and group selection
nPower was assessed by having participants write imaginative stories about eight pictures: ship captain, bicycle race, boxer, women in laboratory, trapeze artists, hooligan attack, nightclub scene and crouching woman with knife. All pictures have been used in previous research on power motivation (McClelland and Steele, 1972; McClelland, 1975; Smith, 1992; Schultheiss et al., 2005b; see Schultheiss and Pang, 2007, for more descriptive information on these pictures). Participants first viewed each picture for 10 s and then had 5 min to write a story. Picture presentation order was randomized across participants. Stories were later coded for motivational imagery independently by two trained scorers using Winter's (1994) Manual for Scoring Motive Imagery in Running Text. According to the manual, power imagery is scored whenever a story character expresses a power concern through strong forceful actions, provides unsolicited help, support or advice, tries to control or regulate others’ behavior; tries to influence, persuade, bribe or argue with another person; tries to impress another person or the world at large, arouses strong, nonreciprocal emotions in others; or shows a concern with reputation and prestige. Each scorer had previously exceeded 85% interrater agreement on calibration materials prescored by an expert, which are contained in the manual. Interrater reliability between the two scorers was good for the overall sample (Pearson r = 0.77) and for the 24 participants included in the final sample (Pearson r = 0.89), and power motive scores were averaged across scorers. On average, the 112 participants wrote 698 (s.d. = 165) words, containing 9.68 (s.d. = 3.64) nPower scores summed across all eight stories. Motive scores were positively correlated with protocol length (r = 0.43); thus, we corrected them for protocol length by regression and converted the residuals to z-scores. Next, we identified the participants from the highest and lowest quartiles of the nPower z-scores and selected 12 participants from each group for the final sample. The primary selection criterion was high- and low-power participants’ availability for a follow-up session at the fMRI scanner, but we also aimed to ensure a roughly balanced sampling from male and female participants in each group. The resulting group of 12 low-power participants (eight women) had an average nPower z-score of −1.28 (s.d. = 0.73, range: −3.13 to −0.72), and the resulting group of 12 high-power participants (six women) had an average nPower z-score of 1.37 (s.d. = 0.80, range: 0.65–3.00), for the z-score difference: t(22) = 8.51, P < 0.00005.
Stimuli and task design
Pictures of 20 individuals (10 male and 10 female) were used in this study. We used three pictures of each individual: one showing a surprised expression (open mouth), one showing an angry expression (bared teeth) and one showing a neutral expression. Faces were color photographs of individuals 1, 2, 3, 5, 6, 7, 8, 9, 10, 17, 22, 23, 26, 27, 29, 32, 34, 36, 37 and 45 taken from the MacBrain Face Stimulus Set (Tottenham et al., 2002). Faces were cropped so that each was visible from cheekbone to cheekbone and hairline to chin, and picture portions below the jawline were blackened. Faces were resized to 12.28 cm height after cropping (width could vary, depending on posers’ physiognomy).
Each facial expression was presented in the middle of the viewing field for 250 ms with a black background, followed by a variable-duration interstimulus interval with an average length of 350 ms (range: 200–500 ms) during which a white fixation cross on black background was presented in the screen's vertical midline, roughly at the height at which the eyes appeared in the face pictures. Presentations of facial expressions were blocked by expression, with pictures of all 20 posers presented in randomized order in every block. Facial expression blocks were presented in alternating order with blocks in which 12.28 cm (height) × 7.8 cm (width) gray squares were presented 20 times instead of faces. Gray-square blocks were the same as facial-expression blocks in all other regards. In both facial expression and gray-square blocks, an X instead of a fixation cross was presented randomly once per block in one half of the blocks and twice in the other half. Facial expression and gray-square blocks were organized into a run as follows (+, gray-square block; N, neutral-face block; S, surprise-face block; A, anger-face block): + N + S + A + S + N + A + N + A + S + A + S + N + S + A + N + A + N + S. Each block lasted 12 s, and each run, consisting of 36 blocks, lasted 7 min 12 s. Participants were instructed to indicate with a response-box key press whenever an X appeared on the screens instead of the regular fixation cross. We used this task to keep participants' attention actively engaged without making them explicitly process the emotions displayed by the faces, a strategy that is in keeping with studies that document stronger effects of emotional faces on motivational brain regions during implicit or incidental processing than during explicit identification or evaluation of the displayed emotion (e.g. Critchley et al., 2000; Taylor et al., 2003; Straube et al., 2004). We also included a passive-viewing condition, counterbalanced across participants with the X-detection oddball task, in our task design. However, this task failed to sufficiently engage participants’ attention, yielded no reliable effects of nPower on brain activation changes and will therefore not be considered in the following.
Scanning parameters
Participants lay supine in a 3.0 Tesla magnet (General Electric, standard quadrature head coil). Head motion was prevented by placement of padding around the head and the use of a soft headband. Stimuli were presented to participants on a 640 × 480 pixel display screen mounted inside the head coil and encompassing their entire visual field. We acquired functional images with a spiral in–out pulse sequence using a 90° flip angle. The field of view (FOV) was 220 × 220 mm, TR (repeat time to accomplish a full volume) was 2500 ms, TE (echo time) was 30 ms and voxel size was 3.44 mm × 3.44 mm × 4 mm. Twenty-nine contiguous horizontal slices of 4 mm thickness were acquired, encompassing the whole brain. Structural images for data presentation and coregistration were acquired in the same slice locations using a T1-weighted gradient echo pulse sequence, with TR = 200 ms, TE = 3.6 ms, FOV = 220 × 220 mm, voxel size = 0.86 mm × 0.86 mm × 4 mm and flip angle = 90°. Each participant underwent two contiguous scanning sessions for the two runs of the X-detection task, yielding 173 functional image volumes per run, excluding discarded initial volumes. Due to a programming error, volume acquisition stopped during the last block (surprise faces) on each run. Data from this block were, therefore, discarded from image analyses.
Image processing
The first four volumes per run were discarded to allow the MRI signal to reach its steady state. Movement correction was performed using the Automated Image Registration package (Woods et al., 1998). The realigned images were normalized to the Montreal Neurological Institute (MNI) template (Evans et al., 1994), and then smoothed with an [8 8 8] mm kernel.
Data analysis
We used SPM2 for subsequent data analyses (Wellcome Department of Cognitive Neurology, London, UK). Data were high-pass filtered at 100 s (0.01 Hz) and fitted to a canonical hemodynamic response function. No global scaling or autocorrelation estimation was used. ROI definitions were selected using the AAL library (Tzourio-Mazoyer et al., 2002), a collection of regions hand-drawn on the MNI single-subject anatomical T1 image. To correct for multiple-voxel comparisons within each ROI while preserving maximum sensitivity, we used an uncorrected P level of 0.005 combined with a 10 voxel threshold for caudate, insula and OFC; an uncorrected P level of 0.001 for amygdala and nucleus accumbens; and an uncorrected P level of 0.001 combined with a 10 voxel threshold for exploratory whole-brain analyses.
We computed the following linear contrasts for our analyses: (i) to test for effects of specific emotions, anger minus neutral and surprise minus neutral; (ii) to test for an overall effect of emotional faces, (anger plus surprise) minus neutral; (iii) to test for differential effects of emotional expressions, anger minus surprise; and (iv) to test whether faces had an effect on brain activation, neutral minus gray. The resulting contrast images were then used in random effect analyses.
RESULTS
Behavioral data
Participants’ accuracy in detecting X-shaped fixation crosses was good across all conditions, M = 91.8%, s.d. = 14.2%. Participants were slightly faster responding to Xs presented in between gray squares (525 ms) than to Xs presented between angry faces (540 ms), neutral faces (543 ms) and surprised faces (546 ms), as indicated by a marginal main effect of the within-subjects factor, P = 0.05
We also tested whether nPower, facial expression or their two-way interaction had an effect on accuracy or response latencies on correct trials, but without significant results. These findings suggest that participants followed instructions on the oddball task and that their performance was not influenced by their power motive or its interaction with stimulus type.
ROI analyses
In sample-level analyses, we first examined whether motivational brain structures showed brain activation changes to (emotional) faces by running analyses on emotional face minus neutral, anger minus surprise and neutral minus gray-square contrasts for the total sample. We then examined at the motive level whether nPower exerted the hypothesized moderating effect on activation in motivational brain structures by testing whether emotional face minus neutral, anger minus surprise and neutral minus gray-square contrasts differed significantly between low- and high-power participants. Results for sample-level analyses are reported in Table 1 and for motive-level analyses in Table 2.
Table 1.
Significant contrasts in ROI analyses at the group level
| K | F | x | y | z | Side | Activation (relative to control) is … | ||
|---|---|---|---|---|---|---|---|---|
| Amygdala | ||||||||
| N–G | 11 | 20.03 | −28 | −6 | −12 | L | Stronger | |
| Insula | ||||||||
| A–N | 19 | 24.91 | −42 | 2 | 4 | L | Weaker | |
| 6 | 16.00 | 36 | −22 | 22 | R | Weaker | ||
| A–S | 14 | 16.78 | 40 | 10 | −14 | R | Weaker | |
| (A + S)–N | 23 | 16.89 | −44 | 0 | 4 | L | Weaker | |
| N–G | 4 | 15.77 | 40 | −12 | 12 | R | Weaker | |
| Caudate | ||||||||
| (A + S)–N | 11 | 11.90 | 14 | 16 | 6 | R | Stronger | |
| Orbitofrontal cortex | ||||||||
| A–N | 67 | 15.19 | 12 | 54 | −14 | R | Stronger | |
| 10 | 11.09 | −8 | 58 | −22 | L | Stronger | ||
| S–N | 87 | 20.48 | −22 | 46 | −16 | L | Stronger | |
| 46 | 17.43 | 32 | 36 | −14 | R | Stronger | ||
| 18 | 15.21 | 14 | 36 | −18 | R | Stronger | ||
| 39 | 14.65 | 12 | 54 | −14 | R | Stronger | ||
| (A + S)–N | 98 | 19.08 | 12 | 54 | −14 | R | Stronger | |
| 45 | 15.69 | −22 | 46 | −16 | L | Stronger | ||
| 22 | 12.70 | 36 | 42 | −18 | R | Stronger | ||
| 11 | 12.28 | 14 | 36 | −18 | R | Stronger | ||
| N–G | 12 | 15.28 | 16 | 8 | −20 | R | Stronger | |
| 14 | 13.56 | −22 | 46 | −16 | L | Weaker |
K, cluster size in voxels; F, F-statistic; x, y and z represent distance in millimeter from the center of the anterior commissure on the dimensions left to right, posterior to anterior and inferior to superior in the Montreal Neurological Institute template brain space. A–N, anger–neutral contrast; S–N, surprise–neutral contrast; A–S, anger–surprise contrast; (A + S)–N, (anger and surprise)–neutral contrast; N–G, neutral–gray contrast.
Table 2.
Significant differences between individuals high or low in nPower in ROI analyses
| K | F | x | y | z | Side | High-power participants activate … | |
|---|---|---|---|---|---|---|---|
| Insula | |||||||
| A–N | 166 | 17.13 | 40 | 14 | −4 | R | More |
| 15 | 13.65 | −40 | −10 | −8 | L | More | |
| 20 | 12.75 | −50 | 8 | −8 | L | More | |
| 13 | 12.05 | −46 | −10 | 4 | L | More | |
| S–N | 28 | 11.49 | 40 | 12 | −4 | R | More |
| (A + S) –N | 98 | 17.01 | 40 | 14 | −4 | R | More |
| 19 | 16.65 | −38 | −14 | −6 | L | More | |
| 12 | 11.24 | −50 | 8 | −8 | L | More | |
| N–G | 20 | 16.18 | 34 | 26 | 10 | R | Less |
| 13 | 14.15 | −38 | −16 | −4 | L | Less | |
| 24 | 13.89 | 32 | 12 | −12 | R | Less | |
| 10 | 13.01 | −36 | −28 | 20 | L | Less | |
| Caudate | |||||||
| A–N | 33 | 20.41 | −18 | 24 | 10 | L | More |
| 122 | 16.32 | 8 | 8 | 16 | R | More | |
| Orbitofrontal cortex | |||||||
| A–N | 14 | 15.09 | −36 | 46 | −16 | L | More |
| A–S | 12 | 11.77 | −12 | 56 | −10 | L | More |
K, cluster size in voxels; F, F-statistic; x, y and z represent distance in millimeter from the center of the anterior commissure on the dimensions left to right, posterior to anterior and inferior to superior in the Montreal Neurological Institute template brain space. A–N, anger–neutral contrast; S–N, surprise–neutral contrast; N–G, neutral–gray contrast.
Sample-level analyses
We observed the following effects across the entire sample: participants showed increased activation of the frontal pole of the OFC, but attenuated activation of posterior portions of the insula in response to angry faces, relative to neutral faces. Surprised faces elicited increased activation bilaterally in medial anterior OFC. Presentation of emotional faces, regardless of specific content, was associated with more activation in right anterior caudate and bilateral medial OFC and with less activation in left posterior insula. Differential responses to angry and surprised faces were observed in the right insula, with relatively lower activation in response to anger than to surprise. Finally, the left amygdala showed a robust increase in activation in response to neutral faces, relative to gray squares, whereas the right insula showed attenuated activation and the OFC showed mixed responses on the same contrast. No significant activation changes were observed in the accumbens. In summary, OFC and caudate activation increased in response to emotional faces, amygdala activation increased in response to neutral faces and insula activation decreased in response to all face stimuli.
Motive-level analyses
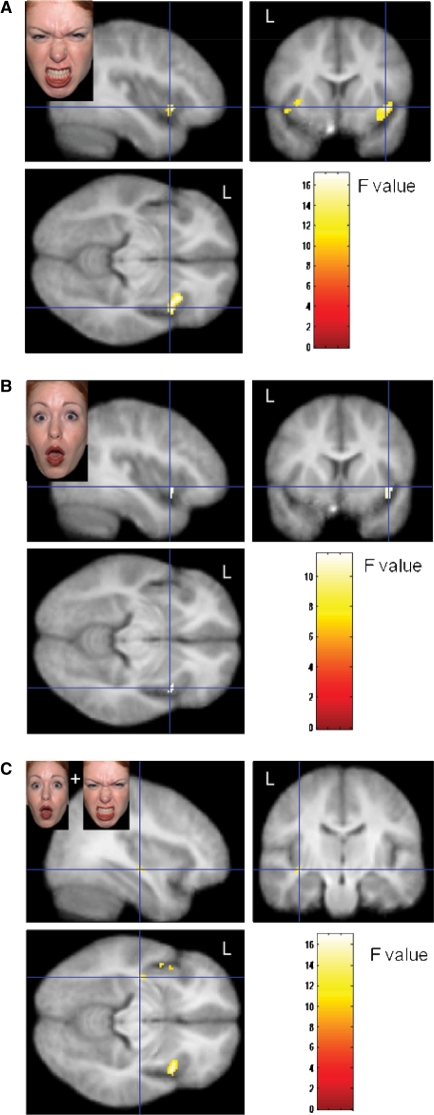
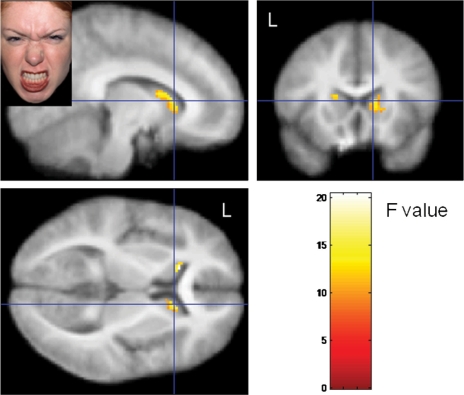
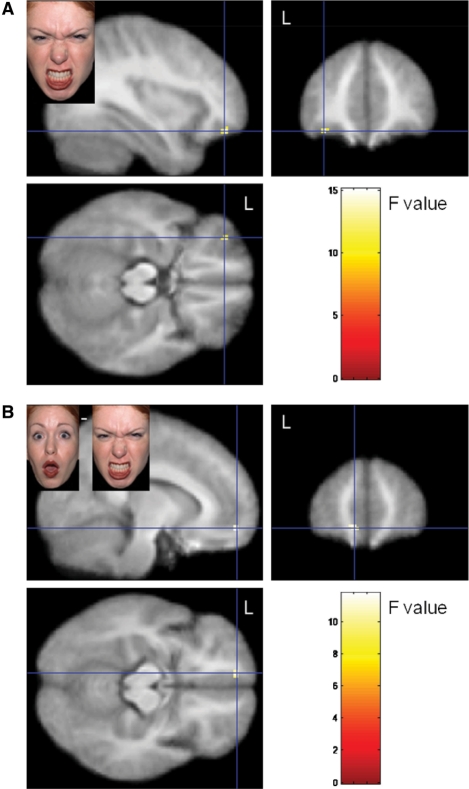
Activation changes in response to angry faces were moderated by nPower in insula, caudate and OFC (Figures 1–3), but not in amygdala or accumbens. High-power participants, compared with low-power participants, showed stronger activation in bilateral caudate (Figure 2) and insula (Figure 1A) as well as in left lateral anterior OFC (Figure 3A) when watching angry faces, relative to neutral faces. On surprise–neutral contrasts, high-power individuals showed greater activation than low-power individuals only in the anterior right insula (Figure 1B). For high-power participants, relative to low-power participants, we also observed greater bilateral insula activation in response to emotional faces generally [(anger + surprise)−neutral contrasts; Figure 1C] and less bilateral insula activation on neutral–gray square contrasts. Finally, a site in the left anteromedial OFC responded differentially to angry and surprised faces, depending on participants’ nPower: high-power participants showed relatively greater activation in response to angry faces, whereas low-power participants showed relatively greater activation in response to surprised faces (cf. Figure 3B).1
Fig. 1.
Effect on nPower on bilateral insula activation on anger–neutral (A; activation maxima left insula: x: −40, y: −10, z: −8; right insula: x: 40, y: 14, z: −4), surprise–neutral (B; activation maximum right insula: x: 40, y: 12, z: −4) and (anger + surprise)−neutral (C; activation maxima left insula: x: −38, y: −14, z: −6; right insula: x: 40, y: 12, z: −4) contrasts.
Fig. 2.
Effect on nPower on bilateral caudate activation on anger–neutral contrasts (activation maxima left caudate: x: −18, y: −24, z: −10; right caudate: x: 8, y: 8, z: 16).
Fig. 3.
Effect on nPower on left lateral OFC activation on anger–neutral contrasts (A; activation maximum: x: −42, y: 42, z: −12) and on left anteromedial OFC activation on anger–surprise (B; activation maximum: x: −12, y: 56, z: −10) contrasts.
Exploratory whole-brain analyses
Results of exploratory motive-level analyses for the whole brain are reported in Table 3. In general, findings from these analyses were consistent with our ROI results (e.g. nPower-dependent activation of caudate and insula in response to emotional faces; greater activation in high-power participants in response to emotional faces, less activation in response to neutral faces). But they also revealed consistent nPower-dependent activation changes in parts of the brain outside of our ROIs that appear to be worthy of further scrutiny in future studies (e.g. widespread decreased activation of posterior brain areas in response to neutral faces relative to gray squares but increased activation of dorsolateral and medial prefrontal areas and the parahippocampal region in response to emotional faces).
Table 3.
Significant differences between individuals high or low in nPower in whole-brain analyses
| K | F | x | y | z | Side | Structure | High-power participants activate … |
|---|---|---|---|---|---|---|---|
| A–N | |||||||
| 30 | 24.20 | 4 | −2 | 28 | R | Medial cingulate | More |
| 17.74 | −4 | −2 | 26 | L | More | ||
| 23 | 20.41 | −4 | −66 | 8 | L | Cuneus | More |
| 13 | 19.58 | 4 | 32 | 40 | R | Medial frontal gyrus | More |
| 25 | 18.63 | 28 | −82 | 20 | R | Middle occipital gyrus | More |
| 33 | 18.32 | −14 | 50 | 10 | L | Medial frontal gyrus | More |
| 31 | 18.24 | 10 | 20 | 12 | R | Anterior caudate | More |
| 14 | 17.87 | −26 | −30 | −8 | L | Parahippocampal gyrus | More |
| 15 | 17.28 | 10 | −72 | 48 | R | Precuneus | More |
| 39 | 17.13 | 40 | 14 | −4 | R | Insula | More |
| S–N | |||||||
| 10 | 21.05 | 22 | −34 | 22 | R | Caudate tail | Less |
| 12 | 17.29 | −50 | 20 | 32 | L | Middle frontal gyrus | More |
| 10 | 16.66 | −26 | −36 | −10 | L | Parahippocampal gyrus | More |
| (A + S)–N | |||||||
| 15 | 27.86 | 18 | −64 | 44 | R | Precuneus | More |
| 57 | 27.33 | −50 | 18 | 32 | L | Middle frontal gyrus | More |
| 14 | 20.42 | 56 | 0 | 16 | R | Precentral gyrus | More |
| 15 | 18.36 | 4 | 30 | 40 | R | Medial frontal gyrus | More |
| 19 | 17.42 | −26 | −32 | −10 | L | Parahippocampal gyrus | More |
| 16 | 17.01 | 40 | 14 | −4 | R | Insula | More |
| A–S | |||||||
| 13 | 22.71 | 20 | −38 | 12 | R | Caudate tail | More |
| N–G | |||||||
| 158 | 29.49 | −10 | −40 | 6 | L | Posterior cingulate | Less |
| 58 | 26.80 | 14 | −52 | 0 | L | Lingual gyrus | Less |
| 23 | 23.55 | −22 | −94 | 4 | L | Middle occipital gyrus | Less |
| 41 | 21.53 | 36 | −30 | −12 | R | Parahippocampal gyrus | Less |
| 22 | 19.47 | −42 | −56 | 8 | L | Middle temporal gyrus | Less |
| 19 | 19.16 | −16 | −34 | −8 | L | Parahippocampal gyrus | Less |
| 15 | 18.97 | 4 | 6 | 40 | R | Anterior cingulate | Less |
K, cluster size in voxels; F, F-statistic; x, y, and z represent distance in millimeter from the center of the anterior commissure on the dimensions left to right, posterior to anterior and inferior to superior in the Montreal Neurological Institute template brain space. A–N, anger–neutral contrast; S–N, surprise–neutral contrast; N–G, neutral–gray contrast.
DISCUSSION
In the present study, we provided a first test for the idea that implicit motives are mediated by brain structures mediating motivation, an idea that was originally proposed by McClelland et al. (1989) and has, more recently, also been endorsed by LeDoux (2002). We had predicted that individual differences in implicit power motivation would modulate activity in motivational brain areas in response to facial expressions signaling another person's anger or surprise (high incentive value), but not in response to neutral facial expressions (low incentive value).
In general, our findings support these predictions. In comparison to low-power participants, individuals high in nPower showed stronger bilateral activation in response to anger faces in the anterior caudate and the anterior insula, suggesting that they were more primed to recruit behavioral routines to cope with the dominance challenge inherent in encountering a threatening anger face and also may have experienced stronger bodily responses to it (Rolls, 1999; Critchley et al., 2004). In addition, high-power participants had stronger lateral activation of the OFC in the left hemisphere than low-power participants, a finding that is consistent with the lateral OFC's propensity to represent negative incentives and to aid behavioral change for coping with them (Kringelbach, 2005). Interestingly, high-power participants also responded with more activation of the left anteromedial OFC to angry faces relative to surprised faces. The medial OFC is dedicated to the representation of the reward values of a variety of incentives (Kringelbach, 2005). We speculate that the coactivation of medial and lateral OFC in response to angry faces reflects high-power participants’ ambivalence about this emotional expression: on the one hand, it signals one's emotional impact on another person (rewarding); on the other, another person's anger also represents a dominance challenge the perceiver needs to cope with somehow (aversive). Clearly, however, angry faces turned out to be a potent stimulus for revealing activation differences between low- and high-power participants in key areas of the motivational brain.
Surprise faces were less effective for eliciting activation responses in high-power participants. High-power participants responded only with increased insula activation to this emotional stimulus, perhaps reflecting a somatosensory or ‘gut’ response (Damasio, 1994). While this interpretation is consistent with the notion that surprise, like anger, should hold incentive value for power-motivated individuals, the lack of activation elicited by surprised faces in other motivational brain areas requires explanation. While we cannot rule out the possibility that anger is simply a stronger incentive for power-motivated individuals than surprise, two other factors may explain the differences in our findings for the two emotions. First, our previous research suggests that surprise is a potent reinforcer for power-motivated individuals only if it is being displayed by a sender of one's own gender, not if it is being displayed by a sender of the opposite gender (Schultheiss et al., 2005a). The design of our present study did not allow us to separate effects of same-gender from opposite-gender surprise faces. Opposite-gender faces may, therefore, have diluted stronger effects that same-gender surprise faces may have had on high-power participants’ brain activation responses. The validity of this explanation could be tested in future studies by varying face gender within subjects in blocked or even event-related designs.
A second reason for the fewer effects found for surprised faces relative to angry faces may reside in the different motivational significance of these social signals for power-motivated individuals. Schultheiss et al. (2005a) and Schultheiss and Hale (2007) have argued that a surprised expression directed at the perceiver is a signal of the perceiver's attained dominance (i.e. he/she has done something that the sender did not expect or have control over) and thus constitutes a reward for a power-motivated perceiver. In a sense, then, another's surprise is the outcome, rather than the start, of a motivational transaction with the environment and therefore does not necessitate any further action, mediated by activation of brain sites involved in response recruitment, such as the striatum. In contrast, an anger face expresses the sender's claim to dominance (e.g. Tiedens, 2001) and signals a challenge to the perceiver (Schultheiss and Hale, 2007). It thus not only constitutes a strong incentive for the high-power perceiver but also prompts some form of counteraction if he/she wants to maintain dominance. This interpretation is consistent with the observation that high-power participants showed specific activation in response to anger faces in the dorsal striatum, a brain area that is involved in the preparation of instrumental behavior (e.g. Delgado et al., 2004).
Contrary to our predictions, we did not observe nPower-dependent activation changes in amygdala and accumbens in response to emotional faces. This paucity of findings also extends to the results at the group level of analysis: we found amygdala activation only in response to neutral faces, relative to gray squares, but not in response to emotional faces, relative to neutral faces, and the accumbens remained silent in these analyses, too. One possible reason for this lack of activation findings in amygdala and accumbens may be insufficient resolution for detecting effects in these small-scale structures; the obvious remedy for this drawback would be to acquire images focusing only on limbic structures in future studies. Another possible reason for the lack of amygdala activation in the present study specifically may be that anger and surprise are less potent elicitors of amygdala activation than the emotional expression of fear (Murphy et al., 2003). Although no studies have been conducted yet on the effects of fearful expressions on power-motivated observers, further inquiry into the effects of nPower on amygdala activation could, therefore, fruitfully employ fearful faces as potent stimuli.
LIMITATIONS AND FUTURE DIRECTIONS
Besides replicating and extending our findings to other implicit motives (affiliation, achievement) and motive-specific incentive stimuli (e.g. affiliative vs hostile facial expressions; other nonverbal motivational signals), we suggest that future research should address the following issues.
First, while nPower predicted increased activation on emotional-face/neutral-face contrasts in targeted motivational brain areas, particularly in response to anger faces, nPower effects were more clear-cut and extensive for anterior insula and anterior caudate than for OFC and particularly for amygdala and accumbens, where we observed no activation differences. The latter three structures are known to be more responsive to new or unpredicted incentive cues and to decrease in their activation in response to repeated exposure to the same stimuli over time (e.g. Rolls et al., 1989; Schultz, 1998; Fischer et al., 2003). We would, therefore, expect to observe nPower-dependent activation of amygdala, accumbens and OFC with greater likelihood in studies using event-related fMRI designs, in which the occurrence of motivational incentive stimuli is less predictable than in our block design.
Second, the present research did not address the question what consequences the observed nPower-dependent activation changes in motivational brain areas had for participants’ thoughts, feelings and behavior. For instance, both activation of the anterior insula and arousal of nPower have been linked to subjective reports of emotional arousal (e.g. Critchley et al., 2004; Fodor et al., 2006), and nPower influences implicit learning, a process that depends on the striatum (cf. Schultheiss et al., 2005b; Seger, 2006). Thus, a fruitful line of future research may be to examine the role of the insula in nPower-dependent subjective arousal changes, to examine the role of the caudate in nPower-dependent implicit learning and, more generally, to explore the cognitive, affective and behavioral correlates of nPower-dependent activation in motivational brain areas.
CONCLUSIONS
In the present research, we explored for the first time the neural basis of an implicit motive using fMRI. Our findings partially supported LeDoux's (2002) hypothesis that implicit motives are mediated by a motivational circuit involving amygdala, striatum, and OFC, a circuit that drives autonomic and behavioral responses to positive and negative incentives. They thereby also corroborate McClelland et al. (1989) claim that implicit motives are rooted in brain structures that, from a phylogenetic perspective, have developed to allow sophisticated and adaptive forms of goal-directed behavior long before the advent of language and verbally mediated forms of behavior. Moreover, the frequently observed independence between implicit motives on the one hand and the motivational needs that individuals explicitly ascribe to themselves on the other may represent a particularly fruitful area for further research on the brain correlates of motivation in humans (e.g. Spangler, 1992; Schultheiss and Brunstein, 2001; Pang and Schultheiss, 2005). We think it would be informative to examine more closely under which conditions, and in which individuals, brain structures mediating explicit goals and brain structures mediating implicit motives show reciprocal activation (indicative of motivational antagonism) or coactivation (indicative of motivational synergy). This type of research would not only help to further validate the idea that human behavior is influenced by two independent motivational systems, one verbal and one nonverbal but it could also contribute a well-developed conceptual perspective to the study of brain correlates of human motivation.
FUNDING
National Science Foundation (BCS-0444301); Office of the Vice President of Research at the University of Michigan.
Acknowledgments
We thank Scott Liening and Alexstine Davis for their help in scheduling and testing participants and Alexi Wisher for coding the PSEs. Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set.
Footnotes
Motive-level analyses indicated that high-power individuals, relative to low-power individuals, showed relatively greater activation in response to emotional faces, and relatively less activation in response to neutral faces, than to gray-square control stimuli (i.e. an emotional face > gray square > neutral face ranking of activation effects). Because of this, stronger effects usually emerged for emotional face minus neutral face contrasts, whereas emotional face minus gray-square contrasts yielded few significant activation differences.
References
- Balleine BW, Dickinson A. The effect of lesions of the insular cortex on instrumental conditioning: evidence for a role in incentive memory. Journal of Neuroscience. 2000;20(23):8954–64. doi: 10.1523/JNEUROSCI.20-23-08954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews: Neuroscience. 2002;3(7):563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in Neuroscience. 2003;26(9):507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Camras LA, Meng Z, Ujiie T, et al. Observing emotion in infants: facial expression, body behavior, and rater judgments of responses to an expectancy-violating event. Emotion. 2002;2(2):179–93. doi: 10.1037/1528-3542.2.2.179. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Conway M, Di Fazio R, Mayman S. Judging others’ emotions as a function of the others' status. Social Psychology Quarterly. 1999;62:291–305. [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews: Neuroscience. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Human Brain Mapping. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error. Emotion, Reason, and the Human Brain. London: Papermac; 1994. [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA. Motivation-dependent responses in the human caudate nucleus. Cerebral Cortex. 2004;14(9):1022–30. doi: 10.1093/cercor/bhh062. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Research Bulletin. 2003;59(5):387–92. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Fodor EM, Wick DP, Hartsen KM. The power motive and affective response to assertiveness. Journal of Research in Personality. 2006;40:598–610. [Google Scholar]
- Knutson B. Facial expressions of emotion influence interpersonal trait inferences. Journal of Nonverbal Behavior. 1996;20:165–82. [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews: Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The Emotional Brain. New York: Simon & Schuster; 1996. [Google Scholar]
- LeDoux JE. The Synaptic Self. New York: Viking; 2002. [Google Scholar]
- Lieberman MD, Jarcho JM, Satpute AB. Evidence-based and intuition-based self-knowledge: an FMRI study. Journal of Personality and Social Psychology. 2004;87(4):421–35. doi: 10.1037/0022-3514.87.4.421. [DOI] [PubMed] [Google Scholar]
- Mason A, Blankenship V. Power and affiliation motivation, stress, and abuse in intimate relationships. Journal of Personality and Social Psychology. 1987;52:203–10. doi: 10.1037//0022-3514.52.1.203. [DOI] [PubMed] [Google Scholar]
- McClelland DC. Power: The Inner Experience. New York: Irvington Publishers; 1975. [Google Scholar]
- McClelland DC. Motivational factors in health and disease. American Psychologist. 1989;44:675–83. doi: 10.1037//0003-066x.44.4.675. [DOI] [PubMed] [Google Scholar]
- McClelland DC, Boyatzis RE. Leadership motive pattern and long-term success in management. Journal of Applied Psychology. 1982;67:737–43. [Google Scholar]
- McClelland DC, Koestner R, Weinberger J. How do self-attributed and implicit motives differ? Psychological Review. 1989;96:690–702. [Google Scholar]
- McClelland DC, Steele RS. Motivational Workshops. New York: General Learning Press; 1972. [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective & Behavioral Neuroscience. 2003;3(3):207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Pang JS, Schultheiss OC. Assessing implicit motives in U.S. college students: effects of picture type and position, gender and ethnicity, and cross-cultural comparisons. Journal of Personality Assessment. 2005;85(3):280–94. doi: 10.1207/s15327752jpa8503_04. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The Brain and Emotion. Oxford: Oxford University Press; 1999. [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10(3):284–94. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. European Journal of Neuroscience. 1989;1(1):53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC. Implicit motives. In: John OP, Robins RW, Pervin LA, editors. Handbook of Personality: Theory and Research. 3rd edn. New York: Guilford; (in press) [Google Scholar]
- Schultheiss OC, Brunstein JC. Assessing implicit motives with a research version of the TAT: picture profiles, gender differences, and relations to other personality measures. Journal of Personality Assessment. 2001;77(1):71–86. doi: 10.1207/S15327752JPA7701_05. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Brunstein JC. Inhibited power motivation and persuasive communication: a lens model analysis. Journal of Personality. 2002;70:553–82. doi: 10.1111/1467-6494.05014. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Dargel A, Rohde W. Implicit motives and sexual motivation and behavior. Journal of Research in Personality. 2003;37:224–30. [Google Scholar]
- Schultheiss OC, Hale JA. Implicit motives modulate attentional orienting to perceived facial expressions of emotion. Motivation and Emotion. 2007;31(1):13–24. [Google Scholar]
- Schultheiss OC, Pang JS. Measuring implicit motives. In: Robins RW, Fraley RC, Krueger R, editors. Handbook of Research Methods in Personality Psychology. New York: Guilford; 2007. pp. 322–44. [Google Scholar]
- Schultheiss OC, Pang JS, Torges CM, Wirth MM, Treynor W. Perceived facial expressions of emotion as motivational incentives: evidence from a differential implicit learning paradigm. Emotion. 2005a;5(1):41–54. doi: 10.1037/1528-3542.5.1.41. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Rohde W. Implicit power motivation predicts men's testosterone changes and implicit learning in a contest situation. Hormones and Behavior. 2002;41:195–202. doi: 10.1006/hbeh.2001.1745. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Wirth MM, Torges CM, Pang JS, Villacorta MA, Welsh KM. Effects of implicit power motivation on men's and women's implicit learning and testosterone changes after social victory or defeat. Journal of Personality and Social Psychology. 2005b;88(1):174–88. doi: 10.1037/0022-3514.88.1.174. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10(3):272–84. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Seger CA. The basal ganglia in human learning. Neuroscientist. 2006;12(4):285–90. doi: 10.1177/1073858405285632. [DOI] [PubMed] [Google Scholar]
- Spangler WD. Validity of questionnaire and TAT measures of need for achievement: two meta-analyses. Psychological Bulletin. 1992;112:140–54. [Google Scholar]
- Stanton SJ, Schultheiss OC. Basal and dynamic relationships between implicit power motivation and estradiol in women. Hormones and Behavior. 2007;52(5):571–80. doi: 10.1016/j.yhbeh.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Wirth MM, Schultheiss OC. CA: Palm Springs; 2006. Effects of Perceivers’ Implicit Power Motivation on Attentional Orienting to Pavlovian-conditioned Cues of Anger and Joy. Paper presented at the Society for Personality and Social Psychology. [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56(12):921–30. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18(3):650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tiedens LZ. Anger and advancement versus sadness and subjugation: the effect of negative emotion expressions on social status conferral. Journal of Personality and Social Psychology. 2001;80:86–94. [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, Nelson CA. Paper presented at the Cognitive Neuroscience Society Annual Meeting. San Francisco: 2002. Categorization of Facial Expressions in Children and Adults: Establishing a Larger Stimulus Set. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Veroff J. Assertive motivations: achievement versus power. In: Stewart AJ, editor. Motivation and Society. San Francisco, CA: Jossey-Bass; 1982. pp. 99–132. [Google Scholar]
- Veroff J, Veroff JB. Reconsideration of a measure of power motivation. Psychological Bulletin. 1972;78:279–91. [Google Scholar]
- Winter DG. The Power Motive. New York: Free Press; 1973. [Google Scholar]
- Winter DG. Measuring personality at a distance: development of an integrated system for scoring motives in running text. In: Ozer DJ, Healy JM, Stewart AJ, editors. Perspectives in Personality. Vol. 3. London: Jessica Kingsley; 1991. pp. 59–89. [Google Scholar]
- Winter DG. 4th edn. Ann Arbor: Department of Psychology, University of Michigan; 1994. Manual for Scoring Motive Imagery in Running Text. [Google Scholar]
- Winter DG, Stewart AJ. Power motive reliability as a function of retest instructions. Journal of Consulting and Clinical Psychology. 1977;45:436–40. [Google Scholar]
- Wirth MM, Welsh KM, Schultheiss OC. Salivary cortisol changes in humans after winning or losing a dominance contest depend on implicit power motivation. Hormones and Behavior. 2006;49:346–52. doi: 10.1016/j.yhbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22(1):139–52. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Zurbriggen EL. Social motives and cognitive power-sex associations: predictors of aggressive sexual behavior. Journal of Personality and Social Psychology. 2000;78:559–81. doi: 10.1037//0022-3514.78.3.559. [DOI] [PubMed] [Google Scholar]