Abstract
The characterization of patients who have acute coronary syndrome (ACS) without critical stenosis is unclear. First, we wanted to learn more about the angiographic and demographic characteristics of patients with non-ST-segment-elevation myocardial infarction who were not in need of percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Second, we wanted to look for further cardiac events during follow-up.
We retrospectively analyzed all patients with ACS plus ischemic-marker elevation from May 2002 through September 2005. Follow-up was obtained primarily by telephone interviews. Of the 1,437 ACS patients who were screened, 127 (8.8%) had noncritical stenosis (study group), and 509 (35.4%) had sufficient stenosis (>50%) to warrant PCI (control group). Patients with noncritical stenosis (≤50%) were significantly younger, were more frequently women, and had fewer risk factors. Myocardial infarction or PCI/CABG occurred in no patients with noncritical stenosis (follow-up, 12.2 mo) and in 5.1% or 16.1% (respectively) of patients with critical stenosis (follow-up, 11.1 mo; P <0.05 for revascularization). Cardiac death was more likely in patients without critical stenosis, but insignificantly so (2.4% vs 1.8%, P=0.6).
Subanalysis: Of patients without critical stenosis, 52.8% had smooth coronary arteries, and 47.3% had mild-to-moderate atherosclerosis (stenosis ≤50%). Follow-up revealed no deaths in the 1st subgroup and 3 cardiac deaths (5%) in the 2nd (P=0.06).
We conclude that the prognosis of patients without significant stenosis was significantly better in regard to revascularization, but statistically the same in regard to mortality.
Key words: Angina, unstable; biological markers; coronary stenosis; myocarditis; syndrome, acute coronary
In patients who have acute coronary syndromes, an elevated level of cardiac troponins (suggestive of myocardial necrosis) is a well-known risk factor for fatal events.1
However, the characterization of and prognosis for patients who present with acute typical anginal pain and elevated ischemic markers—but without critical coronary artery stenosis—remain uncertain. The importance of this problem, which occurs in up to 6% of patients with troponin-positive acute coronary syndrome (ACS) and which seems to be associated with significant morbidity and death,2 appears to be underestimated in clinical practice.
The purpose of our analysis was, first, to compare demographic and clinical findings in patients who have ACS without critical stenosis (Group I) with those same findings in patients who have ACS with stenosis critical enough to warrant percutaneous coronary intervention (PCI) (Group II). Further, we sought to determine whether it is possible before cardiac catheterization to differentiate between patients with or without critical narrowing of the coronary arteries; to test whether a subdivision of the ACS-without-critical-stenosis group could provide more information about this clinical entity; and to compare the prognosis of patients in both ACS groups (with and without critical stenosis) during long-term follow-up.
Patients and Methods
Using our hospital's database, we retrospectively analyzed the records of all consecutive patients who had been admitted from May 2002 through September 2005 with acute typical angina pectoris and elevation of an ischemic marker: troponin I, creatine kinase, or both. All patients had undergone cardiac catheterization within 12 hours of admission to our hospital.
The study population (Group I) consisted of individuals whose coronary angiogram showed noncritical stenosis (≤50%). Such patients were compared with patients in a control group (Group II), who had coronary stenosis substantial enough (>50%) to warrant percutaneous coronary intervention.
Patients were excluded from both groups under the following circumstances: the presence of ST-segment-elevation myocardial infarction (STEMI); a requirement for urgent coronary artery bypass grafting (CABG) after angiography (in consideration of the associated rate of perioperative morbidity and death); refusal by the patient to undergo PCI or CABG after cardiac catheterization; false diagnosis of STEMI as indicated by pacemaker rhythm or bundle-branch block upon electrocardiography (ECG); renal failure, defined here as elevation of creatinine (>220 μmol/L) or as a new or permanent requirement for hemodialysis—which could interfere with measurement of troponin I; sepsis or other infectious disease (clinical signs: fever >38 °C or Creactive protein >100 mg/L); and pulmonary embolism (Fig. 1). Pulmonary embolism was ruled out in each patient of the study group by determining the level of D-dimers; and, whenever D-dimers were elevated, a computed tomographic scan of the lungs was carried out for definitive diagnosis.
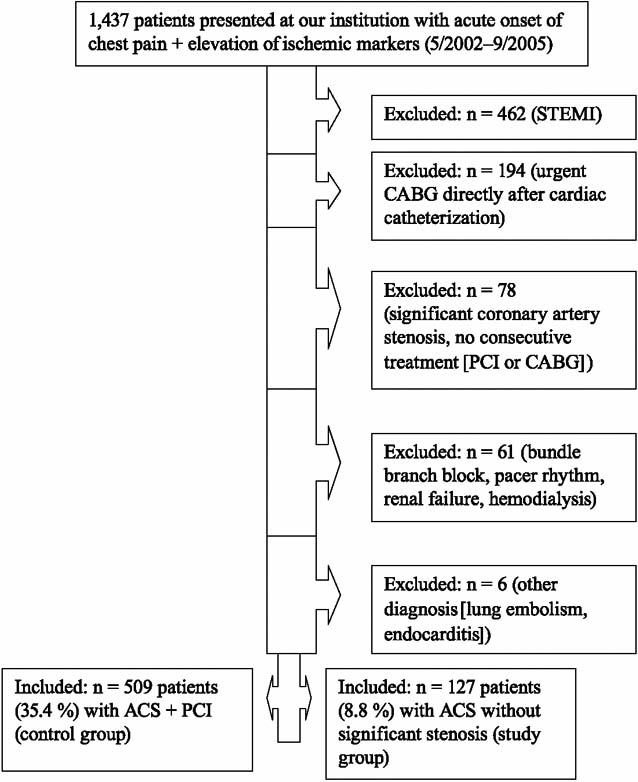
Fig. 1 Source of data for this study.
ACS = acute coronary syndrome; CABG = coronary artery bypass grafting; PCI = percutaneous coronary intervention; STEMI = ST-segment–elevation myocardial infarction
The threshold used to define a positive troponin I test was 0.1 ng/mL and to define a positive test of D-dimers was 0.3 mg/L. Blood was collected in the emergency department. Basic data, previous medications, and laboratory values were recorded on clinical report forms.
Electrocardiographic Analysis
All ECGs were analyzed retrospectively by an independent observer, who recorded ST-segment depression, Q waves, and T-wave inversion.
Cardiac Catheterization
All angiograms—recorded after intracoronary application of nitrates—were analyzed retrospectively by an independent operator. All patients with ACS and stenosis that was visually estimated to be greater than 10% were evaluated further by means of quantitative coronary angiography (Quantcor QCA® V2.0; Pie Medical Imaging; Maastricht, The Netherlands). The percent diameter stenosis, minimal luminal diameter, length of stenosis, and reference diameter were calculated by applying the “worst-view” method.
We isolated and defined 2 subgroups within our Group I study population: patients with no detectable atherosclerosis and patients with atherosclerosis (1%– 50% diameter stenosis).
Intraluminal thrombus was defined as an intraluminal filling defect separate from the adjacent vascular wall, an ulcer was defined as a breakdown of the plaque surface, and vasospasm was defined as a stenosis that could be reversed by the application of nitrates.
Takotsubo-like left ventricular cardiomyopathy was defined as hypokinesis or akinesis from the mid-portion to the apex of the left ventricle together with hyperkinesis in the base, wherever this extended over a territory supplied by more than 1 coronary artery.3
Follow-Up
Follow-up data were obtained by reviewing the patient's hospital charts, by standardized telephone interviews, by contact with the patient's physician, or by periodic outpatient visits. Anginal status was noted, and events were identified as myocardial infarction or as those requiring reintervention or readmission to the hospital. The cause of death was subclassified as cardiac or noncardiac.
Statistics
Continuous variables were expressed as mean ± SD. Comparisons for continuous variables were performed using Student's t test. All P values were 2-tailed, and a P value <0.05 was considered to be statistically significant.
Results
Basic Data
We identified 1,437 consecutive patients with recent onset of chest pain and a serum elevation of troponin I or creatine kinase who were admitted to our hospital from May 2002 through September 2005.
In accordance with our above criteria, we excluded 801 patients (Fig. 1). One hundred twenty-seven patients (8.8% of all screened patients) were found to have ACS and no critical narrowing of a coronary artery (Group I, the study population), and 509 patients (35.4% of all screened patients) were found to have ACS with coronary stenosis substantial enough to warrant PCI (Group II, the control group).
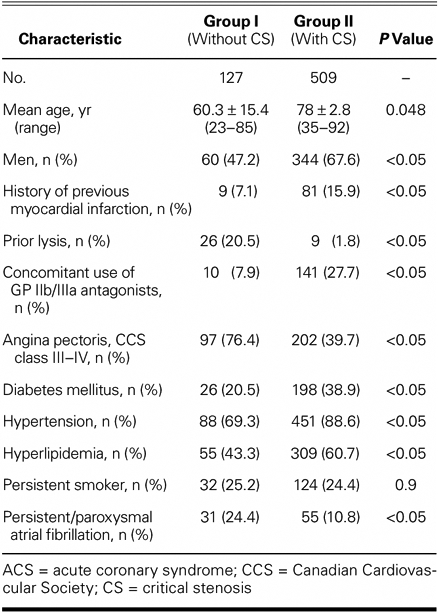
Significant differences between these 2 groups of patients were found in terms of age, sex, history of previous myocardial infarction, concomitant medication, and anginal status, and with regard to risk factors other than current smoking status (Table I). Group I patients were significantly younger and more often female, and they had fewer cardiovascular risk factors. Atrial fibrillation and severe angina (Canadian Cardiovascular Society class III or IV) were more frequent among these patients. Significantly more Group I patients had undergone lysis without ST-segment elevation before transferring to our center, but concomitant use of GP IIb/IIIa antagonists was more frequent among Group II patients. No Group I patient showed any evidence of intravascular thrombotic material.
TABLE I. Basic Data on ACS Patients with and without Critical Stenosis
Laboratory Values
We recorded the maximum levels of creatine kinase (μmol/s/L) during the hospital stay. For Group I, these were: mean, 6.06 ± 8.8; range, 0–54.6. For Group II, these were: mean, 7.1 ± 10.8; range, 0.3–103.9 (P=0.4). We also recorded the initial levels of troponin I (ng/mL). For Group I, these were: mean, 11.83 ± 30.2; range, 0–246. For Group II, these were: mean, 9.98 ± 22.8; range, 0.09–352 (P=0.5). The initial levels (at admission) of C-reactive protein (mg/L) were also examined. For Group I, these were: mean, 21.9 ± 34.2; range, 0.1–219. For Group II, they were: mean, 16.3 ± 33.5; range, 0.1–268 (P=0.09). We found no statistically significant differences between any of these findings.
Electrocardiographic Analysis
Analysis of the ECG for the presence of ST-segment depression (Group I, 27 [21.3%] versus Group II, 79 [15.5%], P=0.2) and the presence of T-wave inversion (Group I, 65 [51.2%] versus Group II, 206 [40.5%], P=0.06) revealed no significant differences. However, Q waves were significantly more frequent among Group I patients: 14 (11%) versus 27 (5.3%) among Group II patients, P=0.03.
Angiographic and Quantitative Coronary Angiographic Analysis
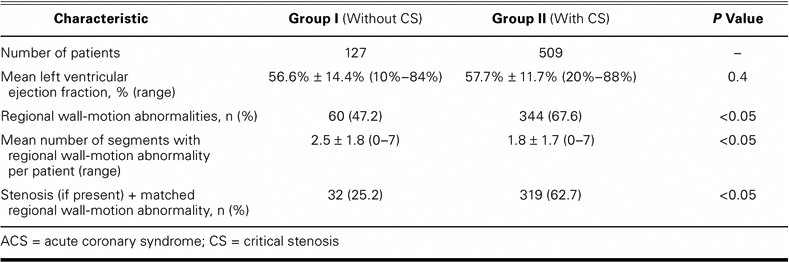
The left ventricular ejection fraction of both groups showed no differences. Analysis of regional wall-motion abnormalities showed significantly more abnormalities in Group II patients, although the number of segments, per patient, with regional wall-motion abnormality was significantly higher among Group I patients. If a stenosis was present, we found a matched regional wall-motion abnormality in 25.2% of Group I patients, versus 62.7% of Group II patients (P <0.05; Table II).
TABLE II. Angiographic Analysis of ACS Patients with and without Critical Stenosis
In Group I patients, we found normal coronary arteries with no suspected atherosclerosis in 52.8% and noncritical stenosis or sclerosis in the remainder (Fig. 2A). When stenosis was present in Group I patients, its distribution was predominantly (61%) in the left anterior descending coronary artery (Fig. 2B).
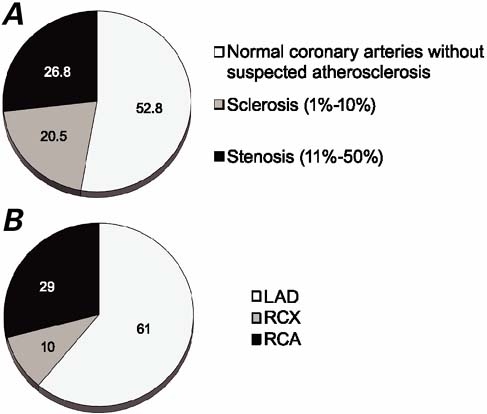
Fig. 2 Angiographic data pertaining to patients who had acute coronary syndrome without critical stenosis (n=127). A) Percentages of patients with no visible stenosis, very little stenosis, or moderate stenosis; and B) locations of moderate stenosis (>10%–50%) in 34 patients (with 41 stenoses).
LAD = left anterior descending coronary artery; RCA = right coronary artery; RCX = right circumflex artery
Quantitative coronary angiographic analysis of the stenoses (11%–50%) in Group I patients showed a mean percentage diameter of 28.2% (range, 19%–37%) for the right coronary artery and 39.3% (range, 31%–46%) for the right circumflex artery, and a mean minimal luminal diameter of 2.25 mm (range, 1.77–3.43 mm) for the right coronary artery and 1.91 mm (range, 1.56– 2.25 mm) for the right circumflex artery.
We found no evidence of any thrombus, ulcer, or vasospasm in any Group I patient.
A takotsubo-like left ventricular cardiomyopathy was identified in 10 Group I patients (7.9%), and a muscular bridge was found in 5 Group I patients (3.9%). No variant of a takotsubo-like left ventricular cardiomyopathy was observed in Group II.
Cardiac Magnetic Resonance Imaging
In the 21 patients who underwent cardiac magnetic resonance imaging (MRI), cine studies of 10 (47.6%) revealed spotted hyperintense lesions (with or without late enhancement) or edema (focal or global) suggestive of an ongoing myocarditis. None of these 10 patients with possible myocarditis had stenosis as we defined it (11%–50%). Nine of these patients (90%) had normal coronary arteries with no suspected atherosclerosis, while the remaining patient had coronary atherosclerosis (1%–10%). Nine of 11 patients (81.8%) without conspicuous MRI findings had normal coronary arteries with no suspected atherosclerosis, while 2 patients (18.2%) did manifest atherosclerosis (1%–10%).
Follow-Up Data
The mean follow-up duration was 12.2 months (range, 0.3–43 mo) in Group I patients and 11.1 months (range, 1–40 mo; P=0.3) in Group II patients.
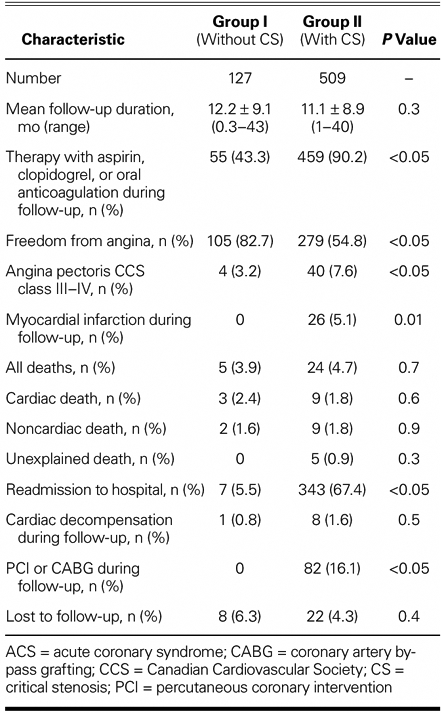
Therapy with aspirin, clopidogrel, or oral anticoagulants was significantly more frequent among Group II patients than among Group I patients. Myocardial infarction was observed in no Group I patient and in 5.1% of Group II patients (P <0.05).
All deaths were homogenously distributed, whether they were of a cardiac, noncardiac, or unexplained nature. Analysis of the deaths of Group I patients revealed that 2 patients died due to cardiogenic shock during the hospital stay, 1 patient died at home due to heart failure, and 2 died of noncardiac causes (intestinal infarction and cancer). We observed a nonsignificant trend towards a higher cardiac mortality rate among Group I patients (2.4% vs 1.8%, P=0.6).
Readmission to the hospital and revascularization were observed in significantly more Group II patients, but the incidence of cardiac decompensation was not different between these 2 groups (Table III).
TABLE III. Follow-Up Data (All Patients)
Subanalysis: Acute Coronary Syndrome with and without Atherosclerosis
We isolated and defined 2 subgroups within our Group I study population: patients with no detectable atherosclerosis and patients with atherosclerosis (noncriticalstenosis of 1%–50% diameter). Patients who had no detectable atherosclerosis were significantly younger (mean, 53.8 ± 16.2 yr; range, 23–84 yr) than were thepatients who had 1%–50% stenosis (mean, 67.5 ± 11 yr; range, 42–85 yr, P <0.05), were mostly female (65.7% vs 38.3%, P <0.05) and manifested significantly fewer risk factors (for diabetes mellitus, 9% vs 33.3%; P <0.05; and for hypertension, 53.7% vs 86.6%; P <0.05), with the exception of hyperlipidemia (35.8% vs 52.6%; P= 0.07). Atrial fibrillation was homogenously distributed (23.9% vs 25%; P=1.0). The left ventricular ejection fraction was significantly lower among Group I patients who had 1%–50% stenosis (mean, 53.26% ± 15.3%; range, 10%–76%) than among Group I patients who had normal angiograms (mean, 59.59% ± 13.1%; range, 26%– 84%; P <0.05).
Study of the follow-up data revealed no deaths among patients who had normal coronary arteries, versus 3 cardiac deaths (5%) among patients who had atherosclerosis (P=0.06). Myocardial infarction was observed in no patient of either group.
Discussion
Patients who display both acute coronary syndrome and elevation of ischemic markers suggestive of myocardial damage are at high risk of fatal events,1 but in some cases angiography reveals no critical stenosis.
After we safely excluded other possible causes of a rise in ischemic markers, we observed this phenomenon in a large patient series at a frequency of approximately 9%, a figure that agrees well with the findings of other studies.2,4
In our attempt to answer the question of whether one can feasibly discriminate, before cardiac catheterization, between these 2 syndromes (ACS with no critical narrowing of a coronary artery vs ACS with coronary stenosis substantial enough to warrant PCI), we used tests that were not able to make such a distinction. For example, we found that C-reactive protein levels increased in troponin-positive patients regardless of whether they had critical stenosis or not, just as Dokainish and associates observed.2
The prognosis of patients with ACS who have no critical narrowing of a coronary artery seems to be better than that of patients with ACS who have coronary stenosis substantial enough to warrant PCI; however, in our study cardiac death and cardiac decompensation occurred in both groups, with no significant differences between groups. Other authors have reported a higher incidence of cardiac death among troponin-positive patients with coronary artery disease,5 or near-identical rates of death, infarction, or readmission for troponin-positive patients with or without coronary artery disease.2 In our study, the prognosis of Group I patients in regard to survival was not different from that of Group II patients, but the Group I prognosis in regard to repeat hospitalization was better.
What causes this elevation of ischemic markers that suggests an ongoing destructive process inside the myocardium? Our analysis of Group I patients revealed 2 subgroups with different characteristics and prognoses —which might shed some light on the pathogenesis of this syndrome.
Approximately half of our Group I patients had normal coronary arteries without any suspected atherosclerosis, whereas the rest showed signs of atherosclerosis (1%–50% diameter stenosis). In these patients without atherosclerosis, we observed a nonsignificant trend towards a lower mortality rate.
Using cardiac MRI as a sensitive approach to the detection of myocarditis, we found signs of myocarditis in some of these patients without atherosclerosis, an observation that is consistent with those of other studies.6–8
Patients with atherosclerosis (stenosis ≤50%) and ACS had a worse prognosis than did those who had smooth coronary arteries. Altogether, these findings support the concept of an insubstantial but malignant stenosis that has a pathogenesis and prognosis resembling that of ACS with coronary stenosis substantial enough to warrant PCI; yet it falls into a different subgroup. This idea was further developed by Scheffold and colleagues.9
Other possible causes of ACS with no critical narrowing of a coronary artery include coronary vasospasm. A positive spasm provocation test3,10,11 and a microvascular dysfunction test12 have been described. We of course were not able to use these in a retrospective study, nor did we find any transitional ST-segment elevation or vasospasm during our study of the angiograms of these patients.
Overlapping syndromes—such as “takotsubo cardiomyopathy” or “stress cardiomyopathy”—have an analogous pathogenesis that is sometimes due to exaggerated sympathetic stimulation; they also play an important causative role in ACS. We observed apical ballooning in 8.7% of our Group I patients.10,13,14
In conclusion, ACS with no relevant narrowing of a coronary artery is a frequently occurring syndrome with a heterogeneous pathogenesis. It therefore demands further evaluation in order that better treatment approaches might be developed.
Limitations
This study was a single, high-volume-center analysis, which was nonrandomized and retrospective. An implicit bias due to loss of patients during follow-up may have played an important role.
Conclusion
Patients with elevated ischemic markers but no critical narrowing stenosis represented approximately 9% of all patients whose records we screened; differentiation, before cardiac catheterization, into patients with critical versus noncritical stenosis was not possible.
Although the prognosis of Group I patients (who had ACS with no critical stenosis) was better than that of Group II patients (who had ACS with coronary stenosis severe enough to warrant PCI), cardiac death occurred without any significant difference between the groups. A subdifferentiation of 2 distinct syndromes of ACS with no critical stenosis seems to be plausible: the 1st subgroup consists of patients with atherosclerosis and an inferior prognosis due to an insubstantial but symptomatic stenosis; and the 2nd subgroup consists of patients with no signs of atherosclerosis, but usually with myocarditis.
Footnotes
Address for reprints: Hubertus von Korn, MD, Department of Cardiology, Krankenhaus Hetzelstift, Stiftstr. 10, 67434 Neustadt/Weinstrasse, Germany. E-mail: h.vonkorn@new.marienhaus-gmbh.de
References
- 1.Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol 2001;38(2):478–85. [DOI] [PubMed]
- 2.Dokainish H, Pillai M, Murphy SA, DiBattiste PM, Schweiger MJ, Lotfi A, et al. Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS-TIMI-18 substudy [published erratum appears in J Am Coll Cardiol 2005;45(11):1911]. J Am Coll Cardiol 2005;45(1):19–24. [DOI] [PubMed]
- 3.Kurisu S, Sato H, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J 2002;143(3): 448–55. [DOI] [PubMed]
- 4.Diver DJ, Bier JD, Ferreira PE, Sharaf BL, McCabe C, Thompson B, et al. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI-IIIA Trial). Am J Cardiol 1994;74(6):531–7. [DOI] [PubMed]
- 5.Maurin T, Zeller M, Cottin Y, Touzery C, Beer JC, L'Huillier I, et al. Long-term prognosis of patients with acute coronary syndrome and moderate coronary artery stenosis. Cardiology 2003;99(2):90–5. [DOI] [PubMed]
- 6.Angelini A, Calzolari V, Calabrese F, Boffa GM, Maddalena F, Chioin R, Thiene G. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart 2000;84(3):245–50. [DOI] [PMC free article] [PubMed]
- 7.Sarda L, Colin P, Boccara F, Daou D, Lebtahi R, Faraggi M, et al. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol 2001;37(3):786–92. [DOI] [PubMed]
- 8.Dec GW Jr, Waldman H, Southern J, Fallon JT, Hutter AM Jr, Palacios I. Viral myocarditis mimicking acute myocardial infarction. J Am Coll Cardiol 1992;20(1):85–9. [DOI] [PubMed]
- 9.Scheffold N, Schongart H, Kaag N, Eisenlohr E, Cyran J. Intravascular ultrasound in recurrent myocardial ischemia and unremarkable coronary angiogram [in German]. Dtsch Med Wochenschr 1998;123(18):556–61. [DOI] [PubMed]
- 10.Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001;38(1):11–8. [DOI] [PubMed]
- 11.Wang CH, Kuo LT, Hung MJ, Cherng WJ. Coronary vasospasm as a possible cause of elevated cardiac troponin I in patients with acute coronary syndrome and insignificant coronary artery disease. Am Heart J 2002;144(2):275–81. [DOI] [PubMed]
- 12.Marroquin OC, Holubkov R, Edmundowicz D, Rickens C, Pohost G, Buchthal S, et al. Heterogeneity of microvascular dysfunction in women with chest pain not attributable to coronary artery disease: implications for clinical practice. Am Heart J 2003;145(4):628–35. [DOI] [PubMed]
- 13.Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart 2003; 89(9):1027–31. [DOI] [PMC free article] [PubMed]
- 14.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352(6):539–48. [DOI] [PubMed]


