Brain injury is a major source of patient morbidity after cardiac surgery and is associated with prolonged hospitalization, excessive operative mortality, high hospital costs, and altered quality of life.[1–4] The frequency and the clinical manifestations depend on multiple factors, including the completeness and timing of neurological testing (Table 1).[1–4] Sensitive diffusion-weighted magnetic resonance imaging of the brain, for example, may reveal brain infarction in 45% of patients after cardiac surgery.[3,5] These ischemic brain infarctions may or may not be associated with stroke or postoperative neurocognitive dysfunction (clinically “silent”), but the long-term implications of these lesions on neurological function have not yet been extensively evaluated. The importance of cardiac surgery on long-term cognition recently has been challenged by controlled studies in which the cognitive function of patients who have undergone coronary artery bypass graft (CABG) surgery with cardiopulmonary bypass (CPB) was compared with that of non-surgical patients who have coronary artery disease and are undergoing medical management and/or percutaneous coronary artery interventions.[2,3,6] Although cognitive deterioration was detectable over the 3 years of follow-up, the rate of cognitive decline was not different between control patients and those who had undergone CABG surgery. These results suggest that the natural progression of cerebrovascular disease in patients with coronary artery disease is the main determinant of subsequent cognitive decline rather than CABG surgery per se. These data further document that in many patients, neurocognitive changes detected after CABG surgery may be transient, with recovery observed by 3 to 6 months after surgery. In this article we will review the current views on the pathophysiologic basis of cerebral injury after cardiac surgery and provide a summary of measures aimed at reducing its occurrence.
Table 1.
Clinical forms of brain injury and relative frequencies
| Clinical manifestation | Frequency |
|---|---|
| Stroke | |
| Low risk patient | ≤ 1% |
| High risk patient | 5% to 16% |
| Encephalopathy | 8.4% to 32% |
| Neurocognitive dysfunction | |
| Hospital discharge | 40% to 75% |
| One Month after surgery | 12% to 30% |
Pathophysiology
Brain injury from cardiac surgery is believed to result from cerebral embolism or hypoperfusion that is exacerbated by inflammatory processes evoked from CPB and/or ischemia/reperfusion injury.[3,7] The manifestations are hypothesized to depend on the extent and location of injury (e.g., motor cortex resulting in clinical stroke versus brain areas involved with cognition resulting in neurocognitive dysfunction). Brain imaging has shown that nearly one-half of strokes after cardiac surgery result from macroemboli, which most likely arise from atherosclerosis of the ascending aorta.[8–12] Microemboli that lead to encephalopathy or neurocognitive dysfunction may arise from the ascending aorta, particulate matter inadvertently introduced into the surgical field, fat globules arising from the epicardial surface, or air entrained into the CPB circuit.[3]
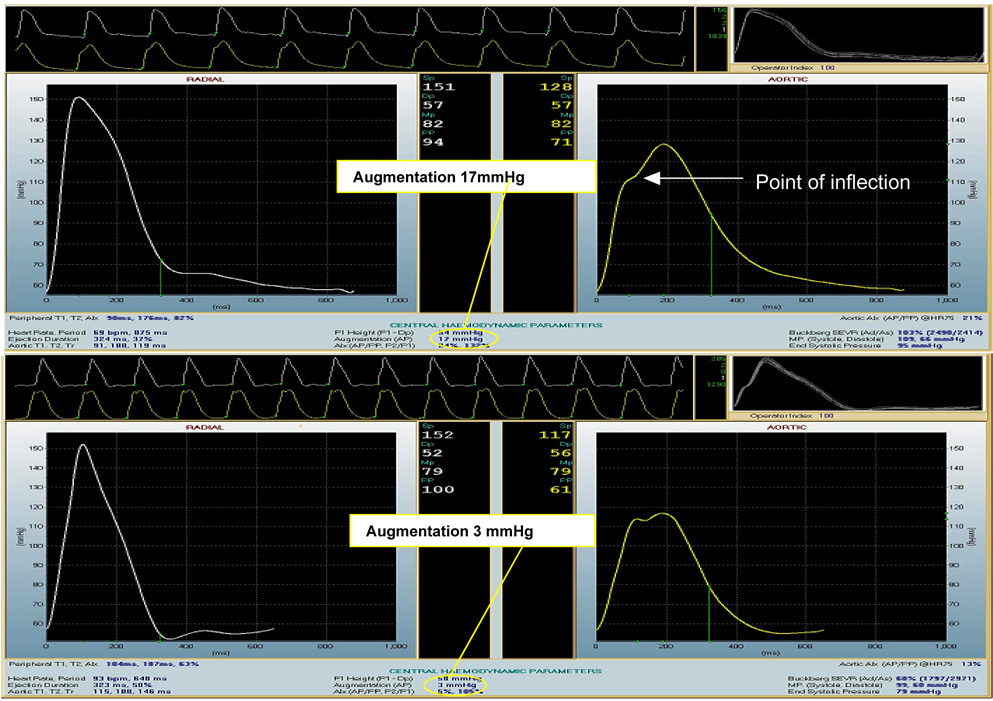
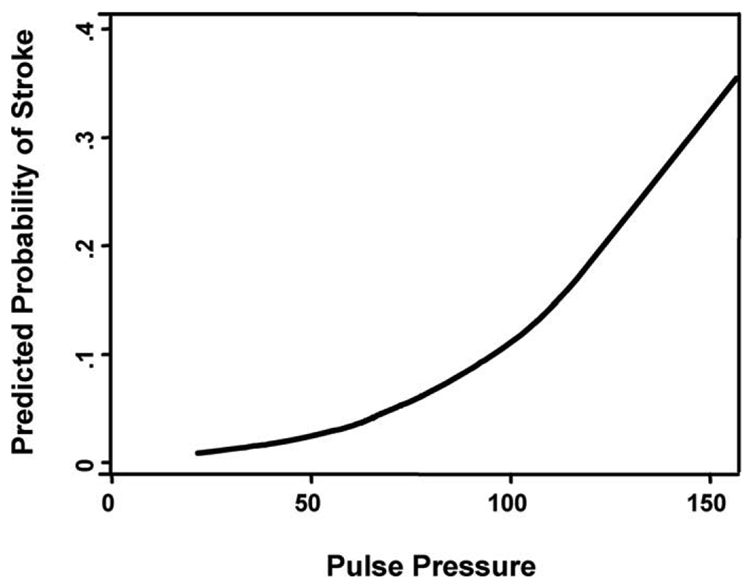
Cerebral blood flow (CBF) autoregulation is maintained during CPB with α-stat pH management.[13] Many patients might have impaired CBF-blood pressure autoregulation due to pre-existing hypertension, cerebral vascular disease, or other causes, and are thus prone to cerebral hypoperfusion and subsequent ischemic injury.[3] Of importance, the population susceptible to cerebral hypoperfusion may grow as the number of elderly surgical patients continues to increase. Aging is associated with structural changes in the arterial tree that result in increasing vascular stiffness in some individuals.[14,15] Vascular stiffening results in early return of reflected arterial waves to the central circulation, causing increased systolic and pulse pressures (Fig. 1, upper panel). Conversely, in a compliant vasculature, reflected pressure waves return to the central vasculature during diastole, augmenting organ diastolic perfusion (Fig. 1, lower page panel). Normally, a compliant vascular tree dampens systolic pressure waves; consequently, with vascular stiffening, the brain is exposed to higher pulse pressure, which leads to microcirculatory damage that may be manifest as lacunar infarction or white matter lesions.[16] Our group has reported recently that pulse pressure is an independent predictor of stroke after cardiac surgery (Figure 2).[17] Aging-associated vascular changes also may be linked to the rising prevalence of cerebral vascular disease in patients presenting for cardiac surgery. Studies in which brain imaging was performed before cardiac surgery have shown that 50% of elderly patients have prior brain infarction, white matter lesions, and/or lacunar infarcts that are clinically asymptomatic.[3,18,19] Further, in a study that used brain SPECT imaging, 75% of patients had areas of abnormal cerebral perfusion before CABG surgery.[20]
Fig. 1.
Illustration of augmentation index calculation in stiff (upper panel) and normal vessels (lower panel). The left panel illustrates tonometrically measured radial artery wave form obtained from a patient prior to CABG surgery (upper panel) and a healthy volunteer (lower panel). The right panels show derived aortic waveforms. The augmentation index is calculated as the quotient of the difference between systolic blood pressure and the pressure measured at the inflection point of the waveform over the pulse pressure. In normal vessels (lower panel) the augmented pressure resulting from reflected waves is small in magnitude and occurs during diastole. In contrast, in stiff vessels (upper panel), the augmented pressure is amplified and occurs during systole, resulting in increased pulsatile load in the cardiovascular system.
Fig. 2.
Relationship between pulse pressure and probability for stroke in a series of 703 patients after cardiac surgery. There was a significant relationship between rising pulse pressure and risk for perioperative stroke. In a Cox model, pulse pressure was an independent predictor of stroke (hazard ratio, 1.32, 95% confidence interval, 1.12 to 1.57 for every 10 mmHg increase in pulse pressure, p = 0.001). (Reprinted with permission from Benjo A, Thompson RE, Fine D, et al. Hypertension 2007;50:630–5.)
The presence of cerebral vascular disease may predispose affected patients to cerebral O2 imbalance during surgery. Indeed, the use of jugular venous bulb monitoring or near infrared spectroscopy has revealed cerebral O2 desaturation in 27–43% of patients during CPB, particularly during rewarming, when cerebral metabolic rate increases.[21,22] The high frequency of watershed type strokes after cardiac surgery further underscores the importance of cerebral hypoperfusion as a source of brain injury in an aging patient population. In a study at The Johns Hopkins Hospital, Gottesman et al[23] found that more than two-thirds of strokes detected with diffusion-weighted MRI after cardiac surgery were watershed strokes. The latter type of strokes occur in border-zone arterial perfusion territories and are indicative of hypoperfusion brain injury. Of importance, a decrease from baseline mean arterial pressure (MAP) of ≥10 mmHg during CPB was associated with a three-fold higher risk for watershed stroke.
The understanding of individual susceptibility to brain injury has been advanced by studies identifying a relationship between genotype and risk for neurological complications after cardiac surgery. This risk was first suggested by data showing that individuals with the apolipoprotein E ε4 allele were at higher risk for postoperative neurocognitive dysfunction than non-carriers.[24] The data linking the latter gene with brain injury has been inconsistent, however.[24–29] Other candidate polymorphisms include genes that encode coagulation and inflammatory pathways, such as minor alleles of C-reactive protein (CRP; 3'UTR 1846C/T), interleukin-6 (IL-6; −174G/C), and platelet glycoprotein IIb/IIIa receptor variants.[30] [31] It is hoped that further knowledge of genetic risk for perioperative brain injury will provide insights into the mechanisms and risk stratification for surgery.
Brain protection
The aims of brain protection during cardiac surgery are to reduce the sources of injury (e.g., embolism and hypoperfusion) and to increase brain tolerance to ischemic insults. Although the intraoperative period surrounding CPB is often the time of greatest focus, brain injury can occur any time perioperatively. In fact, >20% of clinical strokes occur postoperatively.[4,10] In general, protective measures for the brain can be grouped as pharmacologic and non-pharmacologic. A discussion of proposed measures to reduce brain injury will first be discussed followed by a list of recommended strategies using an evidence-based approach.[3]
Atherosclerosis of the aorta
Atherosclerosis of the ascending aorta is an important risk factor for stroke and neurocognitive dysfunction.[1,8,10] Epiaortic ultrasound is the most sensitive method for detecting this disease at the time of surgery and allows physicians to avoid atheroma during surgical aortic manipulations (e.g., cannulating for CPB, applying an aortic crossclamp).[3,8],[32–35] Epiaortic guided surgery is reported to reduce cerebral embolic signals detected with transcranial Doppler and to improve neurological outcomes.[8,36,37] Managing patients with diffuse atherosclerosis of the ascending aorta is more problematic as it may be difficult, or impossible, to identify sites free of atheroma for surgical aortic manipulations. Surgical approaches for this situation might include 1) avoiding CPB by converting to “off-pump” surgery; 2) cannulating for CPB via the axillary artery or other alternative sites; 3) avoiding partial occlusion clamps for proximal bypass graft anastomosis by using a “single cross-clamp” technique; 4) avoiding aorta cross-clamping by using fibrillatory arrest; 5) using all arterial bypass grafts to avoid proximal anastomosis; and 6) performing circulatory arrest and replacing the ascending aorta.[3] Recently, a randomized study of 169 high-risk patients found that a single-cross clamp technique reduced the incidence of cognitive deficits after CABG surgery compared with a traditional approach of using a partial occlusion clamp for proximal bypass graft anastomosis.[38] Even if avoided during aortic manipulations, atheroma might be disrupted or injured during surgery due to the “sandblasting” effects of CPB.[39] Ura et al[40] performed epiaortic ultrasound before and after CPB in a cohort of 472 patients. In 3.4% of patients, mobile lesions of the ascending aorta were discovered after surgery in areas where previously there had been only mild-to-moderate atherosclerosis. The new lesions were usually at the sites of aortic clamping or cannulation and increased the risk for stroke. Injury to aortic atheromatous plaques during surgery might predispose a patient to postoperative athero-thromboembolism, which might explain, in part, the occurrence of delayed stroke following cardiac surgery.
Pericardial suction aspirate
Lipid-laden emboli resulting in small arteriolar capillary dilations (SCADs) are found in the brains of patients at autopsy after cardiac surgery.[41] These SCADs can be reproduced in canines subjected to CPB and appear to arise from fat returned unfiltered to the CPB with cardiotomy suction aspirate.[42] Experimentally, the usual CPB arterial line filters are inefficient in preventing SCADs compared with processing of the cardiotomy suction aspirate with a cell saver.[43] In a human investigation, processing pericardial suction blood with a cell saver before return to the CPB circuit significantly reduced blood lipid content compared with controls (filtering only).[44] However, these results were not reproduced in another human study in which subjecting cardiotomy suction aspirate to a dual filtration method prior to return to the CPB reservoir was found to be more efficient than cell-saver processing for removing lipid material.[45] Thus, some lipid material remains in the pericardial aspirate even after blood processing with a cell saver. Simply discarding pericardial aspirate might be considered when the volume of discarded blood is low. Processing large quantities of blood with a cell saver might lead to thrombocytopenia or dilution of coagulation factors leading to bleeding and high rates of blood transfusion. This possibility is a concern because transfusion of platelets is associated with risk for stroke in patients undergoing cardiac surgery.[46]
Recently, Rubens et al[47] reported the results of a trial of patients undergoing CABG surgery with or without aortic valve replacement. The patients were prospectively randomized into two groups. The first had shed cardiotomy blood processed in a cell saver prior to its return to the CPB circuit (n=132). That group was compared to a group that received standard of care, in which cardiotomy suction aspirate was returned unprocessed to the CPB circuit (n=134). Although there was no difference in the number of patients receiving transfusions, patients in the cell-saver group received significantly more red blood cell (RBC) and non-RBC transfusions than the standard care group (RBC transfusion, 1.24 ± 2.71 units vs 0.81 ± 1.24 units/patient, p = 0.001; non-RBC transfusion, 2.06 ± 7.70 units vs 1.12 ± 4.74 units/patient, p < 0.001). The frequency of neurocognitive dysfunction at hospital discharge (cell-saver group, 45.3% vs standard care, 39.0%) and 3 months after surgery (16.7% vs 15.9%) was not different between the two groups. These results suggest that processing cardiotomy suction blood during CPB before reinfusion to the CPB circuit does not improve neurocognitive outcomes after cardiac surgery but is associated with greater blood product transfusion. These findings are in contrast to a study by Djaiani et al,[48] who found that processing cardiotomy suction with a continuous cell saver during cardiac surgery resulted in a lower frequency of postoperative cognitive dysfunction than did returning pericardial aspirate to the CPB reservoir without processing. The frequency of cognitive dysfunction 6 weeks after surgery was 6% in the cell-saver group compared with 15% in the controls (p < 0.05). Similar to the finding by Rubens et al,[47] the cell-saver group had a significantly higher risk for blood transfusion than did the controls. In both studies, the number of transcranial Doppler-detected cerebral embolic signals did not differ between control patients and those that had had cell-saver processing during CPB.
Blood pressure management
Blood pressure during CPB is often kept at a minimum of 50 mmHg based on the view that intact CBF autoregulation (using α-stat pH management) will ensure adequate brain oxygen and nutrient delivery.[3,13] Further, increasing CBF during CPB is widely believed to pose a risk for brain injury from increased cerebral embolic load.[3,13] Whether this low MAP target during CPB is suitable for contemporary practice that includes caring for an increasing number of patients with pre-existing cerebral vascular disease is unknown. In a prospectively randomized study performed by Gold et al,[49] patients undergoing CABG surgery were managed during CPB with either a “low” or “high” MAP target of 50 to 60 mmHg or 80 to 100 mmHg, respectively. The combined end-point of myocardial infarction and/or stroke was less common in the “high” than in the “low” MAP group (p = 0.026). This study did not have an adequate sample size to detect differences in stroke as a single end-point. There were no differences in the frequency of neurocognitive dysfunction in the different MAP groups. Even though the study by Gold et al[49] has limitations, the data have provided de facto support for maintaining MAP targets higher than 50 mmHg during CPB. Retrospective analysis further supports the notion that higher MAP during CPB reduces the risk of brain injury in high-risk patients, such as those with atherosclerosis of the aorta.[50] Consequently, most institutions keep MAP >70 mmHg in high-risk patients during CPB. A common clinical dictum invoked by many centers is to keep MAP targets within the same numerical value as the decade of the patient’s age (e.g., >70 mmHg for 70-year-olds, >80 mmHg for 80-year-olds).
Mediastinal CO2 insufflation
Most cerebral emboli detected with transcranial Doppler during CPB are composed of air. Because CO2 is more soluble in blood than air is, replacing air in the pericardium during surgery by simply insufflating the wound with CO2 is a strategy often used to increase the rate of absorption of intravascular emboli.14 This method reduces the number of arterial emboli, but it has not been extensively studied as a potential means of brain protection in humans. However, few risks are associated with this practice.
Hemoglobin/hematocrit targets
Hemodilution is used during CPB to reduce the viscosity of blood during hypothermia and to reduce the need for allogeneic blood transfusion. The brain compensates for decreased blood oxygen carrying capacity by increasing CBF and tissue O2 extraction.[51] In dogs undergoing CPB, these processes maintain brain tissue oxygenation to a hematocrit of 12% at 12°C, 15% at 28°C, and 18% at 38°C.[51] However, these targets are likely higher for patients with cerebrovascular disease. In addition, experimentally, ischemic neurons can not compensate for lower tissue oxygen delivery when hematocrit is <30%.[52] Moreover, a CBF that has increased to compensate for anemia could theoretically increase cerebral embolic load and elevate the risk for brain injury. Retrospective analysis has further linked low hematocrit levels during CPB with mortality.[53–55] Habib et al[55] found that a hematocrit <22% on CPB was independently associated with stroke after cardiac surgery. Karkouti et al[56] found that the odds of stroke increased 10% for each 1% decrease in hematocrit during CPB.
The randomized trials that have been conducted in adults undergoing cardiac surgery to evaluate different hematocrit targets for transfusion during CPB have provided very little data. Several small trials using autologous pre-donated red blood cells for transfusion at different hematocrit targets do not provide guidance for intraoperative management during CPB.[57] [58] In a randomized trial of 107 patients undergoing CABG surgery, Mathew et al[59] evaluated a minimal CPB hematocrit target of 27% or 15-to-18% on neurocognitive end-points. The study’s safety committee stopped the trial based on the fact that the adverse event rate was higher in the low hematocrit group. At the same time there was an interaction between age and the lower hematocrit levels and risk for postoperative neurocognitive dysfunction.
The optimal hematocrit level for humans during CPB is not known and is likely to vary between and within patients depending on many factors, including body temperature and the individual risk for ischemic brain injury. Recently released guidelines for blood transfusion from the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists opine that it is “reasonable” to transfuse RBCs during CPB when hemoglobin is <6 g/dL or after surgery when hemoglobin is <7 g/dL.[60] When there is risk for end-organ ischemia, these targets are raised by 1 g/dL to 7 g/dL during CPB. Additionally, the authors of those guidelines emphasize that the patient’s clinical situation is the most important component of the transfusion decision process.
Temperature management
Reducing brain temperature provides protection against ischemic neuronal injury by several mechanisms, including reduced cerebral O2 demands and reduced excitotoxicity.[61] Clinically, hypothermia induced after cardiac arrest has been shown to lead to improved neurological outcomes and survival.[62–64] It has been used during CPB for decades to provide organ protection.[3] Nonetheless, available data are insufficient to support its neuroprotective benefits. In a meta-analysis of randomized trials of patients undergoing CABG surgery, Rees et al[65] reported no differences between the use of hypothermia and normothermia in the risk for stroke. One explanation for the disappointing clinical effects of hypothermia during cardiac surgery might be that any potential benefits might be off-set by inadvertent cerebral hyperthermia during rewarming.[66] The latter can easily occur because the aortic cannula that returns warmed blood to the central circulation is in close proximity to the cerebral vessels. Further, cerebral temperature is often underestimated clinically from normal temperature monitoring sites (e.g., nasopharynx, esophagus).[62,67] In fact, Nathan et al[68] reported that rewarming to a nasopharyngeal temperature of 34°C after hypothermic CPB (32°C) led to a lower frequency of neurocognitive dysfunction 1 week and 3 months after CABG surgery than did rewarming to 37°C. Incomplete rewarming hypothetically may lead to an increase in surgical bleeding and hemodynamic fluctuations. Nonetheless, in the studies by Nathan et al[68], the duration of mechanical lung ventilation, blood loss, blood transfusion rates, and re-operation for bleeding were not different between the partial and complete rewarming groups.
Glycemic control
Blood glucose control as a means for lessening brain injury has been the focus of investigation in both surgical and non-surgical patients. It is well established that there is a relationship between even mild hyperglycemia (i.e., >140 mg/dL) and diminished outcome after stroke that can occur via multiple mechanistic pathways.[3] Interest in strict perioperative glycemic control most recently has been fostered by data from a trial in surgical ICU patients that showed a 34% reduction in mortality (8% vs 4.6%, p = 0.04). This reduction was accomplished by maintaining plasma glucose between 80 and 110 mg/dL with an intensive insulin infusion rather than using insulin therapy when glucose exceeded 215 mg/dL.[69] A caveat to these results, though, is that the improved outcomes were observed only in patients who were in the ICU for more than 5 days. In medical ICU patients, a similar protocol led to lower morbidity but higher mortality compared with controls in patients who stayed in the ICU for fewer than 3 days receiving intensive insulin treatment.[70] However, the benefits of intensive insulin infusion on mortality end-points were observed when the ICU length of stay was more than 3 days.
The risks and benefits of tight intraoperative glycemic control were not compared in the studies described above. In a prospectively randomized trial in non-diabetic patients undergoing CABG surgery, an intraoperative insulin protocol (insulin given when glucose was >100 mg/dL versus when glucose was >200 mg/dL) did not lead to a difference in the frequency of neurological complications 6 weeks or 6 months after surgery.[71] Another investigation in 400 patients undergoing cardiac surgery found that intensive insulin infusion to maintain glucose between 80 and 100 mg/dL was associated with a higher composite outcome of death, cardiac morbidity, stroke, or renal failure than when insulin was given only for glucose >200 mg/dL (p = 0.02).[72] Thus, the benefits to neurological outcome of maintaining tight glycemic control during cardiac surgery are currently unproven.
Hyperglycemia is common after stroke, occurring in more than one-third of non-diabetics and in most diabetics.[73] Persistent hyperglycemia (>200 mg/dL) during the first 24 hours after stroke is an independent predictor of expansion of cerebral infarction.[74] Recommendations for managing hyperglycemia after stroke are included in the general guidelines for management of acute ischemic stroke issued by the American Heart Association.[75] These guidelines acknowledge that the exact glucose level to prompt intervention after stroke is unknown but state that it is reasonable to initiate insulin therapy when glucose is >140 to 185 mg/dL. (Class IIa, level of evidence C). In one prospective study, patients with glucose levels of 108 to 308 mg/dL after acute stroke were randomized to receive either no treatment or a glucose-potassium-insulin (GKI) infusion for 24 hours.[76] There were no differences in the 90-day mortality rate or functional outcome between the GKI-infusion and control groups. This trial has two caveats, however. First, the study included both ischemic and hemorrhagic stroke subtypes, and second, it was stopped prematurely due to low enrollment.
Avoiding CPB during CABG surgery
“Off-pump” CABG surgery has been proposed as a means to reduce the incidence of brain injury, particularly in high-risk patients. It is reasoned that avoiding CPB will prevent many injurious events to the brain, including emboli and systemic inflammation. Early reports from non-randomized case series support this concept. Nonetheless, data from prospectively randomized trials comparing “on” versus “off” CPB CABG surgery have failed to show a markedly reduced frequency of neurological complications with off-pump surgery. In a seminal study, Van Dijk et al[77] found few differences in neurocognitive dysfunction 1 year after surgery in patients prospectively randomized to undergo CABG surgery with or without CPB. These investigators more recently reported that cognitive outcomes remained no different 5 years after surgery between the two randomized groups.[78] Meta-analysis of randomized trials of “off-pump” versus CABG surgery with CPB revealed no difference in risk for stroke between the procedures, but there were too few patients studied to draw conclusions with regard to risk for neurocognitive dysfunction.[79] A substantial limitation of the data gathered thus far is that the prospectively randomized trials have been conducted in mostly young and low-risk patients. Therefore, the benefits of avoiding CPB in older and higher-risk patients have not been substantially examined. Case series, in fact, report that this group of patients might indeed have lower stroke rates when CABG surgery is performed “off pump”.[80] It must be acknowledged, even by proponents of “off-pump” CABG surgery, that embolization is still possible during manipulations of an atherosclerotic ascending aorta, such as during proximal bypass graft anastamosis.[81] Moreover, hemodynamic perturbations during cardiac manipulations that are necessary for distal coronary artery anastamosis might lead to reduced CBF. Thus, more data is needed to evaluate the effects of avoiding CPB during CABG surgery on neurological complications.
Pharmacologic brain protection
Multiple advances toward understanding the basic mechanisms of brain injury have led to the development of pharmacologic strategies for neuroprotection. Based on sound experimental evidence, including data from animal studies, several putative neuroprotective agents have been examined in cardiac surgical patients, but the results have been mostly negative.[3] Agents tested have included those that reduce brain oxygen consumption to increase tolerance to ischemia and those that target established neuroprotective pathways, including the NMDA receptor, calcium channels, oxidant stress, the GABA receptor, and others. Post-hoc analyses of several trials have suggested encouraging neuroprotective effects with remacemide and complement-inhibiting agents.[3] For the most part, however, there are no widely accepted pharmacologic agents with proven efficacy to reduce the extent of brain injury associated with cardiac surgery.
The most encouraging data have evolved from secondary analysis of prospectively randomized clinical trials of aprotinin. In these trials designed to evaluate the efficacy of aprotinin to reduce bleeding and transfusion after cardiac surgery, a lower frequency of stroke has been consistently observed. A meta-analysis of these randomized, placebo-controlled trials involving 3879 patients demonstrated that, compared with placebo, aprotinin reduced the risk for stroke (relative risk 0.53, 95% CI, 0.31–0.90).[82] It must be acknowledged that stroke was a safety end-point for these studies; the studies were not designed to evaluate this end-point primarily. Retrospective analysis of observational data has revealed either a higher rate of stroke or no difference in stroke rate for patients who received aprotinin, as compared with those who received amino-caproic acid, tranexamic acid, or no antifibrinolytic at all.[83,84] Limitations to this approach, despite statistical adjustments, include treatment bias whereby clinicians might have chosen to treat higher-risk patients with aprotinin. Regardless, additional data are needed from clinical trials specifically designed to test the neuroprotective effects of aprotinin. The decision to use aprotinin should be based on its approved indication for reduction of bleeding in cardiac surgical patients.
Neuromonitoring
Reduction in cerebral oxygenation, which can be detected with near infrared cerebral oximetry (NIRS), is associated with risk for stroke and neurocognitive dysfunction.[85,86] Clinical monitoring of NIRS can be performed with two different FDA-approved devices with different methodologies. The INVOS device (Somenetics Corp, Troy, MI) uses a light-emitting diode in two channels to provide a continuous wave spectrometer that measures relative changes in regional O2 saturation of the frontal lobe. The Foresight device (CAS Medical Systems, Branford, CT) uses fiberoptic light in four channels to monitor phase shifts at 754 nm, 785 nm, and 816 nm in reference to 780 nm to measure brain tissue oxygenation.[87] Whether interventions to treat cerebral O2 desaturation will improve neurological outcomes is not yet known. The best current data comes from a randomized study of 200 patients undergoing CABG surgery.[88] In that study, patients received either regimented interventions to treat NIRS-detected O2 desaturation or standard of care. The interventions used to address low cerebral O2 saturation included ensuring adequate CPB flow, raising the MAP, ensuring normocarbia (reducing gas-inflow during CPB or “sweep”), deepening anesthesia, raising the FIO2, and initiating pulsatile CPB flow. The group that received intervention was found to have lower rates of major organ injury (death, myocardial infarction, stroke) and shorter ICU length of stay than the control group.
Evidence-based recommendations
Clinicians are accustomed to evaluating a broad range of evidence when making decisions regarding medical therapeutics and interventions. A structured, evidence-based analysis of the literature is often employed to provide a structure to recommended treatments/interventions.[89] Based on the above review, we have used such an approach to provide recommendations for brain protection during cardiac surgery (Table 2). These evaluations are based on our literature review of human studies that have evaluated the primary outcomes of clinical stroke and neurocognitive dysfunction in patients undergoing cardiac surgery primarily with CPB. We have ranked the quality of the data using methods employed by the American Heart Association as Level A, when the data comes from multiple randomized clinical trials or meta-analysis; Level B, when the data is from a single randomized trial or non-randomized studies; and Level C, case studies, expert opinion, or standard of care. Based on this heirrachy of evidence, recommendations are made as noted. This summary should provide clinicians with a framework on how to interpret the current state of knowledge for brain protection during cardiac surgery.
Table 2.
Recommendations for measures to reduce brain injury during cardiac surgery.[3]
| A membrane oxygenator and an arterial line filter (≤40 µM) should be used for CPB. | Class I (Level A) |
| Epiaortic ultrasound for detection of atherosclerosis of the ascending aorta. | Class I (Level B) |
| Hyperthermia should be avoided during and after CPB. | Class I (Level B) |
| A single aortic cross-clamp technique should be used for patients at risk for atheroembolism. | Class IIa (Level B) |
| During CPB in adults, α-stat pH management should be considered. | Class IIa (Level A) |
| Arterial line temperature during CPB rewarming should be limited to 37°C. | Class IIa (Level B) |
| NIRS monitoring should be considered, especially in high-risk patients. | Class IIb (Level B) |
| Arterial blood pressure should be maintained at >70 mmHg during CPB in high-risk patients. | Class IIb (Level B) |
| Serum glucose should be kept <140 mg/dL with an infusion of insulin. | Class IIb (Level C) |
| Transfusion of packed red blood cells should be considered in high-risk patients when hemoglobin is ≤7 g/dL or higher, depending on other patient-specific considerations. | Class IIb (Level C) |
| Processing cardiotomy suction aspirate with a cell-saver device as a means for preventing neurocognitive dysfunction. | Class Indeterminate (Level A) |
| There are currently no pharmacological neuroprotective agents with proven efficacy in humans. | Class Indeterminate (Level B) |
Note: Class I: always acceptable, proven safe, and definitely useful; Class IIa: acceptable, safe, and useful. Reasonably prudent physicians can choose. Considered the intervention of choice by majority of physicians. Class IIb: acceptable, safe, and useful. Considered optional or alternative treatment by most experts; Class III: no evidence of benefit. Class Indeterminate: Intervention can be used, but evidence is insufficient to support efficacy.
Acknowledgments
Funding: Supported in part by a grant number NHLBI RO1 64600 to Dr. Hogue from the National Institutes of Health, Bethesda, Maryland
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roach GW, Kanchuger M, Mora-Mangano C, et al. Adverse cerebral outcomes after coronary bypass surgery. N Engl J Med. 1996;335:1857–1863. doi: 10.1056/NEJM199612193352501. [DOI] [PubMed] [Google Scholar]
- 2.Newman MF, Grocott HP, Mathew JP, et al. Report of the substudy assessing impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke. 2001;32:2874–2881. doi: 10.1161/hs1201.099803. [DOI] [PubMed] [Google Scholar]
- 3.Hogue CW, Jr, Palin CA, JE A. Cardiopulmonary Bypass Management and Neurologic Outcomes. An Evidence-Based Appraisal of Current Practices Anesth Analg. 2006;103:21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 4.McKhann GM, Grega MA, Borowicz LM, Jr, et al. Stroke and encephalopathy after cardiac surgery: an update. Stroke. 2006;37:562–571. doi: 10.1161/01.STR.0000199032.78782.6c. [DOI] [PubMed] [Google Scholar]
- 5.Leary MC, CL Technology Insight: brain MRI and cardiac surgery-detection of postoperative brain ischemia. Nature Clin Pract: Cardiovasc Med. 2007;4:379–388. doi: 10.1038/ncpcardio0915. [DOI] [PubMed] [Google Scholar]
- 6.Selnes OA, Grega MA, Borowicz LM, et al. Cognitive outcomes three years after coronary artery bypass surgery: a comparison of on-pump coronary artery bypass graft surgery and nonsurgical controls. Ann Thorac Surg. 2005;79:1201–1209. doi: 10.1016/j.athoracsur.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Laffey JG, Boylan JF, DC C. The systemic inflammatory response to cardiac surgery. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Wareing TH, Dávila-Román VG, Barzilia B, et al. Management of the severely atherosclerotic ascending aorta during cardiac operations. J Thorac Cardiovasc Surg. 1992;103:453–462. [PubMed] [Google Scholar]
- 9.Blauth CI, Cosgrove DM, Webb BW, et al. Atheroembolism from the ascending aorta: an emerging problem in cardiac surgery. J Thorac Cardiovasc Surg. 1992;103:1104–1112. [PubMed] [Google Scholar]
- 10.Hogue CW J, Murphy SF, Schechtman KB, et al. Risk factors for early or delayed stroke after cardiac surgery. Circulation. Circulation. 1999;100:642–647. doi: 10.1161/01.cir.100.6.642. [DOI] [PubMed] [Google Scholar]
- 11.Lilosky DS, Marrin CA, Caplan LR, et al. Determinatino of etiologic mechanism of strokes secondary to coronary artery bypass graft surgery. Stroke. 2003;34:2830–2834. doi: 10.1161/01.STR.0000098650.12386.B3. [DOI] [PubMed] [Google Scholar]
- 12.Djaiani G, Fedorko L, Borger M, et al. Mild to moderate atheromatous disease of the thoracic aorta and new ischemic brain lesions after conventional coronary artery bypass graft surgery. Stroke. 2004;35:e356–3358. doi: 10.1161/01.STR.0000138783.63858.62. [DOI] [PubMed] [Google Scholar]
- 13.Murkin JM, Farrar JK, Tweed A, et al. Cerebral autoregulation and flow/metabolism coupling during cardiopulmonary bypass: the influence of Paco2. 1987;66:825–832. [PubMed] [Google Scholar]
- 14.Kass D. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–193. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 15.McEniery CM, Yasmin, Wallace S, et al. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46:221–226. doi: 10.1161/01.HYP.0000165310.84801.e0. [DOI] [PubMed] [Google Scholar]
- 16.O'Rourke MF, ME S. Relationship between aortic stiffening and microvascular disease in brain and kidney. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 17.Benjo A, Thompson RE, Fine D, et al. Pulse pressure is an age-independent predictor of stroke development after cardiac surgery. Hypertension. 2007;50:630–635. doi: 10.1161/HYPERTENSIONAHA.107.095513. [DOI] [PubMed] [Google Scholar]
- 18.Restrepo L, Wityk RJ, Grega MA, et al. Diffusion- and perfusion-weighted magnetic resonance imaging of the brain before and after coronary artery bypass grafting. Stroke. 2002;33:2909–2915. doi: 10.1161/01.str.0000040408.75704.15. [DOI] [PubMed] [Google Scholar]
- 19.Goto T, Baba T, Honma K, et al. Magnetic resonance imaging findings and postoperative neurologic dysfunction in elderly patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2001;72:137–142. doi: 10.1016/s0003-4975(01)02676-5. [DOI] [PubMed] [Google Scholar]
- 20.Moraca R, Lin E, Holmes JH, et al. Impaired baseline regional cerebral perfusion in patients referred for coronary artery bypass. J Thorac Cardiovasc Surg. 2006;131:540–546. doi: 10.1016/j.jtcvs.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 21.Croughwell ND, Newman MF, Blumenthal JA, et al. Jugular bulb saturation and cognitive dysfunction after cardiopulmonary bypass. Ann Thorac Surg. 1994;58:1702–1708. doi: 10.1016/0003-4975(94)91666-7. [DOI] [PubMed] [Google Scholar]
- 22.HL E. Protective effect of neuromonitoring during cardiac surgery. Ann NY Acad Sci. 2005;1053:12–19. doi: 10.1196/annals.1344.002. [DOI] [PubMed] [Google Scholar]
- 23.Gottesman R, Sherman P, Grega M, et al. Watershed strokes after cardiac surgery: diagnosis, etiology, and outcome. Stroke. 2006;37:2306–2311. doi: 10.1161/01.STR.0000236024.68020.3a. [DOI] [PubMed] [Google Scholar]
- 24.Tardiff BE, Newman MF, Saunders AM, et al. Preliminary report of a genetic basis or cognitive decline after cardiac operations. Ann Thorac Surg. 1997;64:715–720. doi: 10.1016/s0003-4975(97)00757-1. [DOI] [PubMed] [Google Scholar]
- 25.Abildstrom H, Christiansen M, Siersma VD, et al. Apolipoprotein E genotype and cognitive dysfunction after noncardiac surgery. Anesthesiology. 2004;101:855–861. doi: 10.1097/00000542-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Steed L, Kong R, Stygall J, et al. The role of apolipoprotein E in cognitive decline after cardiac operation. Ann Thorac Surg. 2001;71:823–826. doi: 10.1016/s0003-4975(00)02511-x. [DOI] [PubMed] [Google Scholar]
- 27.Robson MJ, Alston RP, Andrews PJ, et al. Apolipoprotein E and neurocognitive outcome from coronary artery surgery. J Neurol Neurosurg Psychiatry. 2002;72:675–676. doi: 10.1136/jnnp.72.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askar FZ, Cetin HY, Kumral E, et al. Apolipoprotein E epsilon4 allele and neurobehavioral status after on-pump coronary artery bypass grafting. J Card Surg. 2005;20:501–505. doi: 10.1111/j.1540-8191.2005.2004138.x. [DOI] [PubMed] [Google Scholar]
- 29.Heyer EJ, Wilson DA, Sahlein DH, et al. APOE-epsilon4 predisposes to cognitive dysfunction following uncomplicated carotid endarterectomy. Neurology. 2005;65:1759–1763. doi: 10.1212/01.wnl.0000184579.23624.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grocott HP, White WD, Morris RW, et al. Genetic polymorphisms and the risk of stroke after cardiac surgery. Stroke. 2005;36:1854–1858. doi: 10.1161/01.STR.0000177482.23478.dc. [DOI] [PubMed] [Google Scholar]
- 31.Mathew JP, Podgoreanu MV, Grocott HP, et al. Genetic variants in P-selectin and C-reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49:1934–1942. doi: 10.1016/j.jacc.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 32.Davila-Roman VG, Murphy SF, Nickerson NJ, et al. Atherosclerosis of the ascending aorta is an independent predictor of long-term neurologic events and mortality. J Am Coll Cardiol. 1999;33:1308–1316. doi: 10.1016/s0735-1097(99)00034-0. [DOI] [PubMed] [Google Scholar]
- 33.Tunick PA, Perez JL, I K. Protruding atheromas in the thoracic aorta and systemic embolization. Ann Intern Med. 1991;115:423–427. doi: 10.7326/0003-4819-115-6-423. [DOI] [PubMed] [Google Scholar]
- 34.Katz ES, Tunick PA, Rusinek H, et al. Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol. 1992;20:70–77. doi: 10.1016/0735-1097(92)90139-e. [DOI] [PubMed] [Google Scholar]
- 35.Ribakove GH, Katz ES, Galloway AC, et al. Surgical implications of transesophageal echocardiography to grade the atheromatous aortic arch. Ann Thorac Surg. 1992;53:758–761. doi: 10.1016/0003-4975(92)91431-8. [DOI] [PubMed] [Google Scholar]
- 36.Grega MA, Borowicz LM, WA B. Impact of single clamp versus double clamp technique on neurologic outcome Ann Thorac Surg. 2003;75:1387–1391. doi: 10.1016/s0003-4975(02)04993-7. [DOI] [PubMed] [Google Scholar]
- 37.Gold JP, Torres KE, Maldarelli W, et al. Improving outcomes in coronary surgery: the impact of echo-direct aortic cannulation and preoperative hemodynamic management in 500 patients. Ann Thorac Surg. 2004;78:1579–1585. doi: 10.1016/j.athoracsur.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 38.Hammon JW, Stump DA, Butterworth JR, et al. Single crossclamp improves 6- month cognitive outcome in high-risk coronary bypass patients: The effect of reduced aortic manipulation. J Thorac Cardiovasc Surg. 2006;131:114–121. doi: 10.1016/j.jtcvs.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 39.Swaminathan M, Grocott HP, Mackensen GB, et al. The "sandblasting" effect of aortic cannulation on arch atheroma during cardiopulmonary bypass. Anesth Analg. 2007;104:1350–1351. doi: 10.1213/01.ane.0000264090.24756.08. [DOI] [PubMed] [Google Scholar]
- 40.Ura M, Sakata R, Nakayama Y, et al. Ultrasonographic demonstration of manipulation-related aortic injuries after cardiac surgery. J Am Coll Cardiol. 2000;35:1303–1310. doi: 10.1016/s0735-1097(00)00548-9. [DOI] [PubMed] [Google Scholar]
- 41.Moody DM, Brown WR, Challa VR, et al. Brain microemboli associated with cardiopulmonary bypass: a histologic and magnetic resonance imaging study. Ann Thorac Surg. 1995;59:1304–1307. doi: 10.1016/0003-4975(95)00057-r. [DOI] [PubMed] [Google Scholar]
- 42.Brooker RF, Brown WR, Moody DM, et al. Cardiotomy suction: a major source of brain lipid emboli during cardiopulmonary bypass. Ann Thorac Surg. 1998;65:1651–1655. doi: 10.1016/s0003-4975(98)00289-6. [DOI] [PubMed] [Google Scholar]
- 43.Kincaid EH, Jones TJ, Stump DA, et al. Processing scavenged blood with a cell saver reduces cerebral lipid microembolization. Ann Thorac Surg. 2000;70:1296–1300. doi: 10.1016/s0003-4975(00)01588-5. [DOI] [PubMed] [Google Scholar]
- 44.Jewell AE, Akowuah EF, Suvarna SK, et al. A prospective randomized comparison of cardiotomy suction and cell saver for recycling shed blood during cardiac surgery. Eur J Cardiothorac Surg. 2003;23:633–636. doi: 10.1016/s1010-7940(02)00834-5. [DOI] [PubMed] [Google Scholar]
- 45.Kaza AK, Cope JT, Fiser SM, et al. Elimination of fat microemboli during cardiopulmonary bypass. Ann Thorac Surg. 2003;75:555–559. doi: 10.1016/s0003-4975(02)04540-x. [DOI] [PubMed] [Google Scholar]
- 46.Spiess BD, Royston D, Levy JH, et al. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143–1148. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 47.Rubens FD, Boodhwani M, Mesana T, et al. The Cardiotomy Trial. A randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation. 2007;116:I-89–I-197. doi: 10.1161/CIRCULATIONAHA.106.678987. [DOI] [PubMed] [Google Scholar]
- 48.Djaiani G, Fedorko L, Borger MA, et al. Continuous-Flow Cell Saver Reduces Cognitive Decline in Elderly Patients After Coronary Bypass Surgery Circulation. 2007;116:1888–1895. doi: 10.1161/CIRCULATIONAHA.107.698001. [DOI] [PubMed] [Google Scholar]
- 49.Gold JP, Charlson ME, Williams-Russo P, et al. Improvement of outcomes after coronary artery bypass: a randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110:1305–1311. doi: 10.1016/S0022-5223(95)70053-6. [DOI] [PubMed] [Google Scholar]
- 50.Hartman GS, Yao FS, Bruefach M, et al. Severity of aortic atheromatous disease diagnosed by transesophageal echocardiography predicts stroke and other outcomes associated with coronary artery surgery: a prospective study. Anesth Analg. 1996;83:701–708. doi: 10.1097/00000539-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Cook DJ, Orszulak TA, RC D. Minimum hematocrit at differing cardiopulmonary bypass temperatures in dogs. Circulation. 1998;98:III170–III174. [PubMed] [Google Scholar]
- 52.Dexter F, BJ H. Effect of haemoglobin concentration on brain oxygenation in focal stroke: a mathematical modeling study. Br J Anaesth. 1997;79:346–351. doi: 10.1093/bja/79.3.346. [DOI] [PubMed] [Google Scholar]
- 53.DeFoe GR, Ross CS, Olmstead EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Ann Thorac Surg. 2001;71:769–776. doi: 10.1016/s0003-4975(00)02393-6. [DOI] [PubMed] [Google Scholar]
- 54.Fang WC, Helm RE, Krieger KH, et al. Impact of minimum hematocrit during cardiopulmonary bypass on mortality in patients undergoing coronary artery surgery. Circulation. 1997;96:II194–II199. [PubMed] [Google Scholar]
- 55.Habib RH, Zacharias A, Schwann TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: Should current practice be changed. J Thorac Cardiovasc Surg. 2003;125:1438–1450. doi: 10.1016/s0022-5223(02)73291-1. [DOI] [PubMed] [Google Scholar]
- 56.Karkouti K, Djaiani G, Borger MA, et al. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–1387. doi: 10.1016/j.athoracsur.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 57.Johnson RG, Thurer RL, Kruskall MS, et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. J Thorac Cardio-vasc Surg. 1992;104:307–314. [PubMed] [Google Scholar]
- 58.Bracey AW, Radovancevic R, Riggs SA, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39:1070–1077. doi: 10.1046/j.1537-2995.1999.39101070.x. [DOI] [PubMed] [Google Scholar]
- 59.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. J Am Med Assoc. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 60.Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Ferraris VA, Ferraris SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. J Thorac Cardiovasc Surg. 2007;83:S27–S86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 61.Hogue CW, Jr, Hershey T, Dixon D, et al. Preexisting cognitive impairment in women before cardiac surgery and its relationship with C-reactive protein. Anesth Analg. 2006;102:1602–1608. doi: 10.1213/01.ANE.0000219591.10826.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minamisawa H, Smith ML, BK S. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann Neurol. 1990;28:26–33. doi: 10.1002/ana.410280107. [DOI] [PubMed] [Google Scholar]
- 63.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 64.Group. THACAS. Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 65.Rees K, Beranek-Stanley M, Burke M, et al. Hypothermia to reduce neurological damage following coronary artery bypass surgery. Issue 2. Vol. Chichester, UK: John Wiley & Sons; 2004. (Cochrane Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grocott HP, Mackensen GB, Grigore AM, et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke. 2002;33:537–541. doi: 10.1161/hs0202.102600. [DOI] [PubMed] [Google Scholar]
- 67.Cook DJ, Orszulak TA, Daly RC, et al. Cerebral hyperthermia during cardiopulmonary bypass in adults. J Thorac Cardiovasc Surg. 1996;111:268–269. doi: 10.1016/S0022-5223(96)70425-7. [DOI] [PubMed] [Google Scholar]
- 68.Nathan HJ, Wells GA, Munson JL, et al. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: a randomized trial. Circulation. 2001;104:I-85–I-91. doi: 10.1161/hc37t1.094710. [DOI] [PubMed] [Google Scholar]
- 69.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 70.van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 71.Butterworth J, Wagenknecht LE, Legault C, et al. Attempted control of hyperglycemia during cardiopulmonary bypass fails to improve neurologic or neurobehavioral outcomes in patients without diabetes mellitus undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2005;130:1319–1325. doi: 10.1016/j.jtcvs.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 72.Gandhi FY, Nuttall GA, Abel MD, et al. Intensive Intraoperative Insulin Therapy versus Conventional Glucose Management during Cardiac Surgery. Ann Intern Med. 2007;146:233–243. doi: 10.7326/0003-4819-146-4-200702200-00002. [DOI] [PubMed] [Google Scholar]
- 73.Allport L, Baird T, Butcher K, et al. Frequency and temporal profile of poststroke hyperglycemia using continuous glucose monitoring. Diabetes Care. 2006;29:1839–1844. doi: 10.2337/dc06-0204. [DOI] [PubMed] [Google Scholar]
- 74.Baird TA, Parsons MW, Phanh T, et al. Persistent poststroke hypeglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 75.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the Early Management of Adults With Ischemic Stroke: A Guideline From the American Heart Association/ American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 76.Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurology. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 77.Van Dijk D, Jansen EW, Hijman R, et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery. J Am Med Assoc. 2002;287:1405–1412. doi: 10.1001/jama.287.11.1405. [DOI] [PubMed] [Google Scholar]
- 78.Diederik van Dijk M, PhD, Spoor Monique, MS, Hijman Ron, PhD, Nathoe Hendrik M, MD, PhD, Borst Cornelius, MD, PhD, Jansen Erik WL, MD, PhD, Grobbee Diederick E, MD, PhD, de Jaegere Peter PT, MD, PhD, Kalkman Cor J, MD, PhD for the Octopus Study Group. Cognitive and Cardiac Outcomes 5 Years After Off-Pump vs On-Pump Coronary Artery Bypass Graft Surgery. J Am Med Assoc. 2007;297:701–708. doi: 10.1001/jama.297.7.701. [DOI] [PubMed] [Google Scholar]
- 79.Cheng DC BD, Martin JE, Novick RJ. Evidence-Based Perioperative Clinical Outcomes Research Group.: Does off-pump coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with conventional coronary artery bypass? A meta-analysis of randomized trials. Anesthesiology. 2005;102:188–203. doi: 10.1097/00000542-200501000-00028. [DOI] [PubMed] [Google Scholar]
- 80.Biancari F, Mosorin M, Rasinaho E, et al. Postoperative stroke after off-pump versus on-pump coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2007;133:169–173. doi: 10.1016/j.jtcvs.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 81.Kotoh K FK, Doi T, Nagura S, Misaki T. Predictors of early postoperative cerebral infarction after isolated off-pump coronary artery bypass grafting. Ann Thorac Surg. 2007;83:1679–1683. doi: 10.1016/j.athoracsur.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 82.Sedrakyan A, Treasure T, JA E. Effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: a systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg. 2004;128:442–448. doi: 10.1016/j.jtcvs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 83.Mangano DT, Tudor IC, C D, et al. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 84.Brown JR, Birkmeyer NJ, GT OC. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation. 2007;115:2801–2813. doi: 10.1161/CIRCULATIONAHA.106.671222. [DOI] [PubMed] [Google Scholar]
- 85.Yao FS, Tseng CC, Ho CY, et al. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:552–558. doi: 10.1053/j.jvca.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 86.Goldman S, Sutter F, Ferdinand F, et al. Optimizing intraoperative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incdience of stroke for cardiac surgical patients. Heart Surg Forum. 2004;7:E376–E381. doi: 10.1532/HSF98.20041062. [DOI] [PubMed] [Google Scholar]
- 87.Kurth CD, WS T. A multiwavelength frequency-domain near-infrared cerebral oximter. Phys Med Biol. 1999;44:727–740. doi: 10.1088/0031-9155/44/3/015. [DOI] [PubMed] [Google Scholar]
- 88.Murkin J, Adams SJ, Novick RJ, et al. Monitoring brain oxygen saturation during coronary artery bypass surgery: A randomized, prospective study. Anesth Analg. 2007;104:51–58. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 89.Abildstrom H, Rasmussen LS, Rentowl P, et al. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. Acta Anaesthiol Scan. 2000;44:1246–1251. doi: 10.1034/j.1399-6576.2000.441010.x. [DOI] [PubMed] [Google Scholar]