Abstract
Background/Objective:
To show the efficacy, safety, and tolerability of sildenafil in men with erectile dysfunction (ED) associated with complete or incomplete spinal cord injury (SCI) and to assess its effects on quality of life (QoL) using the Life-Satisfaction Check List.
Methods:
This was a placebo-controlled, multicenter, randomized, double-blind, flexible-dose, 2-way crossover study with a 2-week washout period between each phase. Patients with ED attributable to SCI (Sexual Health Inventory—Male score ≤21) received 50 to 100 mg sildenafil (n = 24) or placebo (n = 26).
Results:
Compared with placebo, sildenafil produced higher levels of successful sexual stimulation, intercourse success, satisfaction with sexual life and sexual relationship, erectile function, overall sexual satisfaction, and an improved Erectile Dysfunction Inventory of Treatment Satisfaction score, with no clinically relevant effects on vital signs. Sildenafil seemed more effective in patients with incomplete SCI than in those with complete SCI, producing significant improvements, compared with placebo, in a number of measures only in patients with incomplete SCI. All patients who expressed a preference selected sildenafil over placebo, although the drug had no effect on patient QoL. Sildenafil was well tolerated, with a profile comparable to that of placebo.
Conclusions:
Compared with placebo, treatment with oral sildenafil safely and effectively improved erectile function in patients with ED attributable to SCI, especially in those with incomplete injury, and was the agent of choice in those who expressed a preference.
Keywords: Sildenafil, Spinal cord injuries, Tetraplegia, Paraplegia, Erectile dysfunction, Impotence
INTRODUCTION
Erectile dysfunction (ED) affects the lives of ∼150 million men worldwide (1,2). The Massachusetts Male Aging Study surveyed 1,709 men 40 to 70 years of age from 1987 through 1989 and reported the prevalence of ED as 52%, with 9.6% of respondents reporting complete ED (2). In 2000, the overall prevalence of ED in this study population was re-estimated to be 44% (3) and, in a recent US survey, the prevalence of moderate to severe ED in 2,173 men older than 40 years of age was estimated to be 22% (4). Numerous factors such as aging, psychological disorders, neurologic disorders, hormonal disorders, vascular disorders, and medications can disrupt the normal physiological mechanisms involved in erection (1,5).
Most of the men with spinal cord injury (SCI) have difficulties with erectile function. These difficulties can include problems getting an erection, maintaining an erection, or both. The impact of SCI on sexual function depends on the severity and location of the injury (6), and an erection is more likely to be obtained if lesions are incomplete rather than complete. A majority of men with complete or incomplete SCI will require treatment for ED. First-line treatments include oral drugs, such as phosphodiesterase inhibitors (sildenafil, tadalafil, and vardenafil) and the dopamine agonist apomorphine, constrictive rings, and vacuum devices. Second- and third-line options include intracavernous injections and penile prosthetic implants (7,8).
Sildenafil is a competitive and selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), found in the corpus cavernosum, vascular smooth muscle, and platelets (9). Sildenafil, as a selective inhibitor of PDE5, facilitates the nitric oxide–driven relaxation of the smooth muscle of the corpus cavernosum and thus enhances the natural physiological erectile response to sexual stimulation (9–11).
In several placebo-controlled studies performed in men with SCI and ED, patients taking sildenafil reported satisfactory erections and expressed a preference for sildenafil over placebo (12–14). In 178 men with SCI and ED, sildenafil improved patients' overall satisfaction with their sex life and sexual relationship and concerns about erectile problems (15). Improvements were also reported in scores for the generic quality-of-life (QoL) parameters of mental health, well being, depression, and anxiety (15).
A majority of these studies did not assess the effects of sildenafil according to the severity of SCI. However, in 1 study (16), sildenafil was found to be an effective, well-tolerated treatment for ED in patients with SCI, regardless of the severity of injury. The aim of this study was to show the efficacy, safety, and tolerability of sildenafil administered orally to men with ED associated with complete or incomplete SCI and to assess its effects on the QoL of these patients.
METHODS
Male patients 19 years of age or older with a diagnosis of traumatic SCI at least 6 months before screening and who were in a stable sexual relationship for at least the past 6 months were eligible for inclusion in the study. Patients had a clinical diagnosis of ED (attributable to injury of the spinal cord) confirmed by a Sexual Health Inventory-Male (SHI-M; Appendix A) score of 21 or less (ie, mild to severe ED) and had some psychogenic or reflexogenic erectile function. Patients who had at least a grade 1 erection (increase in size, but not hard) on the 5-point Qualitative Scale for Subjective Assessment of Erectile Response (Appendix B) were eligible for the study. Written informed consent was obtained from each patient before entering into the study.
The study was designed as a placebo-controlled, multicenter, randomized, double-blind, flexible-dose, 2-way crossover study, with a wash-out period between cross-over phases. The study was conducted according to the revised Declaration of Helsinki (2000) and complied with Turkish legislation, guidelines, and regulations relevant to the conduct of clinical research. The study was evaluated and approved before its start by the Local Ethics Committees of the participating centers.
Study Procedures
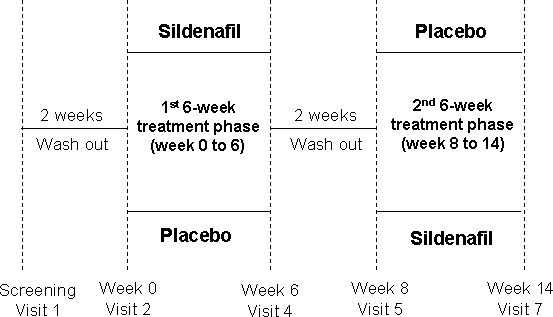
After the screening visit, there was a 2-week run-in period without treatment, during which baseline data on sexual function were collected. Eligible patients were randomized to receive either 50 mg sildenafil or placebo, ∼1 hour before sexual activity was anticipated, for 6 weeks. After another 2 weeks of washout period at the beginning of the second cross-over phase, patients were switched to the alternative treatment for a further 6 weeks, the double blind being preserved. During each cross-over phase, patients returned to the clinic for follow-up visits after 2 and 6 weeks of treatment for evaluation. At the week 2 visit in either cross-over phase, patients who experienced no adverse events with the 50-mg dose of sildenafil or corresponding placebo, but whose ED was not sufficiently improved at this dose had their dose increased to 100 mg. Patients on the 100-mg dose were excluded from the study if they experienced an intolerable or serious adverse effect. Each patient was followed for 16 weeks (Figure 1). Sildenafil dose was switched from 50 to 100 mg in 14 patients during the first phase and in 15 patients during the second phase. There was no withdrawal during this clinical study.
Figure 1. Study design.
Demographic information, a medical history (including the history of spinal cord trauma), information on concomitant medication, and appropriate documentation of ED (from medical records or patient reports and a SHI-M score of ≤21 at screening) were obtained. SCI was evaluated using ASIA impairment scale and standard neurologic classification for spinal cord lesions. A full physical examination including height, body weight, and genital examination was performed, and sitting blood pressure and heart rate were recorded.
Laboratory tests were carried out including complete blood and platelet count, total bilirubin, aspartate and alanine aminotransferases, alkaline phosphatase, blood urea nitrogen, creatinine, sodium, potassium, and blood glucose.
An event log (Appendix C) was issued to each patient for completion every time sexual intercourse was attempted. Patients were requested to note success or otherwise of sexual erectile response and intercourse.
All patients completed the International Index of Erectile Function (IIEF) (17) and Life-Satisfaction Check List (LISAT-8) QoL questionnaire (Appendix D) before the start and at the end of both cross-over phases. The Erectile Dysfunction Inventory of Treatment Satisfaction (EDITS) (18) was completed only at the end of both cross-over phases.
Patients were also asked to complete the Global Efficacy Assessment questionnaire (Appendix E) at the end of each cross-over phase; the primary efficacy variable of the study was the proportion of individuals who indicated a preference for either treatment and who said that the treatment improved their erections.
Statistical Methods
Descriptive statistics were provided for the numerical and categorical variables using mean, SD, and percentage distributions. Baseline values were compared using the Mann-Whitney U and Student's t tests for numerical variables and the χ2 or Fisher tests for categorical variables, where necessary. Analysis of the outcome variables was carried out using mixed linear analysis of variance (ANOVA) models for 2 × 2 cross-over studies to determine the presence of treatment, period, and carryover effects when they were normally distributed. Otherwise, the approach for non-normal quantitative response variables was used to test for the residual effects of treatment. In the absence of a residual treatment effect, the Wilcoxon test was used to assess the period and treatment effects for non-normal distributions. If a nonsignificant (type I error level at 0.05) difference was found between cross-over phases, the primary efficacy variable was analyzed using a binomial test for a single proportion, with the null hypothesis being “the proportion of patients who prefer active treatment is 50%” and the alternative hypothesis being “the proportion of patients who prefer active treatment is higher than 50%.” In calculating sample size, it was assumed that of those subjects whose erections improved, 75% would prefer the active treatment, and 25% would prefer the placebo. Comparing this against the null hypothesis of no treatment difference (with a 5% significance level), the number of patients in the study provides at least 90% power to detect a significant difference between the 2 study groups.
RESULTS
Sociodemographic Characteristics
Of the 71 patients screened by 5 study centers, 50 were recruited and completed the study (17 were ineligible and 4 prematurely withdrew from the study). Mean age of the study participants was 38.9 ± 8.0 years, and mean height and weight were 171.5 ± 6.5 cm and 72.6 ± 13.2 kg, respectively. Alcohol was used regularly by 12.9% of the patients, and 57.1% were cigarette smokers. A majority of the patients were primary school graduates (61.4%); 17.1% graduated from secondary school, 14.3% from high school, and 7.1% from university. There was no significant difference between the sociodemographic characteristics of the 2 randomization groups. All baseline laboratory variables were within normal ranges for all patients (data not shown).
Overall, the mean SHIM score was 9.2 ± 5.0. A majority of patients showed some tumescence but no rigidity (52.9%) or normal tumescence and weakened rigidity (27.1%).
According to the ASIA scale, SCI was complete in 58.5% of patients and incomplete in the remainder (16.9% were classified as ASIA B, 4.6% as ASIA C, and 20.0% as ASIA D). None of the patients was classified as ASIA E (normal).
Effects of Sildenafil vs Placebo
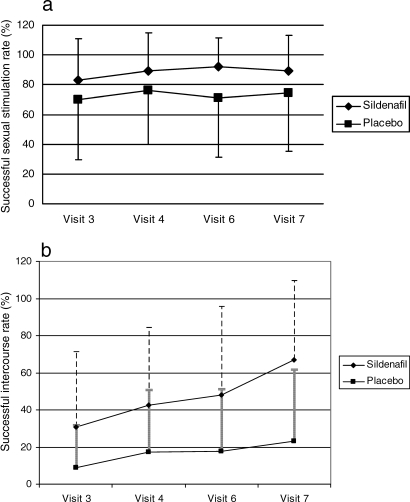
The effect of treatment on success of sexual stimulation and intercourse recorded at each follow-up visit is presented in Figure 2. Successful sexual stimulation (P = 0.008) and intercourse rates (P < 0.001) were significantly higher with sildenafil than with placebo.
Figure 2. (a) The mean successful erectile response rates in sildenafil and placebo groups (no carryover [P = 0.33] or period effects [P = 0.86] were found). The successful erectile response rate was significantly higher with sildenafil than with placebo (P = 0.008, Wilcoxon test). (b) The mean successful intercourse rates in sildenafil and placebo groups (no carryover [P = 0.07] or period effects [P = 0.11] were found). The successful intercourse rate was significantly higher with sildenafil than with placebo (P < 0.001, Wilcoxon test). Vertical bars represent SD.
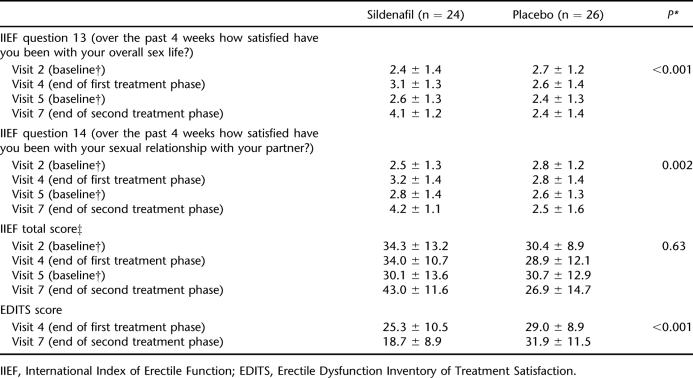
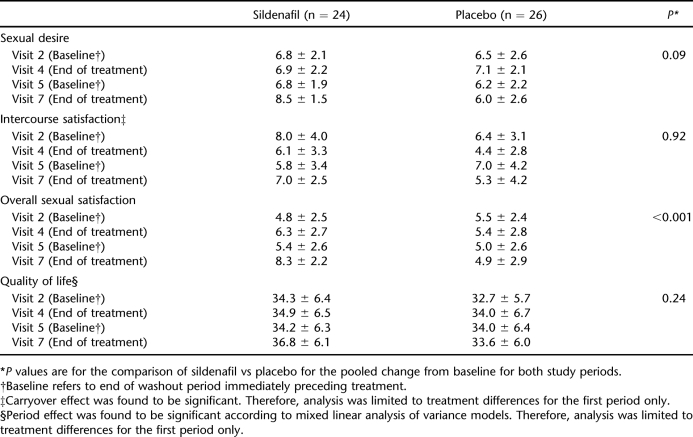
When the effect of treatment on erectile function was evaluated, sildenafil produced greater improvements than placebo in satisfaction with sex life and sexual relationship (IIEF questions 13 and 14) and in EDITS score; however, there was no difference between the 2 groups with regard to IIEF total score (Table 1). Erectile function and overall sexual satisfaction were also significantly improved in the sildenafil group compared with those receiving placebo. However, there was no difference between the 2 study agents with regard to intercourse satisfaction and sexual desire. Patients in study group 1 received sildenafil during visits 2 and 4, whereas they were switched to placebo during visits 5 and 7. Because the study was designed as a 2-way cross-over study, patients in the second study group received sildenafil during visits 5 and 7 and placebo during visits 2 and 4. Clinically, scores 3 to 5 were accepted as responsive regarding IIEF questions 9 and 10. Answers to IIEF questions 9 and 10, which were related to ejaculation and orgasmic experience with or without ejaculation frequencies, were not significantly different between the visits (Wilcoxon test; Tables 2 and 3). Sildenafil therapy also had no significant effect on QoL compared with placebo (Table 4).
Table 1.
Effect of Treatment on IIEF and EDITS Scores
Table 2.
Ejaculatory or Orgasmic Function in Patients in the First Study Group Based on IIEF Scores
Table 3.
Ejaculatory or Orgasmic Function in Patients in the Second Study Group Based on IIEF Scores
Table 4.
Effect of Treatment on Sexual Desire, Erectile and Orgasmic Functions, Intercourse, and Overall Sexual Satisfaction and Quality of Life (International Index of Erectile Function Data)
The proportions of patients who gave a positive response to Global Efficacy Assessment question 1 (Compared to having no treatment at all for your erection problem, has the medication you have been taking over the past 4 weeks improved your erections?) at the end of the first and second cross-over phases were compared for active treatment (76.9% and 87.5%, respectively; Fisher exact test, P = 0.47) and for placebo (34.6% vs 50.0%, χ2 test, P = 0.39); no significant difference was recorded.
The proportion of participants who expressed a preference for active treatment during the first (42.3%) and second cross-over phases (37.5%) did not differ significantly (χ2 test, P = 0.73). This test could not be repeated for the placebo intervention because there were no patients who preferred placebo. Because there was a nonsignificant (type I error level at 0.05) difference between cross-over periods, the primary efficacy variable was analyzed using a binomial test for a single proportion. All of the 20 patients who expressed a preference after completing both cross-over phases selected sildenafil. Sildenafil, therefore, was found to be preferred statistically significantly over placebo (P < 0.001, binomial test).
Effect of Severity of Injury on Outcomes
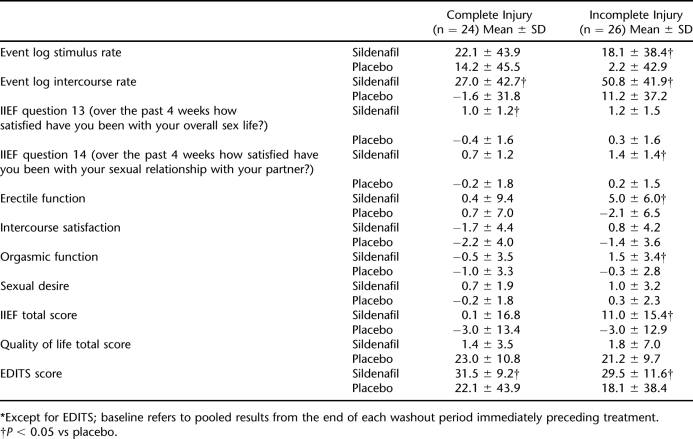
The effect of treatment on several outcomes according to the severity of SCI was also evaluated (Table 5). Sildenafil was significantly more effective than placebo for event log stimulus rate, IIEF question 14, erectile function, orgasmic function, and IIEF total score in patients with incomplete injury, but had no significant effect on these measures in patients whose SCI was complete. However, the effects of sildenafil on intercourse rate, intercourse satisfaction, and EDITS score did not seem to be influenced by the severity of SCI (Table 5).
Table 5.
Change From Baseline* in Outcomes According to the Extent of SCI
Safety Assessment
A comparison of changes in vital signs from baseline between treatment groups showed that the period/carryover effect was significant according to mixed linear ANOVA models; therefore, analysis of a comparison between the 2 treatment groups for vital signs was limited to the first cross-over phase only. In the group receiving sildenafil, diastolic blood pressure decreased by 3 mmHg compared with an average increase of 0.8 mmHg in those receiving placebo (P = 0.035). No significant difference was found in the change from baseline for systolic blood pressure (P = 0.24) or heart rate (P = 0.47) between the 2 study groups.
In general, sildenafil was found to be safe and well tolerated. Headache and mild urinary tract infection were the most common adverse events encountered in both placebo and active treatment groups. Overall, the number of adverse events reported did not differ significantly between the sildenafil and placebo groups (P = 0.19). No drug-related serious adverse event occurred in patients.
DISCUSSION
In accordance with previous studies (7,12,13,19), sildenafil was found to be superior to placebo in patients with ED and SCI with regard to erectile response and intercourse, satisfaction with sex life and sexual relationship, erectile function, overall sexual satisfaction, and EDITS score. Evaluation of the primary efficacy variable showed that, in this double blind study, all patients who expressed a preference selected sildenafil over placebo.
Erectile response rates after treatment with sildenafil have been reported to be generally higher in patients with incomplete vs complete SCI and in men with upper vs lower motor neuron lesions (19). Although it has also been reported that response to treatment with sildenafil is not modified according to ASIA score (16), in this study, we found that sildenafil improved only 3 of 11 measures compared with placebo in patients with complete injury and 7 of 11 measures in patients with incomplete SCI. The proportion of patients with complete and incomplete injuries expressing a preference for sildenafil treatment were not recorded; thus, it was not possible to express the respective ratios of injury types in those patients.
Several formalized sexual questionnaires, such as the IIEF and EDITS, facilitate the detection and grading of the severity of ED. In agreement with studies showing that patients receiving sildenafil and their partners have a significantly higher level of treatment satisfaction, as documented by EDITS score, than those receiving placebo (20), sildenafil treatment in this study also improved patients' EDITS scores significantly overall and in both patients with complete and incomplete SCI.
Hemodynamic studies have shown that therapeutic doses of sildenafil produce only mild and transient changes in mean systolic and diastolic blood pressure and heart rate in healthy men, as well as in those with ischemic heart disease or chronic stable angina (11,21). Overall, PDE5 inhibitors have been shown to be safe and effective in most patient populations, including men with ischemic cardiovascular disease or those receiving antihypertensive agents (11,22). Adverse events that are reported in men with SCI most often include headache and flushing; priapism or symptoms of dysreflexia have not been reported in patients with SCI (12,13,23,24). Although the adverse effects of sildenafil seen in patients with SCI are similar to those seen in the general population, sildenafil may induce significant hypotension in people with cervical level injuries (to a greater degree than in those with thoracic level injuries) and can cause dizziness in both populations (25). The concomitant use of nitrates in patients with ED receiving PDE5 inhibitors can cause a profound decline in blood pressure and is therefore contraindicated (26). Our results, showing that sildenafil had no clinically relevant effect on any vital signs, including blood pressure, are in agreement with the findings of previous studies and may be a further proof of the drug's safety in SCI patients with ED.
In a multinational study evaluating the effect of sildenafil on QoL in patients with ED caused by SCI, improvements were reported in scores for the generic QoL parameters of mental health, well being, depression, and anxiety (14,27). In contrast to these findings, our results showed no significant change in QoL in patients with SCI receiving sildenafil.
CONCLUSION
This study adds to the body of evidence supporting the efficacy and tolerability of oral sildenafil in patients with ED attributable to SCI. Sildenafil was effective in patients, regardless of the severity of SCI, but particularly so in those in whom SCI was incomplete. In addition, all patients who expressed a preference selected sildenafil over placebo.
Acknowledgments
We thank Dr Papatya Togay, Dr Halim Yilmaz, Dr Suheda Bayram, and Prof Dr Ozlen Peker for invaluable contributions during the study and preparation of the manuscript.
APPENDIX A. Sexual Health Inventory-Male (SHIM)
SEXUAL HEALTH INVENTORY FOR MEN
PATIENT INSTRUCTIONS
Sexual health is an important part of an individual's overall physical and emotional well being. Erectile dysfunction, also known as impotence, is one type of very common medical condition affecting sexual health. Fortunately, there are many different treatment options for erectile dysfunction. This questionnaire is designed to help you and your doctor identify if you may be experiencing erectile dysfunction. If you are, you may choose to discuss treatment options with your doctor.
Each question has several possible responses. Circle the number of the response that best describes your own situation. Please be sure that you select one and only one response for each question. SCORE Add the numbers corresponding to questions 1–5. If your score is 21 or less, you may want to speak with your doctor.
OVER THE PAST 6 MONTHS:
APPENDIX B. Five-Point Qualitative Scale for Subjective Assessment of Erectile Response
0—No response (no tumescence and rigidity)
1—Increase in size, but not hard (some tumescence, no rigidity)
2—Hard, but not hard enough for penetration (normal tumescence and weakened rigidity)
3—Hard enough for penetration, but not completely hard (normal tumescence and slightly weakened rigidity, penile angle below 90°)
4—Completely hard (normal tumescence and rigidity, penile angle above 90°)
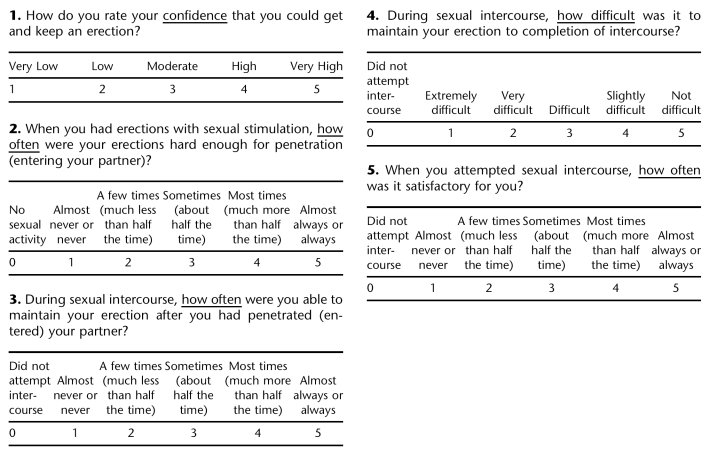
APPENDIX C. International Index of Erectile Function (IIEF)
These questions ask about the effects your erection problems have had on your sex life over the past 4 weeks. Please answer the following questions as honestly and clearly as possible. In answering these questions, the following definitions apply:
sexual activity includes intercourse, caressing, foreplay and masturbation
sexual intercourse is defined as vaginal penetration of the partner (you entered your partner)
sexual stimulation includes situations like foreplay with a partner, looking at erotic pictures, etc
ejaculate: the ejection of semen from the penis (or the feeling of this)
1. Over the past 4 weeks, how often were you able to get an erection during sexual activity?
Please check one box only
[ ] No sexual activity
[ ] Almost always or always
[ ] Most times (much more than half the time)
[ ] Sometimes (about half the time)
[ ] A few times (much less than half the time)
[ ] Almost never or never
2. Over the past 4 weeks, when you had erections with sexual stimulation, how often were your erections hard enough for penetration?
Please check one box only
[ ] No sexual stimulation
[ ] Almost always or always
[ ] Most times (much more than half the time)
[ ] Sometimes (about half the time)
[ ] A few times (much less than half the time)
[ ] Almost never or never
The next three questions will ask about the erections you may have had during sexual intercourse
3. Over the past 4 weeks, when you attempted sexual intercourse, how often were you able to penetrate (enter) your partner?
Please check one box only
[ ] Did not attempt intercourse
[ ] Almost always or always
[ ] Most times (much more than half the time)
[ ] Sometimes (about half the time)
[ ] A few times (much less than half the time)
[ ] Almost never or never
4. Over the past 4 weeks, during sexual intercourse, how often were you able to maintain your erection after you had penetrated (entered) your partner?
Please check one box only
[ ] Did not attempt intercourse
[ ] Almost always or always
[ ] Most times (much more than half the time)
[ ] Sometimes (about half the time)
[ ] A few times (much less than half the time)
[ ] Almost never or never
5. Over the past 4 weeks, during sexual intercourse, how difficult was it to maintain your erection to completion of intercourse?
Please check one box only
[ ] Did not attempt intercourse
[ ] Extremely difficult
[ ] Very difficult
[ ] Difficult
[ ] Slightly difficult
[ ] Not difficult
6. Over the past 4 weeks how many times have you attempted sexual intercourse?
Please check one box only
[ ] No attempts
[ ] 1–2 attempts
[ ] 3–4 attempts
[ ] 5–6 attempts
[ ] 7–10 attempts
[ ] 11+ attempts
7. Over the past 4 weeks, when you attempted sexual intercourse how often was it satisfactory for you?
Please check one box only
[ ] Did not attempt intercourse
[ ] Almost always or always
[ ] Most times (much more than half the time)
[ ] Sometimes (about half the time)
[ ] A few times (much less than half the time)
[ ] Almost never or never
8. Over the past 4 weeks, how much have you enjoyed sexual intercourse?
Please check one box only
[ ] No intercourse
[ ] Very highly enjoyable
[ ] Highly enjoyable
[ ] Fairly enjoyable
[ ] Not very enjoyable
[ ] No enjoyment
9. Over the past 4 weeks, when you had sexual stimulation or intercourse how often did you ejaculate?
Please check one box only
[ ] No sexual stimulation/intercourse
[ ] Almost always or always
[ ] Most times (much more than half the time)
[ ] Sometimes (about half the time)
[ ] A few times (much less than half the time)
[ ] Almost never or never
10. Over the past 4 weeks, when you had sexual stimulation or intercourse how often did you have the feeling of orgasm (with or without ejaculation)?
Please check one box only
[ ] No sexual stimulation/intercourse
[ ] Almost always or always
[ ] Most times (much more than half the time)
[ ] Sometimes (about half the time)
[ ] A few times (much less than half the time)
[ ] Almost never or never
The next two questions ask about sexual desire. Let's define sexual desire as a feeling that may include wanting to have a sexual experience (for example masturbation or intercourse), thinking about having sex, or feeling frustrated because of lack of sex.
11. Over the past 4 weeks how often have you felt sexual desire?
Please check one box only
[ ] Almost always or always
[ ] Most times (much more than half the time)
[ ] Sometimes (about half the time)
[ ] A few times (much less than half the time)
[ ] Almost never or never
12. Over the past 4 weeks how would you rate your level of sexual desire?
Please check one box only
[ ] Very high
[ ] High
[ ] Moderate
[ ] Low
[ ] Very low or none at all
13. Over the past 4 weeks how satisfied have you been with your overall sex life?
Please check one box only
[ ] Very satisfied
[ ] Moderately satisfied
[ ] About equally satisfied and dissatisfied
[ ] Moderately dissatisfied
[ ] Very dissatisfied
14. Over the past 4 weeks how satisfied have you been with your sexual relationship with your partner?
Please check one box only
[ ] Very satisfied
[ ] Moderately satisfied
[ ] About equally satisfied and dissatisfied
[ ] Moderately dissatisfied
[ ] Very dissatisfied
15. Over the past 4 weeks how do you rate your confidence that you can get and keep your erection?
Please check one box only
[ ] Very high
[ ] High
[ ] Moderate
[ ] Low
[ ] Very low
Thanks for completing this questionnaire.
APPENDIX D. Life Satisfaction Check-list (LISAT-8)
How satisfactory are these different aspects of your life? Please indicate the number which best suits your situation.
Where:
1 = very dissatisfying
2 = dissatisfying
3 = rather dissatisfying
4 = rather satisfying
5 = satisfying
6 = very satisfying
Life as a whole is:
My sexual life is:
My partnership relation is:
My family life is:
My contacts with friends and acquaintances are:
My leisure situation is:
My vocational situation is:
My financial situation is:
APPENDIX E. Global Efficacy Assessment Question
Instructions: The purpose of this question is to find out whether the treatment you have been taking during this study improves your erections compared to having no treatment at all. Your answer to this question will not affect your ability to take part in a new study.
Compared with having no treatment at all for your erection problem, has the medication you have been taking over the past 4 weeks improved your erections?
• Yes
• No
Compared with having no treatment at all for your erection problem, has the medication you have been taking over the past 4 weeks improved your ability to have sexual intercourse?
• Yes
• No
Did not attempt intercourse
REFERENCES
- Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- Kleinman KP, Feldman HA, Johannes CB, Derby CA, McKinlay JB. A new surrogate variable for erectile dysfunction status in the Massachusetts Male Aging Study. J Clin Epidemiol. 2000;53:71–78. doi: 10.1016/s0895-4356(99)00150-x. [DOI] [PubMed] [Google Scholar]
- Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts Male Aging Study. J Urol. 2000;163:460–463. [PubMed] [Google Scholar]
- Laumann EO, West S, Glasser D, Carson C, Rosen R, Kang JH. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the Male Attitudes Regarding Sexual Health Survey. J Sex Med. 2007;4:57–65. doi: 10.1111/j.1743-6109.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- Fazio L, Brock G. Erectile dysfunction: management update. CMAJ. 2004;170:1429–1437. doi: 10.1503/cmaj.1020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biering-Sorensen F, Sonksen J. Sexual function in spinal cord lesioned men. Spinal Cord. 2001;39:455–470. doi: 10.1038/sj.sc.3101198. [DOI] [PubMed] [Google Scholar]
- Ramos AS, Samso JV.Specific aspects of erectile dysfunction in spinal cord injury Int J Impot Res. 200416 (suppl 2) S42–S45. [DOI] [PubMed] [Google Scholar]
- Deforge D, Blackmer J, Garritty C, et al. Male erectile dysfunction following spinal cord injury: a systematic review. Spinal Cord. 2006;44:465–473. doi: 10.1038/sj.sc.3101880. [DOI] [PubMed] [Google Scholar]
- Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- Corbin JD.Mechanisms of action of PDE5 inhibition in erectile dysfunction Int J Impot Res. 200416 (suppl 1) S4–S7. [DOI] [PubMed] [Google Scholar]
- Sussman DO.Pharmacokinetics, pharmacodynamics, and efficacy of phosphodiesterase type 5 inhibitors J Am Osteopath Assoc. 2004104 (3 suppl 4) S11–S15. [PubMed] [Google Scholar]
- Maytom MC, Derry FA, Dinsmore WW, et al. A two-part pilot study of sildenafil (VIAGRA) in men with erectile dysfunction caused by spinal cord injury. Spinal Cord. 1999;37:110–116. doi: 10.1038/sj.sc.3100803. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Hultling C, El Masry WS, et al. Randomized trial of sildenafil for the treatment of erectile dysfunction in spinal cord injury. Sildenafil Study Group. Ann Neurol. 1999;46:15–21. doi: 10.1002/1531-8249(199907)46:1<15::aid-ana5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hultling C, Giuliano F, Quirk F, Pena B, Mishra A, Smith MD. Quality of life in patients with spinal cord injury receiving Viagra (sildenafil citrate) for the treatment of erectile dysfunction. Spinal Cord. 2000;38:363–370. doi: 10.1038/sj.sc.3101011. [DOI] [PubMed] [Google Scholar]
- Schmid DM, Schurch B, Hauri D. Sildenafil in the treatment of sexual dysfunction in spinal cord-injured male patients. Eur Urol. 2000;38:184–193. doi: 10.1159/000020278. [DOI] [PubMed] [Google Scholar]
- Sanchez Ramos A, Vidal J, Jauregui ML, et al. Efficacy, safety and predictive factors of therapeutic success with sildenafil for erectile dysfunction in patients with different spinal cord injuries. Spinal Cord. 2001;39:637–643. doi: 10.1038/sj.sc.3101210. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- Althof SE, Corty EW, Levine SB. EDITS: development of questionnaires for evaluating satisfaction with treatments for erectile dysfunction. Urology. 1999;53:793–799. doi: 10.1016/s0090-4295(98)00582-2. [DOI] [PubMed] [Google Scholar]
- Derry F, Hultling C, Seftel AD, Sipski ML.Efficacy and safety of sildenafil citrate (Viagra) in men with erectile dysfunction and spinal cord injury: a review Urology. 200260 (2 suppl 2) 49–57. [DOI] [PubMed] [Google Scholar]
- Lewis R, Bennet CJ, Borkon WD, et al. Patient and partner satisfaction with Viagra (sildenafil citrate) treatment as determined by the Erectile Dysfunction Inventory of Treatment Satisfaction Questionnaire. Urology. 2001;57:960–965. doi: 10.1016/s0090-4295(01)00945-1. [DOI] [PubMed] [Google Scholar]
- Jackson G, Montorsi P, Cheitlin MD.Cardiovascular safety of sildenafil citrate (Viagra): an updated perspective Urology. 200668 (3 suppl) 47–60. [DOI] [PubMed] [Google Scholar]
- Israilov S, Baniel J, Shmueli J, et al. Treatment program for erectile dysfunction in patients with cardiovascular diseases. Am J Cardiol. 2004;93:689–693. doi: 10.1016/j.amjcard.2003.11.049. [DOI] [PubMed] [Google Scholar]
- Monga M, Bernie J, Rajasekaran M. Male infertility and erectile dysfunction in spinal cord injury: a review. Arch Phys Med Rehabil. 1999;80:1331–1339. doi: 10.1016/s0003-9993(99)90039-4. [DOI] [PubMed] [Google Scholar]
- Gans WH, Zaslau S, Wheeler S, Galea G, Vapnek JM. Efficacy and safety of oral sildenafil in men with erectile dysfunction and spinal cord injury. J Spinal Cord Med. 2001;24:35–40. doi: 10.1080/10790268.2001.11753553. [DOI] [PubMed] [Google Scholar]
- Ethans KD, Casey AR, Schryvers OI, MacNeil BJ. The effects of sildenafil on the cardiovascular response in men with spinal cord injury at or above the sixth thoracic level. J Spinal Cord Med. 2003;26:222–226. doi: 10.1080/10790268.2003.11753687. [DOI] [PubMed] [Google Scholar]
- Prisant LM. Phosphodiesterase-5 inhibitors and their hemodynamic effects. Curr Hypertens Rep. 2006;8:345–351. doi: 10.1007/s11906-006-0075-y. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Pena BM, Mishra A, Smith MD. Efficacy results and quality-of-life measures in men receiving sildenafil citrate for the treatment of erectile dysfunction. Qual Life Res. 2001;10:359–369. doi: 10.1023/a:1012270220064. [DOI] [PubMed] [Google Scholar]