Abstract
Background/objective:
To determine the effects of locomotor training (LT) using body weight support (BWS), treadmill, and manual assistance on muscle activation, bone mineral density (BMD), and body composition changes for an individual with motor complete spinal cord injury (AIS B), 1 year after injury.
Methods:
A man with chronic C6 AIS B (motor complete and sensory incomplete) spinal cord injury (SCI), 1 year after injury, completed 2 blocks of LT over a 9-month training period (35-session block followed by 8.6 weeks of no training and then a 62-session block).
Results:
Before training, muscle activation was minimal for any muscle examined, whereas after the 2 blocks of LT (97 sessions), hip and knee muscle activation patterns for the bilateral rectus femoris, biceps femoris, and gastrocnemius were in phase with the kinematics. Mean EMG amplitude increased for all bilateral muscles and burst duration increased for rectus femoris and gastrocnemius muscles, whereas burst duration decreased for the biceps femoris after 62 LT sessions. Before LT, left biceps femoris had a pattern that reflected muscle stretch, whereas after training, muscle stretch of the left biceps femoris could not totally account for mean EMG amplitude or burst duration. After the 62 training sessions, total BMD decreased (1.54%), and regional BMD decreased (legs: 6.72%). Total weight increased, lean mass decreased (6.6%), and fat mass increased (7.4%) in the arms, whereas fat mass decreased (3.5%) and lean mass increased (4%) in the legs.
Conclusions:
LT can induce positive neural and body composition changes in a nonambulatory person with chronic SCI, indicating that neuromuscular plasticity can be induced by repetitive locomotor training after a motor complete SCI.
Keywords: Spinal cord injuries, Tetraplegia, Locomotor training, Kinematic profiles, Electromyography profiles, Neural alterations, Bone and muscle changes, Body composition
INTRODUCTION
During stepping, it has been shown that sensory input associated with alternate limb kinematics and limb loading can provide afferent information to modulate efferent muscle patterns when there is compromised supraspinal input (1,2). Recently, new approaches to facilitate locomotor recovery have been directed away from compensatory strategies and toward locomotor training (LT) that optimizes this afferent sensory information and facilitates activity-dependent plasticity in the spinal cord to control movement (1–3). LT was first proposed by the Montreal group of researchers in the 80s (4–6). The studies on LT using body weight support treadmill training (BWST) in humans after spinal cord injury (SCI) (6–16) are based on extensive research related to animal studies (17–19).
It is well documented that LT facilitates functional walking recovery among persons with chronic incomplete SCI with an ASIA (20) impairment scale (AIS) C and D (6–16), but little is known for individuals who have a chronic motor complete SCI and who have completed LT (14,21–23). Studies have indicated that individuals with complete motor lesions can induce a locomotor-like electromyography (EMG) pattern when stepping on the treadmill using body weight support (BWS) (1,3,23,24). These studies highlighted that decreased body weight support did increase leg muscle activation in one muscle; however, it was difficult to distinguish whether this was simply the effect of greater load bearing or a possible effect of training. For example, Harkema et al (1) determined during one training session that the soleus, medial gastrocnemius, and tibialis anterior muscles were directly related to peak load per step on the lower limb during assisted locomotion. EMG amplitudes were highly dependent on the phase of the step cycle regardless of the level of load. However, to date, no studies have addressed the effect on an extended period of training on the bilateral muscle activation of motor complete individuals.
The primary objective was to investigate the effect of an extended period of LT using BWST and manual assistance with over-ground training on bilateral muscle activation (EMG pattern, amplitude, and burst duration) as recorded during treadmill walking. It was hypothesized that with repetitive step training using BWS for an extended period of time, there would be an increase in EMG amplitudes and alteration in burst duration, which were appropriate to the swing and stance phase of locomotion. This would provide quantitative evidence of neuronal activity changes as seen in a person with chronic motor complete SCI who undergoes LT. Specifically, this article addresses the impact of LT in an individual with chronic, motor complete AIS B SCI. A second objective was to investigate whether LT (using BWST and manual assistance with over-ground training) promoted improvements in the functional ability to stand over ground for an individual with chronic motor complete ASIA B SCI.
Bone Loss and Muscle Atrophy
Bone loss below the level of injury leading to osteoporosis and potential risk of fracture are often consequences after SCI (25,27,28,29). Bone loss is reported to be rapid after injury, and new steady-state levels 1 to 2 years after injury have been reported for patients 18 to 49 years of age at injury onset with motor complete lesions (C7–L1) (29). However, recently, additional data starting at 5 weeks after injury for 10 individuals 19 to 62 years of age suggested that no steady state for bone loss is achieved within 3 years after injury (25). Some research suggests that early mobilization and stand training after SCI may prevent bone mineral density loss at various sites of the lower extremity (31).
Muscle atrophy after SCI has been examined using magnetic resonance imaging (MRI) (26–28) and dual energy X-ray absorptiometry (DPX) (32,28). For example, in individuals with a complete SCI, muscle atrophy was examined at 6, 11, and 24 weeks after injury by MRI of the leg (26). Over the 18-week period, the cross-sectional areas for the quadriceps femoris, hamstring group, gastrocnemius, and soleus decreased significantly (16%, 14%, 24%, and 12%, respectively). In addition, DEXA measurements (32) have shown significant reductions in lean tissue mass for total body (16%) and the lower limb (12%) for individuals with paraplegia matched for age and height and compared with able-bodied controls. A third and minor objective of this study was to describe the body composition and bone changes after an extensive period of LT.
This article describes the neural changes that occur after an extended number of training sessions in a man with chronic AIS B C6 motor complete and sensory incomplete SCI, 1 year after injury, and reports EMG activity from multiple muscles simultaneously and musculoskeletal changes before and after training. There have been no reports to our knowledge of such significant neural or musculoskeletal changes in multiple muscles in an AIS B individual. In fact, even in individuals with acute AIS B reported by Dobkin et al (33), minimal functional changes were shown with early intervention with LT.
MATERIALS AND METHODS
Institutional Review Board approval was received for the study, and the participant signed an informed consent. The requirement was a bone mineral density (BMD) T-score measurement, as recorded by the dual energy x-ray absorptiometry (DPX, Lunar, Madison, WI) (28), greater than −2.5. The World Health Organization defines osteoporosis as total body BMD measurement with a T-score less than −2.5. After the DPX scan, the participant had a physical examination (by the study physician) as an additional measure to monitor the suitability of the participant for the study.
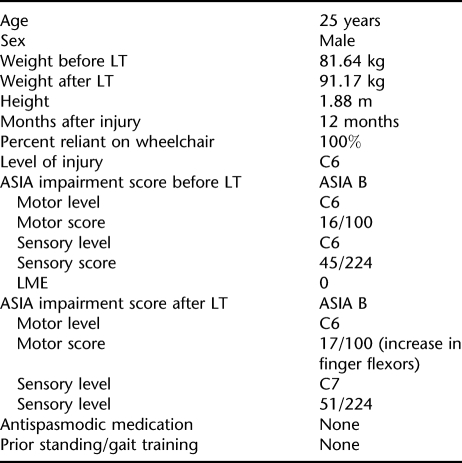
Clinical characteristics are given in Table 1. The participant did not have any prior standing or gait training. He trained for 35 sessions of LT (T1), stopped training for 8.6 weeks (because of equipment problems), and restarted training for another 62 sessions (21 weeks; T2; Figure 1). Training occurred 3 times per week. Testing procedures were conducted before T1 before T3 and after T4. Because of equipment problems, testing could not be completed immediately after T1.
Table 1.
Clinical Characteristics
Figure 1. Training progression with decrease in BWS. Mean % BWS/per session/5 sessions vs number of training sessions. Specific points (T1, T2, T3, and T4) on the training schedule are also identified. (T1) Start of training. (T2) Stopped training for 8.6 weeks (represented by the broken line). (T3) Started training again. (T4) Stopped training after a total of 97 training sessions. Kinematic and EMG data were collected before T1, before T3, and after T4.
Testing Procedure
Figure 1 identifies specific points (T1, T2, T3, and T4) on the training schedule: T1, start of training; T2 stopped training for 8.6 weeks (represented by the broken line in Figure 1); T3, started training again; T4, stopped training after a total of 97 training sessions. Kinematic and EMG data collection occurred before T1, before T3, and after T4.
Specifically, before T1 kinematic and EMG data were collected bilaterally for 60% BWS, while treadmill training occurred at a speed of 0.71 m/s. These parameters determined the initial testing parameters and were based on the ability of the participant to stand with an erect posture and step comfortably in the BWS system. At T2, the BWS was 40%; therefore, before T3, bilateral kinematic and EMG data were collected at 60% BWS (T1 test condition) and 40% BWS (final BWS at the end of T1 training). Collection time per trial was 20 seconds. At T4, kinematic and EMG data were collected bilaterally for 60%, 40%, and 20% BWS. The additional condition after training was studied because the individual was able to bear more weight during assisted stepping, and 20% BWS was the minimum BWS trained. Between stepping bouts, the treadmill was turned off, and the participant stood in place with BWS or rested in a chair. For all test sessions (at T1, T3, and T4), the participant performed 3 independent trials for each BWS condition. Throughout all testing and training session, blood pressures were recorded regularly. During testing, 3 trainers gave assistance at the pelvis and at each knee. Over-ground kinematic or EMG testing did not occur.
Hip, total BMD measurement, and body composition (lean mass and fat mass; DPX-IQ; Lunar) scans were used before (T1) and after training (T4). DPX used a pencil beam x-ray to measure BMD and bone mineral content (BMC) for regional body components including the femoral neck, lumbar spine, and total body. The whole body scans were analyzed using commercially available software from Lunar. Day-to-day DPX variation determined over a 12-month period with the phantom scanned 57 consecutive times was less than 1% error (coefficient of variation [CV] = 0.004).
Instrumentation, Data Acquisition, and Data Analyses
A 7-camera Vicon system (Vicon Workstation 5.0; Vicon Motions Systems, Lake Forest, CA), sampled at 60 Hz, was used to collect kinematic data for T3 and T4 gait analyses. For T1, a 6-camera Vicon system collected (at 60 Hz) the kinematic data. Spherical reflective markers were placed on the right and left second and fifth metatarsals, calcaneus, lateral malleolus, tibial tuberosity, distal thigh shank femoral epicondyle, greater trochanter, anterior superior iliac spine, and the posterior superior iliac spine. Use of the 7-camera system obviated the problem of occlusion of reflective markers caused by leg trainers' positioning. With the 6-camera system, we did have occlusion of the malleoli, so we used markers at the tibia tuberosity and mid-joint line to mathematically determine the malleolus in 3-dimensional space.
The EMG was recorded using the MA-100 EMG system (Motion Lab Systems, Baton Rouge, LA). Surface EMG preamplified electrodes were placed bilaterally on the medial gastrocnemius (below the popliteal crease on medial aspect of the calf), tibialis anterior (below the tibial tuberosity and lateral to the tibial crest), rectus femoris (anterior aspect of thigh, midway between the superior border of patella and the anterior superior iliac spine), and biceps femoris (midway on the line between the lateral epicondyle of the femur and the ischial tuberosity). Gains were applied to all EMG signals and were generally uniform across muscles for the collection periods. EMG data were collected at a bandwidth of 10 to 600 Hz and sampled at 1,500 (T1) and 1,560 (T3 and T4) Hz. Raw EMG signals were filtered at a bandwidth of 30 to 150 Hz and full-wave rectified. Root mean squares (RMSs; defined as the square root of the mean squared value of rectified amplitude) were calculated over a 100-ms window (34) within the stance and swing of the gait cycle. Overall mean during stance was calculated as the sum of the RMS amplitudes from burst onset to burst offset divided by burst duration during each stance phase. Similarly overall mean during swing was calculated. Burst onset/offset was defined as time of onset/offset of EMG burst. Burst duration (BD) was defined as time between the onset of EMG burst to the offset of EMG burst of each muscle within stance and swing phase of gait cycle. BDs were normalized to stance:swing ratios for all test conditions. Normalization of BD would negate the influence of the variance in relative stance on the BD data and allow for comparisons of RMS EMG mean amplitude across all test conditions. Six to 10 gait cycles were analyzed for amplitude and BD data. EMG data were processed using MATLAB (Version 6.1; MathWorks, Natick, Maine). Calculation of sagittal plane segment motion for the thigh, shank, and foot was determined using MATLAB (Version 6.1; MathWorks, Natick Maine). Limb kinematic data were calculated in the local moving plane with calculation of orientation angles for each segment relative to the right horizontal (35). All kinematic data are presented as segment range of motion (ROM) at the thigh, shank, and foot. At least 6 to 10 gait cycles were analyzed for each test condition.
Training Protocol
Locomotor training protocol consists of training on the treadmill, over-ground training, and community (at home) ambulation (12,16).
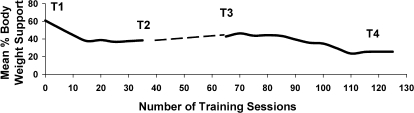
Training on the Treadmill. Three trainers were involved in the step-training component of LT: 1 trainer at the hip/pelvis and 1 trainer at each of the legs. Treadmill sidebars were not used to support the body. The participant was encouraged to swing his arms in a rhythmical motion with the lower limbs. Before being manually moved, the participant would stand with his feet straddled, simulating stride length: one hip extended near midstance and bearing most of the load. Initially, trainers would help to position the legs. The participant was encouraged to shift his body weight forward and laterally toward the opposite leg. Shifting the body weight forward to the front leg allowed for the posterior limb to initiate swing (and push off). During stance, assistance was given to aid in knee extension (not hyperextension) at the patellar tendon and to optimally support body weight. During swing, assistance was given to promote knee flexion at the medial hamstring tendon at the distal femoral region to aid in toe clearance and unloading. Assistance was given to aid in coordination between limbs (ie, simultaneous heel-strike and foot placement of 1 limb concurrently to the movement of the other limb). Figure 1 describes the progression of training for a total of 97 sessions. Over the total training period (97 sessions), trainers reduced assistance at the knee during flexion, during swing, and initiation of swing (Figure 1). Table 2 describes the training profile for the participant including the time walking on the treadmill, total time loading (including standing and walking) on the treadmill, average decrease in BWS per training session, average time standing over ground, and average treadmill speed per training session.
Table 2.
Training Profile for Participants
Over-Ground Training. After each treadmill training session, the participant practiced standing over ground by using a Rolling Stand-up Walker (Allegromedical.com, Brisbane, CA). During this over-ground training, the standing task would progressively increase in the level of difficulty. For example, initially, he would hold on to a walking frame with both arms and change his weight distribution from the right to left leg. With further training, the participant would release both arms from the walker and maintain postural stability while flexing and extending at the shoulder and elbows. Standing time was recorded for each standing bout and the total standing time was defined as the sum of all standing bouts during the session. No lower limb orthoses were used during the over-ground activities. Assistance was given to position the pelvis and knee extension when standing. Two individuals were needed to support at the knees and pelvis during over-ground training. Total training time/session including stretching and harness setup was approximately 2 hours. The participant was expected to complete set tasks at home such as standing or chair-seated exercises. All training and testing sessions were recorded on video. This enabled us to make qualitative assessments on posture, trunk control, and arm position during treadmill training and over-ground training.
RESULTS
Stepping Using BWST
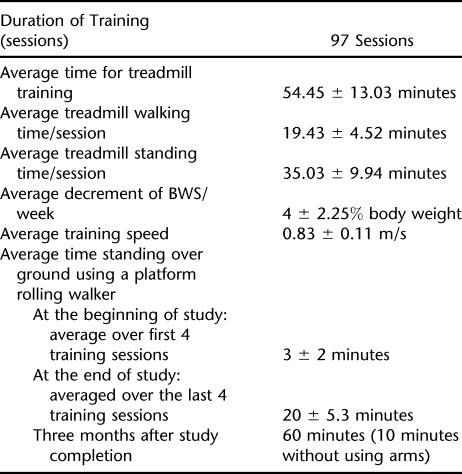
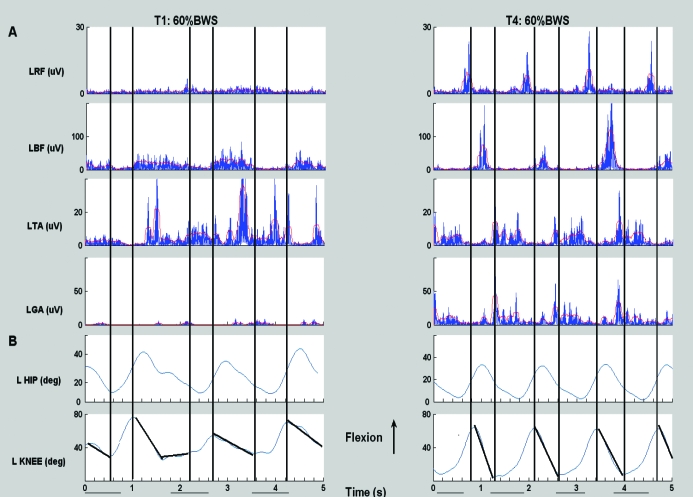
Before training, the participants gait pattern on the treadmill with BWS and with manual assistance was characterized by a flexed posture at the trunk, hip, and knee, indicating a decrease of weight-bearing tolerance when lower limbs were passively moved on the treadmill. After training, the participant gained the ability to walk with less BWS and with the trainer's assistance, was able to produce more consistent lower limb joint kinematics (60% BWS, 40% BWS, and 20% at 0.71 m/s) as illustrated for 60% BWS in Figure 2. After training, (T4), the bilateral limb kinematics and EMG showed a more improved and repeatable interlimb and intralimb coordination compared with T1. This improvement in intralimb coordination is shown in Figure 2, where the EMG profiles are more phasic for the right leg (ie, right rectus femoris [RRF] and right biceps femoris [RBF] show alternate and phasic patterns; similar results are shown for the left rectus femoris [LRF] and left biceps femoris [LBF]). Improvements in the interlimb coordination are also shown in Figure 2, with alternating firing patterns for the LRF and RRF (and similarly for the RBF and the LBF). A more detailed explanation of these improvements follows. Although visual qualitative assessments were not part of the formal analyses, it is relevant that there were visual improvements in contralateral arm swing and head, neck, and trunk postural control within the first few weeks of training.
Figure 2. Kinematic data and EMG data at T1 and T4 for 5 seconds of assisted walking time. Treadmill speed is .71m/sec. Ordinate: Kinematic data for hip and knee. Rectified EMG and EMG RMS amplitude for left and right rectus femoris (LRF, RRF), bicep femoris (LBF, RBF), tibilias anterior (LTA, RTA), gastrocnemius (LGA, RGA). Abscissa: seconds. Data were collected during 20 seconds of continuous stepping. Dotted line represents toe off, solid black line represents foot contact. There are no kinematic data for right side at T1.
Effect of Training on Muscle Activity
Before training, there was no consistent, repeated EMG pattern except for the bilateral biceps femoris (Figure 2). However, after 97 training sessions of LT, considerable changes were observed in EMG profiles of the LRF and RRF and left and right hip and knee flexors (LBF and RBF, respectively; Figure 2). Higher EMG amplitudes and reciprocal patterns between rectus femoris (RF) and the biceps femoris (BF) were observed. These alterations in patterns were observed in late stance and early swing for the LRF and especially the RRF and late swing for the LBF and RBF after training (Figure 2, T4). The reciprocal EMG patterns between the bilateral RF and the BF resulted in better interlimb and intralimb coordination at T4 (Figures 2 and 3) and were not present at T1.
Figure 3. Kinematic data and EMG data at T1 and T4 for 5 seconds of assisted walking time. Treadmill speed is .71m/sec. Ordinate: Rectified EMG and EMG RMS amplitude and kinematic data Abscissa: Time (seconds). (A) EMG Activity recorded at T1 and T4 for (LR, LBF, LTA, LGA respectively). (B) Kinematics data for left hip and left knee are presented for T1 and T4. Left foot contact to left foot contact. Solid horizontal lines below plots represent stance phase of gait cycle. All data were collected for 20 seconds of continuous stepping. Solid vertical lines represent the minimum and maximum points for left knee extension and muscle activation for left bicep femoris.
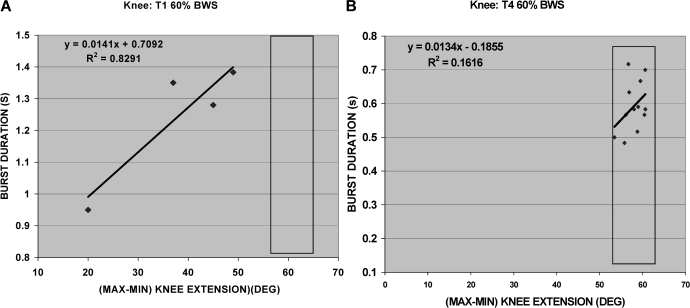
Figure 3 examines the relationship between the EMG pattern of the LBF and left knee kinematics. The BD of the LBF was coincident with the stretch applied to the left hamstrings by the trainer during swing when the knees were passively extended (Figure 3, bold line), as the leg was drawn forward by the trainer at foot contact with the floor. Figures 3 and 4 identify that, with an increase in passive knee extension, BD increases. At T4 (Figure 3), the leg is moved more easily by the trainers, and there is a minimal relationship between knee extension and BD. Figure 4A shows a positive correlation between BDs and knee range of motion (r2 = 0.83) at T1; however, after training, there appeared to be minimal correlation between BD(s) and knee range of motion (r2 = 0.16). Figure 4B shows that, after training with a small change in knee extension, there is a small change in BDs.
Figure 4. (A) T1: LBF correlation between burst duration (seconds) and knee extension (degrees) for 4 points. (B) T4: LBF correlation between burst duration and knee extension is based on 12 points.
Before training, the bilateral TA activity (Figure 2) occurred in the swing phase of the step cycle. However, the right tibialis anterior (RTA) fired simultaneously with the left tibialis anterior (LTA). After training, the bilateral TA burst activity readings were more reciprocal; however, they occurred during the support phase. The left gastrocnemius (LGA) burst activity occurred during support and at the end of swing; however, the LGA and LTA showed more co-contraction rather than alternating agonist and antagonist activity (Figure 2B). The 3-dimensional positional data identified that the toe often landed at foot contact, resulting in clonus-like activity in the right gastrocnemius (RGA; Figure 2B).
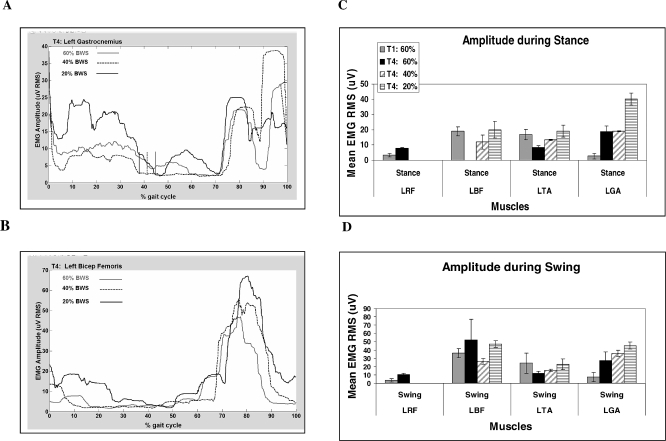
Figure 5 illustrates the mean flexor and extensor muscle EMG RMS amplitude (μV) during stance and swing for 6 to 10 gait cycles for each test condition at T1 and T4. At T4, there was an increase in the LRF mean EMG amplitude. Although the RMSs for LRF are minimal, there was still an increase in amplitude compared with the very low amplitudes at T1 (Figure 2). A greater EMG–amplitude response to training is observed by examining the RRF. After training, EMG amplitude increased for LBF and LGA but not LTA.
Figure 5. An example to show the effect of BWS limb loading on burst duration in the SCI participant. Broken vertical (A) line represents end of stance. (A and B) The effect of limb loading on burst duration for LGA and LBF, respectively, for 1 gait cycle. Rectangular box: transition from stance to swing. (C and D) EMG amplitude during stance and swing.
Effect of Loading on EMG Activity
Figure 5 shows a positive linear response in mean EMG amplitude to body weight (BW) load. Figure 5A and C shows that, after training, the EMG mean amplitudes for LGA were modulated by limb peak load. The responsiveness to loading was most apparent at 20% BWS. The LGA fired during late swing and stance (a similar firing was observed for the RBF). This activation pattern was potentially induced by leg trainers and was consistently preceded the onset of loading. At T1, LBF was active for much of the gait cycle (ie, mean BD during stance was 56% ± 6.1% of the stance cycle and mean BD during swing was 96% of swing cycle), whereas at T4, the BD of the LBF decreased to 20% ± 7% (of swing). This was in contrast with the bilateral activation pattern of the BF at T1 (see above). For the 40% and 20% BWS, the LBF increased its burst amplitude and burst duration during stance and swing (Figure 5).
Clinical Outcomes: ASIA, Wheelchair Mobility, Standing—Functional Outcome
After training, no change was evident in the neurologic level for motor, whereas the sensory level changed to C7 (Table 1). The participant was not loading or standing over ground before entry in the study. He was totally wheelchair reliant. Initially, with the over-ground training, the participant needed assistance at the pelvis, knees (to maintain knee extension), and arms to stand using the walker. After 97 BWST training sessions, the participant improved his ability to stand by using the walker with minimal assistance at the knees and pelvis to maintain postural stability while shifting body weight medial/laterally or anterior/posteriorly. His mean total standing time at the end of the study, averaged over the last 4 training sessions, was 20 ± 5.3 minutes. His standing postural control improved whereby he could remove his hands from the walker to perform forward reaching tasks. Three months after completion of the study, the participant reported standing at home for 20 minutes per standing bout using the Rolling Stand-up Walker without assistance at the knees. This participant continued to stand at home, and 18 months after completing the study, he reported standing for 50 minutes per session. He needed maximum assistance (1 person at the pelvis and 1 person at the knees) to get up and minimal assistance (less assistance at the pelvis and knees) to maintain standing position.
BMD Values and Body Composition
BMD and BMC for pre- and post-training are presented in Tables 3A and 3B. Table 3A presents the total BMD and BMD at the hip (including the femoral neck). After 97 training sessions, total BMD decreased 1.54%. Table 3B presents the BMC and the area for each region. The femoral neck decreased its BMC by approximately 21% (with a 4.2% decrease in area).
Table 3A.
BMD Values
Table 3B.
BMC Values for Hip With Cross-Sectional Area
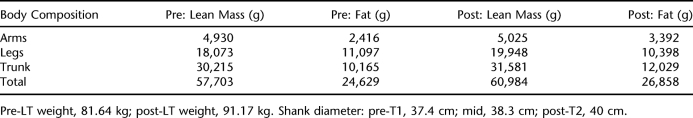
Overall, the participant increased body weight (Table 4). He lost total lean body mass (0.5%) and gained fat (2.7%) over the training period. He gained fat in the arms (7.4%) and trunk (2.5%) and decreased lean body mass in the arms (−6.6%) and trunk (−2.5%). He also gained lean body mass (4%) and decreased fat (−3.5%) in the legs. Additionally, shank circumference increased by 2.6 cm.
Table 4.
Body Composition Values
Adverse events recorded for this participant were 7 occasions of autonomic dysreflexia. On each occasion, training was stopped. No other adverse events occurred.
DISCUSSION
The novel aspect of this study is the detailed analyses over an extended period of LT on bilateral muscle activation (EMG pattern, amplitude, and BD) as recorded during treadmill stepping for an individual who has a chronic motor complete SCI. The changes in inter- and intralimb coordination (kinematics; Figure 2) and the multiple changes in detailed EMG profiles for bilateral muscle groups simultaneously provides quantitative evidence of neuronal activity changes and addresses the impact of LT as a training therapy for an individual with chronic, motor complete ASIA B SCI.
Changes in the EMG Amplitude of Lower Limb Muscles
This participant showed improvements in EMG patterns for all muscles studied after LT (without any improvement in his ASIA motor score). After training, the kinematic profiles at the hip and knee were more regular for several step cycles. Specifically, the increases in mean EMG amplitude and modulations in BD (particularly for the bilateral BF and RF and to a certain extent the LGA) reflected a stepping pattern that was functionally more appropriate for locomotion. Before training, the bilateral BF pattern could be partially explained by the stretch applied to the hamstrings during the passive movement of knee extension. However, after training, it seemed that the BD decreased markedly for the same knee extension. Barbeau and Norman (36) commented on a similar passive stretch response of the hamstrings during LT for 4 participants with complete SCI; however, with the administration of clonidine, a antispasticity medication, the stretch response were completely abolished. It is suggested that these modulations in afferent patterns occurred without supraspinal input available and a modification of the afferent information by the spinal cord, resulting in some plasticity of the spinal circuitry (37).
After training, the ankle joint at times would plantarflex on foot landing where the LTA had difficulty to concentrically contract against gravity to promote ankle dorsiflexion. Continued stepping with ankle plantarflexion at floor contact was likely to compromise afferent propriocpetive sensory input at foot floor contact and early stance. This probably contributed partially to the co-contraction of the LTA and the LGA and clonus of the RGA as shown at T4 (Figure 2). The rhythmic increase of the LBF EMG amplitude during swing (Figures 2 and 5) seemed to be caused by the contralateral limb stepping and not by the loading or ipsilateral limb. Ferris et al (22) cited that the most likely neural mechanism for the repeated and rhythmic EMG patterns is the “contralateral excitation of the spinal locomotor circuits,” (ie, afferent feedback from the loading limb potentially stimulating spinal circuitry).
The LGA did show increased amplitude at T4 with increased loading and decreased BD during swing (Figure 5). The literature (1,22) has established that an increase in afferent loading stimulus and kinematics associated with repetitive stepping resulted in more reciprocal EMG patterns of activity between agonists and antagonists within 1 training bout (for AIS levels A, C, and D). It is suggested that stepping with knee and hip extension/ flexion combined with loading and unloading provided optimal afferent input to the spinal cord to facilitate the motor unit recruitment.
This case study shows that a combination of afferent stimuli during passive rhythmic and repeated movements of lower limbs and an associated increased limb loading in both the ipsilateral and contralateral limb during training will induce neuromuscular plasticity in the lower limbs even in an individual with motor complete SCI. Potentially, these data can be used as baseline data for future research, combining different treatment modalities (such as electrical stimulation or pharmacologic treatments) directed toward the recovery of walking for individuals with motor complete injuries. For any treatment leading to spinal cord regeneration, it only makes sense if the function of the neuronal circuits is improved and retention of muscle mass is preserved or enhanced further recovery of function is possible in motor complete SCI.
BMD and Body Composition
After repetitively loading for 97 sessions, there was very little change in total body BMD. BMC (Table 3) of the femoral neck decreased approximately 21% from before to after the study, a reduction to 79% of normal BMC values. This value is still higher than what is reported in the literature for the decrease in femoral neck BMC 2 years after injury (based on 8 individuals with motor complete SCI) (28). Biering-Sorensen et al (28) has shown that, after 2 years, the femoral neck seems to reach a steady state of approximately 60% to 70% of normal BMC values.
Recently, it has been reported that LT did not prevent bone loss from occurring after acute SCI (38) and chronic SCI (39). For the acute study, participants (mean age, 29.6 ± 8.6 years; 4 individuals were AIS B) completed 48 sessions of LT, and the %BWS at the end of the training period ranged from 66% to 0%. The individual who lost the least amount of BMD walked with the greatest ambulatory capacity and walked on the treadmill at 0% BWS. For the chronic study, the results showed no significant difference in the BMD at the proximal and distal femur, proximal tibia, and lumbar spine after LT (after 144 sessions of training); however, the study had a wide variability for time since injury, and only 2 participants were categorized as chronic AIS B (4.25 ± 3.8 years after injury). Future research needs to focus on the intensity of loading, velocity of treadmill, duration of training at low BWS and the frequency of training per week, and the recruitment of a large number of participants who are matched for age, sex, level of injury, AIS score, and time since injury.
For the participant in this case study, an increase in muscle mass for legs was recorded after the completion of training. This may be an overall training response to the exercise. Additionally, there was an increase in fat in the trunk and arms but a decrease in fat in the legs with an increase in shank circumference. Overall, these skeletal muscle adaptations would have contributed positively to the alterations in the participant's ambulatory capacity and postural control on the treadmill and over ground. Stewart et al (40) trained individuals with SCI (AIS C, n = 9, postinjury mean, 8 ± 2.5 years) for 6 months (3 times per week) and showed changes in muscle phenotype to include hypertrophy and increased oxidative capacity and subsequently reflected a change in ambulatory capacity. Atrophy is a result of SCI and is potentially a source of fatigue because of the large number of motor units needed to perform a walking task. Potentially, LT (and the reduction of BWS to provide a mechanically loading stimulus) would induce relative muscle hypertrophy to reverse the injury-induced atrophy. With continued LT and repetitive load-bearing stepping, the hypertrophy would enhance the torque potential around the hip, knee, and ankle joint, and theoretically, this improved torque would transfer to an improvement in walking or standing ability. In addition to increased hypertrophy, the level of muscle activation would increase (as shown by EMG activity), which is a prerequisite for the task of standing or walking.
Certainly, this participant was able to increase his ability to stand for longer periods of time using a walker. Future research needs to examine whether the muscle hypertrophy in the legs does transfer to a greater torque-generating potential about the joints at the hip, knee, and ankle. Potentially, this information could be used with other therapies to further enhance walking or standing performance on the treadmill and over ground for an individual after SCI. Other authors (41) have suggested that an increase in muscle mass seen with LT may also improve glucose tolerance and increase the metabolic rate, leading to less fat deposits and also benefit improve skin integrity and reduce seating pressures and increase peripheral blow flow to reduce risk for pressure ulcers (42). A potential limitation of the study was the failure by the researchers to collect dietary intake of the participant during the study period. Changes in diet may have contributed to the overall weight gain and may have influenced the increase in lean mass.
Standing—Functional Mobility
Qualitatively, an improvement in upper body strength and/or control was observed whereby the participant developed more dynamic and postural stability for the head, neck, and trunk to maintain a more erect posture when loading at the beginning of stance on the treadmill. Before entry into the study, the participant had very little arm/hand control and could not stand. Progressively, the participant's tolerance improved for standing using a walker (and with assistance at the pelvis and knees) over ground on a flat surface. At the end of training, standing duration was documented to be 20 ± 5.3 minutes. On follow-up (3 months after the completion of the study), the participant was able to stand in a walker at home for 1 hour with the help of 1 assistant. LT allowed him to passively step on a treadmill at a minimum of 20% BWS and improve his trunk control without using his arms to load down. He was able to transfer this control to improve his capacity to stand over ground with the aid of a walker and without using braces or a standing frame. Eng et al (42) have documented the patterns of prolonged standing over time after an SCI. These authors have reported that proportionally more respondents who did not stand were individuals with tetraplegia (vs paraplegia), and consequently, led more sedentary lifestyles. Numerous benefits were reported by those individuals who practiced prolonged standing: 87% of the individuals who practiced standing reported more of a sense of well being, and more than 50% reported improvements in circulation and improvement in bowel and bladder function. Many individuals reported a reduction in spasms, and more than one third reported an improvement in breathing, skin integrity, and fatigue.
This study has allowed us to collect quantifiable treadmill gait data involving bilateral kinematics and EMG on multiple muscles before and after a long period of LT for an individual with a motor complete SCI. Consequently, the data have allowed us to see the effects of training and the effects on decreasing BWS as a result of training on kinematics and EMG activity simultaneously. After training, multiple alterations in EMG and kinematics have indicated neural adaptations to produce improved inter- and intralimb coordination associated with assisted walking on a treadmill. Furthermore, LT has improved the ability to stand for an increased time using a standing frame for an individual that was 100% wheelchair reliant. This study provides the preliminary data to describe the neural effect that LT alone has on an individual with incomplete SCI. Potentially, the validation of our results could be enhanced by increasing the n size. Also, a larger sample size would establish a suitable benchmark to develop future studies where LT could be used with another modality (43,44) such as functional electrical stimulation, pharmacologic treatments, or the a combination of these and other biological treatments under development to evaluate the neurologic response of multiple modalities associated with LT for individuals who have motor complete injuries.
Lack of a control is a limitation in this case study; however, Dobkin et al (33) compared the “efficacy of step training with body weight support on a treadmill (BWSTT) to the efficacy of a defined over-ground mobility therapy (CONT) for patients with incomplete SCI admitted for inpatient rehabilitation.” Participants were graded as AIS B, C, and D with levels of injury from C5 to L3. They receive 12 weeks of BWSTT or CONT. Primary outcomes for AIS B group were the Functional Independence Measure for Locomotion (FIM-L) at 6 months after injury. There was no significant difference between treatment groups at 6 months for the FIM-L. The authors determined that patients who were still graded AIS B at 8 weeks after SCI had a low probability of achieving functional walking or spontaneous recovery with a FIM-L score ≥4 when given either the BWSTT or CONT. In addition, for this case study, we do show bilateral kinematics to validate the EMG data.
It would have been more advantageous to complete testing after 35 sessions of training rather than after the 8.6 weeks of no training. We could have compared 35 sessions of training with the following 62 sessions of training for relative outcome measures such as walking, standing, muscle mass, and bone. Also, it would have been preferable to collect data relevant to dietary intake because the participant increased his weight while in the study (9.5 kg total: 6.4 kg lean mass and 2.2 kg fat mass).
CONCLUSION
LT allows for overhead harness support, progressive loading of body weight, and treadmill speed to promote repetitive stepping. The detailed kinematic and EMG results for bilateral multiple muscles as described in this case study provide evidence that the human nervous system with severely compromised supraspinal input can respond to specific afferent sensory information such as limb loading and rhythmic repeated stepping with manual assistance. Specifically, the study identified the positive neural and body composition changes that occur after LT for an individual with a complete motor SCI (AIS B, 1 year after injury). Furthermore, we provided evidence that the afferent information related to the loaded limb during stepping can facilitate the generation and modulation of the locomotor patterns in the ipsilateral and contralateral limbs. In our case study, these gains in neural activation and alterations in spinal circuitry were shown to transfer to functional outcomes and potentially may benefit overall health and quality of life. In addition, this study provides baseline data as a first step of a series of investigations where LT could be used with another modality (42,43,45) such as functional electrical stimulation, pharmacologic treatments, or a combination of these and other treatments under development for individuals who have motor complete SCI. The ultimate goal is to examine the improvement of functional recovery in motor complete chronic SCI (43,44,46,47).
REFERENCES
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotrauma. 2002;19:1217–1229. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol. 1995;37:574–582. doi: 10.1002/ana.410370506. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Wainberg M, Finch L. Description and application of a system for locomotor rehabilitation. Med Biol Eng Comput. 1987;25:341–344. doi: 10.1007/BF02447435. [DOI] [PubMed] [Google Scholar]
- Visintin M, Barbeau H. The effects of body weight support on the locomotor pattern of spastic paretic patients. Can J Neurol Sci. 1989;16:315–325. doi: 10.1017/s0317167100029152. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Danakas M, Arsenault B. The effects of locomotor training in spinal cord injured subjects. Restor Neurol Neurosci. 1993;5:81–84. doi: 10.3233/RNN-1993-5122. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Fung J. The role of rehabilitation in the recovery of walking in the neurological population. Curr Opin Neurol. 2001;14:735–740. doi: 10.1097/00019052-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Nymark JR, De Forge D, Barbeau H, et al. Body weight support treadmill gait training in the subacute phase of incomplete spinal cord injury. J Neurol Rehabil. 1998;12:119–138. [Google Scholar]
- Wernig A, Nanassy A, Muller S. Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord. 1998;36:744–749. doi: 10.1038/sj.sc.3100670. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC. Spinal cord control of movement: implications for locomotor rehabilitation following spinal cord injury. Phys Ther. 2000;80:477–484. [PubMed] [Google Scholar]
- Protas EJ, Holmes SA, Qureshy H, Johnson A, Lee D, Sherwood AM. Supported treadmill ambulation training after spinal cord injury: a pilot study. Arch Phys Med Rehabil. 2001;82:825–831. doi: 10.1053/apmr.2001.23198. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80:688–700. [PubMed] [Google Scholar]
- Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther. 2002;82:707–715. [PubMed] [Google Scholar]
- Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote EC, Lindley SD, Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther. 2005;29:127–137. doi: 10.1097/01.npt.0000282245.31158.09. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Lawless-Dixon AR, Davis SB, et al. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys Ther. 2005;85:1356–1371. [PubMed] [Google Scholar]
- Rossignol S, Chau C, Brustein E, Belanger M, Barbeau H, Drew T. Locomotor capacities after complete and partial lesions of the spinal cord. Acta Neurobiol Exp (Wars). 1996;56:449–463. doi: 10.55782/ane-1996-1148. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- American Spinal Injury Association. International standards for neurological classification of spinal cord injury J Spinal Cord Med. 200326 (suppl 1) S50–S56. [DOI] [PubMed] [Google Scholar]
- Stewart JE, Barbeau H, Gauthier S. Modulation of locomotor patterns and spasticity with clonidine in spinal cord injured patients. Can J Neurol Sci. 1991;18:321–332. doi: 10.1017/s0317167100031887. [DOI] [PubMed] [Google Scholar]
- Ferris DP, Gordon KE, Beres-Jones JA, Harkema SJ. Muscle activation during unilateral stepping occurs in the nonstepping limb of humans with clinically complete spinal cord injury. Spinal Cord. 2004;42:14–23. doi: 10.1038/sj.sc.3101542. [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Harkema S, Requejo P, Edgerton VR. Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J Neurol Rehabil. 1995;9:183–190. [PubMed] [Google Scholar]
- Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- de Bruin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E. Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord. 2005;43:96–101. doi: 10.1038/sj.sc.3101685. [DOI] [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol. 1999;80:373–378. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- Modlesky GM, Bickel CS, Slade JM, Meyer BA, Cureton KJ, Dudley GA. Assessment of skeletal muscle in men. J Appl Physiol. 2004;96:561–565. doi: 10.1152/japplphysiol.00207.2003. [DOI] [PubMed] [Google Scholar]
- Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–214. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin ED, Frey-Rindova P, Herzog RE, Dietz V, Dambacher MA, Stussi E. Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch Phys Med Rehabil. 1999;80:214–220. doi: 10.1016/s0003-9993(99)90124-7. [DOI] [PubMed] [Google Scholar]
- Jones LM, Goulding A, Gerrard DF. DEXA: a practical and accurate tool to demonstrate total and regional bone loss, lean tissue loss and fat mass gain in paraplegia. Spinal Cord. 1998;36:637–640. doi: 10.1038/sj.sc.3100664. [DOI] [PubMed] [Google Scholar]
- Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen J, Sisto SA, Kirshblum S. Algorithm for the detection of muscle activation in surface electromyograms during periodic activity. Ann Biomed Eng. 2002;30:97–106. doi: 10.1114/1.1430750. [DOI] [PubMed] [Google Scholar]
- Putman C. Interaction between segments during a kicking motion. In: Matsui H, Kobayashi K, editors. Biomechanics. Vol. VIII-B. Champaign, IL: Human Kinetics; 1984. pp. 688–694. [Google Scholar]
- Barbeau H, Norman KE. The effect of noradrenergic drugs on the recovery of walking after spinal cord injury. Spinal Cord. 2003;41:137–143. doi: 10.1038/sj.sc.3101374. [DOI] [PubMed] [Google Scholar]
- Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain. 2004;127:2232–2246. doi: 10.1093/brain/awh252. [DOI] [PubMed] [Google Scholar]
- Giangregorio LM, Hicks AL, Webber CE, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005;43:649–657. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- Giangregorio LM, Webber CE, Phillips SM, et al. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury. Appl Physiol Nutr Metab. 2006;31:283–291. doi: 10.1139/h05-036. [DOI] [PubMed] [Google Scholar]
- Stewart BG, Tarnopolsky MA, Hicks AL, et al. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve. 2004;30:61–68. doi: 10.1002/mus.20048. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Stewart BG, Mahoney DJ, et al. Body-weight-support treadmill training improves blood glucose regulation in persons with incomplete spinal cord injury. J Appl Physiol. 2004;97:716–724. doi: 10.1152/japplphysiol.00167.2004. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Levins SM, Townson AF, Mah-Jones D, Bremner J, Huston G. Use of prolonged standing for individuals with spinal cord injuries. Phys Ther. 2001;81:1392–1399. doi: 10.1093/ptj/81.8.1392. [DOI] [PubMed] [Google Scholar]
- Ladouceur M, Barbeau H. Functional electrical stimulation-assisted walking for persons with incomplete spinal injuries: longitudinal changes in maximal overground walking speed. Scand J Rehabil Med. 2000;32:28–36. doi: 10.1080/003655000750045712. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Norman K, Fung J, Visintin M, Ladouceur M. Does neurorehabilitation play a role in the recovery of walking in neurological populations. Ann N Y Acad Sci. 1998;860:377–392. doi: 10.1111/j.1749-6632.1998.tb09063.x. [DOI] [PubMed] [Google Scholar]
- Fung J, Stewart JE, Barbeau H. The combined effects of clonidine and cyproheptadine with interactive training on the modulation of locomotion in spinal cord injured subjects. J Neurol Sci. 1990;100:85–93. doi: 10.1016/0022-510x(90)90017-h. [DOI] [PubMed] [Google Scholar]
- Ladouceur M, Pepin A, Norman KE, Barbeau H. Recovery of walking after spinal cord injury. Adv Neurol. 1997;72:249–255. [PubMed] [Google Scholar]
- Barbeau H, Ladouceur M, Mirbagheri MM, Kearney RE. The effect of locomotor training combined with functional electrical stimulation in chronic spinal cord injured subjects: walking and reflex studies. Brain Res Brain Res Rev. 2002;40:274–291. doi: 10.1016/s0165-0173(02)00210-2. [DOI] [PubMed] [Google Scholar]