Abstract
Objective:
To provide a systematic review of the studies assessing exercise training and inspiratory muscle training (IMT) in individuals for the improved respiratory function of patients with spinal cord injury (SCI).
Methods:
Thirteen studies (5 exercise training, 8 IMT) were identified. Articles were scored for their methodological quality using the Physiotherapy Evidence Database scores and Downs and Black tools for randomized and nonrandomized studies, respectively. Conclusions were based on the most rigorously executed studies using Sackett's levels of evidence.
Results:
Study comparison was compromised by diverse research designs; small sample sizes; and heterogeneity of studied populations, protocols, and outcome measures. Based on current literature, there is level 2 evidence supporting exercise training as an intervention to improve respiratory strength and endurance and level 4 evidence to support exercise training as an intervention that might improve resting and exercising respiratory function in people with SCI. There is level 4 evidence to support IMT as an intervention that might decrease dyspnea and improve respiratory function in people with SCI.
Conclusions:
There are insufficient data to strongly support the use of exercise training or IMT for improved respiratory function in people with SCI. There is some evidence of efficacy of both regimens; however, the evidence is not of the best possible quality.
Keywords: Spinal cord injuries; Exercise training; Inspiratory muscle training, Rehabilitation; Respiratory dysfunction; Systematic review
INTRODUCTION
The respiratory system, composed of the lungs, respiratory muscles, and associated neuronal controls, is a complex physiological unit that must operate in a cyclical and highly coordinated fashion 24 hours a day in order to sustain life. Spinal cord injury (SCI) impairs neuronal control of respiratory muscles and in turn respiratory function. Despite advances in patient care resulting in significant improvements in acute and long-term survival rates, individuals with SCI continue to have a mortality rate 47% higher than able-bodied people (1). Respiratory complications remain one of the leading causes of morbidity and mortality among individuals with SCI (2,3).
Numerous protocols of respiratory training have been employed as a means of improving respiratory function in individuals with SCI. Two specific training modalities that have been clinically evaluated are exercise training and inspiratory muscle training (IMT). This review systematically assessed the efficacy of exercise training and IMT for the improved respiratory function of patients with SCI to facilitate the identification of optimal clinical care for clinicians who treat patients with SCI. This article is organized into 2 sections: the first section reviews the current understanding of SCI-related respiratory impairment and the mechanisms by which exercise training and IMT are believed to mitigate these impairments; the second section reports the search strategy employed in this review and the results and analyses of studies that have assessed exercise training and IMT.
RESPIRATORY DYSFUNCTION IN SCI
SCI-related respiratory dysfunction is largely dependent on the level of injury, completeness of injury, and time since injury (1,4,5). Higher lesions result in greater denervation of the expiratory and inspiratory muscles, and, thus, injuries to the cervical and upper thoracic regions of the spinal cord result in greater reductions in inspiratory and expiratory function. Complete paralysis of all muscles involved with respiration occurs when the lesion is above C3; this type of injury requires immediate and ongoing ventilatory support in order to sustain life. When the injury is between C3 and C5 (innervation of the diaphragm), respiratory insufficiency occurs via respiratory muscle dysfunction. Although primary and some accessory muscles of inspiration are fully innervated with injuries below cervical lesions, the ability to ventilate at higher levels is still compromised because the intercostals and other chest wall muscles do not provide the integrated expansion of the upper chest wall as the diaphragm descends during inspiration. There is some evidence that the chest wall is pulled in during diaphragm excursion, which could further limit inspiratory capacity. Furthermore, ventilation during exercise can be greatly compromised; the expiratory muscles actively contract in healthy people, whereas in SCI, partial or fully denervated expiratory muscles have impaired contractile activity and thus exhibit diminished exercise ventilation and ventilatory reserve.
Lung volumes, such as inspiratory capacity and expiratory reserve volume, reflect these diminished capacities for full inspiration and forced expiration in people with SCI. Both inspiratory capacity and expiratory reserve volume are progressively smaller in higher cervical lesions vs lower thoracic and lumbar lesions (4). The forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) are usually measured in apparently healthy people to detect airways obstruction and restrictive lung disease. Due to reduced inspiratory muscle force, these measures are diminished in people after SCI with higher lesions and especially in people with tetraplegia (4,5) and demonstrate moderate correlation with injury level (4). Whereas incomplete lesions appear to mitigate FEV1 and FVC losses, smoking and longer duration of injury are associated with greater reductions in FEVs (5).
Although cough is an important defense mechanism against respiratory tract infections and atelectasis, the capacity to generate cough and clear respiratory secretions is severely compromised in most individuals with SCI due to the impaired innervation of the abdominal muscles at most SCI levels. The respiratory system has other important roles, such as speaking and posture-related activities (eg, trunk stability), which can also be negatively affected by the SCI, especially with higher lesions. Respiratory system complications can be exacerbated by preexisting medical conditions, history of smoking, advanced age, and therapeutic measures to manage the resuscitation phase of the injured patient (6–8).
Exercise Training
As with able-bodied individuals, there is strong evidence supporting the use of exercise training for improved cardiovascular health among people with SCI (9). This is important because there is a high incidence of physical inactivity in individuals with SCI and they are at increased risk of secondary conditions, such as cardiovascular disease, diabetes, osteoporosis, and obesity (9).
With respect to respiratory function, exercise training in patients with SCI improves ^both the control of breathing (eg, minute ventilation [V̂E] and tidal volume [Vt]) and respiratory sensations (eg, dyspnea). Specifically, exercise training results in a lowered V̂E at any given absolute oxygen consumption or power output and is associated with decreased feelings of breathlessness. Although the former reduces the work of breathing during exercise, the latter prevents its early termination. These training effects are likely due to a reduction in one or more of the mechanisms (neural and/or humoral) purported to cause the hyperpnea of exercise.
Recent evidence suggests that improvements in respiratory function associated with exercise training may be partly explained by the adaptive response of the respiratory system itself (10–12). Structural adaptations may be muted to a greater extent among individuals with SCI, considering the small amount of muscle mass used in wheelchair propulsion or arm-cranking exercise. In contrast, respiratory muscles have been shown to be both metabolically and structurally plastic. As such, it is possible that the positive effects of exercise training in SCI may reside in an increase in respiratory muscle strength and endurance in addition to adjacent effects of reduced ventilatory demand during exercise via peripheral adaptations. A training response of the respiratory muscles has been demonstrated directly in animal models (10) and indirectly in able-bodied humans (11,12).
Inspiratory Muscle Training
Approximately two thirds of the prevalence of dyspnea in patients with SCI is attributed to inspiratory muscle paralysis (13), with greater severity associated with higher levels of injury. Improved inspiratory muscle strength and endurance could potentially improve cough and maximal exercise ventilation in addition to decreasing dyspnea.
Inspiratory muscles can be trained similarly to the limb muscles with inexpensive devices that increase the resistive or threshold inspiratory load on the inspiratory muscles (14). The 2 main types of devices used to improve the strength and endurance of inspiratory muscles are resistive and threshold trainers. Both of these devices have a 1-way valve that closes during inspiration so that the subject must breathe through a small-diameter hole for the resistive trainer or against a spring-loaded valve for the threshold trainer. The 1-way valve opens during expiration such that no load is imposed during the expiratory phase of respiration.
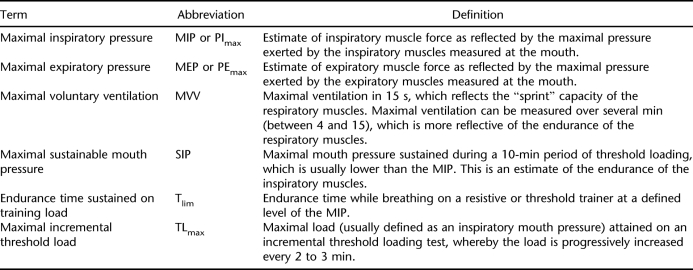
Evidence showing decreased dyspnea and improved strength and endurance after IMT is well documented in people with other health conditions, such as chronic obstructive pulmonary disease (COPD) (14). Table 1 outlines common measures that are indicative of respiratory muscle strength and endurance. In neuromuscular disorders such as SCI, maximal lung volumes that measure inspiratory capacity also can reflect increased inspiratory muscle strength.
Table 1.
Measures of Respiratory Muscle Strength and Endurance
STUDY DESIGN
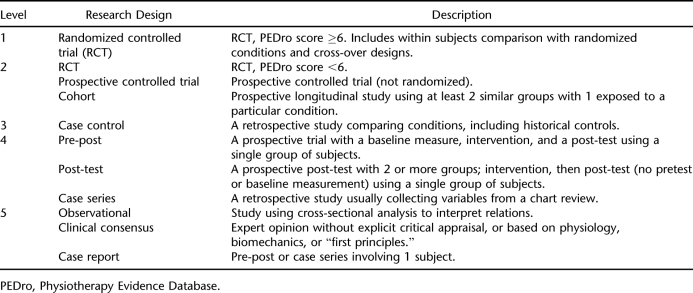
A systematic search of multiple databases (MEDLINE/PubMed, CINAHL, EMBASE, PsycINFO) was executed to identify all relevant literature published from 1980 through 2006. Databases were searched for spinal cord injury, tetraplegia, quadriplegia, or paraplegia paired with the following key words: exercise, ventilation, respiratory muscle, respiratory muscle strength, maximal inspiratory pressure, maximum voluntary ventilation, and pulmonary function. Articles were included if they were published in English and involved human subjects and a specific intervention. To ensure the ability to generalize to a SCI population, we excluded studies in which less than half of the reported sample had SCI. Studies describing the acute responses to exercise in people with SCI were not included nor were studies concerned with competitive athletes. Studies with no measurable outcome associated with the intervention were also not included. References of all identified articles were searched, and relevant articles were retrieved. Articles were scored for their methodological quality using either the Physiotherapy Evidence Database (PEDro) score (15) for randomized controlled trials (RCTs) or the Downs and Black tool (16) for nonrandomized studies. A thorough report of these tools and their psychometric properties is reported elsewhere (17). In brief, PEDro scores range from 0 to 10 and Downs and Black scores range from 0 to 28; higher scores for each tool indicate higher methodological quality. Scoring was executed by 2 independent reviewers, and discrepancies were resolved through discussion or a third independent reviewer. Tables were generated from the extracted data that included sample subject characteristics, nature of the intervention, outcome measures, and key results. Subsequent to individual study assessment, conclusions were drawn about the accumulated studies based on a modified version of Sackett's levels of evidence (Table 2) (18). RCTs received priority when formulating conclusions. If studies addressing the same treatment differed in quality, more weighting was applied to the studies with higher quality scores when deriving the final conclusions.
Table 2.
Five Levels of Evidence
RESULTS
Exercise Training
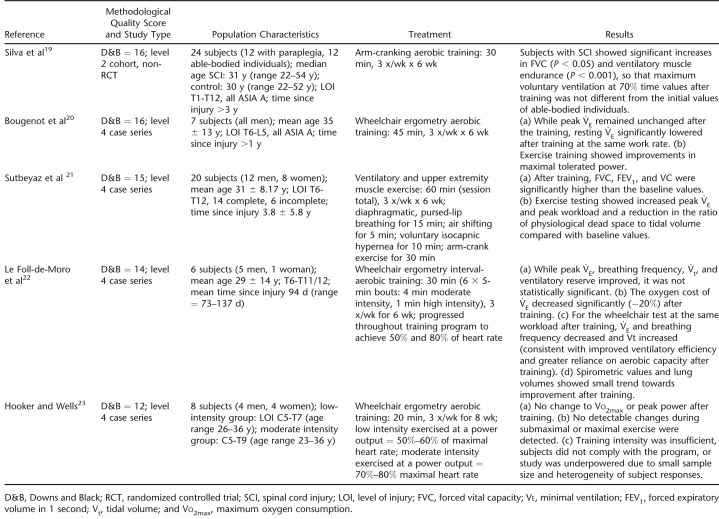
Those studies concerned with exercise training principally included subjects with paraplegia. Five studies assessing the respiratory effects of exercise training in individuals with SCI were identified for review (N = 65) (19–23). Study characteristics and results are listed in Table 3. All studies employed a case series design with the exception of 1 cohort trial (19). As a group, these studies were difficult to interpret because of relatively small sample sizes; differences in the type of exercise modality evaluated (wheelchair vs arm-crank exercise); and inconsistencies in the frequency, intensity, and duration of exercise training used. Despite these limitations, evidence from 2 studies supported the efficacy of exercise training for improved respiratory function (13,19), and trends for its therapeutic potential were suggested by another 2 studies (20,22). Only 1 study failed to demonstrate an effect after exercise training; this study, however, was ranked last according to its Downs and Black methodological score (23). Based on these findings, there is level 2 evidence to support that exercise training improves expiratory capacity and ventilatory muscle endurance (19) and level 4 evidence to support that exercise training is associated with improved lung volumes and FEVs (13). There is some evidence from level 4 studies that VE (20,22), as well as Vt and ventilatory reserve, may be improved after exercise training (22). There were no reports of negative consequences of exercise training.
Table 3.
Exercise Training
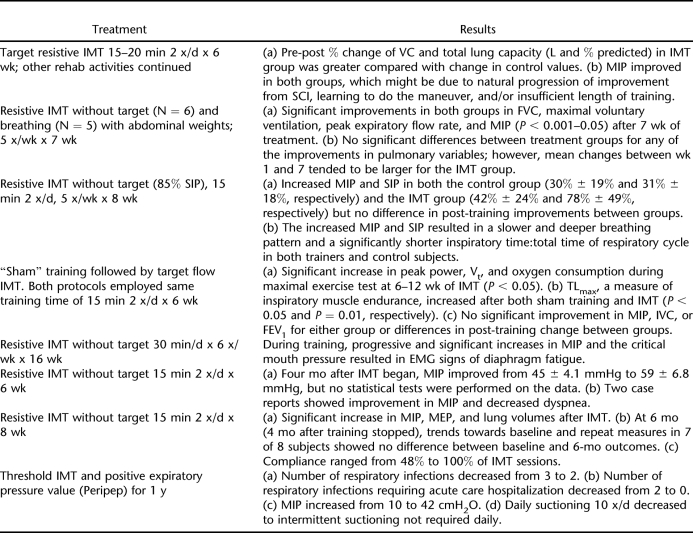
Inspiratory Muscle Training
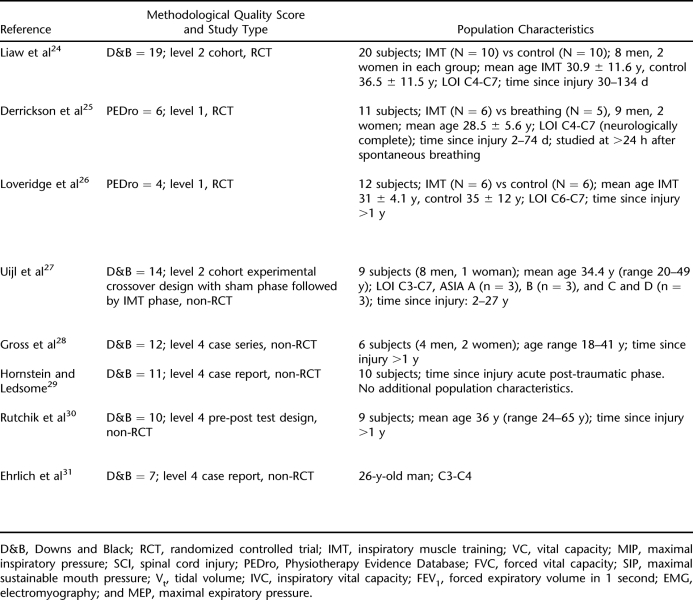
Our search captured 8 studies assessing the respiratory effects of IMT in individuals with SCI (N = 78) (24–31). As with studies assessing exercise training, most studies were not comparable on account of diverse research designs, heterogeneity of subject characteristics, or differences in training techniques (Table 4). Those studies concerned with inspiratory muscle included subjects with both paraplegia and tetraplegia.
Table 4.
Inspiratory Muscle Training
Only 3 studies utilized an RCT design (24–26), 2 of which showed improved measures of inspiratory muscle and lung function in both control (or sham) and training groups (25,26). In contrast, Liaw et al (24) showed significant improvement in total lung capacity. Of the nonrandomized studies, 1 cohort study showed improvements in endurance measures with no change in lung volumes or measures of respiratory capacity (27). One case series (28) and 1 pre-post study (30) demonstrated significant improvements in respiratory muscle strength, findings that were consistent with the positive, nonsignificant trends of 2 other studies (29,31). Of note, follow-up data from Rutchik et al (30) showed respiratory benefits to be lost after the cessation of IMT. Based on these findings, there is only level 4 evidence to support IMT as a means to improve inspiratory muscle function and decrease dyspnea in individuals with SCI.
DISCUSSION
Based on the current literature, there is insufficient evidence to strongly support either exercise training or IMT as a means to improve pulmonary function or ventilatory responses in individuals with SCI. There is some evidence for efficacy of both regimens; however, the evidence is not of the best possible quality.
Exercise training has been evaluated in a limited number of studies that assessed various training protocols. Although the data from these studies suggest the potential of exercise training to improve exercise ventilation and ventilatory efficiency, well-designed RCTs are needed to determine the true impact of exercise training on pulmonary function. Combined with the well-known able-bodied response to upper-limb training, exercise training may offer a strong clinical tool for the management of people with SCI and at least should be encouraged for the maintenance of general cardiorespiratory health in this population.
In contrast to exercise training, IMT has been evaluated by 3 randomized studies. Although the results of these studies failed to support the efficacy of IMT, poor study design may have impeded the demonstration of the true therapeutic potential of IMT. For example, comparable improvements of respiratory function in control and treatment groups may reflect learning of testing maneuvers, benefit from other rehabilitation or lifestyle activities, and/or natural progression of improvement after SCI. All randomized studies used an inspiratory resistive device with no target to control for decreasing resistance with slower flows. As such, the training methods may have induced an alteration in breathing pattern towards slower inspiratory flows rather than a training response against higher inspiratory pressures.
Our assessment of RCTs of IMT confirms the findings of 2 previous systematic reviews of RCTs assessing the efficacy of IMT (32) and respiratory muscle training (33) for individuals with SCI. Although RCTs are the “gold standard” for assessing treatment efficacy, we believe more comprehensive data can be gleaned from the systematic assessment of both randomized and nonrandomized studies. Thus, the strength of our review is the inclusion and assessment of nonrandomized studies of IMT and exclusion of confounding respiratory interventions (eg, expiratory muscle training, pectoralis muscle training) (33).
Nonrandomized studies evaluating IMT demonstrated significant positive findings associated with its utilization in persons with SCI. Although the methodological design of these studies suggests caution when interpreting their results, their positive findings, coupled with the demonstrated efficacy threshold IMT has shown for improving inspiratory muscle strength and endurance in people with COPD, clearly demonstrate the need for a larger and more robust RCT in a SCI population. In addition to random allocation to either a control or a treatment group, such a study should also employ (a) a research design that controls for the influence of learning or recovery from SCI on IMT outcome measures of inspiratory muscle strength and endurance and dyspnea; (b) optimal training techniques of threshold loading, targeted resistive devices, or isocapnic hyperpnea; (c) outcomes of inspiratory muscle strength and endurance, dyspnea, quality of life, and daily function; and (d) a comparison of the relative effectiveness of IMT relative to or as an adjunct to other rehabilitation interventions.
Ideal training regimes for both exercise training and IMT have not been identified. Based on the protocols employed by Silva et al (19) and Sutbeyaz et al (21), improved respiratory function requires a high training intensity performed for 30 minutes 3 times per week for 6 weeks. Parameters to optimize IMT are only available for people with other respiratory conditions, namely COPD. For this population, the optimal IMT protocol should utilize threshold or targeted resistive trainers at an intensity of 30% to 70% of maximal inspiratory pressure, for a duration up to 30 minutes per session, performed continuously or in intervals, 4 to 6 days/week and be continued indefinitely (34). Progression of intensity (maximal inspiratory pressure) should not exceed 5% per week. Until more rigorous research is performed to further delineate the most effective exercise training and IMT protocols, prescription of these training modalities should be approached cautiously. Of equal importance, overly aggressive prescription of IMT has the potential to fatigue and injure the inspiratory muscles, which can increase the person's predisposition to respiratory compromise. Parameters to monitor during IMT in order to avoid untoward responses, such as muscle fatigue and hypercapnia, have been described elsewhere (35).
Finally, additional large-scale, cross-sectional and longitudinal studies are required to fully characterize pulmonary function in SCI. Secondary respiratory complications related to other respiratory pathologies (eg, COPD, asthma) are not well described. In particular, the consequences of aging on pulmonary function are not well defined in SCI. With healthy aging, there is a decline in lung function, primarily because of a loss of elastic recoil. Moreover, additional age-related changes that are known to negatively affect gas exchange are decreased surface area of the lung, decreased pulmonary capillary blood volume, increased dead space ventilation, and decreased distensibility of the pulmonary arterial vasculature. A greater understanding of the interactions among SCI, aging, and the respiratory system is necessary for comprehensive patient management.
It is important to note that most exercise studies included only individuals with paraplegia who have lower-level lesions and minimal deficits in pulmonary function, whereas the respiratory muscle training studies enrolled only persons with cervical injury (Tables 3 and 4). This is an essential consideration from the theoretical and practical viewpoints because cervical injuries cause profound reductions in inspiratory muscle function and are associated with the greatest prevalence of breathlessness and highest risk of death due to respiratory complications. It is in this group that improvements in pulmonary function may have the greatest clinical benefit. Conversely, those with lower-level paraplegia typically have FEVs that are within the “normal” range such that improvements may be (a) difficult to achieve and (b) of limited clinical significance.
CONCLUSIONS
Consistent improvement in respiratory function after respiratory muscle training has not been demonstrated in people with SCI. There is level 2 evidence supporting exercise training as an intervention to improve respiratory strength and endurance and level 4 evidence to support exercise training as an intervention that might improve resting and exercising respiratory function in people with SCI. There is level 4 evidence to support IMT as an intervention that might decrease dyspnea and improve respiratory function in some people with SCI. We conclude that there are insufficient data to strongly support the use of exercise training or IMT for improved respiratory function in people with SCI. There is some evidence of efficacy of both regimens; however, the evidence is not of the best possible quality.
Acknowledgments
We thank the Rick Hansen Man-in-Motion Foundation and Ontario Neurotrauma Foundation for their financial support of the SCIRE project. N. T. Ayas was supported by a scholar award from the Michael Smith Foundation for Health (MSFHR). A. W. Sheel was supported by a scholar award from the MSFHR and a new investigator award from the Canadian Institutes of Health Research.
Table 4.
Extended.
REFERENCES
- Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51:853–868. discussion 869–870. [PMC free article] [PubMed] [Google Scholar]
- DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993;74:248–254. [PubMed] [Google Scholar]
- DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Baydur A, Adkins RH, Milic-Emili J. Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol. 2001;90:405–411. doi: 10.1152/jappl.2001.90.2.405. [DOI] [PubMed] [Google Scholar]
- Linn WS, Adkins RH, Gong H, Jr, Waters RL. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil. 2000;81:757–763. doi: 10.1016/s0003-9993(00)90107-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine. 2001;26:426–430. doi: 10.1097/00007632-200102150-00020. [DOI] [PubMed] [Google Scholar]
- Reiss SJ, Raque GH, Jr, Shields CB, Garretson HD. Cervical spine fractures with major associated trauma. Neurosurgery. 1986;18:327–330. doi: 10.1227/00006123-198603000-00012. [DOI] [PubMed] [Google Scholar]
- Urdaneta F, Layon AJ. Respiratory complications in patients with traumatic cervical spine injuries: case report and review of the literature. J Clin Anesth. 2003;15:398–405. doi: 10.1016/s0952-8180(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Warburton DER, Eng JJ, Krassioukov A, Sproule S, SCIRE Research Team. Cardiovascular health and exercise rehabilitation in spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;13:98–122. doi: 10.1310/sci1301-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabas IS, Dodd SL, Powers SK, et al. Endurance training reduces the rate of diaphragm fatigue in vitro. Med Sci Sports Exerc. 1999;31:1605–1612. doi: 10.1097/00005768-199911000-00017. [DOI] [PubMed] [Google Scholar]
- Clanton TL, Dixon GF, Drake J, Gadek JE. Effects of swim training on lung volumes and inspiratory muscle conditioning. J Appl Physiol. 1987;62:39–46. doi: 10.1152/jappl.1987.62.1.39. [DOI] [PubMed] [Google Scholar]
- Robinson EP, Kjeldgaard JM. Improvement in ventilatory muscle function with running. J Appl Physiol. 1982;52:1400–1406. doi: 10.1152/jappl.1982.52.6.1400. [DOI] [PubMed] [Google Scholar]
- Spungen AM, Grimm DR, Lesser M, Bauman WA, Almenoff PL. Self-reported prevalence of pulmonary symptoms in subjects with spinal cord injury. Spinal Cord. 1997;35:652–657. doi: 10.1038/sj.sc.3100489. [DOI] [PubMed] [Google Scholar]
- Geddes EL, Reid WD, Crowe J, O'Brien K, Brooks D. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: a systematic review. Respir Med. 2005;99:1440–1458. doi: 10.1016/j.rmed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the physiotherapy evidence database (PEDro) Aust J Physiother. 2002;48:43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng JJ, Teasell R, Miller WC, et al. Spinal cord injury rehabilation evidence: methods of the SCIRE systematic review. Top Spinal Cord Inj Rehabil. 2007;13:1–10. doi: 10.1310/sci1301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-Based Medicine: How to Practice and Teach EBM. Toronto, Ontario, Canada: Churchill Livingstone; 2000. [Google Scholar]
- Silva AC, Neder JA, Chiurciu MV, et al. Effect of aerobic training on ventilatory muscle endurance of spinal cord injured men. Spinal Cord. 1998;36:240–245. doi: 10.1038/sj.sc.3100575. [DOI] [PubMed] [Google Scholar]
- Bougenot MP, Tordi N, Betik AC, et al. Effects of a wheelchair ergometer training programme on spinal cord-injured persons. Spinal Cord. 2003;41:451–456. doi: 10.1038/sj.sc.3101475. [DOI] [PubMed] [Google Scholar]
- Sutbeyaz ST, Koseoglu BF, Gokkaya NK. The combined effects of controlled breathing techniques and ventilatory and upper extremity muscle exercise on cardiopulmonary responses in patients with spinal cord injury. Int J Rehabil Res. 2005;28:273–276. doi: 10.1097/00004356-200509000-00012. [DOI] [PubMed] [Google Scholar]
- Le Foll-de Moro D, Tordi N, Lonsdorfer E, Lonsdorfer J. Ventilation efficiency and pulmonary function after a wheelchair interval-training program in subjects with recent spinal cord injury. Arch Phys Med Rehabil. 2005;86:1582–1586. doi: 10.1016/j.apmr.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Hooker SP, Wells CL. Effects of low- and moderate-intensity training in spinal cord-injured persons. Med Sci Sports Exerc. 1989;21:18–22. doi: 10.1249/00005768-198902000-00004. [DOI] [PubMed] [Google Scholar]
- Liaw MY, Lin MC, Cheng PT, Wong MK, Tang FT. Resistive inspiratory muscle training: its effectiveness in patients with acute complete cervical cord injury. Arch Phys Med Rehabil. 2000;81:752–756. doi: 10.1016/s0003-9993(00)90106-0. [DOI] [PubMed] [Google Scholar]
- Derrickson J, Ciesla N, Simpson N, Imle PC. A comparison of two breathing exercise programs for patients with quadriplegia. Phys Ther. 1992;72:763–769. doi: 10.1093/ptj/72.11.763. [DOI] [PubMed] [Google Scholar]
- Loveridge B, Badour M, Dubo H. Ventilatory muscle endurance training in quadriplegia: effects on breathing pattern. Paraplegia. 1989;27:329–339. doi: 10.1038/sc.1989.50. [DOI] [PubMed] [Google Scholar]
- Uijl SG, Houtman S, Folgering HT, Hopman MT. Training of the respiratory muscles in individuals with tetraplegia. Spinal Cord. 1999;37:575–579. doi: 10.1038/sj.sc.3100887. [DOI] [PubMed] [Google Scholar]
- Gross D, Ladd HW, Riley EJ, Macklem PT, Grassino A. The effect of training on strength and endurance of the diaphragm in quadriplegia. Am J Med. 1980;68:27–35. doi: 10.1016/0002-9343(80)90157-6. [DOI] [PubMed] [Google Scholar]
- Horstein S, Ledsome JR. Ventilatory muscle training in acute quadriplegia. Physiother Can. 1986;38:145–149. [Google Scholar]
- Rutchik A, Weissman AR, Almenoff PL, Spungen AM, Bauman WA, Grimm DR. Resistive inspiratory muscle training in subjects with chronic cervical spinal cord injury. Arch Phys Med Rehabil. 1998;79:293–297. doi: 10.1016/s0003-9993(98)90009-0. [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Manns PJ, Poulin C. Respiratory training for a person with C3-C4 tetraplegia. Aust J Physiother. 1999;45:301–307. doi: 10.1016/s0004-9514(14)60359-7. [DOI] [PubMed] [Google Scholar]
- Brooks D, O'Brien K, Geddes EL, Crowe J, Reid WD. Is inspiratory muscle training effective for individuals with cervical spinal cord injury? A qualitative systematic review. Clin Rehabil. 2005;19:237–246. doi: 10.1191/0269215505cr856oa. [DOI] [PubMed] [Google Scholar]
- Van Houtte S, Vanlandewijck Y, Gosselink R. Respiratory muscle training in persons with spinal cord injury: a systematic review. Respir Med. 2006;100:1886–1895. doi: 10.1016/j.rmed.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Geddes EL, Reed WD, Brooks D, Crowe J, O'Brien K. A Primer on Inspiratory Muscle Trainers: Buyers Guide for the European Respiratory Society. Lausanne, Switzerland: European Respiratory Society; 2006. [Google Scholar]
- Reid WD, Geddes EL, Brooks D, O'Brien K, Crowe J. Inspiratory muscle training in chronic obstructive pulmonary disease. Physiother Can. 2004;56:128–142. [Google Scholar]