Abstract
Previous research on single joint movements has lead to the development of models of control that propose that movement speed and distance are controlled through an initial pulsatile signal that can be modified in both amplitude and duration. However, the manner in which the amplitude and duration are modulated during the control of movement speed and distance remains controversial. We now report two studies that were designed to test and refine the pulse-step model of movement control. In our first study, participants move at a series of speeds to a single spatial target. In this task, acceleration duration (pulse-width) varied substantially across targets, and was negatively correlated with peak acceleration (pulse-height). In a second experiment, we removed the spatial target, but required movements at three speeds similar to those used in the first study. In this task, acceleration amplitude varied extensively across the speed targets, while acceleration duration remained constant across the three speeds. Taken together, our current findings demonstrate that pulse-width measures can be modulated independently from pulse-height measures, and that a positive correlation between such measures is not obligatory, even when sampled across a range of movement speeds. In addition, our findings suggest that pulse-height modulation plays a primary role in controlling movement speed and specifying target distance, whereas pulse-width mechanisms are employed to correct errors in pulse-height control, as required to achieve spatial precision in final limb position.
Keywords: Pulse-step model, trajectory control, position control
Introduction
Studies of single joint movements and isometric tasks have provided powerful models for understanding the neural mechanisms that underlie control of movement speed and distance. In particular, pulse-step control (Ghez, 1979) describes the relationship between force or torque profiles and movement amplitude. This model emerged from initial studies examining targeted isometric force tracking tasks in cats (Ghez & Vicario, 1978a, 1978b) and was later expanded to isometric studies in humans (Gordon & Ghez, 1984, 1987a, 1978b). Brown and Cooke extended this model to single joint movements (Brown & Cooke, 1981a, 1981b, 1984, 1986), an extension that was further validated by a number of studies (Corcos, Gottlieb, & Agarwal, 1989; Gottlieb, Corcos, & Agarwal, 1989; Hoffman & Strick, 1986; Mustard & Lee, 1987; Krakauer, Gordon, Veytsman, & Ghez, 2002; Sainburg & Schaefer, 2004). The pulse-step model proposes that rapid limb movements are governed by an initial pulsatile output that determines the amplitude and duration of the initial acceleration impulse. Under constant inertial conditions, peak acceleration scales with, and thus predicts movement velocity and distance. Intended distance and velocity are thus thought to be specified by modifications in control pulse amplitude. Brown and Cooke (1981a, 1981b, 1984, 1986) showed that acceleration amplitude is determined by initial agonist burst amplitude, and that both measures are resistant to peripheral sensory events evoked by mechanical perturbations. They concluded that pulse-height control is largely due to preplanning processes.
The pulse-step model also includes modulation of the width of the initial pulse, which is reflected by agonist EMG burst duration, antagonist burst latency and force, torque, or acceleration duration (Brown & Cooke, 1981a, 1981b, 1984, 1986; Gordon & Ghez, 1987b). In a targeted isometric task, Gordon and Ghez (1984, 1987a, b) reported negative correlations between peak force (pulse-height) and force rise time (pulse-width), when data were assessed within a single force target. More recently in a multijoint reaching task, Krakauer et al. (2002) reported negative correlations between acceleration duration and acceleration amplitude, also when assessed within a single target amplitude. However, in these studies, when data were grouped across targets, strong positive correlations between acceleration duration and peak acceleration were reported. These authors suggested that within targets, pulse-width can be independently modulated to compensate planning errors in pulse-height control. The idea that such compensatory pulse-width control might be modulated by sensory feedback is supported by studies by Brown and Cooke (Brown & Cooke, 1981a, b, 1984, 1986). These studies showed that initial agonist duration, but not amplitude, is substantially influenced by sensory stimuli evoked by mechanical perturbations delivered prior to or during movement onset, thus demonstrating that pulse-height and pulse-width can be differentially modulated.
Regardless of the evidence described above, the proposition that pulse-height and pulse-width control can be executed independently remains controversial. Gottlieb and colleagues (Corcos et al., 1989; Gottlieb et al., 1989) proposed a model of single joint control that was similar to the pulse-step model, in which the control ‘pulse’ was explicitly modeled as a square wave of motoneuron pool activation. These authors studied movements under varied conditions, and measured torque, which varies with inertial load conditions, rather than acceleration. Both the amplitude and duration of the initial torque profiles scaled together when variations in speed were an explicit task requirement. They proposed that variation in torque rise time was a passive consequence of torque amplitude control, thus suggesting that the duration of the torque pulse cannot be independently varied from its amplitude when movement speed is controlled. This idea contrasts with the suggestion that pulse-width control is a distinct, compensatory control mechanism, as put forth by Ghez and colleagues and Brown and colleagues. Thus, the exact role of pulse-height (torque or acceleration amplitude) and pulse-width (torque or acceleration duration) measures and whether these can be independently varied during the control of movement speed and amplitude remains controversial.
We now present two studies that were designed to test the hypothesis that pulse-height and pulse-width control are not obligatorily coupled and in fact can be independently modulated even over a larger range of peak acceleration. We further test the hypothesis that modulation of pulse-height measures is employed to specify movement speed and target distance, whereas pulse-width modulation is utilized for refining final steady state limb position around the target. In our first study, participants achieved three different speeds while moving to a single spatial target. These speeds were matched to the average speeds produced in a previous study (Sainburg & Schaefer, 2004), in which participants showed strong positive correlations between acceleration amplitude and duration and movement distance. If the aforementioned hypotheses are correct, acceleration amplitude should vary directly with peak velocity and movement distance (pulse-height control), indicating the role of pulse-height mechanisms in controlling movement speed. However, under these conditions, we predict that acceleration duration (pulse-width control) will show a negative correlation with movement speed. This prediction is derived from the previously described work of Gordon and Ghez (1984, 1987a, b) and likely reflects compensatory adjustments to errors in the specified pulse height, so that the desired final position accuracy is achieved. In addition, this would also confirm that variations in acceleration duration are not a necessary feature of variations in acceleration height over a range of speeds, which has been previously shown to elicit strong positive correlations across target amplitudes.
In our second experiment, participants achieved similar ranges of peak velocities, but in the absence of a spatial target. We expect to observe a strong scaling of peak acceleration with peak velocity under these conditions, confirming the role of pulse-height control in specifying movement velocity. However, because no final spatial target position is specified and adjustments to accurately achieve a final position become irrelevant to task goals, we also expect that acceleration duration will not vary across speed conditions. This would confirm that pulse-width modulation is specifically adapted for control of spatial features of the task.
Methods
Participants were 10 neurologically intact adults aged between 20 to 30 years, such that 5 participants performed each task. Only right-handers were selected; handedness was determined using a 12-item version of the Edinburgh inventory (Oldfield, 1971). Participants gave informed consent prior to participation. Informed consent had been approved by the Institutional Review Board of the Pennsylvania State University (Interlimb Differences in Control of Multijoint Dynamics, IRB # 00M0863).
Experimental Setup
Fig. 1 illustrates the general experimental setup used in both studies. Participants sat facing a table with their hand supported over the horizontal surface positioned just below shoulder height (adjusted to participants' comfort) by an air jet system, which minimized friction. A cursor representing finger position, a start circle and a velocity feedback bar were projected on a horizontal back projection screen positioned above the arm. A mirror placed parallel and below this screen reflected the visual display so as to give the illusion that the display was in the same horizontal plane as the fingertip. Calibration of the display assured that this projection was veridical. This virtual reality setup assured that participants had no visual feedback of their arm during an experimental session. All joints distal to the elbow were immobilized using an adjustable brace. Position and orientation of each segment was sampled using a Flock of Birds (Ascension Technology) electro-magnetic 6-degree of freedom (DOF) movement recording system. The position of the index fingertip, the lateral epicondyle of the humerus and the acromion, directly posterior to the acromio-clavicular joint were recorded using a stylus that was rigidly attached to a 6- DOF Flock of Birds (FOB) sensor. One 6-DOF sensor was then attached to the upper arm segment by means of a plastic arm cuff, while the other sensor was attached to the air sled upon which the forearm was fixed. The sensors were positioned at approximately the center of each segment. As sensor data were received from the FOB, the 3-D position of the above mentioned landmarks was computed by our custom software, with the X-Y plane parallel to the tabletop. We thus used our computed X-Y coordinates of the fingertip to define the projected cursor position.
Fig. 1.
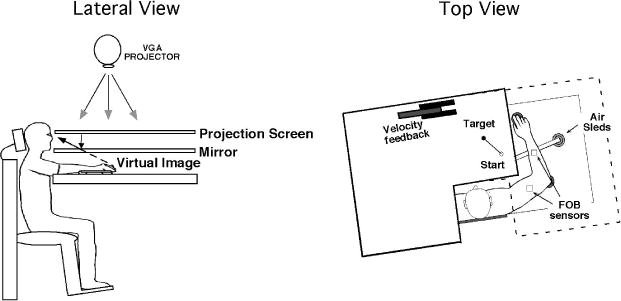
Experimental setup
Digital data was collected at 103 Hz using a Macintosh computer, which controlled the sensors through separated serial ports. This data were stored on disk for further analysis. Custom computer algorithms for experimental control and data analysis were written in REAL BASIC (REAL Software) and Igor Pro (Wavemetrics, Inc) respectively.
Experimental Tasks
We designed two experimental tasks to test our hypotheses. In both tasks, peak speed was specifically targeted, using real-time speed feedback on the computer generated display. In our first task, participants were required to move at three targeted speed ranges while aiming for a single spatial target (2 cm diameter). In our second task, the speed ranges achieved by our participants during experiment 1 were targeted, but in the absence of a spatial target. Both tasks were performed using elbow extension. Figs. 2A and 2B illustrate the tasks for experiment 1 and experiment 2, respectively.
Fig. 2.
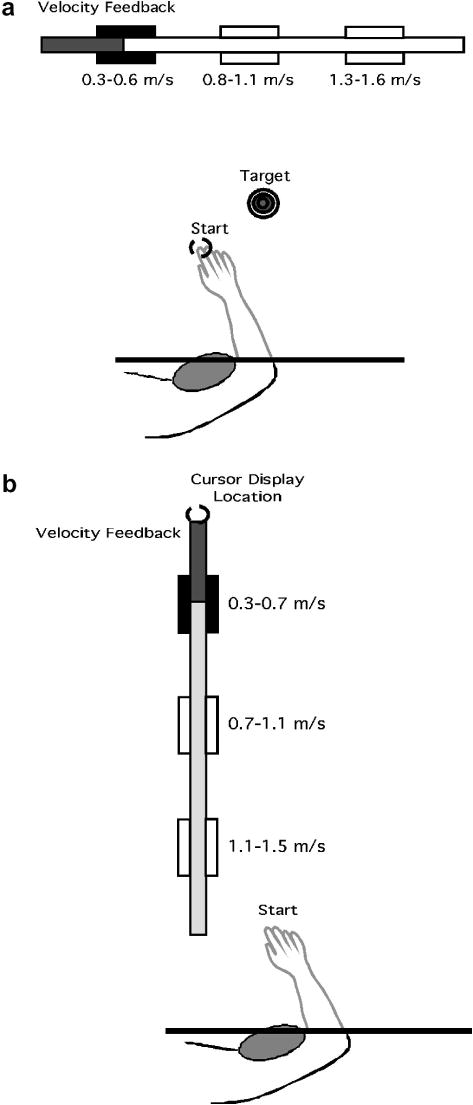
Experimental Tasks A: Experiment 1: Moving at three speeds to a single spatial target. B: Moving at three speeds without a spatial target.
Experiment 1 (Fig. 2A)
Participants were required to make rapid elbow extension movements at three different speed ranges, while stopping at a single spatial target. For all participants, the starting shoulder position was maintained at 20° flexion by a brace, while the starting elbow angle (angle formed between upper arm and forearm) was 80°. The location of the target was designed to require 23° of elbow extension. Across participants, the target distance was about 15 cm. The task required the participants to match their tangential hand velocity within three different ranges displayed on the velocity gauge and stopping on the spatial target. The three target velocity ranges were set at .3 m/s to .6 m/s (speed target1), .8 m/s to 1.1 m/s (speed target 2) and 1.3 m/s to 1.6 m/s (speed target 3).
Prior to and between trials, participants could view their finger position as a screen cursor. To initiate a trial, the participant placed this cursor within the start circle. After .5 s within the start circle, an audiovisual stimulus (simultaneous ‘go’ tone and change of target color) signaled participants to move to the target using a single uncorrected elbow extension motion. At movement initiation, the cursor was blanked. At this time, participants could see the ‘thermometer’ type display of their instantaneous velocity at the top of the screen. They were instructed to move the hand to the target, and received points only if their peak velocity fell within the targeted velocity range (black bars above the target display). At the end of every trial, they received knowledge of results in the form of peak velocity feedback, a circle representing final position, and points based on accuracy. The points were given purely for motivation, and were awarded only if the movement occurred within the specified velocity range. Participants received 10 points for errors of less than 1 cm, or 3 points for errors less than 2 cm, or 1 points for errors less than 3 cm.
Experiment 2 (Fig. 2B)
In this task participants were required only to match a speed target without any spatial accuracy constraint. Because there was no explicit spatial display, there was no start circle or target. We provided a vertical bar to represent velocity in order to prevent participants from erroneously interpreting velocity feedback as positional feedback. That is, the tangent to hand motion with elbow extension was roughly 20°, and thus, the velocity display was nearly orthogonal to that motion. As in experiment 1, the shoulder position was maintained at 20° by a brace, and the elbow start position was 80°. On any given trial, a single velocity target appeared on the screen along the velocity gauge bar. The three target velocity ranges were set at .3 m/s to .7 m/s (target1), .7 m/s to 1.1 m/s (target 2) and 1.1 m/s to 1.5 m/s (target 3).
Because there was no spatial feedback, participants initiated a trial by moving their elbow into an 80° position. When this was achieved, a velocity slider was shown at the top of the velocity gauge. After .5 s, a ‘go’ tone was sounded, at which time participants were to move using a rapid elbow extension motion in order to achieve the targeted speed. The velocity slider provided real-time instantaneous velocity feedback during the movement. The aim of the task was thus to get the slider within the specified target range. After each trial, participants received knowledge of results in the form of a ‘thermometer’ style display along the velocity gauge. Points were also awarded for motivational purposes, such that participants received 10 points if their movements fell within the specified velocity range, and zero points for movements outside the target range. Velocity targets were presented in a pseudorandom order.
For both tasks, participants performed 150 movements, with randomized velocity targets. In order to allow for task familiarity, the first 39 trials were not subjected to kinematic analysis. Previous studies with similar tasks have demonstrated that stable task performance is achieved within the first 30 trials (Bagesteiro & Sainburg, 2003; Sainburg & Schaefer, 2004), which was verified visually in the current study. Kinematic analysis was performed on the remaining trials.
Kinematic Analysis
The 3D position of the index finger and elbow were calculated from sensor position and orientation data. Since movements were restricted to the horizontal plane, only the horizontal plane positions of shoulder, elbow and hand were considered. All kinematic data were low pass filtered at 8 Hz (3rd order, dual pass Butterworth) and differentiated to yield angular velocity and acceleration values. Each trial usually started with the hand at zero velocity, but small oscillations of the hand sometimes occurred within the start circle. In this case, the onset of movement was defined as the last cross zero point prior to the maximum in the index finger's tangential acceleration profile. Movement termination was defined as the first minimum (below 5% maximum tangential hand velocity) following the peak in tangential hand velocity. Data were also subjected to visual inspection to confirm that movement start and end points were accurate. If the final position of the hand was not well reflected by our automatic routine, the final position was marked by hand. This was required on less than 5% of the trials, and reflected a small oscillation at the end of some movements.
Data and Statistical Analyses
Measures of Task Performance
Five dependent measures of task performance were calculated from the kinematic data: peak tangential finger velocity, peak tangential finger acceleration, tangential finger acceleration duration, movement distance at movement end, and movement distance at peak velocity. Distance was calculated as the Euclidean distance between the position of the index finger tip at movement onset and the position of the index finger tip at a given point. The acceleration duration was calculated as the duration of the initial acceleration impulse, which ended at acceleration cross zero, or the time of peak tangential finger velocity.
Statistical Analysis
The data from each experiment were analyzed separately, using a repeated measures ANOVA, with subject as the randomized factor, and speed target as the within subject factor. We conducted linear regression analysis for select sets of data using the FitLine routine in JMP (SAS Institute). Effect sizes were obtained in the form of Cohen's d values where appropriate.
Results
Experiment 1: Moving at three speeds to a single target
Fig. 3 shows sample handpaths (Fig. 3A), tangential velocity profiles (Fig. 3B (left)), and tangential acceleration profiles (Fig. 3B (right)) for a representative participant. The velocity profiles were asymmetrical as has been previously observed in tasks performed in the horizontal plane and during which movement accuracy was emphasized (Sainburg & Schaefer 2004, Gottlieb et al., 1989; Corcos et al., 1989). As reflected by the distance between the points in Fig. 3A, and by the amplitude of the velocity profiles in Fig. 3B (left), participants successfully scaled movement speed as the task required. The scaling of peak velocity was achieved by scaling acceleration amplitude, as reflected by the acceleration profiles in Fig. 3B (right). This result was consistent across all participants. The mean distance moved under all three speed conditions, which while fairly consistent, showed a small but systematic variation across speeds. Whereas the slow movements were within millimeters (Mean = 14.62 cm, SE = .17 cm) of the 15 cm target, the fast movements tended to overshoot the target by less than 1 centimeter (Mean = 15.70 cm, SE = .28 cm). Thus, overall, participants were fairly accurate in achieving the target distance. Peak velocity showed a large systematic variation across speed targets (Mean = 0.43 m/s, SE = .02 m/s for the slow target; Mean = 0.69 m/s, SE = .02 m/s for the intermediate target; Mean = 0.88 m/s, SE = .008 m/s for the fastest target), and peak accelerations also showed substantial scaling (Mean = 4.87 m/s2, SE = .46 m/s2 for the slow target; Mean = 8.69 m/s2, SE = .47 m/s2 for the intermediate target; Mean = 11.77 m/s2, SE = .59 m/s2 for the fastest speed target) with these speed variations. When these measures were subjected to repeated measures ANOVA, a main effect of speed target occurred for all dependent measures (distance: F = 28.73, p = .0002; peak velocity: F = 119.43, p < .0001; peak acceleration: F = 120.73, p < .0001).
Fig. 3.
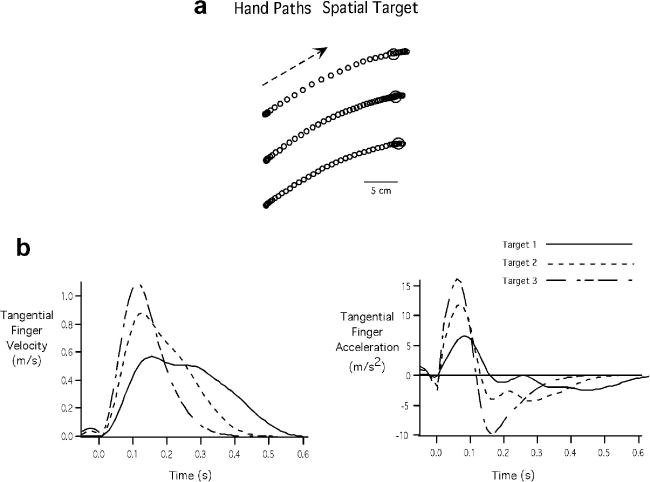
Kinematic measures for Experiment 1 for a representative participant. A: Handpaths. B: Tangential finger velocity profiles (left) and Tangential finger acceleration profiles (Right)
Gordon and Ghez (1987b) previously showed that when aiming for a single spatial target, small variations in initial acceleration or force amplitude were compensated by variations in acceleration duration or force rise time. However, in their studies, these variations never occurred across large ranges of initial forces or accelerations. As can be seen by the acceleration plots in Fig. 3B (right), an inverse relationship between acceleration duration (cross zero) and acceleration amplitude occurred, such that the smaller accelerations (speed target 1) were sustained longer, and the larger accelerations (speed target 3) were terminated sooner (ANOVA: F = 48.81, p < .01). Fig. 4A shows the mean and standard error of acceleration duration across participants for each speed target. The negative correlation between acceleration duration and acceleration amplitude is shown by the scatterplot in Fig. 4B, in which a single participant's data are shown, along with the regression lines for all participants. As shown, all participants showed negative relationships with an average r2 value of .71. The average change in acceleration duration across the fastest and the slowest targets was significant and on average 40 ms (Cohen's d = 2.942). The correlation between movement distance and acceleration amplitude is fairly weak as can be seen from Fig. 4C, which shows also shows data from a single participant with regression lines from all participants overlaid. The maximum r2 value in this case was .26 suggesting that acceleration amplitude alone is an insufficient predictor of movement distance therefore requiring other adjustments in acceleration in order to achieve a desired final position. Such adjustments might include in the form of modulation of acceleration duration, thus giving rise to the aforementioned negative correlation between pulse height and pulse width measures.
Fig. 4.
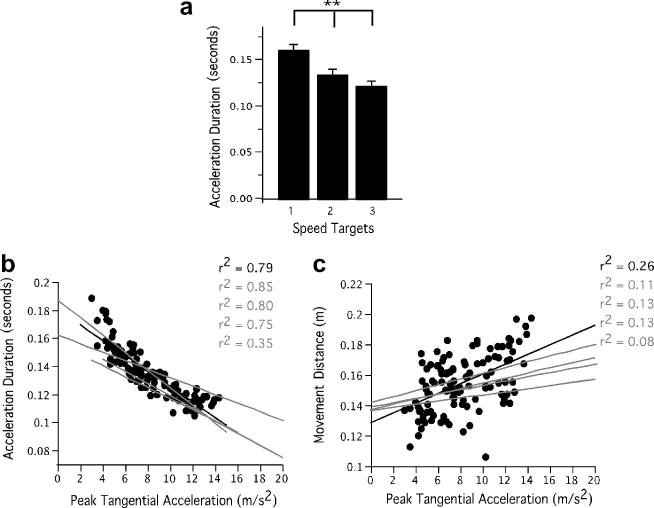
Acceleration duration measures for experiment 1. A: Acceleration duration across participants and conditions. B: Correlation of acceleration duration with acceleration amplitude with data for one participant (black) and regression lines for all participants (gray). C: Correlation of movement distance with acceleration amplitude with data for one participant (black) and regression lines for all participants (gray).
Nevertheless, the findings described here indicate that acceleration duration does not show an obligatory positive coupling with acceleration amplitude. Instead, changes in acceleration duration can compensate variations in acceleration amplitude when aiming to a single target location, even when the ranges of accelerations are substantial.
Experiment 2: Moving at three speeds without any spatial target
Fig. 5 shows the handpaths (Fig. 5A), velocity profiles (Fig. 5B (left)) and acceleration profiles (Fig. 5B (right)) for the movements made with varying speeds in the absence of any spatial target for a representative participant. It can be seen that the distance covered by each arm scaled with target speed, as reflected by the handpaths. The mean distance achieved across participants was 8.92 cm (SE = 1.82 cm) for the slowest target, 15. 11 cm (SE = 2.33 cm) for the intermediate speed target and 19.31 cm (SE = 2.21cm) for the fastest target. Peak velocity (Mean = 0.29 m/s, SE = .02 m/s for the slow target; Mean = 0.56 m/s, SE = .02 m/s for the intermediate target; Mean = 0.79 m/s, SE = .008 m/s for the fastest target) and peak acceleration (Mean = 3.38 m/s2, SE = .06 m/s2 for the slow target; Mean = 6.88 m/s2, SE = .62 m/s2 for the intermediate target; Mean = 10.16 m/s2, SE = .63 m/s2 for the fastest speed target) also varied in accord with the speed targets. In this case, the variation in speed was achieved primarily by scaling in the initial acceleration impulse. Our repeated measures ANOVA indicated a significant main effect for speed target suggesting that there is substantial scaling across all these variables (distance: F = 28.67, p = .0002; peak velocity: F = 290.82, p < .0001; peak acceleration: F = 216.90, p < .0001).
Fig. 5.
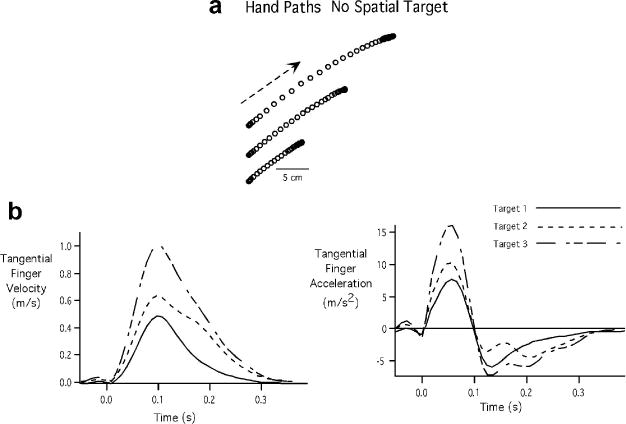
Kinematic measures for Experiment 2 for a representative participant. A: Handpaths. B: Tangential finger velocity profiles (left) and Tangential finger acceleration profiles (right)
Our results also indicate that there was no significant change in the duration of the acceleration pulse with change in speed. This is reflected by the time to peak velocity in the velocity profile or cross zero of the acceleration profile, which did not show any significant variation. The mean difference in the acceleration duration between the slowest and the fastest speed target was about 1 ms. Thus the target speed was achieved primarily by scaling of acceleration amplitude and even when acceleration amplitude scaled, there was no scaling of acceleration duration. This result was consistent across participants as depicted by the barplots of Fig. 6A. Our ANOVA (F = .1329, p = .8775) showed no significant difference in acceleration duration across speed targets. However, movement distance scaled strongly with peak acceleration as can be observed from the scatterplot of Fig. 6C. In this case, the maximum r2 was .66. Thus, acceleration amplitude alone was a good predictor of both movement speed and distance. Acceleration duration, in this case was weakly correlated with acceleration amplitude as reflected in our scatter plot of Fig. 6B, which shows data for one participant with regression lines for all participants. The average r2 values across all participants was .096, indicating a weak relationship between acceleration amplitude and acceleration duration. Thus, in the absence of a spatial target, pulse-width control does not vary across speed conditions.
Fig. 6.
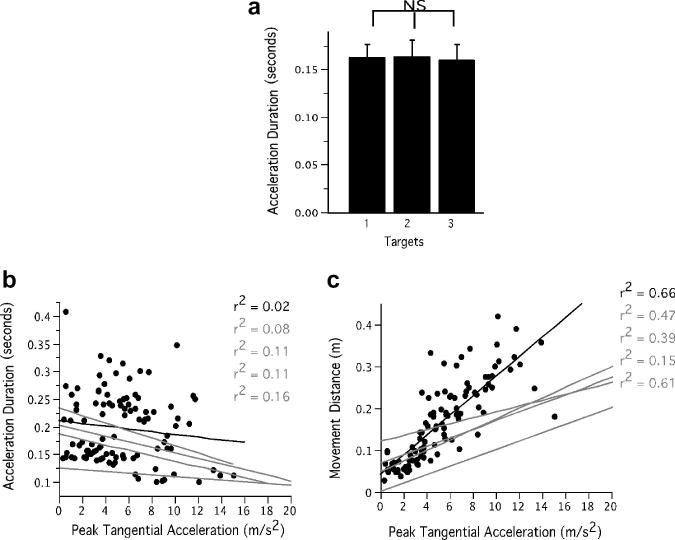
Acceleration duration measures for Experiment 2. A: Acceleration duration across participants and conditions. B: Correlation of acceleration duration with acceleration amplitude with data for one participant (black) and regression lines for all participants (gray). C: Correlation of movement distance with acceleration amplitude with data for one participant (black) and regression lines for all participants (gray).
Discussion
We conducted two studies that were designed test and refine the pulse-step model for control of single joint movements. We tested the hypothesis that pulse-height modulation is specifically adapted to control movement speed, and that pulse-width control is modulated to regulate final position around a specified target position. In the first experiment, participants targeted three movement speeds to a single final position target. Under these conditions, acceleration amplitude varied directly, while acceleration duration varied inversely with speed target. In our second study, participants targeted the same speeds, but in the absence of a spatial target. In both cases, acceleration amplitude varied directly with speed condition and strongly scaled with measured peak velocity, confirming the role of pulse-height mechanisms in control of movement speed. However, acceleration duration did not show a positive correlation with acceleration amplitude. This contrasts the model of Gottlieb and colleagues (Corcos et al., 1989; Gottlieb et al., 1989), who reported an increase in torque duration as a consequence of an increase in torque amplitude. Rather, when there was no spatial target, acceleration duration remained constant across speed conditions. These findings confirm that pulse-width control, as measured by variations in acceleration duration, is not obligatorily positively coupled with variations in pulse-height, as measured by variations in acceleration amplitude. The fact that acceleration duration and acceleration amplitude can be negatively correlated in one case, but uncorrelated in the other case provides evidence that these two features of control can be executed independently. Our findings also suggest that pulse-width mechanisms may be specifically adapted for regulating final steady state limb position in relation to a spatial target. In the absence of a spatial target, movement distance and velocity scaled with acceleration amplitude, implicating the role of pulse-height mechanisms in specifying these parameters. More importantly however, acceleration duration was constant across speed conditions, indicating that pulse-width control was not evoked in the absence of spatial targets. In contrast, pulse height and pulse width were strongly negatively correlated for movements made to a spatial target. Taken together, these findings suggest that pulse width modulation may be employed only for fine tuning final steady state limb position around a target location. Pulse height mechanisms on the other hand, appear to reflect the plan to propel the limb along a distance that is consistent with the general vicinity of the target.
Pulse-height and pulse-width modulation reflect distinct control mechanisms
The seminal studies of Gordon and Ghez (1984, 1987a, b) established the pulse-step model in controlling force amplitude in single joint isometric tasks. When participants targeted a range of peak forces, the peak rate of change of force scaled with and thus predicted peak force. However, when data were analyzed within a single force target, small variations in the peak rate of change of force occurred between trials, and force rise time varied inversely with variations in the rate of change of force. The authors (Gordon & Ghez, 1987b) interpreted this finding as evidence that pulse-width control can be regulated independent of pulse-height modulation, and that the former can be employed to compensate for inaccuracies in the latter. However, when data were grouped across force targets, and thus across larger variations in peak rate of change of force, a strong positive relationship was seen between peak force-rate-change and force rise time. This suggested that modulation of rise time might not be independent of peak force-rate-change, when the range of forces sampled was large. In other words, pulse-width control might be limited to very small ranges of force. Consistent with this idea, Gottlieb and colleagues (Corcos et al., 1989; Gottlieb et al., 1989) showed that in a targeted movement task, variations in initial torque amplitude (at 30 ms) and torque rise time are strongly coupled. They proposed a control scheme in which the two variables are obligatorily coupled when controlling movement speed. Our current results extend both lines of work, indicating that a positive relationship between pulse-height and pulse-width measures is not obligatory, and that pulse-width measures can be actively regulated across a large range of movement speeds and accelerations. Pulse-width control also appears to be adapted for fine tuning final position rather than speed.
Although the terminology used by Gottlieb and colleagues (Corcos et al., 1989; Gottlieb, 1996, 1998; Gottlieb, Chen, & Corcos, 1995, 1996; Gottlieb et al., 1989; Gottlieb, Corcos, Agarwal, & Latash, 1990) was different than that of the Ghez group, the control models proposed by both groups were quite similar to one another. Both described a pulsatile output that can be modulated in both amplitude and duration. However, each group used a slightly different experimental paradigm, the Ghez group studying isometric force pulse production, and the Gottlieb group studying targeted elbow movements. In Ghez's studies, peak force rate of change, their measure of pulse-height, always varied directly with the force target. However, in Gottlieb's studies, torque amplitude, their measure of pulse-height, only varied when movement speed was an explicit task requirement. In addition, under these conditions, torque rise time always varied proportionately with torque amplitude. Under conditions in which distance was targeted, without any explicit speed requirements, only variation in torque rise time occurred. These authors therefore hypothesized that pulse-height modulation was employed only to control movement speed, whereas, pulse-width modulation could be employed for control of movement distance, in the absence of speed requirements. However, it should be noted that under speed control conditions, torque rise time was always positively correlated with amplitude, leading the authors to suggest an obligatory coupling of torque rise time with torque amplitude, but not vice versa. The major differences between the models were that Ghez proposed that pulse-width control could be employed independently of pulse-height control, and could be used to compensate errors in the latter, while Gottlieb hypothesized that pulse-width was a passive consequence of pulse-height control. In addition, Gottlieb proposed pulse-height modulation as a speed control strategy, whereas, Ghez did not specify between speed and distance control.
Our current findings integrate and expand the pulse-step model of Ghez and colleagues (Ghez, 1979; Gordon & Ghez, 1984, 1987a, b) with the model of Gottlieb and colleagues (Corcos et al., 1989; Gottlieb, 1996, 1998; Gottlieb et al., 1989, 1990, 1995, 1996). Our findings provide support for the idea that during targeted movement tasks, pulse-height mechanisms specify movement speed, whereas pulse-width mechanisms appear to be adapted for modulation of final limb position. It should be stressed that events occurring in the deceleration phase of movement are critical to achieve positional accuracy. However, because the pulse-step model is based only on the acceleration phase of movement, prior to peak velocity, we have not considered movement deceleration in our analysis. The strong positive relationship between peak velocity and movement distance in this and previous studies (Krakauer et al., 2002; Sainburg & Schaefer, 2004) further emphasizes the importance of the acceleration phase in task performance.
Differential control of trajectory and position
Whereas much previous attention has been focused on control of movement speed and distance, recent electrophysiological and behavioral findings have focused on two analogous features of control, trajectory and position. Scott and colleagues (Kurtzer, Herter, & Scott, 2005) have reported primate motor cortex cells whose activities vary with loads applied during either the postural or trajectory phases of movement. Importantly, many cells showed activities that varied with posture, but were not sensitive to loads applied during movement. In contrast, other cells, the activity of which varied with trajectory loads did not show variations with postural loads. Cells that showed variation with both posture and movement indicated independent tuning functions for each type of load (Kurtzer et al., 2005). These results provide strong support to the idea that trajectory and posture are independently coded and controlled in the CNS. Consistent with these findings, Ebner and colleagues (Fu, Suarez, & Ebner, 1993; Fu, Flament, Coltz, & Ebner, 1995) previously reported cells in premotor and motor cortex whose activity patterns correlated with the initial direction, final position and amplitude of movements. However, the pattern of these correlations changed during the course of movement. Prior to movement, cell activities correlated well with movement direction, whereas correlations with final positions and distance evolved during the course of movement. These findings are consistent with the idea of independent control mechanisms, in which trajectory control is employed mainly through feedforward mechanisms, and positional control employs feedback mechanisms that evolve during the course of motion.
The idea that movement trajectory and final position are differentially controlled is also consistent with studies that have examined adaptation to novel forces (DiZio & Lackner, 1995; Lackner & DiZio, 1994) and visuomotor rotations (Sainburg & Wang, 2002; Wang & Sainburg, 2003, 2004, 2005). In the studies by Lackner and DiZio (DiZio & Lackner, 1995; Lackner & DiZio, 1994), participants reached to a target when adapting to Coriolis force fields, without any visual feedback. When participants first experienced these forces, their handpaths were curved and inaccurate. With practice, these handpaths became straighter and directed towards the target, only when the participants were allowed to touch the target at the end of the movement. However, when no haptic information was available, movements became straighter but were still inaccurate. In a subsequent study of interlimb transfer, DiZio and Lackner (1995) demonstrated that only final position information transferred to the non-exposed limb. This clearly suggests that trajectory and position seem to be differentially controlled. More recent studies investigating interlimb transfer of adaptation to visuomotor rotations have revealed similar distinctions in transfer of trajectory and final position information across limbs, thus lending further support to the idea that these features may be differentially encoded (Sainburg & Wang, 2002; Wang & Sainburg, 2003, 2004, 2005).
Our current findings support a model of control that is consistent with the idea that trajectory and final position are differentially controlled. Trajectory or speed is specified through pre-programming of torque amplitude, whereas final position is modulated largely through feedback control of torque duration. We expect that these two different aspects of control might correspond to the differences in trajectory and positional control that have recently been identified in the electrophysiological and behavioral studies reviewed above. It is not yet known how pulse-height and pulse-width mechanisms might be expressed in multijoint motion, when torque profiles are not linearly related to acceleration profiles. However, preliminary research (Krakauer et al., 2002; Schaefer & Sainburg, 2004) suggests that analogues of pulse-step control occur in multijoint reaching movements. Work from our laboratory indicates that pulse-height and pulse-width mechanisms are not expressed in acceleration amplitude and duration for multijoint movements. Instead, the acceleration profile tends to be biphasic, with the early and later acceleration impulses reflecting largely anticipatory control and modulation of that control respectively (Schaefer & Sainburg, 2004).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagesteiro LB, Sainburg RL. Handedness: Nondominant arm advantages in load compensation during rapid elbow joint movements. Journal of Neurophysiology. 2003;90:1503–1513. doi: 10.1152/jn.00189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Responses to force perturbations preceding voluntary human arm movements. Brain Research. 1981a;220:350–355. doi: 10.1016/0006-8993(81)91224-5. [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Amplitude and instruction dependent modulation of movement related electromyogram activity in humans. Journal of Physiology. 1981b;316:97–107. doi: 10.1113/jphysiol.1981.sp013775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Initial agonist burst duration depends on movement amplitude. Experimental Brain Research. 1984;55:523–527. doi: 10.1007/BF00235283. [DOI] [PubMed] [Google Scholar]
- Brown SH, Cooke JD. Initial agonist burst is modified by perturbations preceding movement. Brain Research. 1986;377:311–322. doi: 10.1016/0006-8993(86)90874-7. [DOI] [PubMed] [Google Scholar]
- Corcos DM, Gottlieb GL, Agarwal GC. Organizing principles for single joint movements. II. A speed-sensitive strategy. Journal of Neurophysiology. 1989;62:358–368. doi: 10.1152/jn.1989.62.2.358. [DOI] [PubMed] [Google Scholar]
- Dizio P, Lackner JR. Motor adaptation to Coriolis force perturbations of reaching movements: Endpoints but not trajectory adaptation transfers to the nonexposed arm. Journal of Neurophysiology. 1995;74:1787–1792. doi: 10.1152/jn.1995.74.4.1787. [DOI] [PubMed] [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Temporal encoding of movement kinematics in the discharge of primate primary motor and premotor neurons. Journal of Neurophysiology. 1995;73:836–854. doi: 10.1152/jn.1995.73.2.836. [DOI] [PubMed] [Google Scholar]
- Fu QG, Suarez JI, Ebner TJ. Neuronal specification of direction and distance during reaching movements in the superior precentral premotor area and primary motor cortex of monkeys. Journal of Neurophysiology. 1993;70:2097–2116. doi: 10.1152/jn.1993.70.5.2097. [DOI] [PubMed] [Google Scholar]
- Ghez C. Contributions of central programs to rapid limb movement in the cat. In: Asanuma H, Wilson VJ, editors. Integration in the nervous system. Tokyo New York: Igaku-Shoin; 1979. [Google Scholar]
- Ghez C, Vicario D. The control of rapid limb movement in the cat. I. Response latency. Experimental Brain Research. 1978a;33:173–189. doi: 10.1007/BF00238058. [DOI] [PubMed] [Google Scholar]
- Ghez C, Vicario D. The control of rapid limb movement in the cat. II. Scaling of isometric force adjustments. Experimental Brain Research. 1978b;33:191–202. doi: 10.1007/BF00238059. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghez C. EMG patterns in antagonistic muscles during isometric contractions in man: Relations to response dynamics. Experimental Brain Research. 1984;55:167–171. doi: 10.1007/BF00240511. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghez C. Trajectory control in targeted force impulses. II. Pulse height control. Experimental Brain Research. 1987a;67:241–252. doi: 10.1007/BF00248546. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghez C. Trajectory control in targeted force impulses. III. Compensatory adjustments in initial errors. Experimental Brain Research. 1987b;67:253–269. doi: 10.1007/BF00248547. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL. On the voluntary movement of compliant (inertial-viscoelastic) loads by parcellated control mechanisms. Journal of Neurophysiology. 1996;76:3207–3229. doi: 10.1152/jn.1996.76.5.3207. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL. Muscle activation patterns during two types of voluntary single-joint movement. Journal of Neurophysiology. 1998;80:1860–1867. doi: 10.1152/jn.1998.80.4.1860. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Chen CH, Corcos DM. ‘Adequate control theory’ for human single-joint elbow flexion on two tasks. Annals of Biomedical Engineering. 1995;23:388–398. doi: 10.1007/BF02584439. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Chen CH, Corcos DM. Nonlinear conrol of movement distance at the human elbow. Experimental Brain Research. 1996;112:289–297. doi: 10.1007/BF00227647. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC. Organizing principles for single joint movements. I. A speed-insensitive strategy. Journal of Neurophysiology. 1989;62:342–357. doi: 10.1152/jn.1989.62.2.342. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC, Latash ML. Organizing principles for single-joint movements. III. Journal of Neurophysiology. 1990;63:625–636. doi: 10.1152/jn.1990.63.3.625. [DOI] [PubMed] [Google Scholar]
- Hoffman DS, Strick PL. Step tracking movements of the wrist in humans. I. Kinematic analysis. Journal of Neuroscience. 1986;6:3309–3318. doi: 10.1523/JNEUROSCI.06-11-03309.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Gordon J, Veytsman M, Ghez C. Contributions of planning and updating to accuracy of reaching movements in normals and stroke. Society for Neuroscience Abstract. 2002;169 [Google Scholar]
- Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nature Neuroscience. 2005;8:498–504. doi: 10.1038/nn1420. [DOI] [PubMed] [Google Scholar]
- Lackner JR, Dizio P. Rapid adaptaion to Coriolis force perturbations of arm trajectory. Journal of Neurophysiology. 1994;72:299–313. doi: 10.1152/jn.1994.72.1.299. [DOI] [PubMed] [Google Scholar]
- Mustard BE, Lee RG. Relationship between EMG patterns and kinematic properties for flexion movements at the human wrist. Experimental Brain Research. 1987;66:247–256. doi: 10.1007/BF00243302. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Schaefer SY. Handedness: Interlimb differences in control of movement extent. Journal of Neurophysiology. 2004;92:1374–1383. doi: 10.1152/jn.00181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: Independence of direction and final position information. Experimental Brain Research. 2002;145:437–447. doi: 10.1007/s00221-002-1140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer SY, Sainburg RL. Interlimb differences in control of movement extent during multijoint reaching. Society for Neuroscience Abstract. 2004;871 doi: 10.1152/jn.00181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Experimental Brain Research. 2003;149:520–526. doi: 10.1007/s00221-003-1392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Limitations in interlimb transfer of visuomotor rotations. Experimental Brain Research. 2004;155:1–8. doi: 10.1007/s00221-003-1691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Adaptation to visumotor rotations remaps movement vectors, not final positions. Journal of Neuroscience. 2005;25:4024–4030. doi: 10.1523/JNEUROSCI.5000-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


