Abstract
Researchers have proposed that females and males differ in the structure of their moral attitudes, such that females tend to adopt care-based moral evaluations and males tend to adopt justice-based moral evaluations. The existence of these gender differences remains a controversial issue, as behavioral studies have reported mixed findings. The current study investigated the neural correlates of moral sensitivity in females and males, to test the hypothesis that females would show increased activity in brain regions associated with care-based processing (posterior and anterior cingulate, anterior insula) relative to males when evaluating moral stimuli, and males would show increased activity in regions associated with justice-based processing (superior temporal sulcus) relative to females. Twenty-eight participants (14 females) were scanned using fMRI while viewing unpleasant pictures, half of which depicted moral violations, and rated each picture on the degree of moral violation that they judged to be present. As predicted, females showed a stronger modulatory relationship between posterior cingulate and insula activity during picture viewing and subsequent moral ratings relative to males. Males showed a stronger modulatory relationship between inferior parietal activity and moral ratings relative to females. These results are suggestive of gender differences in strategies utilized in moral appraisals.
Keywords: gender, moral sensitivity, posterior cingulate, anterior insula
The existence of gender differences in moral reasoning has been an issue of much controversy and debate. Gilligan (1977) claimed that men and women speak in a different ‘moral voice’. Specifically, women are believed to typically approach moral dilemmas with a care-based orientation that emphasizes maintenance of interpersonal relationships and is guided by social emotions including empathy and altruism (Robertson et al., 2007), whereas men typically approach moral dilemmas with a justice-based orientation that emphasizes maintenance of order and adherence to rules and obligations. However, despite numerous behavioral studies comparing moral decision-making in males and females, little evidence has been found to support Gilligan's arguments (Jaffee and Hyde, 2000; Hyde, 2005; but see Skoe et al., 2002).
Previous research investigating gender differences in moral reasoning has been limited primarily to behavioral techniques such as coding verbal responses to hypothetical moral dilemmas (Jafee and Hyde, 2000). Complementary techniques, such as neuroimaging, have the potential to yield new insight into gender differences in moral reasoning, since behavior can be considered the sum result of all neural activity (Canli and Amin, 2002). In other words, patterns of brain activity during moral processing might differ for males and females despite similar behavioral outcomes. Although researchers have begun investigating the neural basis of moral emotion and cognition (Greene et al., 2001, 2004; Moll et al., 2002a, 2002b; Heekeren et al., 2003, 2005; Harenski and Hamann, 2006; Schaich Borg et al., 2006), the neural basis of gender differences in moral processing has not been explored.
Functional neuroimaging studies of moral appraisal have identified a consistent set of brain regions that are involved in processing different types of moral stimuli including moral dilemmas, statements and pictures (Greene and Haidt, 2002; Moll et al., 2005; Raine and Yang, 2006). These stimuli typically describe or show examples of ‘moral violations’, such as one person intentionally causing harm to another. Tasks that involve processing these types of stimuli reliably activate regions of inferior parietal cortex, including the posterior superior temporal sulcus and temporo-parietal junction, which may represent the contribution of theory of mind processes to moral decision-making, and the medial prefrontal cortex, which may represent the integration of emotional responses into moral decision-making (Greene and Haidt, 2002; Moll et al., 2005; Koenigs et al., 2007). Another region, the posterior cingulate (and adjacent precuneus region), has been implicated in moral appraisal in some studies (Greene et al., 2001, 2004; Heekeren et al., 2005; Harenski and Hamann, 2006) but not others (Moll et al., 2002a, 2002b; Heekeren et al., 2003). It has been suggested that this region may represent emotion and memory processes in the context of moral appraisal (Greene and Haidt, 2002). This region has also been implicated in other affective and cognitive processes including autobiographical emotional recall (Fink et al., 1996; Maddock, 1999), and is believed to link emotion and memory processes (Maratos et al., 2001; Maddock et al., 2003). More recently, activity in this region has been shown to contribute to experiential self reflection (Johnson et al., 2006).
Posterior cingulate activity in response to moral stimuli may be related to the gender composition of the study sample, such that females are more likely to show activity in this region in response to moral stimuli than are males (Harenski and Hamann, 2006). This hypothesis fits well with a recent study in which participants showed increased posterior cingulate activity in response to care-based moral dilemmas relative to justice-based moral dilemmas (Robertson et al., 2007). In contrast, increased activity during justice-based relative to care-based dilemmas occurred in the posterior superior temporal sulcus. Care-based dilemmas were designed to represent concerns regarding the welfare of others, and to elicit empathic emotions such as compassion and benevolence. Justice-based dilemmas were designed to represent concerns regarding the liberation of others from injustice, and to elicit attitudes of fairness and impartiality. Although the participants in this study were male, and gender differences were not explored, if females indeed have stronger care-based orientations to moral stimuli relative to males they should show greater posterior cingulate activity associated with moral processing (when making moral appraisals that are not explicitly designed to elicit care- or justice-based judgments). Since care-based moral appraisals are also expected to invoke empathic responses, females should also show increased neural activity associated with empathic processes. Recent studies investigating the neural correlates of empathy have implicated two primary regions; the anterior insula and dorsal anterior cingulate cortex (Botvinick et al., 2005; Singer et al., 2004, 2006; Jackson et al., 2005, 2006; Saarela et al., 2007; Lamm et al., 2007). Regarding males, if they indeed have stronger justice-based orientations to moral stimuli relative to females, they should show greater posterior superior temporal sulcus activity during moral processing. The current study tested these hypotheses.
In the current study, we used functional magnetic resonance imaging (fMRI) to examine gender differences in neural responses to pictures depicting moral violations. We also assessed gender differences in behavioral moral evaluations by obtaining online ratings of the severity of depicted moral violations. In contrast to most prior neuroimaging studies of moral appraisal, which have typically compared averaged neural responses to a set of ‘moral’ and ‘non-moral’ stimuli, the current study design allowed us to explore the association between neural activity in response to individual moral pictures and subsequent moral violation severity ratings. Specifically, we conducted a parametric modulation analysis in which we explored brain regions whose activity during moral picture viewing positively modulated subsequent moral violation severity ratings. Thus, the current design is sensitive to individual variation in responses to diverse moral stimuli. We explored whether activity in specific brain regions was positively correlated with moral severity ratings, and whether these regions differed for males and females. The primary hypothesis was that females would show a greater positive modulation of moral severity ratings by activity in the posterior cingulate, anterior insula, and dorsal anterior cingulate during moral picture viewing relative to males. In addition, we hypothesized that males would show a greater positive modulation of moral severity ratings by activity in posterior superior temporal sulcus during moral picture viewing relative to females. Whether we would observe gender differences in other neural correlates of moral processing, such as medial prefrontal cortex, was an open question.
METHODS
Participants
Thirty healthy, right-handed adults were recruited (16 females) from the Olin Neuropsychiatry Research Center (Hartford, CT, USA) and a nearby liberal arts college (Trinity College, Hartford, CT, USA). One female participant was excluded due to excessive head motion during scanning (>10 mm), and another female participant was excluded due to poor task performance (the participant missed several ratings across both experimental runs). Analyses are presented on the remaining 28 participants, 14 females (mean age 24.5 years) and 14 males (mean age 25.8 years). All participants provided written informed consent, and the study was conducted in accordance with institutional ethical standards.
Stimuli and task
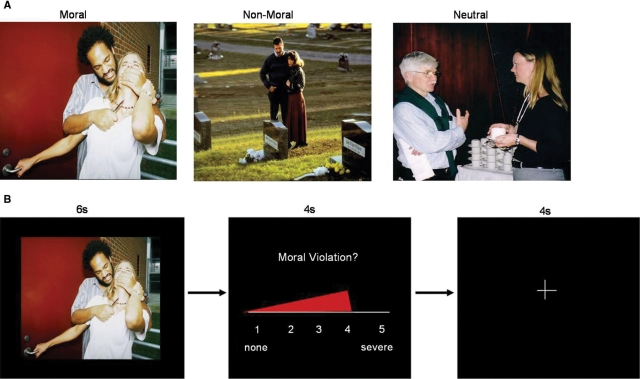
Three sets of pictures (25 moral, 25 non-moral and 25 neutral) were selected mostly from the International Affective Picture System (IAPS) (Lang et al., 1995), and supplemented with pictures from the popular media (the full picture set can be viewed at the following website: www.mrn.org/mrt_stimuli). All moral pictures depicted unpleasant social scenes indicating a specific moral violation. Non-moral pictures depicted unpleasant social scenes without moral content. Neutral pictures depicted affectively neutral social scenes without moral content. Moral and non-moral pictures were matched for social content by equating the number of individuals present and types of social situations. Specifically, a one-to-one matching approach was taken, such that if a moral picture depicted a male individual interacting with a female person in a negative manner (e.g. an abusive situation), the corresponding non-moral ‘pair’ picture also contained a male and a female interacting in a negative manner (e.g. an argument), and the corresponding neutral picture contained a male and a female interacting in a neutral manner (e.g. a conversation). An example picture set is shown in Figure 1A. Moral and non-moral pictures were matched on emotional arousal (Harenski and Hamann, 2006).
Fig. 1.
(A) Example moral, non-moral and neutral picture set. (b) Example ‘moral’ trial.
Participants were informed that during the task they would see a series of different pictures depicting people and events. For each picture, they were instructed to determine whether it represented a moral violation (i.e. an action or attitude that the participant considered to be morally wrong) and to rate the severity of the moral violation on a scale from 1 (none) to 5 (severe). If the picture did not represent a moral violation, participants were instructed to give a rating of 1. If they were unsure whether the picture represented a moral violation, they were instructed to give low to moderate ratings. Emphasis was placed on asking the participants to make ratings based on their own system of moral values, not what others or society would think was a moral violation. Due to technical error, online ratings were not obtained for one female participant.
Following the instructions, participants entered the scanner and, prior to the experimental task, completed five practice trials to ensure that they understood how to perform the task. In each trial, a picture was first displayed for 6 s, during which the participant determined whether the picture represented a moral violation. Next, a rating scale was shown. The rating scale was displayed in continuous presentation format, such that a red bar began at ‘1’ (none) and progressed to ‘5’ (severe) over a period of 4 s (Figure 1B). The participant pressed a button to stop the bar when it reached the appropriate rating that they wished to give. Following the rating, a rest period occurred during which a black screen with a white fixation cross was displayed. Moral, non-moral and neutral picture trials were presented in a randomized order, and were interspersed with ‘null’ fixation trials of the same duration as picture trials. The 100 total trials were presented across two separate runs. Images were rear-projected into the scanner using an LCD projector, controlled by a personal computer. Tasks were designed and presented and responses were recorded using the program Presentation (version 10.78, http://nbs.neuro-bs.com).
Following scanning, participants completed an open-ended questionnaire in which they were asked to indicate what types of information and strategies they used in determining violation severity ratings. Although the questionnaire was not designed to conduct a formal coding analysis on the content of the responses, there was a clear distinction between the usage of ‘internal’ subjective information (e.g. ‘how the picture made me feel’) vs the usage of ‘external’ objective information (e.g. ‘whether someone appeared to be intentional hurting someone else’). We explored whether females and males showed a differential tendency to use each type of information in their ratings. Four males and four females did not complete the questionnaire; results are reported for the 10 males and 10 females who provided this information.
MRI data acquisition and analysis
Whole-brain imaging data were obtained using a Siemens 3T Allegra MRI scanner. The gradient-echo planar sequence (TR = 1500 ms, TE = 27 ms, FA = 65, FOV 24 × 24 cm, 64 × 64 matrix, 3.44 by 3.44 mm in plane resolution, 5 mm slice thickness, 30 slices) effectively covered the entire brain (150 mm). A total of 480 scans were obtained in each of two scan runs. Head movement was limited by padding and restraint. Functional images were motion corrected, normalized to a standard template and spatially smoothed (eight FWHM) using SPM2 (www.fil.ion.ucl.ac.uk/spm). Low frequency noise was removed using a high-pass filter.
Individual participant data were analyzed using a mixed-effect model in SPM2. Picture presentations (moral, non-moral and neutral) and the rating period were modeled as separate events. The primary event of interest, picture presentation, was modeled with the standard hemodynamic response function with 6 s duration. Neural responses associated with individual violation severity ratings were assessed by a parametric modulation analysis in which the participant's rating associated with each picture from the moral condition was entered as a covariate in the first-level analysis.
Gender differences were compared for: (i) the summary images resulting from the moral > non-moral contrast, which revealed brain regions whose activity during moral picture viewing was significantly greater than during non-moral picture viewing; and (ii) the summary images from the parametric modulation analyses of moral pictures, which revealed brain regions whose activity during moral picture viewing significantly modulated the subsequent violation severity rating. Unless otherwise indicated, statistical maps at the group level for each contrast were thresholded at P < 0.001 (uncorrected) with an extent threshold of five contiguous voxels. Gender differences in hemodynamic activity were assessed by a second-level, mixed-effect analysis with participants as the random effects factor, using an independent sample t-test on the individual participant-specific contrast images. Statistical maps were thresholded at P < 0.005 (uncorrected) with an extent threshold of five contiguous voxels. Results are overlaid on a structural T1-weighted image from the SPM2 canonical image set, registered to Montreal Neurological Institute (MNI) space. All coordinates are reported in MNI space.
RESULTS
Moral severity ratings
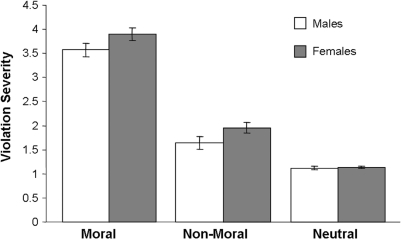
Females and males rated moral pictures higher in moral severity than non-moral pictures [F1,26 = 506.69, P < 0.0001] and neutral pictures [F1,26 = 754.79, P < 0.0001] (Figure 2). There was a marginal gender effect, reflecting a tendency for females to rate all pictures higher on violation severity than males [F1,25 = 3.83, P < 0.07]. The interaction between gender and stimulus type was not significant [F2,50 = 2.03, ns].
Fig. 2.
Online ratings of moral violation severity across picture stimuli in males and females.
Imaging results
Regions activated during moral vs non-moral picture viewing
When viewing moral relative to non-moral pictures, female and male participants showed increased activity in brain regions previously implicated in moral processing, including medial prefrontal cortex [Brodmann area (BA 10/11)], posterior cingulate (BA 31) and inferior parietal cortex (BA 39) (Table 1). Additional activated regions included the parahippocampal gyrus (BA 36) and superior prefrontal (BA 8/10) and parietal (BA 7) cortex. The unpaired t-test comparing activity during moral picture viewing in females vs males did not reveal significant differences in any of these regions.
Table 1.
Gender differences in brain regions activated during moral vs non-moral picture viewing
| BA | x | y | z | All Participants |
Females |
Males |
Females vs Males | Males vs Females | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| z(k) | P | z(k) | P | z(k) | P | |||||||
| Medial prefrontal Cortex | 10/11 | 12 | 45 | −12 | 5.06 (691) | 0.001 | 4.7 (156) | 0.001 | – | – | NS | NS |
| (12, 45, −9) | ||||||||||||
| – | – | 4.17 (85) | 0.001 | 3.55 (7) | 0.001 | NS | NS | |||||
| (3, 60, 6) | (−6, 54, 6) | |||||||||||
| Posterior cingulate | 23/31 | −6 | −54 | 9 | 4.84 (380) | 0.001 | 3.02 (25) | 0.005 | 4.07 (86) | 0.001 | NS | NS |
| (−6, −57, 24) | (−9, −54, 9) | |||||||||||
| 12 | −48 | 3 | 4.05 (29) | 0.001 | – | – | – | – | NS | NS | ||
| 9 | −48 | 6 | – | – | – | – | 3.81 (48) | 0.001 | NS | NS | ||
| – | – | – | – | 3.74 (23) | 0.001 | NS | NS | |||||
| (−6, −60, 36) | ||||||||||||
| −3 | −36 | 36 | – | – | – | – | 3.66 (12) | 0.001 | NS | NS | ||
| Inferior parietal cortex | 39 | −51 | −72 | 24 | 4.52 (98) | 0.001 | 2.87 (13) | 0.005 | 3.61 (31) | 0.001 | NS | NS |
| (−54, −60, 27) | ||||||||||||
| 54 | −66 | 33 | 3.69 (48) | 0.001 | 3.24 (22) | 0.005 | – | – | NS | NS | ||
| (51, −69, 36) | ||||||||||||
| Parahippocampal Gyrus | 36 | 21 | −33 | −18 | 4.02 (65) | 0.001 | 3.03 (11) | 0.005 | 4.13 (19) | 0.001 | NS | NS |
| (−24, −36, −18) | (21, −36, 51) | |||||||||||
| −24 | −30 | −18 | – | – | – | – | 3.32 (8) | 0.001 | NS | NS | ||
| Superior prefrontal cortex | 8 | −24 | 36 | 51 | 3.98 (10) | 0.001 | – | – | – | – | NS | NS |
| Superior parietal cortex | 7 | −12 | −72 | 60 | 3.86 (46) | 0.001 | 3.11 (21) | 0.005 | 3.22 (25) | 0.005 | NS | NS |
| (−6, −66, 57) | ||||||||||||
| Superior prefrontal cortex | 9 | −21 | 6 | 69 | 3.46 (6) | 0.001 | – | – | 3.33 (30) | 0.005 | NS | NS |
| (−21, 6, 72) | ||||||||||||
| Midbrain | 0 | −12 | −15 | 3.39 (6) | 0.001 | – | – | 3.92 (17) | 0.001 | NS | NS | |
| (9, −24, −15) | ||||||||||||
| – | – | – | – | 3.59 (11) | 0.001 | NS | NS | |||||
| (0, −15, −15) | ||||||||||||
| Middle temporal cortex | 19 | 54 | −72 | 12 | – | – | – | – | 3.66 (10) | 0.001 | NS | NS |
| Pons | −9 | −33 | −36 | – | – | – | – | 3.36 (7) | 0.001 | NS | NS | |
Z, z-score; k, extent threshold.
Brain regions modulating violation severity ratings
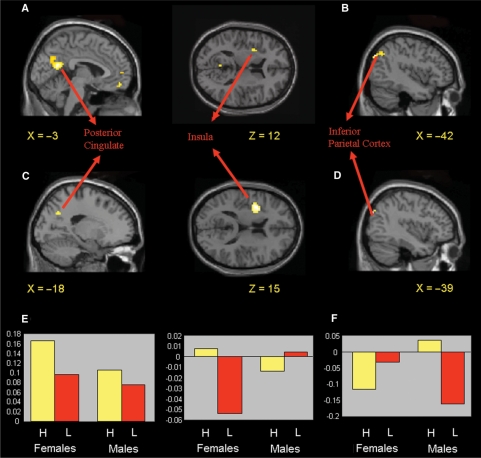
The parametric modulation analysis, which revealed brain regions whose activity during moral picture viewing positively modulated subsequent moral violation severity ratings in females and males, showed increased modulation by posterior cingulate (BA 31) and medial prefrontal cortex (BA 11) activity, and, at a reduced threshold (P < 0.005, uncorrected), inferior parietal cortex (BA 39) and the amygdala-hippocampal junction (Table 2). Gender differences were also observed in several regions. In females, a positive modulation of violation severity ratings occurred in the posterior cingulate (BA 31), as predicted. At a reduced statistical threshold (P < 0.005, uncorrected), a positive modulation also occurred in the anterior insula (BA 13) (Table 2, Figure 3A). The modulation by these regions was significantly greater in females than males (Figure 3C). Although males did not show a positive modulation of violation severity ratings in the superior temporal sulcus, as was hypothesized, there was a positive modulation in a region of inferior parietal cortex slightly posterior to the superior temporal sulcus (BA 19/39) (Table 2, Figure 3B). The modulation by this region was significantly greater in males than females (Figure 3D). Males also showed a negative modulation of violation severity ratings in the anterior insula (BA 13). Thus, distinct neural correlates were predictive of violation severity ratings for females and males.
Table 2.
Gender differences in brain regions modulating violation severity ratings
| Positive | BA | x | y | z | All Participants |
Females |
Males |
Females vs Males |
Males vs Females |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| z(k) | P | z(k) | P | z(k) | P | z(k) | P | z(k) | P | |||||
| Posterior cingulate/ | 31 | 6 | −51 | 27 | 3.94 (122) | 0.001 | 3.87 (48) | 0.001 | – | – | 2.90 (7) | 0.005 | NS | NS |
| Precuneus | (−3, −57, 21) | (−18, −63, 33) | ||||||||||||
| 3.34 (7) | 0.001 | – | – | NS | NS | NS | NS | |||||||
| (−12, −57, 30) | ||||||||||||||
| Medial frontal cortex | 11 | 0 | 51 | −15 | 3.20 (32) | 0.001 | – | – | – | – | ||||
| Inferior parietal cortex | 19/39 | −45 | −75 | 36 | 3.24 (17) | 0.005 | – | – | 4.00 (5) | 0.001 | NS | NS | 3.42 (8) | 0.005 |
| (−42, −78, 36) | (−39, −81, 33) | |||||||||||||
| Amygdala/ | −21 | −3 | −27 | 2.87 (11) | 0.005 | – | – | – | – | NS | NS | NS | NS | |
| Hippocampus | ||||||||||||||
| Anterior insula | 13 | −30 | 6 | 12 | – | – | 3.23 (14) | 0.005 | – | – | 3.54 (46) | 0.005 | NS | NS |
| (−33, 6,15) | ||||||||||||||
| Negative | ||||||||||||||
| Anterior insula | 13 | 36 | 21 | 3 | 4.26 (57) | 0.001 | – | – | 3.52 (46) | 0.001 | NS | NS | NS | NS |
| Inferior frontal cortex | 9 | 42 | 9 | 27 | 3.95 (55) | 0.001 | – | – | – | – | NS | NS | NS | NS |
| Cerebellum | 0 | −36 | −42 | 3.63 (7) | 0.001 | – | – | – | – | NS | NS | NS | NS | |
| Occipital cortex | 19 | −45 | −72 | 15 | 3.37 (5) | 0.001 | – | – | – | – | NS | NS | NS | NS |
| Dorsomedial prefrontal cortex | 8 | 3 | 18 | 51 | – | – | 3.39 (11) | .001 | – | – | NS | NS | NS | NS |
Fig. 3.
(A) Positive modulation of moral violation severity ratings in females by posterior cingulate (BA 31) and anterior insula (BA 13) activity as revealed by the parametric modulation analysis. Statistical map thresholded at P < 0.005, uncorrected, to highlight insula activity and extent of posterior cingulate activity. (B) Positive modulation of moral violation severity ratings in males by inferior parietal (BA 19/39) activity in the same analysis. (C) Increased modulation of moral violation severity ratings by posterior cingulate and anterior insula activity in females relative to males. (D) Increased modulation of moral violation severity ratings by inferior parietal activity in males relative to females. (E and F) Parameter estimates of posterior cingulate, anterior insula and inferior parietal activity in response to the 10 highest rated pictures, and the 10 lowest rated pictures, on moral violation severity in females and males.
It is noteworthy that the positive modulation of violation severity ratings by insula activity in females occurred in the left hemisphere, whereas the negative modulation in the same region in males occurred in the right hemisphere. To further explore this apparent asymmetry, a gender × hemisphere interaction analysis was conducted using methods developed in our laboratory (Stevens et al., 2005). The results revealed a significant interaction effect in the anterior insula (x = −39, y = 0, z = 15, BA 13), confirming a significant left greater than right hemisphere asymmetry in females compared to males for the positive modulation (P < 0.001, uncorrected). However, a significant right greater than left hemisphere asymmetry in males compared to females for the negative modulation was not observed. No other asymmetry effects between gender were observed for regions of interest, (e.g. posterior cingulate, inferior parietal cortex and medial prefrontal cortex) for either the modulation analysis or the moral > non-moral contrast.
Post-scan questionnaire
We examined responses to the questionnaire that asked participants what types of information and strategies they utilized in determining violation severity ratings. Most responses focused on the content of the pictures and what type of situation was depicted, with references to context (e.g. drinking while driving), symbols (e.g. Nazi paraphernalia) and intentions (e.g. an abusive situation). Indeed, all participants with the exception of one female reported using this type of information in their ratings. However, several individuals reported basing their ratings primarily on how the picture made them feel, i.e. whether what was being depicted in the picture made elicited a strong emotional reaction. Of the 10 females who completed the questionnaire, five reported primarily using this type of information, while another female reported using only this type of information. In contrast, only one of the 10 males who completed the questionnaire reported using this type of information in their judgments.
DISCUSSION
Gender differences in moral reasoning have been a controversial issue for decades. This study was designed to explore whether different neural systems are predictive of moral sensitivity in females and males. Consistent with hypotheses, females’ violation severity ratings were predicted by increased posterior cingulate and anterior insula activity during moral picture viewing. Males’ violation severity ratings were predicted by increased inferior parietal activity. These results indicate that while females and males show similar behavioral evaluations of moral stimuli, they engage different neural systems in generating these evaluations.
Several previous neuroimaging studies of moral appraisal have included a mixed gender sample; however, gender differences in neural responses to moral stimuli have not been previously reported. Although gender differences may not have been examined in these studies, it is also possible that prior studies of moral processing have failed to observe gender differences because they compared neural responses to a generalized set of stimuli categorized as ‘moral’ by the experimenter. Since an individual might have a very strong reaction to one type of moral violation but less of a reaction to another type of moral violation, assessing a person's average neural response across all types of moral stimuli might obscure patterns of brain activity that represent increased sensitivity or stronger responses to specific moral violations. The current study utilized a design that was sensitive to individual differences in evaluations of different types of moral violations, which proved effective in elucidating significant gender differences in brain activity associated with judging the violation severity of pictorial stimuli.
Females showed a positive modulation of violation severity ratings by posterior cingulate activity. This effect was hypothesized based on research showing that posterior cingulate activity is increased when individuals evaluate care-based moral dilemmas relative to justice-based dilemmas (Robertson et al., 2007) and Gilligan's (1977) theory that females typically adopt care-based approaches to moral stimuli relative to males. Thus, greater modulation of moral ratings by posterior cingulate activity in females is suggestive of greater care-based evaluations in females relative to males. However, since the current results do not directly demonstrate greater care-based evaluations in females, it is useful to consider other potential functions of this region that might contribute to moral sensitivity in females. Posterior cingulate has been linked to emotional and self-reflective processing (Fink et al., 1996; Maddock, 1999; Damasio et al., 2000; Vogt and Laureys, 2005; Johnson et al., 2006) and has been proposed to represent emotional and memory processes in the context of moral judgments (Greene and Haidt, 2002). Thus, it is possible that moral sensitivity in females is associated with the strength of their emotional response to the pictures, and/or on reflections of their own subjective reactions to the pictures.
In light of these possibilities, it is important to consider the increased modulation by anterior insula activity that females also showed relative to males. The role of this region in empathic processing has been well established (Botvinick et al., 2005; Singer et al., 2004; Jackson et al., 2005, 2006; Saarela et al., 2007; Singer et al., 2006; Lamm et al., 2007), and many of the moral picture stimuli involved depictions of individuals in distress (i.e. victims of a moral transgression), which likely induced empathic responses. Thus, increased insula activity in females is consistent with our hypothesis that moral sensitivity in females would be associated with greater empathy-related neural activity. It is noteworthy, however, that females did not show increased activity in dorsal anterior cingulate, which has also been implicated in empathic processing. This may suggest dissociable roles of dorsal anterior cingulate and anterior insula in empathic processing, though this has not yet been demonstrated (only anterior–posterior distinctions within each region representing observed and experienced pain, respectively; see Decety and Lamm, 2006, for a review). An alternate possibility is that increased insula modulation does not represent specifically empathic processing, but may represent the influence of the broader spectrum of being subjectively aware of one's emotional states during the processing of moral stimuli. Indeed, such a role for this region in general emotion processing has been demonstrated (Damasio et al., 2000).
Interestingly, males showed a negative modulatory relationship between anterior insula activity and violation severity ratings. This indicates that while insula activity positively influenced moral sensitivity in females, predicting higher violation severity ratings, insula activity negatively influenced moral sensitivity in males, predicting lower violation severity ratings. Put in another way, violation severity ratings were higher in males when insula activity during moral picture viewing was low. If the modulation of moral ratings by insula activity indeed represents the association between empathic responses and/or awareness of one's emotional states and moral sensitivity, this result suggests that these types of responses did not have a role in males’ evaluations of violation severity, or even that males suppressed such processing during their evaluations. It is worth noting, however, that the insula is not a brain region that has typically been implicated in moral appraisal in prior studies. This may be a function of task design. Insula activity in the current study was specifically related to the degree of rated violation severity for individual pictures. As such, the insula might not be active in response to all types of moral stimuli, only those that invoke a strong reaction of moral outrage, and thus would not likely show significant activity across a diverse set of moral stimuli. Future research exploring neural correlates of moral sensitivity to different types of moral stimuli will be helpful in exploring this hypothesis.
Although moral sensitivity in males was not positively influenced by activity in brain regions associated with affective and empathic processing, males showed a greater positive modulation of violation severity ratings by inferior parietal activity relative to females. This region, located in BA 19/39, lies slightly behind the posterior subregion of the superior temporal sulcus (BA 39), but is notably different than the subregion of the superior temporal sulcus that was activated in the justice-based moral condition in Robertson et al. (2007), which represents the anterior portion of this region (BA 22). Thus, caution should be taken in associating these findings with greater justice-based evaluations in males. A recent study that examined neural correlates of different types of moral judgments found that the BA 19/39 region was active across all types of moral judgment, whereas BA 22 activity was modulated by different types of moral judgments (Schaich Borg et al., 2006). The more posterior activity that we observed may be due to the representation of a wide variety of ‘moral violations’ that were judged in our picture set. Consistent with this suggestion is a finding that the BA 39 region of superior temporal sulcus is particularly involved in judging ‘difficult’ personal moral dilemmas (Greene et al., 2004), and the suggestion that this region represents ‘thought-provoking, first-time moral judgment that requires executive resources’ (Schaich Borg et al., 2006, p. 811). In other words, males might have utilized a great deal of cognitive resources incorporating a substantial amount of contextual information that varied across pictures in generating their ratings. In summary, males may have used executive resources to evaluate multiple contextual aspects of pictures in evaluating violation severity, whereas females may have focused more on the perception of individuals in distress, e.g. the target of a moral transgression.
In an attempt to explore whether different types of information were indeed associated with moral sensitivity in females and males, we asked participants after scanning what they looked for in the pictures to make their violation severity ratings. One striking difference in the types of information that participants reported using was found in certain participants who reported basing their ratings primarily on subjective reactions to pictures, such as ‘how the picture made me feel’, and others who based their ratings less on subjective reactions and more on objective information, such as ‘whether it looked like someone was intentionally hurting someone else’. While all participants reported utilizing objective information in evaluating violation severity, only one of the 10 males also utilized subjective reactions, in contrast to six of the 10 females who reported utilizing this type of information. There is some evidence, then, that females had a heightened tendency to reflect on their personal feelings in response to the pictures, and this influenced their ratings to a greater extent than males.
It should be emphasized, however, that since the post-scan questionnaire was presented in free response format, it is possible that males were less forthcoming with personal and emotional reactions to moral pictures relative to females. This is an important issue, given that prior studies have typically not observed gender differences in behavioral measures of moral appraisal. Whether such gender differences in moral appraisal exist in certain contexts but not others is a question for future research. It would also be useful to collect other types of behavioral data associated with moral appraisals, such as reaction time, to explore whether gender differences occur in these domains, and if so, whether these are associated with differences in patterns of brain activity underlying moral appraisal across gender.
Finally, it is interesting to consider whether an interaction might exist between participant gender and the gender of victims or perpetrators depicted in moral pictures. In the pictures used in our study, the gender of the victim or perpetrator was sometimes not apparent (e.g. in a picture of a pregnant woman smoking, or of a neglected child, respectively). However, in the 12 moral pictures that depicted a clear victim, half were females and half were males. In contrast, in the 16 pictures that depicted a clear perpetrator, 14 were males. Whether neural responses to moral violations might differ to those perpetrated by males relative to females, and whether such a difference would be gender dependent, is unknown. Further research is needed to explore these possibilities more directly.
REFERENCES
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expression of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–9. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Canli T, Amin Z. Neuroimaging of emotion and personality: scientific evidence and ethical considerations. Brain and Cognition. 2002;50:414–31. doi: 10.1016/s0278-2626(02)00517-1. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. Human empathy through the lens of social neuroscience. The Scientific World Journal. 2006;6:1146–63. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. Cerebral representation of one's own past: neural networks involved in autobiographical memory. Journal of Neuroscience. 1996;16:4275–82. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan C. In a different voice: women's conceptions of self and of morality. Harvard Educational Review. 1977;47:481–517. [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–8. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Greene J, Haidt J. How (and where) does moral judgment work? Trends in Cognitive Sciences. 2002;6:517–23. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–24. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Schwintowski HP, Villringer A. An fMRI study of simple ethical decision-making. Neuroreport. 2003;14:1215–9. doi: 10.1097/00001756-200307010-00005. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Prehn K, Schwintowski HP, Villringer A. Influence of bodily harm on neural correlates of semantic and moral decision making. Neuroimage. 2005;24:887–97. doi: 10.1016/j.neuroimage.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Hyde JS. The gender similarities hypothesis. American Psychologist. 2005;60:581–92. doi: 10.1037/0003-066X.60.6.581. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others: a window into the neural processes involved in empathy. Neuroimage. 2005;24:771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jaffee S, Hyde JS. Gender differences in moral orientation: a meta-analysis. Psychological Bulletin. 2000;126:703–26. doi: 10.1037/0033-2909.126.5.703. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KM, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self reflection. Social, Cognitive, and Affective Neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, et al. Damage to the prefrontal cortex increases utilitarian moral judgments. Nature. 2007;446:908–11. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS) Bethesda, MD: National Institute of Mental Health Center for the Study of Emotion and Attention; 1995. [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends in Neurosciences. 1999;22:310–6. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence detection task. Human Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain. 2001;124:1156–70. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39:910–20. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, et al. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience. 2002a;22:2730–6. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Bramati IE, Grafman J. Functional networks in emotional moral and non-moral judgments. Neuroimage. 2002b;16:696–703. doi: 10.1006/nimg.2002.1118. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Nature Reviews: Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social, Cognitive, and Affective Neuroscience. 2006;1:203–13. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Snarey J, Ousley O, et al. The neural processing of moral sensitivity to issues of justice and care. Neuropsychologia. 2007;45:755–66. doi: 10.1016/j.neuropsychologia.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams AC, Schurmann M, Kalso E, Har R. The compassionate brain: humans detect intensity of pain from another's face. Cerebral Cortex. 2007;17:230–7. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- Schaich Borg J, Hynes C, Van Horn J, Grafton S, Sinnott-Armstrong W. Consequences, action, and intention as factors in moral judgments: an fMRI investigation. Journal of Cognitive Neuroscience. 2006;18:803–17. doi: 10.1162/jocn.2006.18.5.803. [DOI] [PubMed] [Google Scholar]
- Skoe EEA, Cumberland A, Eisenberg N, Hansen K, Perry J. The influences of sex and gender-role identity on moral cognition and prosocial personality traits. Sex Roles. 2002;46:295–309. [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not the sensory components of pain. Science. 2004;303:1157–61. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Calhoun VD, Kiehl KA. Hemispheric differences in hemodynamics elicited by auditory oddball stimuli. Neuroimage. 2005;26:782–92. doi: 10.1016/j.neuroimage.2005.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in Brain Research. 2005;150:205–17. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]