Abstract
Antiphospholipid syndrome is a well-defined entity that is characterized by spontaneous abortion, thrombocytopenia, and recurrent arterial and venous thromboses. A partially calcified right atrial thrombus mimicking myxoma with recurrent pulmonary embolism has not been previously reported in a patient who also had systemic lupus erythematosus and secondary antiphospholipid syndrome.
Herein, we describe the case of a 37-year-old woman with systemic lupus erythematosus and secondary antiphospholipid syndrome who was admitted to the hospital with progressive exertional dyspnea. Ventilation-perfusion scanning showed multiple parenchymal defects in the lungs that portended pulmonary embolism. In addition, the scanning revealed normal regional ventilation. Transthoracic and transesophageal echocardiography showed a right atrial mass that was highly suggestive of myxoma, and the patient subsequently underwent surgery. A histologic examination showed an organized, partially calcified thrombus.
Intracardiac thrombus has been rarely reported as a complication of antiphospholipid syndrome. In our patient, the preoperative investigations could not differentiate the partially calcified right atrial thrombus from a myxoma, and the diagnosis was made postoperatively.
Key words: Antibodies, anticardiolipin/blood; antiphospholipid syndrome/complications; autoimmune diseases/complications; coronary thrombosis/complications/diagnosis/epidemiology/radiography/surgery; heart atria; heart neoplasms/diagnosis; lupus erythematosus, systemic/complications; myxoma/diagnosis; recurrence; thrombosis/complications/diagnosis/etiology/pathology/prevention & control/surgery
Antiphospholipid syndrome is characterized by spontaneous abortion, thrombocytopenia, and recurrent arterial and venous thromboses in association with medium-to-high titers of antiphospholipid antibodies or positive lupus anticoagulant test results. The disorder is referred to as primary antiphospholipid syndrome when it occurs alone1; however, it can also be found in association with systemic lupus erythematosus (SLE). Less well known is the association between antiphospholipid antibodies and primary intracardiac thrombosis.
Right atrial thrombus mimicking myxoma with recurrent pulmonary embolism (PE) has not, to the best of our knowledge, been previously reported in a patient who also had SLE and secondary antiphospholipid syndrome. Here, we report the case of a 37-year-old woman who presented with these conditions.
Case Report
In July 2007, a 37-year-old woman who had SLE and secondary antiphospholipid syndrome was admitted to the hospital with progressive exertional dyspnea. The patient was deemed to be in New York Heart Association functional class III.
Due to malar rash, arthralgia, photosensitive rash, and positive tests for antinuclear antibodies, the patient was diagnosed with SLE in 1987, and she underwent treatment with steroids.
In 2006, she was diagnosed with antiphospholipid syndrome, because she had elevated levels of antiphospholipid antibodies and positive lupus anticoagulant test results. Also, she had a history of recurrent fetal losses of less than 10 weeks' gestation. She began oral anticoagulation (international normalized ratio [INR] levels, 2–3) in 2006.
At the 2007 presentation, physical examination revealed a blood pressure of 140/60 mmHg, a heart rate of 80 beats/min, and respiration of 16 breaths/min. All heart sounds were regular and distant; no murmurs or rubs were noted. The jugular venous pressure was normal. The patient's lungs were clear, her abdomen was soft, and no peripheral edema was found. An electrocardiogram showed sinus tachycardia but no other abnormalities. A chest radiograph showed a normal-sized heart with a prominent pulmonary artery: a finding that suggested pulmonary artery hypertension. Color Doppler and duplex-scan ultrasonography of the lower limbs ruled out deep venous thrombosis. The INR was 2.7, and the hematocrit level was 35%.
Ventilation-perfusion scanning of the lungs showed multiple parenchymal defects, which portended a high probability of PE (Fig. 1). In addition, the scanning showed normal regional ventilation.
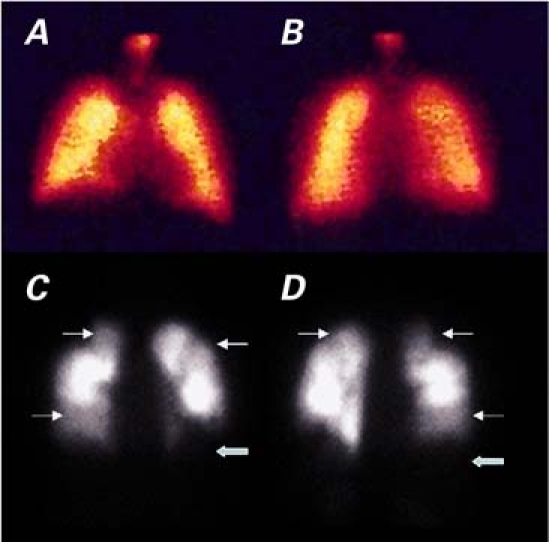
Fig. 1 Lung perfusion scanning in A) anterior and B) posterior views shows a normal appearance in a normal patient. By comparison, multiple perfusion defects in C) anterior and D) posterior views suggest the high probability of a pulmonary embolus in our patient. The thick arrows indicate areas of severe perfusional defects; the thin arrows indicate areas of hypoperfusion.
Two-dimensional transthoracic echocardiography revealed normal left ventricular and left atrial dimensions. A polypoid, partially calcified, mobile mass (3.85 × 1.15 cm), which was attached to the free wall of the right atrium, highly suggested a myxoma (Fig. 2A). Doppler echocardiography revealed a systolic pulmonary artery pressure of 55 mmHg. In view of these findings, which suggested recurrent PE that was refractory to oral anticoagulation (INR levels, 2–3), transesophageal echocardiography was performed, and this confirmed the presence of a highly mobile and friable mass that adhered to the free wall of the right atrium (Fig. 2B).
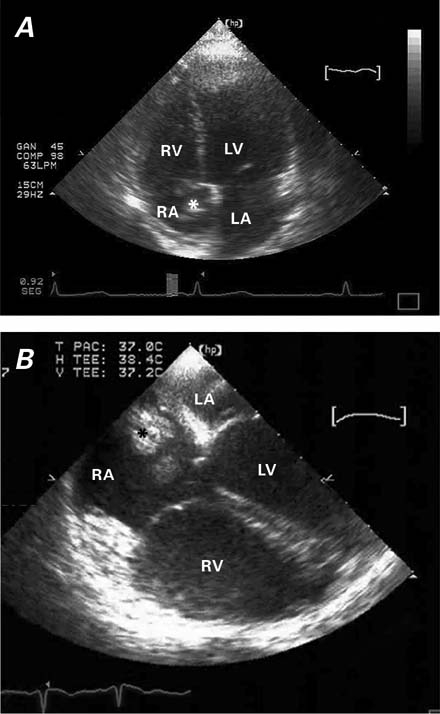
Fig. 2 A) Transthoracic and B) transesophageal echocardiography show a polypoid mass (*) that is attached to the free wall of the right atrium (RA); the mass impinges upon the tricuspid septal valve.
LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle
Real-time motion images are available at texasheart.org/journal.
The patient subsequently underwent surgery. After the institution of cardiopulmonary bypass, the right atrium was opened, and the large mass was removed from the free wall. Gross examination of the operative specimen revealed multiple masses with irregular surfaces (Fig. 3A); histologic examination showed an organized thrombus with a fibrin network and focal calcifications (Fig. 3B).
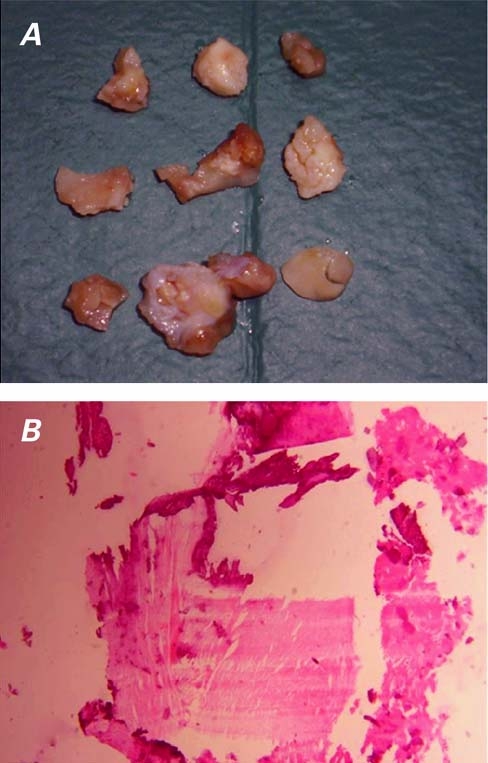
Fig. 3 A) A photograph of the operative specimen reveals multiple masses with irregular surfaces. B) Histologic examination shows an organized thrombus with a fibrin network and focal calcifications (H & E, orig. ×4).
The patient experienced no postoperative complications. However, 3 weeks later, 2-dimensional transthoracic echocardiography showed recurrence of an intracardiac mass: a new thrombus, 1.5 cm in diameter, was attached to the free wall of the right atrium. After this finding, we added 100 mg/day of aspirin to the patient's medical therapy and increased the dosage of warfarin to that of high-intensity therapy (INR levels, 3–4). She had an incomplete response to 1st-line therapy, which led us to add rituximab (1 dose weekly at 375 mg/m2 for 4 weeks).
The patient responded to this 2nd-line therapy and was discharged from the hospital 27 days after surgery, on a regimen of prednisone (5 mg/d), ibuprofen (1,200 mg/d), aspirin (100 mg/d), and warfarin (INR, 3–4).
Discussion
The differential diagnosis of right atrial mass includes vegetation, tumor, and thrombus. The most frequent echocardiographic findings in antiphospholipid syndrome are either valve vegetations or thickening of the valve leaflets. Although tricuspid vegetations can mimic a right atrial mass, they are typically attached to the valve leaflet. These findings are more frequent than that of intracardiac thrombus.
The most common primary cardiac tumor is the myxoma. Fifteen percent of atrial myxomas arise in the right atrium, and they are usually attached to the interatrial septum. Although in our patient the attachment site was the free wall of the right atrium, atrial myxoma was a possible diagnosis.
Right atrial thrombi can develop in several situations. First, they may arise from venous emboli that have become entrapped in the right heart. These thrombi often appear as mobile, irregular masses that float freely in the right atrium. Our patient had a highly mobile and friable mass, but it was not floating freely in the right atrium. In addition, a right atrial thrombus may develop in situ under low-flow conditions. These thrombi are typically immobile, and this too was not the hemodynamic situation of our patient.
The risk of intracardiac thrombosis or thromboembolism is increased in patients who have SLE and who either show positive test results for lupus anticoagulant activity or have medium or high levels of anticardiolipin antibodies.2
A retrospective study of 637 patients3 found that venous thrombosis with PE was more frequent in persons who had lupus anticoagulant activity; while coronary, cerebrovascular, and peripheral arterial events were more likely in individuals who had elevated levels of IgG or IgM anticardiolipin antibodies.
The presence of calcium in our patient's mass helped to narrow the diagnosis. The calcification of an atrialmyxoma appears to occur more frequently when the tumor is in the right atrium4; however, the calcification of thrombi in all cardiac chambers has been reported.5–8
Patients who have SLE and secondary antiphospholipid syndrome can develop intracardiac thrombi. In our patient, preoperative investigations could not differentiate such a thrombus from a myxoma; consequently, the diagnosis was made postoperatively.
It remains unknown whether prolonged heparin administration, thrombolysis, high-intensity anticoagulation with warfarin, intravenous administration of rituximab (an anti-CD20 monoclonal antibody), or surgical excision is the best therapeutic approach. Regardless, the great size of this mass and its polypoid and mobile appearance on 2-dimensional echocardiography—which seemed to place our patient at high risk for PE—led us to choose surgical excision. Moreover, the pathologic features of an organized thrombosis with early calcified deposits, which could constitute the nucleus for further thrombus deposition, further supported the surgical approach.
Recurrent events are common in antiphospholipid syndrome. Most observers have noted that initial arterial thrombosis tends to be followed by an arterial event, and initial venous thrombosis, by a venous event.9–11 The factors that determine whether the affected circulation will be venous or arterial are not known.
Among patients who have antiphospholipid syndrome, the recurrence rate of thrombotic events varies. A prospective study of 360 patients (326 with lupus anticoagulant and 185 with anticardiolipin antibodies) determined that 34 (9%) experienced a recurrent thrombotic event during a median follow-up period of approximately 4 years. Some of these events occurred despite oral anticoagulation therapy,10 as was the case with our patient during the immediate postoperative period.
In other series, higher recurrence rates have resulted. One prospective study of 81 patients who had antiphospholipid antibodies found that 31% experienced recurrent ischemic strokes; patients with high titers of anticardiolipin antibodies (>100 GPL units) and patients with intracardiac thrombus had a shorter time until recurrence.12,13
Conclusion
Intracardiac thrombus has rarely been reported as a complication of antiphospholipid syndrome. Partiallycalcified right atrial thrombus mimicking myxoma with recurrent PE in a patient who also had SLE and secondary antiphospholipid syndrome has not, to our knowledge, been previously reported. In our patient, preoperative investigations could not differentiate such athrombus from a myxoma, and the diagnosis was madepostoperatively.
Patients who have antiphospholipid syndrome and SLE are at high risk of developing intracardiac thrombi; however, the thrombi can be successfully resected. To preclude thrombus formation, warfarin treatment is indicated for all patients who have antiphospholipid syndrome. If thrombotic events recur, aspirin therapy should be added, and warfarin should be increased by means of high-intensity therapy. Our patient had an incomplete response to 1st-line therapy, but she responded to additional therapy with rituximab and was released from the hospital on the medical regimen described above.
Supplementary Material
Footnotes
Address for reprints: Tomás F. Cianciulli, MD, FACC, Echocardiography Laboratory, Department of Cardiology, Hospital of the Government of the City of Buenos Aires “Dr. Cosme Argerich,” Alte. Brown 240 (C1155ADP), Buenos Aires, Argentina. E-mail: tcianciulli@fibertel.com.ar
Drs. Cianciulli and Saccheri are researchers for the Secretary of Health, Government of the City of Buenos Aires.
References
- 1.Ames PR, Khamashta MA, Hughes GR. Clinical and therapeutic aspects of the antiphospholipid syndrome. Lupus 1995; 4 Suppl 1:S23–5. [DOI] [PubMed]
- 2.Galli M, Luciani D, Bertolini G, Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood 2003;101(5):1827–32. [DOI] [PubMed]
- 3.Soltesz P, Veres K, Lakos G, Kiss E, Muszbek L, Szegedi G. Evaluation of clinical and laboratory features of antiphospholipid syndrome: a retrospective study of 637 patients. Lupus 2003;12(4):302–7. [DOI] [PubMed]
- 4.de Roos A, Weijers E, van Duinen S, van der Wall EE. Calcified right atrial myxoma demonstrated by magnetic resonance imaging. Chest 1989;95(2):478–9. [DOI] [PubMed]
- 5.Morales C, Bernal JM, Rabasa JM, Gutierrez F, Val F, Revuel-ta JM. Calcified pedicled thrombus in the left ventricle: the fine art of nature. J Thorac Cardiovasc Surg 1997;114(3): 491–2. [DOI] [PubMed]
- 6.Patel AK, Kroncke GM, Heltne CE, Kosolcharoen PK, Thomsen JH. Multiple calcified thrombi (rocks) in the right ventricle. J Am Coll Cardiol 1983;2(6):1224–7. [DOI] [PubMed]
- 7.Minatoya K, Okabayashi H, Yokota T, Hoover EL. Calcified ball thrombus in the left atrium. Ann Thorac Surg 1996;61 (5):1513–4. [DOI] [PubMed]
- 8.Noma M, Itoh T. Images in cardiovascular medicine. Constrictive pericarditis with right atrial thrombus. Circulation 1996;94(7):1786. [DOI] [PubMed]
- 9.Rosove MH, Brewer PM. Antiphospholipid thrombosis: clinical course after the first thrombotic event in 70 patients. Ann Intern Med 1992;117(4):303–8. [DOI] [PubMed]
- 10.Finazzi G, Brancaccio V, Moia M, Ciaverella N, Mazzucconi MG, Schinco PC, et al. Natural history and risk factors for thrombosis in 360 patients with antiphospholipid antibodies: a four-year prospective study from the Italian Registry. Am J Med 1996;100(5):530–6. [DOI] [PubMed]
- 11.Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med 1995;332(15): 993–7. [DOI] [PubMed]
- 12.Levine SR, Brey RL. Neurological aspects of antiphospholipid antibody syndrome. Lupus 1996;5(5):347–53. [DOI] [PubMed]
- 13.Turiel M, Sarzi-Puttini P, Peretti R, Rossi E, Atzeni F, Parsons W, Doria A. Thrombotic risk factors in primary antiphospholipid syndrome: a 5-year prospective study. Stroke 2005;36(7):1490–4. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


