Abstract
Cardiac support with a ventricular assist device is among the few treatments for heart-failure patients who have profound cardiogenic shock unresponsive to vasopressors and intra-aortic balloon pumps. The TandemHeart® percutaneous ventricular assist device can provide temporary support until another device can be placed or a donor heart becomes available.
We examined the TandemHeart's effect on cardiac index, central venous pressure, mixed venous oxygen saturation, creatinine, mean arterial pressure, urine output, and 30-day mortality rate in 5 heart-failure patients (2 with nonischemic and 3 with ischemic cardiomyopathy; mean preoperative left ventricular ejection fraction, 0.17 ± 0.056). Two patients were undergoing cardiopulmonary resuscitation when the device was inserted. The average duration of TandemHeart support was 7.6 ± 3.2 days; all patients were successfully bridged to transplantation.
The TandemHeart improved the cardiac index (1.9 ± 0.3 vs 3.5 ± 0.8 L/[min·m2], P= 0.01), mean arterial pressure (69 ± 12.5 vs 91 ± 4.3 mmHg, P=0.009), mixed venous oxygen saturation (45.4 ± 14.3 vs 71.4 ± 7.5, P=0.009), and urine output (1,861 ± 988 vs 4,314 ± 1,346 mL/hr, P=0.01). The device decreased central venous pressure (21.2 ± 7.4 vs 12.8 ± 5.9 mmHg, P=0.02) and pressor requirements (2.4 ± 1.1 vs 1.0 ± 0.7 agents, P=0.02). Average long-term follow-up after heart transplantation was 8.4 ± 9.9 months, with no deaths.
We conclude that the TandemHeart can provide hemodynamic support for patients with profound, refractory cardiogenic shock. Furthermore, the device can bridge patients to cardiac transplantation and can be placed percutaneously, without invasive surgery.
Key words: Assisted circulation/instrumentation/methods; blood vessel prosthesis implantation; cardiac output; cardiomyopathies/complications/mortality/therapy; equipment design; heart-assist devices; heart failure/complications/therapy; hemodynamics; preoperative care; shock, cardiogenic/mortality/physiopathology/therapy; treatment outcome
Despite improvements in the medical treatment of heart failure and the well-established usefulness of the intra-aortic balloon pump (IABP),1–3 the mortality rate of patients with cardiomyopathy and cardiogenic shock remains considerable.4 Treatment options for this high-risk group include ventricular assist devices (VADs), but these are associated with substantial morbidity, especially in acute circumstances. In addition, the invasive procedure necessary to implant VADs requires cardiopulmonary bypass (CPB), which significantly increases the risk of morbidity and death.
A newly available option for these patients is the TandemHeart® percutaneous VAD (pVAD) (CardiacAssist, Inc.; Pittsburgh, Pa)—an extracorporeal, axial-flow pump connected to a catheter that crosses the atrial septum and aspirates blood from the left atrium. The blood is then returned to the body through an outflow cannula that is inserted into the femoral artery. This pump can be placed quickly in the cardiac catheterization laboratory and requires only femoral access procedures. Unlike the IABP, the pVAD can generate ventricular unloading at flow rates of up to 4 L/min.
The 1st described use of the pVAD was in 18 patients who experienced cardiogenic shock after myocardial infarction.5 The device provided adequate support that resulted in improved blood pressure, cardiac index, pulmonary capillary wedge pressure, and central venous pressure. Although the pVAD restored systemic perfusion, the high-risk group of patients experienced a 30-day mortality rate of 44%.
The pVAD has also been used in the catheterization laboratory for hemodynamic support during high-risk coronary interventions.6–11 In addition to its having provided successful temporary support for patients with acute myocarditis,12,13 the TandemHeart has served as a bridge to cardiac transplantation in 1 patient.14
Long-term implantable VADs, including the pulsatile pumps and the newer generation of axial-flow devices, can provide adequate support for patients with end-stage cardiomyopathy and can serve as a bridge to transplantation.15 However, these pumps have a less clear role in patients who are experiencing acutely decompensated heart failure. Available mechanical support options for these patients include extracorporeal pumps that require open surgical cardiac cannulation. In contrast, the TandemHeart can be placed percutaneously, and it affords immediate cardiac support.
We studied the TandemHeart's use in 5 patients who were experiencing end-stage cardiomyopathy and cardiogenic shock. Although this pVAD was intended to support these patients only until a longer-term device could be implanted, all experienced substantial improvement in cardiac function and were therefore listed for urgent cardiac transplantation.
Patients and Methods
From June 2005 through May 2007, 5 heart-failure patients with existing cardiomyopathy underwent pVAD placement at our institution for immediate cardiac support in the presence of acute cardiogenic shock (Table I). These patients were unresponsive to multiple vasopressors and continued to experience cardiogenic shock despite maximal support. Criteria for pump placement included evidence of end-organ failure, elevated left ventricular filling pressures, cardiac index <2.0 L/(min·m2), the use of pressor agents, and IABP support. Before pVAD insertion, informed written consent was obtained from each patient's surrogate decision-maker.
TABLE I. Clinical Experience with the TandemHeart pVAD as a Bridge to Transplantation
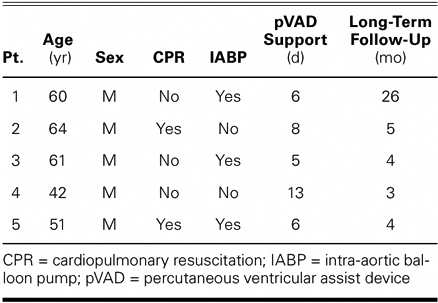
Data that were collected before and after pVAD placement included hemodynamic values (cardiac index, pulmonary capillary wedge pressure, central venous pressure, mean arterial pressure, and mixed venous oxygen saturation) and laboratory data (blood urea nitrogen and creatinine levels, and urine output). Additional data included preoperative left ventricular ejection fraction (LVEF), the number of inotropic drugs prescribed for each patient, the duration of pVAD support, 30-day mortality rates, and long-term follow-up data.
All 5 patients were men (mean age, 56 ± 9 yr; range, 42–64 yr) and had an average LVEF of 0.17 ± .006 before pVAD implantation. The case of patient 1 (60 years of age) was described earlier.14 Immediately before the pVAD was implanted, 2 patients were undergoing cardiopulmonary resuscitation, and 3 patients had IABPs inserted. The average time between the onset of cardiac shock and pVAD placement was 52.3 hours (range, 1.5–132 hr).
The TandemHeart's implantation procedure has been described elsewhere.5 In our patients, these devices were inserted by interventional cardiologists in the cardiac catheterization laboratory. A 21F cannula was positioned in the left atrium by means of a venous transseptal puncture, which enabled the aspiration of blood into the pVAD. The blood was then returned via another cannula (15F–17F) that was positioned in the femoral artery. Flow rates for the pVAD were maintained at 3 to 4 L/min, and anticoagulation was achieved with intravenous heparin, with a target activated partial thromboplastin time of 60 to 80 seconds.
Statistical Analysis
Data are expressed as mean ± SD or as median and range. Paired Student t tests were used to compare hemodynamic values and laboratory results that were recorded before and after pVAD placement. All analyses were performed with use of SAS version 9.1 (SAS Institute; Cary, NC) on a Windows platform.
Results
After the TandemHeart devices were placed, all 5 patients showed significant increases in cardiac index, mean arterial pressure, mixed venous oxygen saturation, and urine output, and significant decreases in central venous pressure and pressor requirements (Table II). No significant differences were noted in pulmonary capillary wedge pressures, blood urea nitrogen levels, or creatinine levels.
TABLE II. Hemodynamic Values Before and After TandemHeart Placement
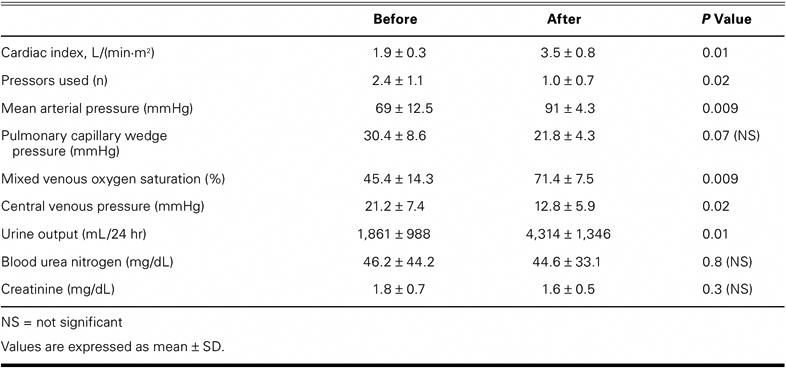
The average duration of TandemHeart support was 7.6 ± 3.2 days (range, 5–13 d). After 48 hours of pVAD support and progressive clinical improvement, all 5 patients were listed for urgent cardiac transplantation. Once organs became available, orthotopic heart transplantation was performed (by use of CPB), with simultaneous removal of the pVAD cannulae.
No deaths or major medical sequelae occurred during the 30-day postoperative period. The average duration of long-term follow-up after transplantation was 8.4 ± 9.9 months (range, 3–26 mo), with no deaths during that time.
Discussion
The development of cardiogenic shock in patients with existing heart failure is a challenging problem with few potential solutions. Because donor hearts are scarce and not always immediately available, options for stabilizing these patients are limited. Although the IABP and pharmacologic therapy can stabilize some patients, others will require mechanical circulatory support. Options in the past have included the insertion of extracorporeal assist devices, which require invasive surgical procedures and result in high mortality rates.16 These devices have been used to bridge heart-failure patients to an implantable left ventricular assist device (LVAD) (“bridge to bridge”) or to heart transplantation. Pagani and associates,17 who used extracorporeal devices in several such patients, reported in-hospital mortality rates of greater than 50%. This is not surprising, considering the already-poor prognosis of this select patient group. Now, with the availability of the pVAD, the additional stresses of surgery and CPB can be avoided.
According to prior reports, the TandemHeart has been used to treat cardiogenic shock and acute myocarditis and, in 1 patient, has served as a bridge to transplantation.9,12,14 In addition, Thiele and colleagues18 compared this device with the IABP in the treatment of 41 patients who were in shock after acute myocardial infarction and who required interventional cardiac catheterization. The investigators found that, although the pVAD normalized hemodynamic values more effectively than did the IABP, the choice of device did not affect 30-day mortality rates. However, unlike our patients, Thiele's patients underwent coronary interventions that certainly affected long-term survival and led to the eventual removal of the device. In contrast, in our heart-failure patients, the pVAD had to serve as a bridge to other treatment for a terminal condition, with no long-term treatment options other than heart transplantation or long-term VAD support.
Our use of the TandemHeart device in these 5 patients was initially planned as a bridge to another, longer-term implantable device. However, all of the patients showed remarkable improvements in clinical status and hemodynamic indices within the first 48 hours of pVAD implantation (Table II). Because clinicians at our institution have documented cases in which patients were supported for up to 2 weeks by the pVAD with minimal complications,9 we listed our patients for urgent cardiac transplantation. By chance, a donor heart became available for each patient within a relatively short time (7.6 ± 3.2 d).
At our institution, not all pVAD-supported heart-failure patients with cardiogenic shock have been bridged to cardiac transplantation. During the period of this study, 9 other patients were successfully bridged to long-term axial-flow LVAD support.19 Supporting a patient with a long-term LVAD until heart transplantation confers advantages over using the much shorter-term pVAD. Longer ventricular support times lead to cardiac improvement and—more important—to sustained improvement in end-organ function, which is likely to reduce morbidity and death after eventual heart transplantation.
Another percutaneously inserted device, the Impella® (ABIOMED, Inc.; Danvers, Mass), has been used as a bridge to transplantation in 6 patients.20 However, 2 of these patients required additional LVAD support, and they died. The authors concluded that once the patient has regained suitable end-organ function and better clinical status, the Impella should serve as a “double bridge”—first as a bridge to the long-term device and then as a bridge to transplantation.
For this reason, most heart-failure patients at our institution who require urgent pVAD support are supported until clinical improvement enables the placement of a long-term implantable device. As a rule, such patients are not listed for cardiac transplantation. However, those few patients whose hemodynamic and end-organ functions dramatically improve with pVAD support—as was the case with our 5 patients—may be listed for urgent transplantation, with the plan of using an implantable LVAD if a donor heart does not immediately become available.
Acknowledgments
Stephen N. Palmer, PhD, ELS, and Angela Townley Odensky, MA, contributed to the editing of this manuscript.
Footnotes
Address for reprints: Igor D. Gregoric, MD, Texas Heart Institute, MC 2-114A, P.O. Box 20345, Houston, TX 77225-0345. E-mail: igregoric@hotmail.com
References
- 1.Norkiene I, Ringaitiene D, Rucinskas K, Samalavicius R, Baublys A, Miniauskas S, Sirvydis V. Intra-aortic balloon counterpulsation in decompensated cardiomyopathy patients: bridge to transplantation or assist device. Interact Cardiovasc Thorac Surg 2007;6(1):66–70. [DOI] [PubMed]
- 2.Rosenbaum AM, Murali S, Uretsky BF. Intra-aortic balloon counterpulsation as a ‘bridge’ to cardiac transplantation. Effects in nonischemic and ischemic cardiomyopathy. Chest 1994;106(6):1683–8. [DOI] [PubMed]
- 3.Scheidt S, Wilner G, Mueller H, Summers D, Lesch M, Wolff G, et al. Intra-aortic balloon counterpulsation in cardiogenic shock. Report of a co-operative clinical trial. N Engl J Med 1973;288(19):979–84. [DOI] [PubMed]
- 4.Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS; NRMI Investigators. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294(4): 448–54. [DOI] [PubMed]
- 5.Thiele H, Lauer B, Hambrecht R, Boudriot E, Cohen HA, Schuler G. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation 2001; 104(24):2917–22. [DOI] [PubMed]
- 6.Aragon J, Lee MS, Kar S, Makkar RR. Percutaneous left ventricular assist device: “TandemHeart” for high-risk coronary intervention. Catheter Cardiovasc Interv 2005;65(3):346–52. [DOI] [PubMed]
- 7.Bonvini RF, Hendiri T, Camenzind E, Verin V. High-risk left main coronary stenting supported by percutaneous left ventricular assist device. Catheter Cardiovasc Interv 2005;66(2): 209–12. [DOI] [PubMed]
- 8.Giombolini C, Notaristefano S, Santucci S, Fortunati F, Savino K, Sindaco FD, et al. Percutaneous left ventricular assist device, TandemHeart, for high-risk percutaneous coronary revascularization. A single centre experience. Acute Card Care 2006;8(1):35–40. [DOI] [PubMed]
- 9.Kar B, Adkins LE, Civitello AB, Loyalka P, Palanichamy N, Gemmato CJ, et al. Clinical experience with the TandemHeart percutaneous ventricular assist device. Tex Heart Inst J 2006;33(2):111–5. [PMC free article] [PubMed]
- 10.Kar B, Butkevich A, Civitello AB, Nawar MA, Walton B, Messner GN, et al. Hemodynamic support with a percutaneous left ventricular assist device during stenting of an unprotected left main coronary artery. Tex Heart Inst J 2004;31(1): 84–6. [PMC free article] [PubMed]
- 11.Kar B, Forrester M, Gemmato C, Civitello A, Loyalka P, Myers T, Delgado R. Use of the TandemHeart percutaneous ventricular assist device to support patients undergoing high-risk percutaneous coronary intervention. J Invasive Cardiol 2006;18(4):A6. [PubMed]
- 12.Chandra D, Kar B, Idelchik G, Simpson L, Loyalka P, Gregoric ID, et al. Usefulness of percutaneous left ventricular assist device as a bridge to recovery from myocarditis. Am J Cardiol 2007;99(12):1755–6. [DOI] [PubMed]
- 13.Khalife WI, Kar B. The TandemHeart pVAD in the treatment of acute fulminant myocarditis. Tex Heart Inst J 2007;34(2): 209–13. [PMC free article] [PubMed]
- 14.La Francesca S, Palanichamy N, Kar B, Gregoric ID. First use of the TandemHeart percutaneous left ventricular assist device as a short-term bridge to cardiac transplantation. Tex Heart Inst J 2006;33(4):490–1. [PMC free article] [PubMed]
- 15.Kirklin JK, Holman WL. Mechanical circulatory support therapy as a bridge to transplant or recovery (new advances). Curr Opin Cardiol 2006;21(2):120–6. [DOI] [PubMed]
- 16.Pennington DG, Smedira NG, Samuels LE, Acker MA, Curtis JJ, Pagani FD. Mechanical circulatory support for acute heart failure. Ann Thorac Surg 2001;71(3 Suppl):S56–9. [DOI] [PubMed]
- 17.Pagani FD, Aaronson KD, Swaniker F, Bartlett RH. The use of extracorporeal life support in adult patients with primary cardiac failure as a bridge to implantable left ventricular assist device. Ann Thorac Surg 2001;71(3 Suppl):S77–85. [DOI] [PubMed]
- 18.Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, Schuler G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26 (13):1276–83. [DOI] [PubMed]
- 19.Gregoric ID, Jacob LP, La Francesca S, Bruckner BA, Cohn WE, Loyalka P, et al. The TandemHeart as a bridge to a long-term axial-flow left ventricular assist device (bridge to bridge). Tex Heart Inst J 2008;35(2):125–9. [PMC free article] [PubMed]
- 20.Garatti A, Colombo T, Russo C, Lanfranconi M, Milazzo F, Catena E, et al. Left ventricular mechanical support with the Impella Recover left direct microaxial blood pump: a single-center experience. Artif Organs 2006;30(7):523–8. [DOI] [PubMed]


