Abstract
A facet of emotional resilience critical for adapting to adversity is flexible use of emotional resources. We hypothesized that in threatening situations, this emotional flexibility enables resilient people to use emotional resources during appropriately emotional events, and conserve emotional resources during innocuous events. We tested this hypothesis using functional magnetic resonance imaging in a repeated recovery from threat task with low- and high-trait resilient individuals (LowR and HighR, respectively, as measured by ER89). In an event-related design, 13 HighR and 13 LowR participants viewed ‘threat’ cues, which signaled either an aversive or neutral picture with equal probabilities, or ‘nonthreat’ cues, which signaled a neutral picture. Results show that when under threat, LowR individuals exhibited prolonged activation in the anterior insula to both the aversive and neutral pictures, whereas HighR individuals exhibited insula activation only to the aversive pictures. These data provide neural evidence that in threatening situations, resilient people flexibly and appropriately adjust the level of emotional resources needed to meet the demands of the situation.
Keywords: resilience, anticipation, recovery, emotion regulation, neuroimaging, threat
Resilience is the ability to cope effectively and adapt in the face of loss, hardship or adversity (Block and Kremen, 1996). Resilient people endure less protracted grief symptoms after a loved one dies (Bonanno et al., 2002), report fewer depressive symptoms in response to a national crisis (Fredrickson et al., 2003) and experience less mental distress after combat (Florian et al., 1995). One psychological mechanism hypothesized to underlie resilient people's ability to adapt successfully to ever-changing environments is emotional flexibility: the flexible use of emotional resources needed to precisely match the demands of the situation (Block and Block, 1980; Block and Kremen, 1996; Bonanno et al., 2004; Charney, 2004). For example, when faced with the prospect of having to give a public speech, both high- and low-resilient participants exhibited similar cardiovascular reactivity. However, when the threat of giving the speech was later removed, high-resilient participants’ cardiovascular reactivity recovered more quickly (Tugade and Fredrickson, 2004). Resilient participant's emotional flexibility is reflected by their use of physiological resources when needed (when facing the threat of giving a speech), and conservation of resources when no longer needed (the threat of the speech was over). A recent study confirmed that this emotional flexibility can indeed be a strategic process. Participants who were better able to both strategically enhance and suppress their emotions to match the demands of the situation experienced less distress in the aftermath of the 11 September terrorist attacks (Bonanno et al., 2004).
The benefit of emotional flexibility is especially apparent in unpredictable situations, such as those in which anticipated negative events are sometimes realized and other times not. In these repeated threat situations, the emotional flexibility hypothesis suggests that resilient people should exhibit appropriate emotional and physiological responses when the negative events occur, and appropriate nonemotional responses when the negative events do not occur (Waugh et al., 2008). For example, in a ‘repeated recovery from threat’ paradigm, participants viewed cues that signaled either a neutral picture (‘no-threat’) or signaled the equal probability of viewing an aversive or neutral picture (‘threat’). High-resilient participants exhibited more complete self-reported affective recovery when anticipated negative events did not occur, but equivalent affective responses (relative to low-resilient participants) when the negative events did occur (Waugh et al., 2008).
In the current functional magnetic resonance imaging (fMRI) study, we adopted the above ‘repeated recovery from threat paradigm’ to examine the neural correlates of the proposed emotional flexibility characteristic of trait resilience. Trait resilience was measured with the same ego-resilience questionnaire (ER89; Block and Kremen, 1996) used in the above studies (Tugade and Fredrickson, 2004; Waugh et al., 2008), designed to tap emotional flexibility. For example, the ER89 includes items such as ‘I enjoy dealing with new and unusual situations’, and ‘I quickly get over and recover from being startled’. The ER89 is also a well-validated measure of ecological resilience—those participants who scored high on this measure before the terrorist attacks on 9/11 exhibited fewer depressive symptoms post-9/11 (Fredrickson et al., 2003). Informed by our behavioral results in previous studies, we hypothesized that brain activation differences between high- and low–trait-resilient participants would be most apparent in response to neutral stimuli that could have been aversive. It is on these trials that resilient participants’ emotional flexibility would be most beneficial, leading to decreased activation in affective regions in those moments when there is no longer the threat of a negative event.
The affective regions we specifically targeted for investigation included the insula, amygdala and orbitofrontal cortex (OFC). Prior work, including several meta-analyses, have ascribed prominent roles to these structures in the generation and regulation of emotion (Phan et al., 2002; Wager et al., in press). The insula, particularly its anterior portion, has been associated with anticipatory anxiety (Ploghaus et al., 2003; Carlsson et al., 2006; Nitschke et al., 2006b), and activity in the insula is augmented in people high in anxiety (Chua et al., 1999; Simmons et al., 2006), a trait associated with low resilience (Fredrickson et al., 2003). The insula's functions include both representing visceral states (particularly the ventral anterior and posterior portions) and conscious feeling states related to interoceptive processes (in the dorsal anterior region; Craig, 2003; Critchley et al., 2004).
Activation of the amygdala is similarly associated with emotionally salient stimuli. The amygdala is responsive to potentially threatening context cues (Herry et al., 2007) and stimuli (Whalen et al., 1998), and serves as a key component in the physiological response to threat (Phelps and LeDoux, 2005). In addition, the amygdala has been shown in depressed people to exhibit sustained activity to emotional events (Siegle et al., 2002). Portions of the amygdala have also been associated with relief from threat (Seymour et al., 2005), the interaction between positive affectivity and positive stimuli (Canli et al., 2002) and both positive and negative expectancies (Paton et al., 2006). Given its robust association with emotion, it is clear that the amygdala is a key region in which to examine our hypothesis that resilient people are characterized by a flexible use of emotional resources. However, we have no explicit hypotheses regarding the direction of activation given the amygdala's association with both positive and negative emotional stimuli.
The OFC is a region implicated broadly in the integration of emotion and cognition (Rolls, 1999), and more specifically in the expectation of negative outcomes (O’Doherty et al., 2001), especially when that outcome is relatively more negative than an alternative (Ursu and Carter, 2005). The lateral OFC tracks negative prediction error, exhibiting increased activation with expectation of an aversive stimulus, and sharp drops in activation when the expected aversive stimulus does not occur (Seymour et al., 2005). High- and low-resilient people have been shown to differ in their appraisals of impending stressful events (Tugade and Fredrickson, 2004), therefore the OFC may also be a key region to track these differences between high- and low-resilient participants in expectations of and recovery from threat.
The critical period for testing the emotional flexibility hypothesis is the response to neutral stimuli that could have been aversive, and we have hypothesized that differential activity between high- and low-resilient participants to these neutral stimuli may speak to the neural underpinnings of trait resilience. Past research has found that high- and low-trait resilient people differ in the duration but not magnitude of their cardiovascular responses after threat (Tugade and Fredrickson, 2004). It becomes important then to distinguish response generation from response duration, and we do so in the current study by separately estimating the height and width of the blood oxygenation level (BOLD) response (Bellgowan et al., 2003; Lindquist and Wager, 2007). Although, these separate parameters are not fully able to differentiate the underlying neural and/or psychological processes hypothesized to be associated with them (Lindquist and Wager, 2007), there is evidence that the different components of psychological processes can be inferred from the different parameters of the BOLD response (Bellgowan et al., 2003).
METHODS AND MATERIALS
Participants
Healthy participants, without active medical or psychiatric illness, were recruited through community advertisements to complete a web-based screening. After answering questions about general eligibility to participate in an fMRI study (e.g. no metals inside body, no history of neurological disorders), 242 people completed the emotional resilience scale (ER89; Block and Kremen, 1996). Participants were contacted if they exhibited a score in the upper (raw score >50) or lower (raw score <42) quartiles of the sample.1 Potential participants were excluded for signs of current depression (scored > 25 on Center for Epidemiologic Studies Depression Scale; Radloff, 1977).2 Of the 100 (45 high resilient, 55 low resilient) eligible subjects, 30 participated in the experiment (15 high resilient, 15 low resilient), matched on age, gender, ethnicity, education and socioeconomic status (Table 1).3 This study was approved by the University of Michigan Institutional Review Board.
Table 1.
Demographics and screening data for participants
| High resilient (n = 15) | Low resilient (n = 15) | |
|---|---|---|
| ER89 (s.d.) | 50.4 (1.4) | 37.8 (2.3) |
| Age (s.d.) | 20.2 (1.37) | 20.5 (2.0) |
| Gender | 5 M, 10 F | 5 M, 10 F |
| Education | 15 College | 14 College, 1 Graduate Student |
| Depression (CESD) | 14.8 ± 2.6 | 16.4 ± 3.8 |
| Family income | 6.64 (∼65 000) | 5.96 (∼60 000) |
ER89 is the only index above on which groups differed significantly, t(28) = 17.8, P < 0.001.
Written measures
Prior to entering the MRI scanner, participants completed several personality scales shown to correlate with the trait resilience measure (ER89) in previous studies, and/or shown in previous studies to be associated with affect regulation (Taylor et al., 2008). These scales included optimism (Scheier et al., 1994); neuroticism, extraversion and openness (Costa and McCrae, 1992); behavioral activation sensitivity (BAS) and behavioral inhibition sensitivity (BIS; Carver and White, 1994); satisfaction with life (Diener et al., 1985); and an emotions questionnaire (Differential Emotions Scale; Izard, 1977; Modified DES; Fredrickson et al., 2003), which asked participants to rate 20 different emotions terms to reflect how they have felt during the last two weeks. As has been found in previous studies, high trait resilience was associated with higher optimism, openness to experience, BAS and positive feelings in the last two weeks, and lower neuroticism, behavioral inhibition and negative feelings (see Table 2 for means and correlation coefficients of these personality scales for participants included in study).
Table 2.
Means of personality variables for high- and low-resilient participants and correlation between personality variables and resilience scale (ER89)
| ER89 (n = 26) | High resilient (n = 13) |
Low resilient (n = 13) |
|||
|---|---|---|---|---|---|
| Personality variable | r | M | s.d. | M | s.d. |
| Optimism | 0.64** | 3.2a | 0.51 | 2.2b | 0.62 |
| Extraversion | 0.37 | 3.8a | 0.62 | 3.4a | 0.43 |
| Neuroticism | −0.53** | 2.2a | 0.57 | 2.8b | 0.50 |
| Openness | 0.38 | 3.8a | 0.87 | 3.4a | 0.48 |
| BIS | −0.09 | 2.8a | 0.50 | 2.9a | 0.49 |
| BAS—reward responsiveness | 0.53** | 3.7a | 0.34 | 3.4b | 0.31 |
| BAS—drive | 0.44* | 3.3a | 0.38 | 2.8b | 0.42 |
| BAS—fun-seeking | 0.43* | 3.4a | 0.46 | 2.9b | 0.43 |
| Positive feelings (last 2 weeks) | 0.50** | 3.1a | 0.55 | 2.4b | 0.53 |
| Negative feelings (last 2 weeks) | −0.53** | 0.5a | 0.28 | 1.0b | 0.49 |
| Satisfaction with life | 0.24 | 4.6a | 0.95 | 4.0a | 0.92 |
For correlation coefficients, **P < 0.01, *P < 0.05. For high- and low- resilient (high and low ER89, respectively) groups, means in the same row that have different subscripts are significantly different from each other at P < 0.05.
Task design
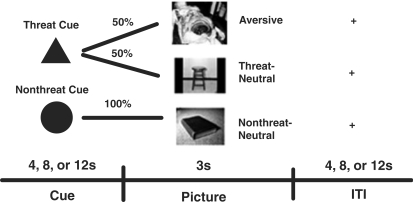
The task consisted of passively viewing images from the International Affective Picture System (IAPS; Lang et al., 1997), preceded by a cue (Figure 1). The ‘threat’ cue (two-third of trials) could be followed by an aversive or neutral picture [50/50 frequency (participants were not informed of this ratio)]; the ‘no-threat’ cue (one-third of trials) was always followed by a neutral picture. We chose aversive pictures normatively rated as highly negative (M = 2.01, s.d. = 0.65) and arousing (M = 6.42, s.d. = 0.57), and relatively specific to disgust (Lang et al., 1997; Mikels et al., 2005). The neutral pictures were selected as being normatively rated as neutral and matched to the aversive pictures on brightness (Mikels et al., 2005). The neutral pictures were randomly divided between the trials in which they were preceded by the threat cue (‘threat-neutral’) or no-threat cue (‘nonthreat-neutral’), resulting in each condition having equivalent valence (M = 4.99 and 5.36, respectively), and arousal (M = 2.85 and 3.07, respectively) ratings.
Fig. 1.
Participants received one of two cues; a ‘threat’ cue indicated that an aversive picture might appear (P = 0.5, unknown to participants), or a neutral (threat-neutral) picture might appear instead. The ‘nonthreat’ cue signified that the participants would see a neutral (nonthreat-neutral) picture (P = 1.0).
After acquiring the structural MRI images, participants were provided with verbal descriptions of the cue-outcome associations and given several trials to train on these associations so that they had thoroughly learned the associations before acquiring the functional images. We counterbalanced between participants whether the triangle or circle represented the threat and nonthreat cues. The cue appeared for 4, 8 or 12 s (equal frequencies, pseudorandomized to optimize design power), followed by picture presentation (3 s), and an intertrial interval, also 4, 8 or 12 s in length (pseudorandomized). The length of the cue and intertrial periods were jittered to enable deconvolution of the hemodynamic response for each epoch. Each run contained 18 trials, and there were five total runs for each participant's session for a total of 90 trials. The participants were reminded of the cue-outcome associations before each run. After participants completed the task in the scanner, they were debriefed on the purpose of the study, paid according to the amount of time the experiment had taken and thanked.
fMRI technical specifications
We measured BOLD in a 3T GE scanner. For the functional images, we collected 40 oblique-axial slices (FOV = 20, slice thk/sp = 3/0), prescribed to be approximately parallel to the AC-PC line (same locations as structural scans), using a T2*-weighted, single-shot, reverse spiral acquisition (Noll et al., 1995; gradient-recalled echo, TR = 2000, TE = 30, flip angle = 90°, 64 × 64), a sequence designed to enable good signal recovery in areas of high susceptibility artifact (e.g. OFC; Yang et al., 2002). The slices were acquired contiguously to optimize the effectiveness of the movement post-processing algorithms. Offline image reconstruction included processing steps to remove distortions caused by magnetic field inhomogeneity and other sources of misalignment to the structural data. Prior to acquisition of functional data, high resolution T1 SPGR images were acquired to enable anatomic normalization.
Image analysis
Images were first realigned and corrected for movement. Next, the realigned images were normalized to a standard single subject T1 template and then smoothed with a [8 8 8] mm kernel. Four subjects were excluded from the data analysis because of excessive movement, or the inability to warp their anatomic images to stereotactic space (two high resilient and two low resilient; leaving nine females in both groups). SPM2 (Wellcome Department of Cognitive Neurology) was used, along with custom routines, to estimate the hemodynamic response function (HRF) to each event in each voxel. We used a finite impulse response (FIR) model of the HRFs to estimate responses without assuming a predefined hemodynamic response shape (Glover, 1999). Using the FIR estimated HRFs in each voxel, we estimated the height (H), width (W) and time-to-peak (T) of the fitted HRF for the cues and pictures separately (Bellgowan et al., 2003; Lindquist and Wager, 2007).
To estimate H, T and W, HRFs for each condition were deconvolved from the BOLD time series using a (FIR) model with a 32 s kernel and 2 s bins. FIR estimates within the first 12 s of the response were smoothed with a 6 s exponential kernel from which H, T, W were estimated from the fitted responses using a simple peak-finding algorithm (Lindquist and Wager, 2007). Next, we calculated contrasts in H and W for four contrasts: [‘threat’—‘nonthreat’ cue], [‘aversive’—‘nonthreat-neutral’ picture], [‘aversive’—‘threat-neutral’ picture], [‘threat-neutral’—‘nonthreat-neutral’ picture].
For the group analysis, we used robust regression at the second level (Wager et al., 2005) to perform random effects analyses on response parameter contrasts (e.g. [‘threat height’—‘nonthreat height’ cue]) and contrast magnitude × resilience interactions. Robust regression minimizes the influence of outliers, at a small cost in power relative to ordinary least squares when statistical assumptions are met. Resilience between observers was contrast coded ([1 −1]) so that the intercept of the second level model tested the response parameter in the group, and the resilience regressor tested the effect of resilience on the response parameters. To restrict our analyses to potential sites of interest and also minimize Type II errors, we used an ‘emotional brain’ mask derived from a meta-analysis of emotion and fMRI studies (Wager et al., in press), and separate regions of interest (ROIs) for the insula, amygdala and OFC, which were created from voxels that overlapped between the emotional brain mask and ROI masks from the AAL library (Tzourio-Mazoyer et al., 2002). Results were thresholded by using Monte Carlo simulations to establish the minimum cluster size of voxels (P < 0.005) for each ROI that exceeded a corrected P-value of 0.05 (Ward, 2000). This led to a minimum cluster size of 29 voxels for the ‘emotional brain’ mask and 11, 5, 13 voxels for the insula, amygdala and OFC ROIs, respectively.
RESULTS
Height and width differences to threat–neutral pictures
To test our hypothesis that emotional flexibility in high-resilient participants would lead to less brain activation in affective regions when recovering from threat, we compared high- and low-trait resilient participants’ response to the threat–neutral pictures, i.e. neutral pictures following a threat cue, in comparison to the nonthreat–neutral pictures. Across all participants, regions exhibiting a higher response peak to the threat–neutral pictures were the left lateral prefrontal cortex, and left middle temporal gyrus (Table 3).
Table 3.
Height and width activations for threat–neutral vs nonthreat–neutral pictures
| Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region of activation | Brodmann's area | x | y | z | Volume (mm3) | Z-score |
| Height | ||||||
| Threat-neutral > nonthreat-neutral | ||||||
| Inferior frontal gyrus | 9 | −44 | 12 | 24 | 967 | 4.36 |
| Middle temporal gyrus | 22 | −50 | −34 | 0 | 850 | 4.61 |
| High > low resilient—no clusters | ||||||
| Low > high resilient—no clusters | ||||||
| Nonthreat-neutral > threat-neutral | ||||||
| No clusters at threshold | ||||||
| High > low resilient—no clusters | ||||||
| Low > high resilient—no clusters | ||||||
| Width | ||||||
| Threat-neutral > nonthreat-neutral | ||||||
| No clusters at threshold | ||||||
| High > low resilient—no clusters | ||||||
| Low > high resilient | ||||||
| Anterior insula | 13 | 38 | 22 | 3 | 381 | 3.65 |
| Nonthreat-neutral > threat-neutral | ||||||
| No clusters at threshold | ||||||
| High > low resilient—no clusters | ||||||
| Low > high resilient—no clusters | ||||||
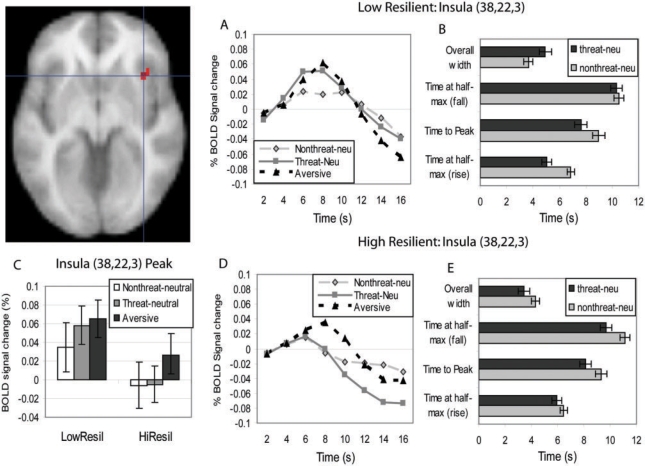
Trait resilience did not predict differences in response height, but it did predict differences in response width to the threat–neutral (vs nonthreat–neutral) pictures. Consistent with predictions, low-trait resilient participants exhibited greater insula response width in response to the threat–neutral (vs nonthreat–neutral) pictures (Figure 2). To further explore this resilience difference, we extracted the average response width in the insula cluster for each participant in each condition. Separate within-group t-tests suggest that this interaction can be explained by low-resilient participants exhibiting wider responses to the threat–neutral (vs nonthreat–neutral) pictures, t(12) = 4.24, P = 0.001, and to a lesser extent high-resilient participants exhibiting narrower responses to the threat–neutral (vs nonthreat–neutral) pictures, t(12) = 3.1, P < 0.01 (Figure 2). There were no differences in response height between the resilient groups at this threshold; however, it is still a possibility that subthreshold differences in response height could partially explain the difference in response width. To examine this possibility, we extracted the average height of the response in the insula cluster for each participant, and subjected these average responses to a 2 (Group: low resilient, high resilient) × 2 (Picture: nonthreat–neutral, threat–neutral) analysis of variance (ANOVA). At a lower threshold, there was a significant main effect of group, such that low-resilient participants (vs high-resilient participants) exhibited a significantly higher response peak across both pictures, F(1, 24) = 4.53, P < 0.05 (Figure 2C); however, there was no interaction between group and picture type. In addition, the relationship between trait resilience and width of insula response remained significant in an ANCOVA with insula response height as the covariate, F(1, 23) = 26.8, P < 0.0001, suggesting that the insula response width difference was not due to differences in response height.
Fig. 2.
Width of activation in the right anterior insula (38, 22, 3) differentiates high- and low-trait resilient participants. (A) Low-trait resilient participants show prolonged activation in the insula in response to the threat–neutral pictures (vs nonthreat–neutral pictures), whereas (D) high-trait resilient participants do not. (B and E) Temporal characteristics of the insula activation including width, time-to-half peak (rise), time-to-peak, and time-to-half peak (fall). The differential width effect in the insula [time at half-peak (fall–rise)] seems to be due to both (B) low-resilient participants exhibiting earlier times at half-peak (rise) and (E) high-resilient participants exhibiting earlier times at half-peak (fall). (C) There was only a main effect of resilience on insula activation height across all pictures. The HRF estimates are deconvolved, and this figure shows the estimates of the HRF response in % BOLD signal change. Bars are SEM.
To further characterize this response width difference in the insula, we extracted the cluster average in each participant for time-to-peak, time-to-half peak (rise) and time-to-half peak (fall; Figure 2B and E), and subjected each of these measures to a repeated-measures ANOVA with picture type(nonthreat–neutral, threat–neutral) as the within-subjects variable and trait resilience as the between-subjects variable. For time-to-peak, there was only a main effect of picture type, F(1, 24) = 11.89, P < 0.003: both low- and high-resilient participants exhibited earlier peaks to threat–neutral than nonthreat–neutral pictures, t = 2.92, 2.08, P 's = 0.01, 0.06, respectively. For time-to-half peak (rise) times, there was an interaction with trait resilience, F(1, 24) = 4.6, P < 0.05: low-resilient participants exhibited earlier time-to-half peak (rise) times to the threat–neutral vs nonthreat–neutral pictures, t(12) = 4.72, P < 0.001. For time-to-half peak (fall) times, there was a marginal interaction with resilience, F(1, 24) = 3.48, P = 0.074; characterized by high-resilient participants exhibiting earlier time-to-half peak (fall) times to the threat–neutral vs nonthreat–neutral pictures, t(12) = 2.62, P < 0.03. These results suggest that the insula response width difference may have been due to both an earlier response onset for low-resilient participants, and an earlier response offset for high-resilient participants.
Responses to anticipatory threat
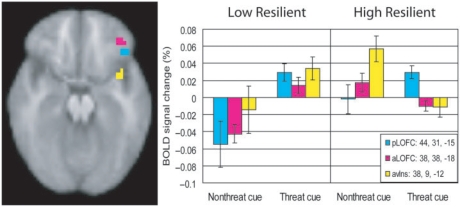
To test for possible trait resilience differences during anticipation, we examined height responses to the threat vs nonthreat cue.4 Across all participants, a region in the right posterior lateral OFC (OFC; 44, 31, −15) exhibited a higher response peak to the threat cue than to the nonthreat cue (Table 4; Figure 3). Two additional, nonoveralapping regions were moderated by trait resilience; an additional region in the lateral OFC (38, 38, −18), slightly anterior to the previously mentioned region and another region in the anteroventral insula (38, 9, −12). Specifically, low-trait resilient people showed a similar differentiation in response height (high during the threat cue, low during the nonthreat cue) in these additional regions of the right lateral OFC and anteroventral insula as they did in the posterior portion of the lateral OFC. In contrast, high-trait resilient participants showed a reverse pattern of activation (nonthreat cue >threat cue) in the anterior lateral OFC and anteroventral insula (Figure 3).
Table 4.
Activations for threat vs nonthreat cue
| Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region of activation | Brodmann's area | x | y | z | Volume (mm3) | Z-score |
| Threat > nonthreat | ||||||
| Posterior LOFC | 47 | 44 | 31 | −15 | 498 | 4.50 |
| High > low resilient | ||||||
| No clusters at threshold | ||||||
| Low > high resilient | ||||||
| Anterior LOFC | 11 | 38 | 38 | −18 | 381 | 4.13 |
| Nonthreat > threat | ||||||
| No clusters at threshold | ||||||
| High > low resilient | ||||||
| Anteroventral Insula | 13 | 38 | 9 | −12 | 439 | 5.83 |
| Low > high resilient | ||||||
| No clusters at threshold | ||||||
Fig. 3.
(A) Height of peak activation in two subregions of the lateral OFC (pLOFC; 44, 31, −15; aLOFC; 38, 38, −18—each image plane taken at the orthogonal coordinate) and anteroventral insula (38, 9, −12) during the threat cue (vs nonthreat cue). Low-resilient participants showed significantly higher peak activation to the threat cue vs nonthreat cue in all three subregions. High-resilient participants showed a similar threat vs nonthreat cue height activation difference in the pLOFC, but the reverse pattern (nonthreat vs threat cue) in the aLOFC and anteroventral insula. Bars are standard error of the mean.
Responses to aversive stimuli
Next, we assessed the regions that were responsive to the aversive stimuli to verify that our experiment elicited regions similar to previous experiments using these stimuli, and to test the second portion of the emotional flexibility hypothesis which predicts there to be little to no differential activation by trait resilience in response to the aversive stimuli. Consistent with previous findings, across all participants, there were several regions that exhibited higher response peak to the aversive pictures than to the nonthreat–neutral pictures, including amygdala, occipital cortex, bilateral insula, left inferior frontal gyrus (IFG), lateral OFC and dorsomedial prefrontal cortex (dMPFC; Table 5). The occipital cortex also exhibited wider responses to the aversive pictures. Supporting the emotional flexibility hypothesis, trait resilience did not predict differences in height or width of activity to the aversive stimuli.
Table 5.
Height and width activations in aversive vs nonthreat-neutral pictures
| Coordinates |
||||||
|---|---|---|---|---|---|---|
| Region of activation | Brodmann's area | x | y | z | Volume (mm3) | Z-score |
| Height | ||||||
| Aversive > nonthreat-neutral | ||||||
| DMPFC | 9 | 6 | 56 | 30 | 1143 | 6.08 |
| LOFC | 47 | 44 | 25 | −9 | 850 | 3.98 |
| OFC/Inferior insula | 47 | −41 | 19 | −12 | 3018 | 4.77 |
| Inferior frontal gyrus | 9 | −47 | 9 | 27 | 1084 | 4.21 |
| Anterior insula | 13 | 38 | 9 | −9 | 2871 | 4.66 |
| Middle insula | 13 | −38 | 0 | 3 | 850 | 4.30 |
| Amygdala | NA | 31 | −3 | −15 | 352 | 4.17 |
| Thalamus/Midbrain | NA | 6 | −19 | 0 | 7178 | 5.47 |
| Occipitotemporal cortex | 37 | 50 | −66 | −3 | 11719 | 6.31 |
| Occipitotemporal cortex | 37 | −47 | −69 | 3 | 12803 | 7.05 |
| Middle temporal gyrus | 39 | 53 | −69 | 12 | 469 | 4.89 |
| Occipital cortex | 17 | −6 | −97 | −9 | 2344 | 3.94 |
| High > low resilient—no clusters | ||||||
| Low > high resilient—no clusters | ||||||
| Nonthreat-neutral vs aversive | ||||||
| No Clusters at threshold | ||||||
| High > low resilient—no clusters | ||||||
| Low > high resilient—no clusters | ||||||
| Width | ||||||
| Aversive > nonthreat-neutral | ||||||
| Inferior occipital gyrus | 17 | −12 | −100 | 0 | 967 | 5.29 |
| High > low resilient—no clusters | ||||||
| Low > high resilient—no clusters | ||||||
| Nonthreat-neutral vs aversive | ||||||
| No clusters at threshold | ||||||
| High > low resilient—no clusters | ||||||
| Low > high resilient—no clusters | ||||||
Personality correlates of insula response width
Lastly, we tested whether the relationship between trait resilience and the insula response width to the threat–neutral vs nonthreat–neutral pictures could be explained by more basic personality traits. We found that when entered in regression equations separately, several personality traits did predict insula response width, including optimism, BAS—reward responsiveness, and BAS—fun-seeking (β's = −0.45, −0.40, −0.44, respectively; all P's < 0.05), as well as positive and negative feelings experienced in the last two weeks (β's = −0.41, 0.43, respectively). However, resilience remained a significant predictor of insula response width controlling for each of these trait or emotion indices, suggesting that this finding is explained better by the meta-construct resilience that incorporates portions of each of these more core traits, rather than by any of the traits alone.
DISCUSSION
This study provides neural evidence in support of the hypothesis that trait resilience may be characterized in part by emotional flexibility. After being threatened with the possibility of viewing an aversive picture, the emotional flexibility characteristic of high-trait resilient participants was reflected by appropriate responses in affective regions (insula, amygdala) to the aversive pictures and an appropriate lack of response in these regions to the neutral pictures (threat–neutral). On the other hand, the emotional inflexibility of low-trait resilient participants was reflected by responses in affective regions both to the aversive and neutral pictures. The main contrast of interest showed that low-trait resilient participants exhibited greater insula response width to the threat–neutral pictures than high-trait resilient participants. This differential width response seemed to be explained by two patterns: (i) low-resilient participants exhibited an earlier rise in activation suggesting that their response to these threat–neutral pictures was characterized by an earlier spike in insula activity; and (ii) high-resilient participants exhibited an earlier fall of their activation suggesting that their response to these threat–neutral pictures was characterized by insula activity more quickly returning to baseline. Although, response time-to-onset can be an unreliable parameter to estimate (Miezen et al., 2000), this pattern of neural data is consistent with psychophysiological and behavioral findings showing that after being threatened with a possible negative experience, high-resilient people recover quicker (Tugade and Fredrickson, 2004) and more completely (Waugh et al., 2008) than low-trait resilient people.
The current insula finding is striking given that the differential insula responses were to neutral pictures. The insula has been associated with processing affective stimuli including pain (Chua et al., 1999; Ploghaus et al., 1999), disgust faces (Phillips et al., 1997) and pictures (Wright et al., 2004), with the anterior insula being important for representing the conscious awareness of the visceral states associated with these emotions (Craig, 2002; Critchley et al., 2004; Singer et al., 2004; Critchley, 2005). However, in this study, the differential insula responses were to neutral stimuli that could have been aversive, raising the possibility that activation in the insula reflected integration of prior expectations with subsequent outcomes. Negative expectations may have produced affective responses in the insula to otherwise nonaffective stimuli—an interpretation consistent with data showing that affective responses in the insula can be influenced by prior expectations (Nitschke et al., 2006a; Sarinopoulos et al., 2006). An alternative, more speculative, explanation for the insula findings may be that when viewing a neutral picture that could have been aversive, low-resilient participants imagined or remembered an aversive scene that they might have seen. Support for this alternative explanation is provided by the fact that we used disgusting scenes as our aversive stimuli and the insula is strongly activated in response to disgust stimuli (Phillips et al., 1997; Wright et al., 2004). Although intriguing, we caution that both the ‘insula as integrating expectations’ and ‘insula as imagining alternatives’ explanations are post hoc and should be subject to further investigation.
During the anticipatory period, high- and low-trait resilient participants showed different patterns of activation in two regions of the lateral OFC and the anteroventral insula. Previous research implicates both the OFC and anterior insula in anticipating possible negative events (Porro et al., 2002; Carlsson et al., 2006; Herwig et al., 2007), with the OFC also being involved in calculating the probability of some event occurring (Knutson et al., 2005) and generating expectancies in the face of an uncertain outcome (Critchley et al., 2001). Besides generating expectancies, the lateral OFC has also been associated with emotion regulation (Lieberman et al., 2007; Phillips et al., 2008) via its connections with lateral prefrontal cortices and the amygdala (Stein et al., 2007). However, in our study, there was no amygdala activation during the anticipation period, suggesting that the OFC's role in generating expectancies may be a more parsimonious explanation for the current results. Low-trait resilient participants showed higher response peak in all three regions to the threat vs nonthreat cue, suggesting that they recruited these regions only during expectations of threat. Although, high-trait resilient participants showed similarly higher threat vs nonthreat cue response peak in the posterior OFC, they exhibited the opposite pattern (nonthreat vs threat) of activation in the anterior OFC and the anteroventral insula, suggesting that high-trait resilient participants recruited subregions of the lateral OFC to represent both expectations of threat and safety. This additional representation of safety for the high-resilient participants is consistent with findings showing that high-resilient people can coactivate positive and negative appraisals of a stressful situation (Folkman and Moskowitz, 2000; Fredrickson et al., 2003; Tugade and Fredrickson, 2004; Ong et al., 2006), leading to faster physiological recovery from stress (Tugade and Fredrickson, 2004), and better mental health outcomes during chronic stress (Folkman and Moskowitz, 2000).
The only significant activation in the amygdala was in response to aversive pictures. This finding is unsurprising given the amygdala's central role in affective processing (LeDoux, 1996), including determining salience (Fitzgerald et al., 2006), heightening vigilance to possible threats (Herry et al., 2007) and emotional memory (Dolcos et al., 2005). What is perhaps surprising is that resilience did not moderate amygdala activity given the finding that resilience moderated insula activity to the pictures that could have been aversive. Although, it is difficult to interpret null findings, one possible explanation is that if the amygdala is responsible for tracking the emotional salience of a stimulus or context, the difference between high- and low-resilient participants was not in the perceived emotional salience of the neutral pictures, but rather in their integration of negative expectations or imagining of negative alternatives—functions perhaps more associated with the insula.
It is also important to note that no regions exhibited differences in activation between high- and low-resilient participants in response to the aversive pictures. This finding further supports the emotional flexibility hypothesis by suggesting that the difference between high- and low-trait resilient people is not a blunted response to affective stimuli—a conclusion consistent with data showing that even when high-resilient individuals exhibit faster cardiovascular recovery from a threat, they do not differ from low-resilient individuals in the degree of experienced adversity while the threat is still present (Tugade and Fredrickson, 2004).
The current study presents a novel way of studying emotional responding in fMRI by examining the width of the hemodynamic response separate from the height of that response. Width of response in this study was measured by fitting a parameter to the HRF that represented the width (in TRs) of the HRF curve at half of the peak height. Although, there is slightly biased parameter estimation in the FIR model (Lindquist and Wager, 2007), separately estimating these parameters presents a unique way of examining the neural correlates of emotion that seems to be more consistent with how emotion theorists conceptualize emotions as having both intensity and duration (Frijda et al., 1992). In addition to providing greater theoretical specificity (Bellgowan et al., 2003), deconvolving the HRF also provides a more flexible model of the data, the accuracy of which is less influenced by task and brain region differences in the shape of the HRF (Lindquist and Wager, 2007). However, much is still unknown about the relationship between these different parameters and brain function. For example, it is not clear whether the time scale with which we measured width of the hemodynamic response in the current study (seconds) captures true behavioral/physiological differences between high- and low-trait resilient participants. This small difference in the duration of neural activity may be sufficient to influence thinking, feeling and downstream physiological responding. Our study was designed to introduce this possibility, not necessarily to answer the question. Future work is needed to extend the current results and more specifically examine the functional relationship between the height and duration of brain activation and emotional phenomena and traits.
The primary limitation of the current study was that we did not measure behavioral responses or subjective feeling states during the fMRI task. We elected to have participants view the stimuli passively in light of the previous evidence that responses in core emotional areas such as the amygdala and insula can be attenuated by attention-demanding tasks (Pessoa et al., 2005), and are thus strongest during passive viewing of emotional stimuli (Taylor et al., 2003). As a result, although our results were consistent with behavioral findings from different samples (Waugh et al., 2008), in the current sample we were unable to correlate behavioral data with brain activations. Also, the neutral and aversive stimuli were not matched for faces and figures, differences that may have confounded differences in BOLD signal. Yet, because our main contrast of interest was between two sets of neutral stimuli, our principal findings should not be affected. Finally, although our self-report measure of resilience did predict insula response width controlling for other more ‘core’ personality traits, such as optimism and BAS, this measure as well as our repeated recovery from threat paradigm examine only a limited slice of the meta-construct ‘resilience’. Future studies will need to examine possible implications of laboratory findings such as these for how-resilient people navigate both major and minor real-life stressors.
In summary, the present study adds neural data to the literature on the mechanisms underlying resilience. We investigated differences in brain activations between low- and high-trait resilient people when viewing innocuous events in the midst of threat. The overall pattern of data is consistent with previous studies and suggests that what differentiates high from low-trait resilient people in times of threat is their flexible use of emotional resources. This neural evidence provides valuable insight into the cognitive and emotional foundations of resilience under threat and lays the groundwork for future research on this consequential individual difference variable. This study also highlights the value of including measures of BOLD activation width in addition to the oft-studied activation magnitude when studying emotional phenomena and traits.
Acknowledgement
This work was made possible by a Pilot Grant from the UM fMRI Lab to S.F.T./C.E.W., National Institutes of Health (R01-MH64148 to S.F.T., R01-MH59615 to B.L.F., R01-DA 15410 to D.C.N.), Positive Psychology Microgrant to C.E.W. and Dissertation Thesis Grant to C.E.W. Portions of this research were submitted as a dissertation by C.E.W. at the University of Michigan. The authors thank Elizabeth Anderson for her help in running the participants.
Footnotes
Although, a shortcoming of this approach is that it does not provide information on the center of the distribution (Preacher et al., 2005), we chose to limit the sample to extreme groups to maximize power given the small sample sizes available in neuroimaging studies (Kagan et al., 1998). Subsequent studies with larger sample sizes are needed to investigate whether effects generalize to participants who scored in the middle portion of the scale.
We did not assess lifetime psychiatric disorders.
Adding participant's gender as a covariate did not influence the pattern of any of the reported results, so is not reported any further.
We do not report response width to anticipation, because unlike pictures which were presented for 3 s, the anticipatory period consisted of the entire period from cue to picture presentation, and the duration of this period varied randomly throughout the experiment making the width of the response relatively uninterpretable.
References
- Bellgowan P.SF, Saad ZS, Bandettini PA. Understanding neural system dynamics through task modulation and measurement of functional MRI amplitude, latency, and width. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1415–9. doi: 10.1073/pnas.0337747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J, Block JH. The role of ego-control and ego-resiliency in the origination of behavior. In: Collings WA, editor. The Minnesota Symposia on Child Psychology. Vol. 13. Hillsdale, NJ: Erlbaum; 1980. pp. 39–101. [Google Scholar]
- Block J, Kremen AM. IQ and ego-resiliency: conceptual and empirical connections and separateness. Journal of Personality and Social Psychology. 1996;70(2):349–61. doi: 10.1037//0022-3514.70.2.349. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Papa A, Lalande K, Westphal M, Coifman K. The importance of being flexible: the ability to both enhance and suppress emotional expression predicts long-term adjustment. Psychological Science. 2004;15(7):482–7. doi: 10.1111/j.0956-7976.2004.00705.x. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Wortman CB, Lehman DR, et al. Resilience to loss and chronic grief: a prospective study from preloss to 18-months postloss. Journal of Personality and Social Psychology. 2002;83(5):1150–64. doi: 10.1037//0022-3514.83.5.1150. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitefield SL, Gotlib IH, Gabrieli J.DE. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. NeuroImage. 2006;32(4):1804–14. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319–33. [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. American Journal of Psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9(6 Pt 1):563–71. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Normal personality assessment in clinical practice: the NEO personality inventory. Psychological Assessment. 1992;4(1):5–13. [Google Scholar]
- Craig AD. Opinion: how do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology. 2005;493(1):154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29(2):537–45. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. Journal of Personality Assessment. 1985;49:71–5. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(7):2626–31. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30(4):1441–8. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Florian V, Mikulincer M, Taubman O. Does hardiness contribute to mental health during a stressful real-life situation? The roles of appraisal and coping. Journal of Personality and Social Psychology. 1995;68(4):687–95. doi: 10.1037//0022-3514.68.4.687. [DOI] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist. 2000;55(6):647–54. doi: 10.1037//0003-066x.55.6.647. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crisis? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology. 2003;84(2):365–76. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijda NH, Ortony A, Sonnemans J, Clore GL. The complexity of intensity: issues concerning the structure of emotion intensity. Review of Personality and Social Psychology. 1992;13:60–89. [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9(4):416–29. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, et al. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27(22):5958–66. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Kaffenberger T, Baumgartner T, Jancke L. Neural correlates of a ‘pessimistic’ attitude when anticipating events of unknown emotional valence. NeuroImage. 2007;34(2):848–58. doi: 10.1016/j.neuroimage.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Izard CE. Human Emotions. New York: Plenum Press; 1977. [Google Scholar]
- Kagan JJ, Snidman N, Arcus D. The value of extreme groups. In: Cairns RB, Bergman LR, Kagan JJ, editors. Methods and Models for Studying the Individual. Thousand Oaks, CA, US: Sage Publications Inc.; 1998. pp. 65–82. [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. Journal of Neuroscience. 2005;25(19):4806–12. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention: University of Florida; 1997. [Google Scholar]
- LeDoux JE. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York, NY, US: Simon & Schuster; 1996. [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lindquist MA, Wager TD. Validity and power in hemodynamic response modeling: a comparison study and a new approach. Human Brain Mapping. 2007;28(8):764–84. doi: 10.1002/hbm.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezen FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–59. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Mikels JA, Fredrickson BL, Larkin GR, Lindberg CM, Maglio SJ, Reuter-Lorenz PA. Emotional category data on images from the international affective picture system. Behavior Research Methods, Instruments, and Computers. 2005;37:626–30. doi: 10.3758/bf03192732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Dixon GE, Sarinopoulos I, et al. Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nature Neuroscience. 2006a;9(3):435–42. doi: 10.1038/nn1645. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. NeuroImage. 2006b;29(1):106–16. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Noll DC, Cohen JD, Meyer CH, Schneider W. Spiral K-space MR imaging of cortical activation. Journal of Magnetic Resonance Imaging. 1995;5(1):49–56. doi: 10.1002/jmri.1880050112. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Ong AD, Bergeman CS, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. Journal of Personality and Social Psychology. 2006;91(4):730–49. doi: 10.1037/0022-3514.91.4.730. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–70. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–55. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008 doi: 10.1038/mp.2008.65. [Epub-ahead of print; 24 June 2008]; doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Becerra L, Borras C, Borsook D. Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends in Cognitive Sciences. 2003;7(5):197–200. doi: 10.1016/s1364-6613(03)00061-5. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;284(5422):1979–81. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, et al. Does anticipation of pain affect cortical nociceptive systems? Journal of Neuroscience. 2002;22(8):3206–14. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K.JK, Rucker DD, MacCallum RC, Nicewander WA. Use of the extreme groups approach: a critical reexamination and new recommendations. Psychological Methods. 2005;10(2):178–92. doi: 10.1037/1082-989X.10.2.178. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rolls ET. The Brain and Emotion. Oxford: Oxford University Press; 1999. [Google Scholar]
- Sarinopoulos I, Dixon GE, Short SJ, Davidson RJ, Nitschke JB. Brain mechanisms of expectation associated with insula and amygdala response to aversive taste: implications for placebo. Brain, Behavior, and Immunity. 2006;20(2):120–32. doi: 10.1016/j.bbi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67(6):1063–78. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Koltzenburg M, et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nature Neuroscience. 2005;8(9):1234–40. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry. 2006;60(4):402–9. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan R, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–61. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychosocial resources. Journal of Personality and Social Psychology. 2008;95(1):197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage. 2003;18(3):650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology. 2004;86(2):320–33. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ursu S, Carter CS. Outcome representations, counterfactual comparisons and the human orbitofrontal cortex: implications for neuroimaging studies of decision-making. Cognitive Brain Research. 2005;23(1):51–60. doi: 10.1016/j.cogbrainres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF, Bliss-Moreau E, et al. Lewis M, editor. The neuroimaging of emotion. In. Handbook of Emotion. in press. [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. NeuroImage. 2005;26(1):99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 Retrieved August, 2007, from http://afni.nimh.nih.gov/afni/docpdf/AlphaSim.pdf.
- Waugh CE, Fredrickson BL, Taylor SF. Adapting to life's slings and arrows: individual differences in resilience when recovering from an anticipated threat. Journal of Research in Personality. 2008;42:1031–46. doi: 10.1016/j.jrp.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, He G, Shapira NA, Goodman WK, Liu Y. Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport. 2004;15(15):2347–51. doi: 10.1097/00001756-200410250-00009. [DOI] [PubMed] [Google Scholar]
- Yang YH, Gu W, Zhan S, Xu D, Silbersweig DA, Stern E. Simultaneous perfusion and BOLD imaging using reverse spiral scanning at 3T: characterization of functional contrast and susceptibility artifacts. Magnetic Resonance in Medicine. 2002;48(2):278–89. doi: 10.1002/mrm.10196. [DOI] [PubMed] [Google Scholar]