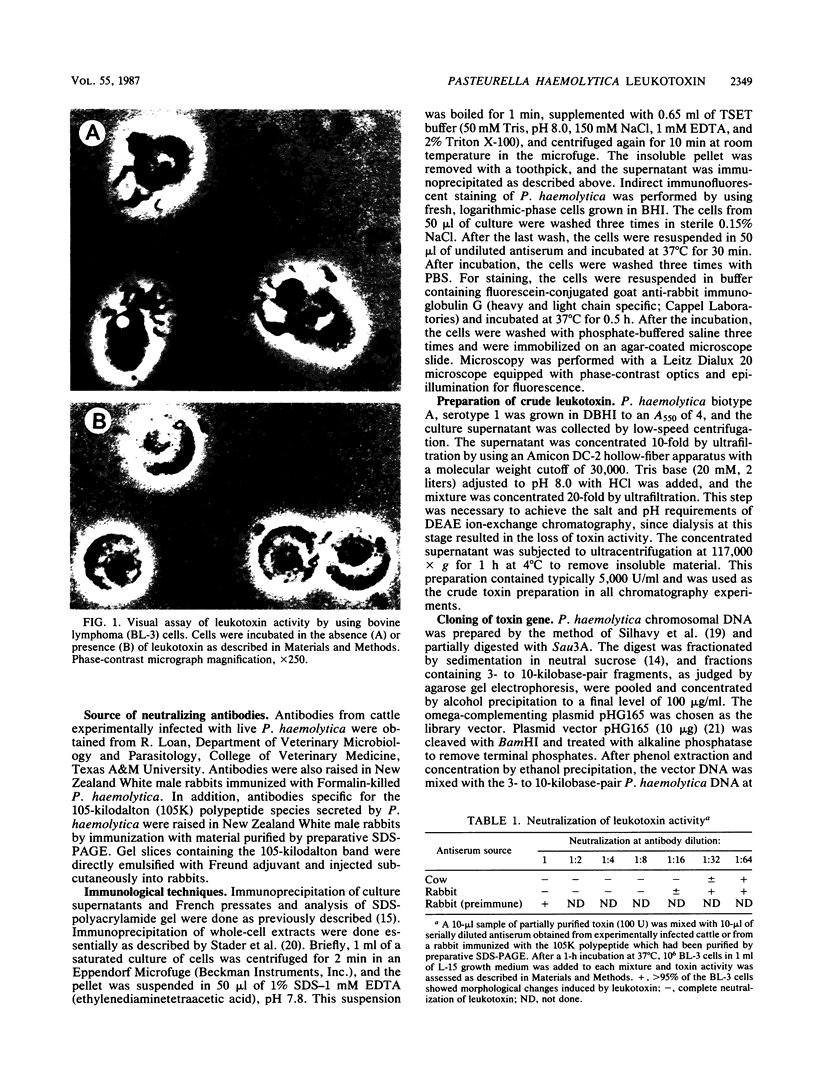
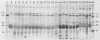Abstract
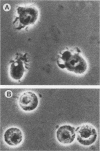
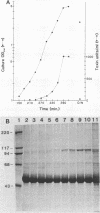
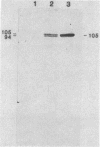
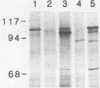
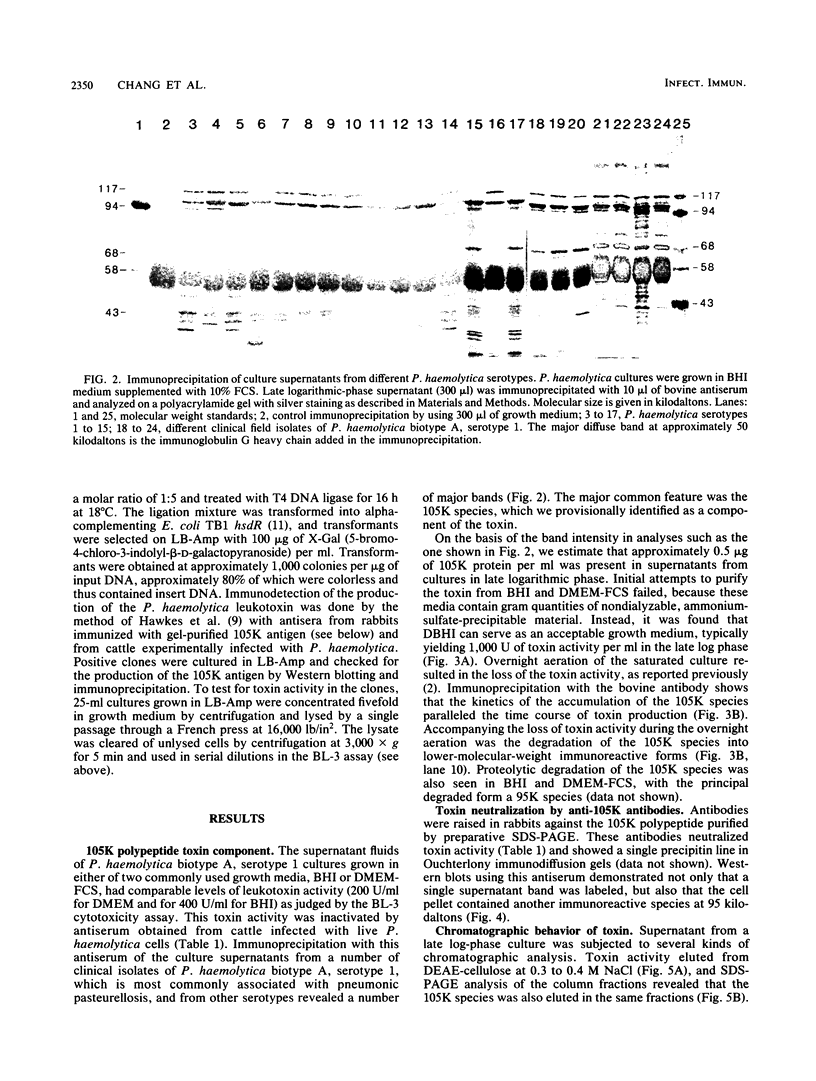
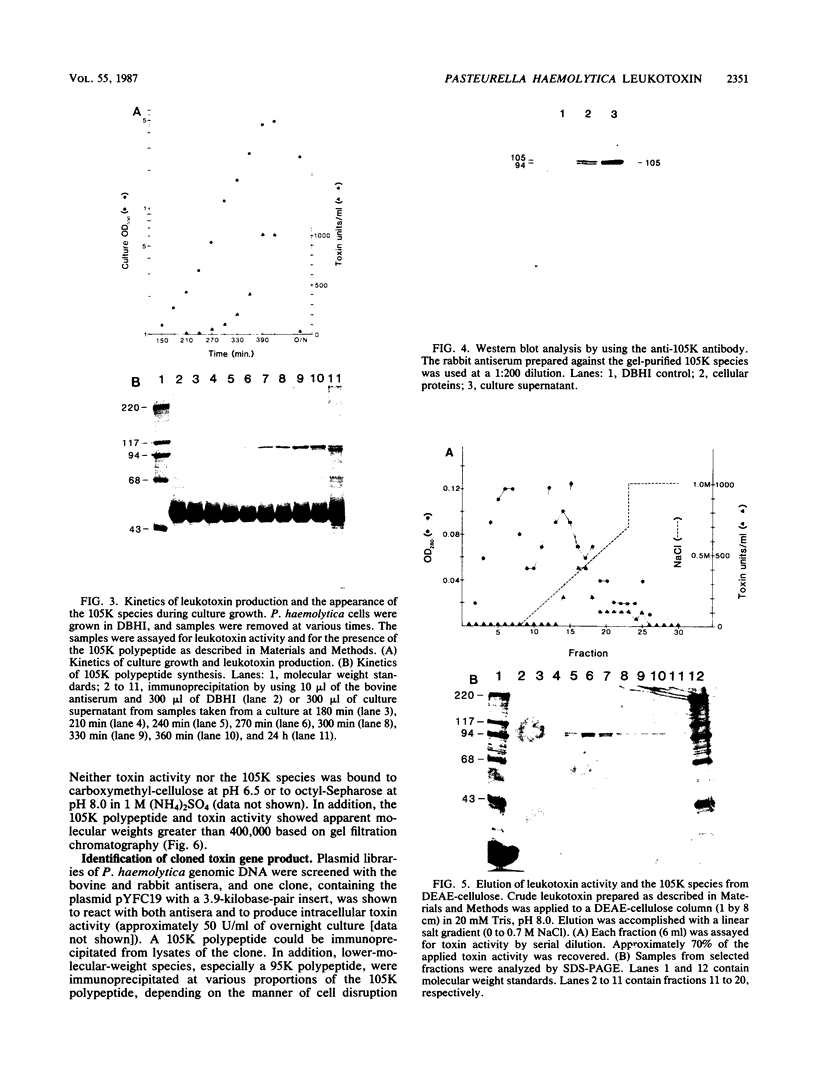
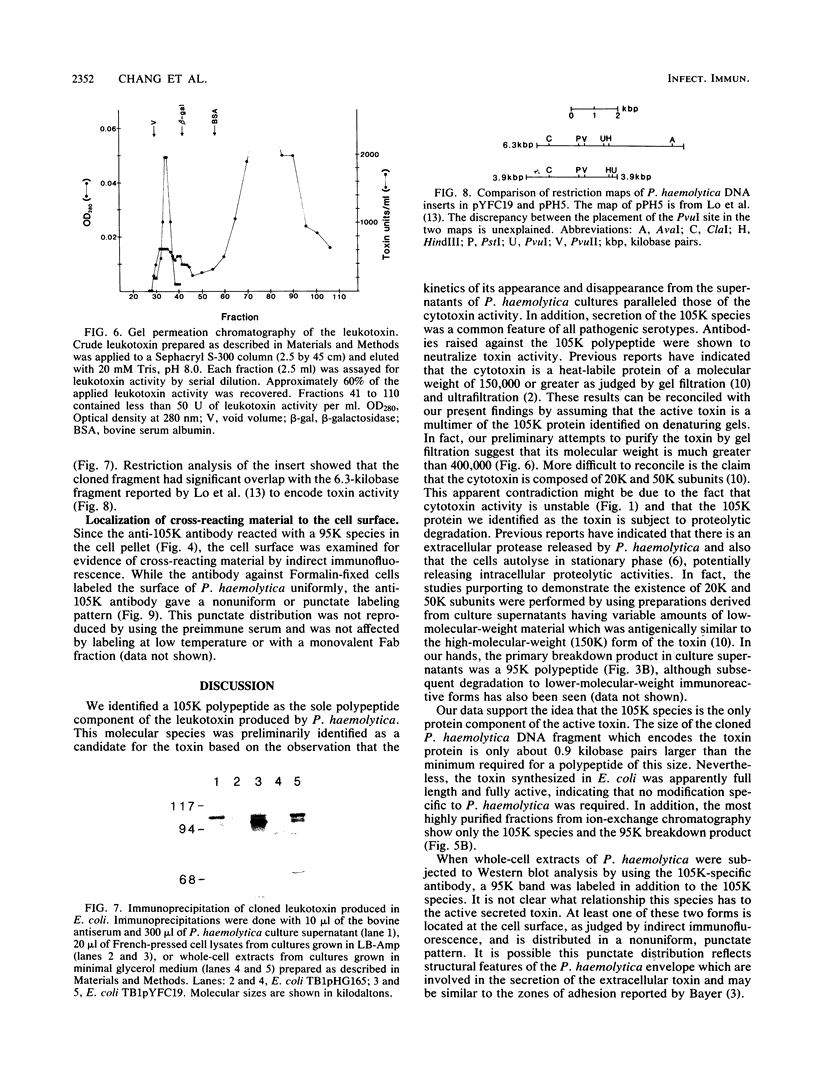
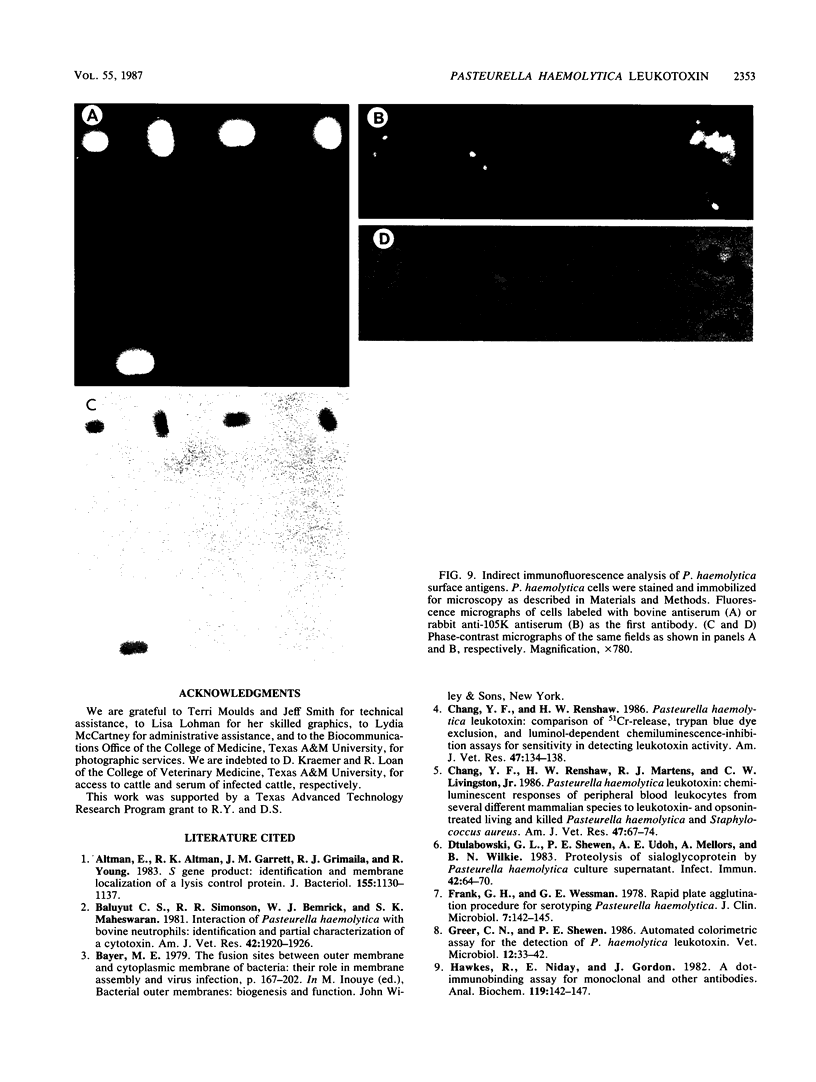
The identification and chromatographic characterization of the leukotoxin of Pasteurella haemolytica is described. The toxin, which has an apparent native molecular weight of greater than 400,000 as judged by gel exclusion chromatography, has a 105-kilodalton (105K) polypeptide as its major protein component. The proteolytic degradation of the 105K polypeptide could be correlated with the loss of toxin activity in aging cultures of P. haemolytica. Antisera raised against purified 105K polypeptide neutralized toxin activity. A 3.9-kilobase-pair fragment of the P. haemolytica genome cloned into a plasmid vector resulted in the production of intracellular toxin in Escherichia coli host cells. The restriction map of this clone shows significant overlap with the map of a previously reported leukotoxin clone (R. Y. C. Lo, P. E. Shewen, C. A. Strathdee, and C. N. Greer, Infect. Immun. 50:667-671, 1985). Finally, antisera raised against the 105K species labeled the P. haemolytica cell surface in a nonuniform, punctate manner.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Altman R. K., Garrett J. M., Grimaila R. J., Young R. S gene product: identification and membrane localization of a lysis control protein. J Bacteriol. 1983 Sep;155(3):1130–1137. doi: 10.1128/jb.155.3.1130-1137.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluyut C. S., Simonson R. R., Bemrick W. J., Maheswaran S. K. Interaction of Pasteurella haemolytica with bovine neutrophils: identification and partial characterization of a cytotoxin. Am J Vet Res. 1981 Nov;42(11):1920–1926. [PubMed] [Google Scholar]
- Chang Y. F., Renshaw H. W., Martens R. J., Livingston C. W., Jr Pasteurella haemolytica leukotoxin: chemiluminescent responses of peripheral blood leukocytes from several different mammalian species to leukotoxin- and opsonin-treated living and killed Pasteurella haemolytica and Staphylococcus aureus. Am J Vet Res. 1986 Jan;47(1):67–74. [PubMed] [Google Scholar]
- Chang Y. F., Renshaw H. W. Pasteurella haemolytica leukotoxin: comparison of 51chromium-release, trypan blue dye exclusion, and luminol-dependent chemiluminescence-inhibition assays for sensitivity in detecting leukotoxin activity. Am J Vet Res. 1986 Jan;47(1):134–138. [PubMed] [Google Scholar]
- Frank G. H., Wessman G. E. Rapid plate agglutination procedure for serotyping Pasteurella haemolytica. J Clin Microbiol. 1978 Feb;7(2):142–145. doi: 10.1128/jcm.7.2.142-145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer C. N., Shewen P. E. Automated colorimetric assay for the detection of Pasteurella haemolytica leucotoxin. Vet Microbiol. 1986 Jun;12(1):33–42. doi: 10.1016/0378-1135(86)90039-8. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Himmel M. E., Yates M. D., Lauerman L. H., Squire P. G. Purification and partial characterization of a macrophage cytotoxin from Pasteurella haemolytica. Am J Vet Res. 1982 May;43(5):764–767. [PubMed] [Google Scholar]
- Johnston T. C., Thompson R. B., Baldwin T. O. Nucleotide sequence of the luxB gene of Vibrio harveyi and the complete amino acid sequence of the beta subunit of bacterial luciferase. J Biol Chem. 1986 Apr 15;261(11):4805–4811. [PubMed] [Google Scholar]
- Kaehler K. L., Markham R. J., Muscoplat C. C., Johnson D. W. Evidence of species specificity in the cytocidal effects of Pasteurella haemolytica. Infect Immun. 1980 Nov;30(2):615–616. doi: 10.1128/iai.30.2.615-616.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Shewen P. E., Strathdee C. A., Greer C. N. Cloning and expression of the leukotoxin gene of Pasteurella haemolytica A1 in Escherichia coli K-12. Infect Immun. 1985 Dec;50(3):667–671. doi: 10.1128/iai.50.3.667-671.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maratea D., Young K., Young R. Deletion and fusion analysis of the phage phi X174 lysis gene E. Gene. 1985;40(1):39–46. doi: 10.1016/0378-1119(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Mosier D. A., Lessley B. A., Confer A. W., Antone S. M., Gentry M. J. Chromatographic separation and characterization of Pasteurella haemolytica cytotoxin. Am J Vet Res. 1986 Oct;47(10):2233–2241. [PubMed] [Google Scholar]
- Otulakowski G. L., Shewen P. E., Udoh A. E., Mellors A., Wilkie B. N. Proteolysis of sialoglycoprotein by Pasteurella haemolytica cytotoxic culture supernatant. Infect Immun. 1983 Oct;42(1):64–70. doi: 10.1128/iai.42.1.64-70.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982 Jan;35(1):91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stader J., Benson S. A., Silhavy T. J. Kinetic analysis of lamB mutants suggests the signal sequence plays multiple roles in protein export. J Biol Chem. 1986 Nov 15;261(32):15075–15080. [PubMed] [Google Scholar]
- Stewart G. S., Lubinsky-Mink S., Jackson C. G., Cassel A., Kuhn J. pHG165: a pBR322 copy number derivative of pUC8 for cloning and expression. Plasmid. 1986 May;15(3):172–181. doi: 10.1016/0147-619x(86)90035-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]