Abstract
Hurricane Katrina was one of the most catastrophic natural disasters to hit the United States. It had a major impact on health care in New Orleans, LA and the surrounding region, not only in relation to acute illness but also chronic disease. When Hurricane Katrina struck New Orleans on August 29, 2005, there were 193 participants being followed in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial at Tulane University Health Sciences Center. In the immediate aftermath of the storm, the Tulane University ACCORD Study site, in collaboration with the Study Coordinating Center and the Southeast Clinical Center Network office of the trial at Wake Forest University Health Sciences in North Carolina, took several actions in order to locate the participants, ensure their safety, and maintain the scientific integrity of the trial. We describe the actions taken and the relative success/failure of such actions.
INTRODUCTION
Hurricane Katrina was one of the most catastrophic natural disasters to hit the United States. It had a major impact on health care in New Orleans, LA and the surrounding region, not only in relation to acute illness (1–3) but also chronic disease (4,5). Other disasters have been shown to have an impact on chronic disease like diabetes and hypertension (6). However, many lessons have been learned that may help to better prepare for managing health-related problems in future disasters (7). There have been no descriptions of the impact of these disasters on clinical trials and guidelines on management of trials in a disaster do not exist.
Very little attention has been given to the fact that in any major disaster, a large number of clinical trial participants may be seriously affected. A disaster may have an impact not only on participants and patient care, but also on the integrity of a clinical trial. We describe our experience with the impact of Hurricane Katrina on a major clinical trial from which we have learned many lessons that could lead to specific recommendations in disaster preparedness for investigators, sponsors, participants, institutional review boards, and others involved in clinical trials.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial (8) is a major National Heart, Lung and Blood Institute (NHLBI) initiative to test the hypothesis that intensive glycemic control will prevent cardiovascular events in people with type II diabetes. In addition, in a double 2 × 2 factorial design, the impact of intensive modification of blood pressure and lipids is being tested. The trial is fully enrolled with 10,251 participants across the United States and Canada at 77 clinical sites. Further details can be found at www.accordtrial.org.
When Hurricane Katrina struck New Orleans on August 29, 2005, there were 193 participants being followed in the ACCORD Trial at Tulane University Health Sciences Center. The primary institution and the trial site (and NIH funded general clinical research center (GCRC) at Charity Hospital, Medical Center of Louisiana)) were severely damaged. All communications were lost and access to the clinical site became impossible for several months. Unfortunately, no back up records or participant contact information had been kept off –site. Due to the use of a central laboratory no biological specimens were left on site prior to evacuation, or else they would have been lost (as happened in several other studies at the institution).
In the immediate aftermath of the storm, the Tulane University ACCORD Study site, in collaboration with the Study Coordinating Center (CC) and the Southeast Clinical Center Network (SE CCN) office of the trial at Wake Forest University Health Sciences in North Carolina, took several actions in order to locate the participants, ensure their safety, and maintain the scientific integrity of the trial.
We describe below the actions taken and the relative success/failure of such actions. In order to maintain the integrity of the main trial, we do not report here the impact of the hurricane on the clinical features, laboratory results or hospitalization of participants or study related “adverse events”. These data may be reported on completion of the ACCORD Study.
METHODS
Strategies to locate participants
Immediate efforts
Table 1 summarizes the variety of strategies used in agreement with the CC, SE CCN, NHLBI, and both the Wake Forest and Tulane University Institutional Review Boards (IRBs) in order to attempt to locate and ensure the safety of study participants. The WFUHS IRB chairs unanimously agreed that contact by the CC and SE CCN staff was appropriate and in line with protecting the health and welfare of the study subjects.
Table 1.
Strategies to find participants after a disaster – listed in chronological order. Figures relate to participants found at 1 year post hurricane. Pre-disaster 193 patients were randomized
| Search Strategy | Number found | Comment |
|---|---|---|
| Staff visits to multiple shelters | 2 | Unproductive and emotionally draining |
| Calls to home/cell phone numbers (from diabetes supplier contact list) | 30 | Phone service disabled in area but multiple contacts made outside of New Orleans area |
| Newspaper advertisements | 31 | Very expensive |
| Radio advertising | 0 | Local radio program remained operational throughout storm and aftermath but unsuccessful in locating patients |
| Internet sites on how to find local physicians | 1 | Limited internet access for patients |
| Internet “people-finder” sites | 0 | Contact information available but out of date |
| Initial mailing | 28 | Very successful |
| Subsequent mailing | 44 | Multiple mailings. Low yield but relatively inexpensive |
| Subsequent phone calls | 38 | Possible only after cell services resumed. Multiple phone call attemptsRelatively inexpensive |
| Flyers to FEMA trailer parks (dropped at each trailer) | 0 | Time consuming a |
| Participant contacting coordinating center | 13 | Infrequent but may reflect lack of phone/internet access immediately after the storm |
| Total number contacted | 187 |
The very first study contacts came as participants called the study Drug Distribution Center through contact information listed on study medications. This information was forwarded to the CC who forwarded it to the SE CCN for follow-up. Because ACCORD supplies the majority of the Tulane participants with all of their diabetes medications, including insulin, there was an immediate need for developing a system for establishing contact and arranging medication delivery. This was particularly difficult in light of the displacement of the site Principal Investigator, Study Coordinator, site staff, and participants into a widespread, multiple-state region. According to a DHHS Office for Civil Rights Bulletin released September 2, 2005, “Providers can share patient information with anyone as necessary to prevent or lessen a serious and imminent threat to the health and safety of a person or the public…”— DHHS Office for Civil Rights Bulletin released September 2, 2005. Therefore, the CC began to investigate ways to contact participants. . A total of 13 participants contacted the coordinating center directly.
The ACCORD website homepage was changed to provide direct contact information for the SE CCN and CC and to allow participants to enter their contact information online. Additionally, a toll-free line that had previously been set up for recruitment purposes was reactivated, converted, and restructured to enable participants to contact the CCN without incurring long distance charges.
Once means were in place for participants to contact the study, the CC began working with the diabetic materials supplier, who kept contact information centrally as part of the study (also provided for in the informed consent). Information for the Tulane clinical site was distributed to SE CCN staff so they could begin attempting to locate participants through cell phones and back-up contacts. A call-script form was developed and distributed to all other regional networks to ensure accurate and complete information gathering in the case of participant contact.
Cell phone service failed significantly around New Orleans following the storm, but because many participants had relocated, 30 were contacted by the SE CCN in the first two weeks through cell and home phone data provided by the Study’s diabetes supplies contractor. Those contacted through home phones lived just outside the New Orleans metro area and did not need to evacuate or were able to return to their homes soon after the storm ended. Approximately 6 weeks later local call services were resumed, and ongoing attempts on participant’s cell phone numbers resulted in an additional 38 contacts.
The Tulane study staff also visited multiple shelters across three states with signs designed to attract ACCORD and other clinical trial participants. Two participants were found in such shelters, but overall, it was felt that this effort was not cost effective, and emotionally draining for site staff. However, the two participants located were extremely relieved and grateful for the efforts. We do not have an accurate account of time spent in these efforts but can only suggest that this strategy was time consuming, expensive and not cost effective. Attempts to find individuals were also made through various relief organizations, including The Red Cross and the Federal Emergency Management Agency (FEMA). Flyers with study contact information were dropped off at various FEMA temporary housing communities, but did not result in contact with any study participants.
Newspaper advertisements were also placed in multiple newspapers in cities around the region where a large number of evacuees were thought to be located. The overall cost of this approach was significant (~$20,000) although 31 subjects were found. Radio advertising was also conducted in the New Orleans, LA area. Although none of the participants found mentioned the radio advertising, it is possible that some of those who stated they had seen a newspaper advertisement had also heard the radio messages which were, at times, run concurrently. In addition, various public internet websites were set up following the hurricane to provide information on locating area physicians and the physicians in the section of endocrinology at Tulane University Health Sciences Center were listed. This led to only one individual and was considered unsuccessful, partly because of limited internet access for participants following their evacuation. The site staff also searched various ‘people finder’ websites and found contact information for 10 study participants but were unable to contact any of them due to the information being outdated. The time spent on these efforts by the staff was not recorded but is estimated to be substantial. The ACCORD Study also modified its website homepage to list information about the study and provided a toll-free number which participants could call. The website information had been provided to patients in the recruitment brochure. This resulted in 13participants being located (these are included in Table 1, under “Participants contacting coordinating center”).
Trial Disaster Plan
A formal Disaster Plan from the Coordinating Center was finalized on September 2, 2005. This plan provided guidance for medication priorities, determining urgent need, and setting up a temporary pharmacy. Because of their immediate need, glycemia medications were given highest priority, then blood pressure and lipid medications. To ensure participant safety during this period of limited follow-up, blinded study medications were not shipped. All participants were treated as if on the standard protocol, which equated to standard-of-care, to minimize risk of hypoglycemia and other adverse effects. Urgent need was defined as having less than five days of medication on hand. Those with immediate needs within the storm affected damaged areas were instructed to proceed to the nearest shelter because of the lack of consistency with shipping.
Later efforts
Approximately two months after the storm, limited (only a few people allowed in for a few hours) access was available to the study clinic for the first time. Without electricity and operational elevators, staff visited the clinic, having to use stairs and flashlights. The most important item retrieved was an index of contact information that contained addresses and phone numbers of participants and alternate contacts. For logistical reasons (essentially lack of electricity in the building) it was not possible to retrieve any other study records, computers etc. Information cards about the study that included the current contact information of the staff were mailed to these addresses and despite local mail interruptions and delays resulted in an additional 28 participants being identified. Next of kin information was also available within the database but calls to these numbers did not result in any participant contacts.
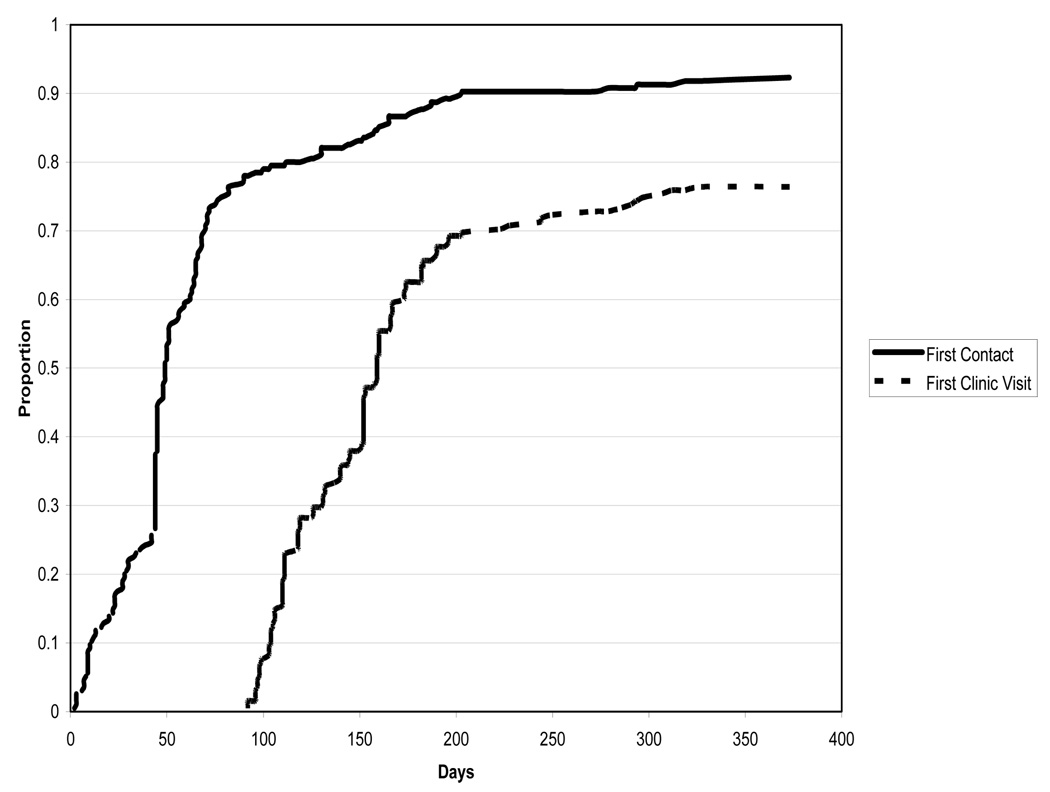
Over the course of the next year, additional mailings (certified mail) were also used at intervals at a cost of approximately $300 per mailing initially (costs declined with subsequent mailings as additional participants were located). While somewhat time consuming, this method was inexpensive and resulted in contact with 44 participants. In summary, the Tulane University ACCORD Study site randomized 193 participants. One year after the storm 5 had transferred to other sites and of the remaining 188, nine were lost to follow up (6 never contacted and 3 who became lost after an initial contact) and 160 had been seen in clinic. Of the remaining 19 participants, all were in contact with the study team but unable to make clinic visits due to their geographical location or other issues. All of the latter indicate a desire for clinic visit follow up if circumstances permit in the future. The study is currently exploring solutions to financial and logistical difficulties in bringing these participants to a study clinic, specifically arranging and paying for transportation. Figure 1 summarizes the timeline for contacting study participants. As indicated, there was a rapid acquisition of participant contacts which gradually leveled off with time.
Figure 1.
Time to first participant contact and first clinic visit after Hurricane Katrina
Study Medications/Supplies Plan
While the Tulane clinic was not operating and as participants were located in the weeks immediately following the hurricane, medication needs were assessed and triaged in order to accommodate the most urgent requests. A temporary pharmacy was initially set-up using inventory from another clinical site within the SE CCN, but as the number of participants located increased, it became apparent that an independent pharmacy was needed. In collaboration with the Drug Distribution Center, the SE CCN established a limited pharmacy to supply located participants with medications. Study medications, including insulin, were distributed as well as medication related supplies. Participants with less than a week’s worth of any medication were considered high-priority with an estimated turn-around time of 24 hours or less. All participant needs were met within a maximum of two weeks time from initial contact. Those participants who reported no need for medications were re-contacted three to four weeks later to determine need. All participant medications were shipped via overnight courier to the address provided, but there were limitations to the delivery system. National courier services like Federal Express, U.S. Postal Service, and United Parcel Service were only operating in limited areas following the storm, making package tracking and delivery confirmation impossible in some areas of the New Orleans metropolitan area.
Clinic Re-establishment
As previously mentioned the hurricane flooded the Tulane University ACCORD Study site and due to the damage incurred the hospital facility that housed the clinic was closed permanently. The first step in rebuilding a clinic site for the study was to establish a location for the limited site staff that remained to begin work. Without options at Tulane a temporary office was set-up in early October of 2005 within the study coordinator’s home, located in Picayune, Mississippi. This enabled the site to take over the process of tracking participants and to begin to plan for a new clinic location. After much time and effort a location was found within a hospital in Covington, LA. Located north of New Orleans, this location was convenient as many New Orleans residents had located to this area after the storm. This clinic saw its first study patient on November 30, 2005. As more and more participants were located it was soon evident that another location was needed within New Orleans. Thus a 2nd clinic opened in January, 2006. Two clinical sites, while convenient for participants that remained in the area, were a severe strain for the clinic staff to operate. In April, 2006, the present single clinical site was opened in downtown New Orleans very close to its original location. Figure 1 presents the timeframe for first clinic visits after the hurricane struck. Similar to participant contact data, there was a dramatic increase in visits early in the recovery process (once a clinic facility was available) followed by a gradual slowdown.
Once Tulane University was able to begin seeing participants in first one and then two temporary clinics, medications were managed by an independent pharmacy located in Picayune, Mississippi. After obtaining an appropriate stock of study medications, staff began the process by faxing prescription orders into the pharmacy, and on the following day picking up those orders, checking the orders, adding syringes or other ancillary materials as needed and mailing the medication and supplies to participants. This method of medication supply continued until the clinical site relocated into the current New Orleans location at Tulane. Less than 5% of shipments were returned as “undeliverable” or reported by participants as “not received”. Nevertheless, almost all participants reported gaps in their supplies of study medications. Many obtained alternative medications through shelters, humanitarian clinics, other medical providers etc. It is impossible for us to quantify the exact duration and extent of gaps in treatment for most participants.
Once back at Tulane, the pharmacy was incorporated into our clinic with the addition of a part time independent pharmacist. At that time the site was again able to supply medications as participants came in for visits rather than through the mail.
Financial implications
A large, upfront expense was the need to establish a temporary clinic. In the aftermath of the hurricane space was difficult to find and more costly. In addition, due to the wide dispersal of study participants in the area, a 2nd clinical site was opened. Increased staff was needed in order to begin the process of rebuilding the site(s) along with much greater phone and mailing costs primarily due to the participant tracking process. Transportation costs were a major component of increased study costs as well. Prior to the disaster subjects were paid a minimal stipend to cover local transportation. Subsequent costs rose dramatically due to the lack of public transportation and the long distances study participants and staff needed to travel. Additional staff time required to track participants and arrange clinic visits (and often participant travel) also contributed to increased costs. As many previously existing services were not operating, some services had to be outsourced (medications dispensation, GCRC supports, etc). In addition, due to the intensive management requirements of the ACCORD Study protocol, additional participant support services were needed with resultant additional costs. All of these issues continue to be challenges. Remarkably, the overall cost of operations over the first year following the disaster was approximately only 10% higher than the estimated costs had all patients been seen according to protocol, using a “fee for service” model. Although the initial site budget was constructed using such a model, this was adapted following the disaster to reimbursement of documented costs, albeit very little of it being related to actual patient visits. Thus, for little incremental cost we have maintained the integrity of the trial and ensured patient safety and continuity of care. An administrative supplement from NIH was sought and provided to offset the additional costs.
Barriers to locating and managing patients
Barriers to locating and managing ACCORD Study participants are listed in Table 2. It is important to note that with time a variety of these barriers were gradually removed through changes in infrastructure. The most important of these were improvement in cell phone service that occurred after about four weeks, and limited improvement in landline phone service occurred after about ten weeks. Similarly, limited postal service began in New Orleans after approximately ten weeks post hurricane. Another important barrier was lack of access to critical study records at the site due to closure of the study clinic and lack of access to the facility for security reasons. Approximately 2 months after the storm coordinators were able to enter the clinic, pack patient charts and source documents into cartons and remove study records from the clinical site. All of this had to be accomplished without benefit of electricity or elevators. Records were moved to a temporary and secure facility. It is important to note that 18 months after the disaster most medical records remain unavailable, except for participants recruited from the New Orleans Veterans Administration hospital due to their nationally available electronic medical record.
Table 2.
Barriers to locating and managing participants
| BARRIERS | RESOLUTION/ PROBLEMS |
|---|---|
| Non-functioning phone service | Gradual improvements with time |
| Lack of cooperation and leadership at shelters | Abandoned searching shelters |
| Poor tracking records of participants from shelters | Records exist up to certain points in time then drop off without explanation |
| “People-finder” websites | Poor construction, lack of updated contact information |
| Poorly functioning postal system | Some areas with no delivery at several months post hurricane |
| Lack of clinical site facilities | Issues are lack of availability, increased costs |
| Lack of clinic staff (many displaced) | Gradually improved with time as population returned to area |
| Lack of access to study records (participant contact data) | Resolved with limited access at 10 weeks |
| Approval of regulatory authorities for search methods | Disallowed use of investigative search service to find patients |
| Local area recovery (multiple issues)- | Slow recovery hampers efforts to see and intensively treat participants per protocol including transportation challenges and lack of support services |
| Additional stress/depression among population | Area conditions hamper efforts to implement ACCORD protocol |
The ACCORD Study clinic was previously located within an NIH funded General Clinical Research Center within the Tulane University Medical Center of New Orleans and utilized the pharmacy services of that institution. Finding and opening alternate clinic sites was an extremely time consuming process amidst the chaos in the area. Although a satellite GCRC reopened a few months after the disaster, pharmacy services at the institutions remain closed, and the research center is no longer funded by NIH. Thus the site had to develop alternative processes (and hire additional staff) in order to provide these services to study participants after the storm.
All clinic staff suffered consequences of the disaster with either personal loss or prolonged mandatory evacuation. Not surprisingly several staff members relocated permanently to other cities, further exacerbating the problem of finding participants and reinstituting protocol related procedures. Staff suffered serious disruption of their routines and a significant increase in workload in their attempt to help the study recover. This included disruption in living quarters, longer travel times to study facilities and dealing with a much less efficient area infrastructure.
The pace of local area recovery from Katrina also affected the recovery of the Tulane ACCORD site and its ability to implement the study protocol. The current dispersed location of many study participants and lack of local infrastructure forced the study to spend additional resources. Local public transportation is still operating below pre-storm levels. As such, it takes additional time, staff and effort to schedule and often arrange transportation for clinic study visits. Any missed or rescheduled visits add to the site’s burden. Nutrition challenges are also a problem for some participants who had to deal with contaminated food/water and high calorie “meals ready to eat” just after the storm. These challenges continued as participants with limited financial means moved into trailer parks with limited shopping and cooking facilities. Nearly two years after the storm, the problem persists for many participants due to a lack of access to more appropriate food choices including fresh fruits and vegetables, in some areas of the city. Participant stress and depression, following significant personal losses, also remains a study management barrier but participation in the trial continues. Depression is common among study patients, while mental health facilities in the New Orleans area have been greatly diminished; essentially unavailable to people without appropriate health insurance. In order to address these issues the site has instituted group sessions with social workers with expertise in psychological problems and post – traumatic stress. This initiative was funded by the study sponsor (separate from the main trial funding).
DISCUSSION
We describe above our experience in managing clinical trials following a major disaster. Our experience has important implications for conduct of future research in relation to disaster planning. In general, no contingency plans exist for most clinical trials, and some of the lessons we have learned may help in future planning for disaster preparedness. Thanks to the strong support from NIH/NHLBI, the CC and SE CCN, one year after a major disaster 187 out of 193 (97%) study participants followed before the hurricane struck had been contacted. Over 86% of these participants have made study clinic visits since the storm. While challenges remain this is a remarkable achievement within a large and complicated clinical trial.
Table 3 summarizes our recommendations for planning for future trials, based on our experience. We believe that it is imperative that every multicenter clinical trial develop a disaster preparedness plan. Most important in this would be a provision to have offsite contact information stored on a computer that is available to the site investigators and coordinators should the primary site be shut down. Other off site storage might include study data and laboratory samples Storage of biological samples may be problematic for single site studies and some health systems that may not allow such storage may need to consider changes to their policies.
Table 3.
Lessons learned – Recommendations for disaster preparedness for clinical trials
| Anticipate potential disasters |
| Ensure clinical sites have a basic disaster plan which includes alternate contact information for participants |
| Advise participants to prepare a study related disaster kit (medications, supplies, contact info, etc) |
| Obtain alternative contact information from participants |
| Update participant contact information frequently |
| Store a copy of participant contact information at an alternate site |
| Consider a secure off site server to store and back up data |
| Set up a toll free number for participants to call in case of emergency |
| Have basic plans for an alternative clinical site should the primary site become unavailable |
| Develop plans in advance for participant tracking and retention should participants be displaced |
| Store biological samples in secure sites with backup electricity. |
| Study sponsors should be flexible in budgeting and study procedures (documentation, protocol deviations, etc.) in case of disruption |
Contact information for participants should be updated at intervals and include, if possible, next of kin or other relatives outside the immediate city or region. Consider a toll-free telephone contact as part of trial structure as well. The Tulane University Health Sciences Center IRB has set up a toll free telephone number for participants to call in case of Medical Center shutdown. Having a basic plan for an alternate site location would be helpful (but perhaps impractical) along with advance plans in case tracking and retaining of study participants is needed. The disaster also had significant financial implications for the sponsors, institution and participants and contingency plans should therefore include a budget to deal with these issues. Of course, the first priority for a study affected by a natural disaster is to ensure participant safety at all times.
We hope that many of these suggestions will be considered in the planning of future clinical trials, to ensure continuity of care, integrity of trial specimens and data and most importantly patient safety. Sponsors of clinical trials need to realize that flexibility in terms of opening satellite sites and shipping medications may be critical in maintaining patient’s health status and study confidence in the face of an emergency. We remain confident that the lessons learned will lead to an improvement in the management of future clinical trials so that major destruction will not lead to any harm for participants and/or a disruption in the scientific integrity of the trials.
Acknowledgements
The ACCORD study is supported by NHLBI. This study was also supported in part by NIH Grants # 5M01RR05096 and RR-00827 in support of the General Clinical Research Center from the Division of Research Resources, National Institutes of Health (GCRC). Diabetes research at Tulane University Health Sciences Center is supported in part by Susan Harling Robinson Fellowship in Diabetes Research and the Tullis-Tulane Alumni Chair in Diabetes. Dr Fonseca and the Tulane section of Endocrinology are also supported in part by the American Diabetes Association grant “Impact of Hurricane Katrina on Diabetes and Co-Morbidities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Berggren RE, Curiel TJ. After the storm--health care infrastructure in post-Katrina New Orleans. N Engl J Med. 2006;354:1549–1552. doi: 10.1056/NEJMp068039. [DOI] [PubMed] [Google Scholar]
- 2.Hamm LL. Personal observations and lessons from Katrina. Am J Med Sci. 2006;332:245–250. doi: 10.1097/00000441-200611000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Walsh TJ, Orsega S, Banks D. Lessons from Hurricane Rita: organizing to provide medical care during a natural disaster. Ann Intern.Med. 2006;145:468–470. doi: 10.7326/0003-4819-145-6-200609190-00137. [DOI] [PubMed] [Google Scholar]
- 4.Cefalu WT, Smith SR, Blonde L, Fonseca V. The Hurricane Katrina Aftermath and Its Impact on Diabetes Care: Observations from "ground zero": lessons in disaster preparedness of people with diabetes. Diabetes Care. 2006;29:158–160. doi: 10.2337/diacare.29.1.158. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Mokdad AH, Link MW, Garvin WS, McGuire LC, Jiles RB, Balluz LS. Chronic disease in health emergencies: in the eye of the hurricane. Prev.Chronic.Dis. 2006;3:A46. [PMC free article] [PubMed] [Google Scholar]
- 6.Inui A, Kitaoka H, Majima M, Takamiya S, Uemoto M, Yonenaga C, Honda M, Shirakawa K, Ueno N, Amano K, Morita S, Kawara A, Yokono K, Kasuga M, Taniguchi H. Effect of the Kobe earthquake on stress and glycemic control in patients with diabetes mellitus. Arch.Intern.Med. 1998;158:274–278. doi: 10.1001/archinte.158.3.274. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Statement on Emergency and Disaster Preparedness: a report of the Disaster Response Task Force. Diabetes Care. 2007;30:2395–2398. doi: 10.2337/dc07-9926. [DOI] [PubMed] [Google Scholar]
- 8.Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC, Jr, Grimm RH, Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]