Abstract
Transcript quantification techniques usually rely on purified mRNAs. We report here a solution-based sandwich hybridization assay for the quantification of mRNAs from Escherichia coli without the need of prior RNA isolation. This assay makes use of four DNA oligonucleotide probes adjacently hybridizing to target RNA in clarified cell extracts. Two helper probes facilitate the hybridization of a detection and a capture probe. The latter is biotin labeled, allowing binding to streptavidin-coated paramagnetic beads and the separation of the RNA-DNA hybrid from cellular constituents. Added antidigoxigenin Fab fragments conjugated to alkaline phosphatase bind to the digoxigenin-labeled detection probe, completing the sandwich of the paramagnetic bead, mRNA, probes, and alkaline phosphatase. The target transcript can be quantified by assessing phosphatase activity on a substrate that is converted into a fluorescent product. The amount of target mRNA is calculated from the fluorescence output and from a calibration curve for a known concentration of in vitro-synthesized target mRNA. This technique was used in time course experiments to investigate the expression of three genes responsible for the copper resistance of E. coli. The induction of gene expression by copper cations was rapid, but under aerobic conditions, the levels of expression returned to low, prestress levels within minutes. In anaerobiosis, high-level expression continued for at least 1 h. When cultures were shifted from anaerobiosis to aerobiosis, expression levels were diminished within minutes to prestress levels. The improved technique presented here is relatively simple, has very high degrees of sensitivity and robustness, is less laborious than other RNA quantification methods, and is not negatively affected by genomic DNA. These characteristics make it a powerful complementary application to genetic reporter fusions and to reverse transcription-PCR.
Several methods for the quantification of transcripts in different organisms are available. These techniques differ largely in the amounts of time and expense required for data acquisition. In general, these methods can be grouped into those that target single transcripts and those that target whole transcriptomes. Northern hybridization has the disadvantage of a rather low level of sensitivity, limited quantification potential, and a large time commitment but is comparatively inexpensive. On the other hand, global and quantitative assays like transcriptome microarray analysis call for major investments in equipment and disposables, aside from limitations in view of quantitative data evaluation/expression analysis. For the quantification of single transcripts, reverse transcription (RT)-PCR is currently the method of choice. RT-PCR can be performed as a quantitative or semiquantitative assay depending on whether absolute transcript numbers are required or whether relative expression levels are investigated.
A major drawback of RT-PCR is the necessity of purified high-quality RNA that is virtually free of contaminating DNA. The preparation of such mRNA is in general laborious and time-consuming. Another negative aspect is that parts of the molecules are lost during the purification process, a problem for low-population mRNA species. All mRNAs together account for ∼50% of the synthesized RNA but only 2 to 3% of the total RNA mass (9), whereas rRNA constitutes up to 80% of the total RNA in a cell. Especially challenging is the isolation of mRNA from prokaryotes, which usually lack polyadenylated mRNA, typically present in eukaryotes. While mRNAs in eukaryotes can be selectively separated from other RNA species by affinity chromatography, total RNA from bacteria is usually purified. The further purification of mRNAs requires additional steps and increases the overall costs, as well as the processing time. In addition to the preparation time needed, total-mRNA isolation procedures require the inactivation of ribonucleases and particular attention to rapid sample acquisition. Prokaryotic mRNAs have short half-lives, averaging 6.8 min in Escherichia coli (23), prohibiting extensive centrifugation steps for preparation, which may cause partial mRNA degradation and altered gene expression results.
RNA-targeting bead-based sandwich hybridization is becoming more of an established method for the analysis of bacterial rRNAs (8, 11, 14) or for the analysis of dynamic changes in mRNA levels (13, 17, 19, 22). Although sandwich hybridization can be applied for the quantitative detection of rRNAs in crude cell extracts (8, 11), so far the method has been inefficient in detecting mRNA transcripts in crude cell lysates with good sensitivity and a high degree of robustness (19).
Here, we present an improved solution-based sandwich hybridization (SBSH) method for the rapid quantitative analysis of transcripts in bacteria. With this method, it is possible to measure absolute transcript levels in crude cell extracts without prior mRNA isolation. As proof of principle, we investigated the responses of three E. coli genes involved in copper homeostasis to copper ions. High (millimolar) concentrations of copper cations are lethal to E. coli. Consequently, specialized mechanisms in this bacterium and in others have evolved to counter the detrimental effects of copper toxicity. E. coli possesses three well-characterized copper detoxification systems. The P-type ATPase CopA transports Cu(I) from the cytoplasm to the periplasm at the expense of ATP hydrolysis (20). Periplasmic Cu(I) is detoxified by two independent systems. The proton motive force-driven CusCFBA complex spans the cytoplasmic and outer membranes. Cus-mediated Cu(I) ion efflux into the extracellular space probably originates from the periplasm, not from the cytoplasm (6). The Cus-independent periplasmic multicopper oxidase CueO oxidizes Cu(I) into the less toxic Cu(II) species (15, 24). This enzymatic reaction circumvents excessive Fenton-like reactions and, thus, diminishes oxidative stress.
The copA and cueO genes are regulated predominantly by CueR in response to rising cytoplasmic copper levels. Apo-CueR binds to nonoptimum −35 and −10 spacing operator structures upstream of copA and cueO and prevents transcription when copper is low (25). Upon Cu(I) binding, the CueR dimer is converted into an activator, which bends the DNA. Consequently, the spacing between the −35 and −10 sites is optimized and transcription can be initiated (25).
The transcription of cusCFBA is regulated by the two-component system CusRS, probably as a result of elevated periplasmic copper concentrations (5). The signal is transduced as a phosphate group by the histidine kinase CusS to the response regulator CusR, which binds to the cus operator and facilitates transcription initiation (5, 12).
In contrast to RT-PCR, the improved SBSH method presented here does not require extensive specialized equipment. Running costs are largely limited to standard plasticware, a set of four DNA oligonucleotides, streptavidin-labeled paramagnetic beads, and digoxigenin (DIG) labeling and detection chemicals.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli cells of wild-type strain W3110 or its derivatives were incubated overnight at 37°C on Luria-Bertani (LB) agar plates. For each experiment, a single colony of E. coli from an LB agar plate was inoculated into LB broth for 16 h at 37°C with shaking and cultured to stationary growth phase.
For aerobic growth, overnight cultures were diluted 1:20 in 20 ml of fresh LB broth and grown until the optical density at 600 nm (OD600) reached 1. For anaerobic growth, overnight cultures were diluted 1:150 in 300 ml of fresh LB broth supplemented with 0.5% glucose in a serum bottle and grown at 37°C until the OD600 reached 0.3. Samples were taken and the exact cell density was determined before the addition of CuCl2 (at time zero) and, after that, at the time points indicated in the figures. Cultures were mixed with ice-cold stop solution (5% [vol/vol] phenol-95% [vol/vol] ethanol at a final concentration of 10%, precooled at −20°C) to inhibit all cellular functions, including RNA degradation. The mixtures were centrifuged for 5 min at 20,800 × g and 4°C, and the supernatant was discarded. Pellets were frozen in liquid nitrogen and stored at −20°C. RNase inhibitors such as RNALater (Ambion, Austin, TX) were not used since they did not significantly improve the overall performance of the assay.
Generation of in vitro-synthesized mRNA for calibration curves.
For the quantification of cellular mRNAs, reference transcripts were generated in vitro using phage T7 RNA polymerase. An internal fragment of about 500 bp of the target gene was PCR amplified, and a T7 RNA promoter sequence was introduced at the 5′ end of the truncated gene (Table 1). The PCR product was column purified by using a QIAquick kit (Qiagen, Hilden, Germany) and eluted with diethylpyrocarbonate (DEPC)-treated water. For mRNA synthesis, the PCR product was transcribed in vitro with T7 RNA polymerase by using a MAXIscript T7 kit according to the instructions of the manufacturer (Ambion, Austin, TX). The quality of in vitro-generated RNA transcripts was checked on a denaturing urea polyacrylamide gel, the concentration was determined using the Quant-IT RiboGreen RNA assay kit (Invitrogen, Karlsruhe, Germany), and aliquots (5 μl) were frozen in liquid nitrogen and stored at −20°C until further use.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide | Target gene | Function | Sequencea (5′→3′) |
|---|---|---|---|
| Probes | |||
| 692 copA h1 | copA | Helper | ACCATCACTGCCAGGGTTATC |
| 671 copA BIO | copA | Capture | AGGCCGATAACCAACCACAGG |
| 650 copA DIG | copA | Detection | CTGCGGTTGTCAGCGGTGAC |
| 630 copA h2 | copA | Helper | CATCATGTTATCGCCGATCATC |
| 678 cueO h1 | cueO | Helper | GGCAGCGTGTTGCGGGTA |
| 660 cueO BIO | cueO | Capture | GATTGCACCGTTGGTCAGC |
| 641 cueO DIG | cueO | Detection | AACGTATCGCCAAACCAGC |
| 622 cueO h2 | cueO | Helper | CCACGGCGGCGGTCATC |
| 266 cusA help1 | cusA | Helper | CCAAACTGTGAGAAACCGCG |
| 246 cusA BIO | cusA | Capture | CACAGTCTTCGCGCCAGGC |
| 227 cusA DIG | cusA | Detection | ACCGACAACATGGTGGTGG |
| 208 cusA help2 | cusA | Helper | TTAGCGGATAAGTCACCTGAT |
| 216 rpoZ H1 | rpoZ | Helper | CTGTTCCTGGCGTTCGCG |
| 195 rpoZ BIO | rpoZ | Capture | GTCGAGGATCTGGTTGTTGA |
| 174 rpoZ DIG | rpoZ | Detection | CAGACCTTCTTCGATTTCGC |
| 153 rpoZ h2 | rpoZ | Helper | CAGCGCGATTACAGTGGTTT |
| PCR primers | |||
| 165 copA T7 newD | copA | T7 promoter forward primer | CTAATACGACTCACTATAGGGAGATCACAGCCAGTCAAAACTGTC |
| 852 copA U | copA | Reverse primer | AAGATGTCGCGCTCCCATCGG |
| 106 cusA T7newD | cusA | T7 promoter forward primer | CTAATACGACTCACTATAGGGGGCCTGTTCCTGATTGATTCC |
| 449 cusA U | cusA | Reverse primer | TTACCGCTGCGATCCACCAGT |
| 113 cueO T7newD | cueO | T7 promoter forward primer | CTAATACGACTCACTATAGGGTGTGTCTGCGGCTTGACCTTC |
| 1352 cueO U | cueO | Reverse primer | TGCGTGCCGTGGATATGGAAC |
| 9 rpoZ T7 D | rpoZ | T7 promoter forward primer | GAAATTAATACGACTCACTATAGGGAACGTAACTGTTCAGGACGCTG |
| 270 rpoZ U | rpoZ | Reverse primer | ACGACGACCTTCAGCAATAG |
Underlining indicates the T7 polymerase promoter sequence.
Determination of RNA concentrations.
For the quantification of mRNAs in samples, in vitro-generated mRNA was always measured in parallel in triplicate (see below). In order to determine the concentration of the in vitro-generated mRNA, the Quant-IT RiboGreen RNA assay was performed according to the instructions of the assay kit manufacturer (Invitrogen, Karlsruhe, Germany). The kit was originally developed for cuvette-sized samples. Therefore, volumes were recalculated for use with 96-well plates. A calibration curve from dilutions of E. coli rRNA (100 mg/ml) as part of the kit was used as a standard. Fluorescence was measured in a microplate reader (Infinite M200; Tecan, Crailsheim, Germany) at an excitation λ of 480 nm and an emission λ of 525 nm. As a basis to calculate the concentrations of specific mRNAs, the number of transcripts was calculated from the relation of the OD600 to the cell number, where an OD600 of 1 was equal to 6 × 108 cells per ml (18). The molar concentrations of transcripts, based on the in vitro standards, could thus be directly calculated as the numbers of molecules per cell.
Probe design and labeling of oligonucleotides.
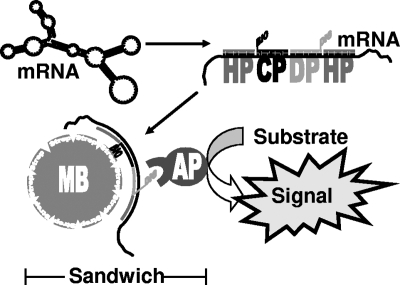
Probes (Table 1) were designed using the CloneManager5 program (Scientific & Educational Software, Cary, NC) followed by an NCBI BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/) (1) against the E. coli genome to exclude alignments with any other gene within E. coli, as described in reference 13. Probes hybridized to a continuous stretch within the target transcript and had a melting temperature of approximately 60°C, with a 1-nucleotide gap between each probe. A melting temperature of 60°C for the probes enables a subsequent hybridization step at 50°C, a temperature at which chromosomal DNA in its double-stranded form will be inaccessible to the probes. Figure 1 indicates the overall architecture of the probes with their labels and the order in which they hybridized to a target transcript.
FIG. 1.
Schematic representation of the SBSH assay. Four DNA oligonucleotide probes hybridizing to a target transcript are added to cleared cell lysate. The tertiary structure of the target mRNA is disrupted upon the binding of two helper probes (HP), a capture probe (CP), and a detection probe (DP). The capture probe is 5′ end labeled with biotin (BIO), allowing specific binding to streptavidin (Strep)-coated paramagnetic beads. After the beads are washed, only the captured target mRNA remains bound to the beads. Added anti-DIG Fab fragments conjugated to alkaline phosphatase (AP) bind to 3′-end-DIG-labeled detection probes, completing the sandwich of the paramagnetic bead (MB), mRNA, probes, and alkaline phosphatase. The amount of target mRNA can be calculated from the signal output generated by phosphatase activity and from a calibration curve for in vitro-synthesized target mRNA.
Helper probes remained unlabeled. The use of helper probes alongside capture and detection probes increases the hybridization efficiency in sandwich hybridization by 15- to 40-fold as demonstrated previously (2). Detection probes were generated by 3′ end labeling of oligonucleotides by using a second-generation DIG oligonucleotide 3′-end-labeling kit according to the instructions of the manufacturer (Roche, Mannheim, Germany). Labeled detection probes were resuspended in DEPC-treated water. Lyophilized 5′-end-biotin-labeled capture probes were purchased from a commercial manufacturer (Metabion, Martinsried, Germany). Labeled capture probes were resuspended in DEPC-treated water.
SBSH assay.
For transcript quantification, the original sandwich hybridization assay (13, 19) was modified and optimized for bacterial transcripts. The frozen cell pellet was resuspended in ice-cold TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.0) containing 10% stop solution and sonicated for 3 s at 60 W and 20 kHz with a Desintegrator GM 60 HD device (Uni Equip, Martinsried, Germany). The lysate was centrifuged for 3 min at 20,800 × g and 4°C. For each replicate (n = 3), 20 μl of the cleared supernatant was used for subsequent steps.
For hybridization buffer II, a master mix was generated. The master mix contained (per 90-μl sample) 86 μl of hybridization buffer I (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 20% [vol/vol] formamide, 3% [wt/vol] dextran sulfate, 0.2% [vol/vol] Tween 20, 0.02% [vol/vol] Ficoll 400, 0.02% [wt/vol] polyvinylpyrollidone, and 1% [vol/vol] blocking reagent [Roche, Mannheim, Germany] in 100 mM maleic acid with 150 mM NaCl [pH 7.5], all mixed in DEPC-treated water), 1 μl of a 1-pmol/μl concentration of each helper probe, 1 μl of a 0.05-pmol/μl concentration of a DIG-labeled detection probe, and 1 μl of a 5-pmol/μl concentration of a biotin-labeled capture probe. As a negative control, a preparation of hybridization buffer II containing a mismatched capture probe that would hybridize not to the target but to another transcript was set up.
A volume of 90 μl of hybridization buffer II was aliquoted into transparent 96-well plates with round bottoms (Greiner Bio-One, Frickenhausen, Germany). The plates were incubated for 5 min at 50°C with shaking at 900 rpm by a DTS-2 shaker (Neolab, Heidelberg, Germany), and 20-μl aliquots of clarified supernatant (two parallels) or in vitro-generated RNA for a calibration curve (three parallels) were added. As a positive control, a known amount of in vitro-generated mRNA can be mixed with clarified supernatant from a strain with a deletion of the target gene and the mixture can be added to hybridization buffer II.
A calibration curve was established by diluting in vitro-generated RNA at several concentrations in triplicate. The hybridization of the DNA probes with the mRNA was carried out for 30 min at 50°C with shaking (900 rpm).
The immobilization of RNA-DNA hybrids was accomplished by using streptavidin-coated magnetic beads (streptavidin MagnaSphere paramagnetic particles; Promega, Mannheim, Germany). Prior to use, the bead solution was transferred into a 1.5-ml plastic tube, the tube was fixed to a MagnaSphere magnetic separation stand, and the beads were washed three times with 5× SSC and resuspended in 5× SSC to the original bead volume. Aliquots of 15 μl of the washed-bead solution were added to the hybridization wells, and the RNA-DNA hybrids were immobilized for 30 min at 50°C with shaking (900 rpm). After the immobilization of the hybrids, the microwell plate was mounted onto a MagnaBot 96 magnetic separation device (Promega, Mannheim, Germany) and the magnetic beads were allowed to collect on the bottoms of the wells for 1 min. The supernatant was discarded by pipetting, and wash solution (1× SSC-0.01% sodium dodecyl sulfate in DEPC-treated water) was added. The beads were washed for 2 min at 50°C with shaking (900 rpm). Washing was repeated twice at 30°C, and the final supernatant was carefully removed.
For sandwich generation, 100 μl of a conjugate solution (1× SSC, 0.1% [vol/vol] Tween 20, 1:10,000 anti-DIG Fab fragment-alkaline phosphatase conjugate [Roche, Mannheim, Germany]) was added to each well and the plate was incubated for 30 min at 30°C with shaking (900 rpm). The samples were washed three times with 120 μl of wash buffer at 30°C each time by using the MagnaBot, and the final supernatants were carefully removed.
For signal detection, 100 μl of the substrate BBTP [2′-(2-benzothiazolyl)-6′-hydroxybenzothiazole phosphate; AttoPhos] from the AttoPhosAP fluorescent substrate system (Promega, Mannheim, Germany) was added to each well and the plate was incubated for 20 min at 37°C with shaking (900 rpm). For the complete resuspension of the magnetic beads, shaking was started at 1,300 rpm for about 5 s. For the separation of the magnetic beads, the well plate was mounted onto the MagnaBot device for 1 min. A volume of 90 μl of the reaction mixture was transferred into new black flat-bottom microwell plates (Nunc, Wiesbaden, Germany). Substrate turnover to inorganic phosphate (Pi) and the fluorescent product BBT [2′-(2-benzothiazolyl)-6′-hydroxybenzothiazole] (21) was measured as a fluorescence signal (excitation λ, 430 nm; emission λ, 560 nm) in a microplate reader (Infinite M200; Tecan, Crailsheim, Germany). The concentrations of specific transcripts were calculated from a calibration curve derived from in vitro-generated target mRNA as described above.
RNA isolation and RT-PCR.
Total RNA from frozen cell pellets was extracted with the NucleoSpin RNA/protein kit by following the protocol of the manufacturer (Macherey-Nagel, Düren, Germany) for bacteria but omitting incubation with lysozyme to avoid RNA degradation. The synthesis of cDNA was performed with SuperScript II reverse transcriptase (Invitrogen, Karlsruhe, Germany) and gene-specific primers 630 copA h2 and 153 rpoZ h2. The reaction mix contained the following: 1.5 μg of RNA, 2 pmol of primers, 2.5 mM (each) deoxynucleoside triphosphates, and 1 μl of polymerase in a 10-μl total volume. RT was carried out for 50 min at 42°C. PCR amplification of cDNAs was done with primers 165 copA T7 newD and 630 copA h2 for copA as well as with 9 rpoZ T7 D and 153 rpoZ h2 for rpoZ in a single reaction with 30 cycles. The PCR mix contained 1 μl of cDNA (diluted 1:10), 10 pmol of primers, 2.5 mM (each) deoxynucleoside triphosphates, and 1 μl of Taq polymerase (1 U/μl; Roche, Mannheim, Germany) in a total volume of 50 μl. PCR products were run on a 2% agarose gel and visualized using ethidium bromide. RNA isolation from independent cultures and RT were performed twice on different days, with similar results.
RESULTS AND DISCUSSION
Sandwich hybridization methods have incorporated several major improvements within the last couple of years. The addition of helper probes facilitates the hybridization of capture and detection probes to target RNAs, resulting in greater sensitivity of the assay (2). This higher degree of sensitivity is probably owed to the helpers' assistance in opening up the secondary structure around the target where the probe is to bind. Additionally, there is an effect of base stacking between two adjacent terminal nucleotides from two oligonucleotides hybridizing next to each other (2). The shift away from bead-based sandwich hybridization toward SBSH has resulted in greater sensitivity of the assay and has facilitated liquid handling (17, 19). Here, we present a next level in the improvement of the SBSH method, demonstrating the usefulness of this approach in direct quantification of low-population mRNA transcripts in crude cell extract samples without prior RNA separation (Fig. 1). The rationale behind this strategy is the property of a single-stranded oligonucleotide of hybridizing to complementary single-stranded mRNA but not to the double-stranded DNA of the bacterial chromosome. This pattern makes direct measurements of mRNA transcripts possible even in the presence of double-stranded DNA and a large amount of other cell material.
The addition of a biotin-labeled capture probe, a DIG-labeled detection probe, and two helper probes results in extensive DNA-RNA heteroduplexes, in which all four probes hybridize to a continuous stretch of the target mRNA (Fig. 1). In the SBSH assay, the number of transcripts of a given specific mRNA species is proportional to an enzymatically generated output signal. Alkaline phosphatase and the substrate BBTP were used in this study. BBTP is cleaved into Pi and the fluorescent product BBT (21).
Previously, SBSH was used to quantify a single mRNA from Saccharomyces cerevisiae. However, this SUC2 mRNA is highly abundant in S. cerevisiae. The hybridization assay was not very sensitive and had only a low degree of robustness (8, 11, 14). Major challenges to address before the usefulness of SBSH could be extended to low-abundance transcripts were high background levels of fluorescence in the fluorescence substrate reaction and the aggregation of the beads. Several cell lysis methods (sonication, the use of a French press or bead mill, and chemical and enzymatic cell lysis) were compared. Sonication was found to be superior to the others, because of its ease and handling speed. A short sonication pulse was sufficient to disrupt the cells. Disruption efficiency was high, as sonicated cell lysates failed to yield any CFU after plating (data not shown).
High concentrations of detergent as in the original assay (8, 11, 14) were found not only to hamper sonication but also to lead to clumping of the magnetic beads. Lower detergent concentrations in buffers prevented bead aggregation and also facilitated the resuspension of the beads and diminished sticking of the beads to plastic surfaces during the assay. By solving the aggregation problem, the low signal-to-background ratio was also successfully countered, enabling the detection of low-abundance transcript species. Finally, the addition of blocking reagent strongly reduced the background fluorescence levels to very low values. With a combination of these improvements, a detection limit of below 1 fmol of RNA per well for crude extracts was reached, corresponding to approximately 3 to 5 transcripts/cell.
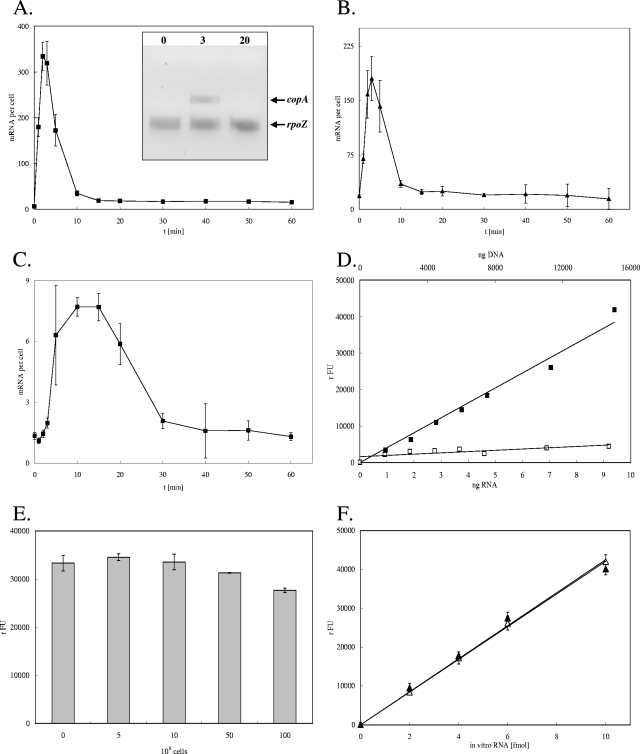
Previously, it was demonstrated that the transcription of all three copper resistance systems of E. coli responds specifically to copper and silver ions. However, the majority of earlier studies investigated concentration-dependent responses only (4, 12, 16, 25). In a single study, S1 nuclease mapping with purified total RNA was used to monitor copper-dependent gene expression 0, 5, 10, and 30 min after copper exposure (26). However, with SBSH, a time course profile of copA, cueO, and cusA expression can be plotted. Thus, it is easily possible to follow changes in expression patterns mediated by copper stress dynamically (Fig. 2). The expression of copA and cueO responded very rapidly to the addition of 1 mM CuCl2. Within 1 min, a 28- or 4-fold induction of copA or cueO expression, respectively, was observed, and after 2 to 3 min, already a maximum induction of 53- or 10-fold was obtained. The quantity of copA transcripts was calculated to be 334/cell, and that of cueO transcripts was calculated to be 180/cell. Soon after the maximum induction, the quantities of copA and cueO transcripts declined. After only 15 min, a transcription level approximately the same as that before induction was reached (Fig. 2 A and B). Additional RT-PCR analysis of copA expression from purified and reverse-transcribed RNA at 0, 3, and 20 min confirmed that a rapid increase in transcript numbers occurred before a preinduction level was reestablished (Fig. 2 A, inset). The expression of cus commenced somewhat more slowly than that of cueO or copA. After 3 min, only a mild induction was observed. Induction progressed rapidly from that point on before reaching the maximum (sixfold induction, yielding 8 transcript copies/cell) after 15 min. A stable low level of cus expression, approximately the same as that in the prestress situation, was established after 30 min (Fig. 2C).
FIG. 2.
Time-dependent profile of the induction of copper homeostasis genes of E. coli by copper. (A to C) E. coli strain W3110 grown aerobically to mid-log phase was challenged with 1 mM CuCl2 (at time [t] zero), and the concentrations of mRNA transcripts of the genes copA (A), cueO (B), and cusA (C) (expressed as signal-giving units per cell) in samples at several time points were determined by SBSH. For comparison, a gel-based RT-PCR analysis of copA transcripts and those of the copper-independent housekeeping gene rpoZ at 0, 3, and 20 min after copper challenge is presented (inset in panel A). (D) As controls, calibration curves from in vitro-generated copA mRNA mixed with copA DNA (PCR product) (▪) or DNA alone (□) were generated. The signal strength is indicated as relative fluorescence units (rFU). (E and F) As additional controls, crude extracts derived from increasing numbers of cells with the deletion of cueO as the target gene, all assayed with 8 fmol of in vitro-generated cueO mRNA and cueO probes (gray columns) (E), and crude extracts of cells with the deletion of cueO (▴) or buffer (▵) spiked with increasing concentrations of in vitro-generated cueO transcripts (F) were also investigated. Shown are averages with standard deviations (error bars) of results for at least three independent cultures with three SBSH replicates each.
The differences in the induction of the cue regulon (copA and cueO) and the cus operon are probably due to their different regulation modes. Apo-CueR is a repressor protein binding to the operator regions upstream of cueO and copA (25). As soon as copper is bound, CueR turns into an activator and gene expression can immediately commence. Therefore, the observed increase in transcripts equals the rate of biosynthesis. In contrast, cus is regulated by a two-component system (5, 12). Upon sensing periplasmic copper through its sensor domain, the histidine kinase CusS must first autophosphorylate and then the histidine-bound phosphoryl group from P-CusS must be passed to the conserved aspartate residue in the receiver domain of the response regulator CusR. Only then, P-CusR diffuses and binds to a sequence upstream of the cusCFBA genes, allowing their expression. The slightly delayed response observed (Fig. 2C) most probably reflects the multiple steps of the sophisticated cus system. Alternatively, different copper concentrations within the compartments of the cell after the onset of copper stress reflect the different expression patterns. The cytoplasm may be the compartment that fills first with copper until a critical level is exceeded, leading to the expression of copA and cueO. Then the metal is relocated to the periplasm by CopA, and CueO oxidizes Cu(I). Finally, cus is expressed when the copper level in the periplasm has begun to exceed some critical periplasmic threshold.
Overall, the results suggested that the response to copper stress was fast and vigorous but that within minutes, further transcription initiation quickly diminished. Probably, a new stable balance of the cytoplasmic and periplasmic copper concentrations for which the number of copper detoxification systems was then sufficient was reached. Additionally, the rapid decline in transcript numbers after the initial copper shock suggested that mRNAs of copper detoxification systems are rather unstable. This observation strongly correlates with values reported in the literature for the average half-life of bacterial transcripts of about 6.8 min (23).
As a control, the SBSH assay was performed with increasing concentrations of a nontranscribed copA PCR product (Fig. 2D). This procedure demonstrated that no significant signal was generated with DNA but that a proportional signal increase was observed with in vitro-synthesized copA transcripts (Fig. 2D). Further control experiments using increasing numbers of cells with the deletion of an SBSH assay target gene, copA, cueO, or cusA, indicated that other cellular components interfered with the assay only when cell numbers above those for the standard assay were used (Fig. 2E; data for cueO are shown). Moreover, spiking crude cell extracts (from a strain with the deletion of a target gene) with target mRNA gave results very similar to those obtained when the same amount of target mRNA in a buffer was measured (Fig. 2F). The results presented in Fig. 2 are in good agreement with the results of S1 nuclease mapping with purified total RNA to monitor copper-dependent gene expression (26).
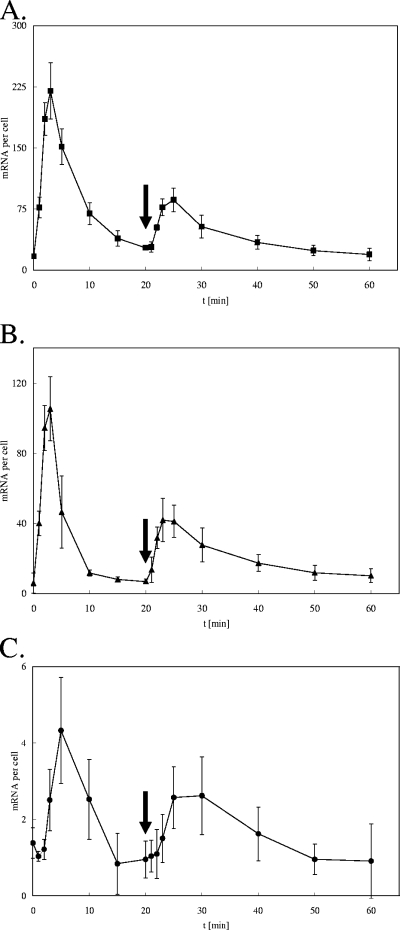
To investigate the response of previously preadapted cells to high-copper stress, the above-described experiment was repeated with an initial stress of only 0.1 mM CuCl2 for 20 min to provide time for the expression of the copper resistance systems. As expected, the maximum transcription levels were lower than those at 1 mM CuCl2 while the overall expression pattern was very similar (Fig. 3). At 20 min, a second, strong stress pulse was provided by the addition of 1 mM CuCl2. The preadapted cells responded with a much smaller increase in transcription than that in naïve cultures when 1 mM CuCl2 was added (Fig. 2), probably because the copper defense proteins persisted and less de novo synthesis was required for the response to the second challenge. Under these conditions, however, cells needed a longer time to adapt to the copper stress and the transcript concentration decreased again. Vice versa, when cells were first shocked with 1 mM CuCl2 and after 20 min with 0.1 mM CuCl2, no additional expression maximum was observed and expression patterns were very similar to those after a 1 mM CuCl2 shock only (data not shown).
FIG. 3.
Induction profile of copper homeostasis genes upon repeated copper challenge. E. coli strain W3110 grown aerobically to mid-log phase was challenged with 0.1 mM CuCl2 (at time [t] zero) and again with 1 mM CuCl2 (at 20 min; arrows). The concentrations of mRNA transcripts of the genes copA (A), cueO (B), and cusA (C) (expressed as signal-giving units per cell) in samples at several time points were determined by SBSH. Shown are averages with standard deviations (error bars) of results for at least three independent cultures with three SBSH replicates each.
Previously, it was observed that during anaerobiosis cells contain more copper than they do when oxygen is present (15). Copper stress dose-response experiments yielded different expression patterns for cus, cueO, and copA under aerobic and anaerobic conditions (15). Under anaerobic conditions, Cu(I) is the predominant species of copper cation and Cu(I) is more toxic than Cu(II) (3, 15). Consequently, the expression of cus, cueO, and copA during anaerobiosis was different from that during aerobiosis. Under anaerobic conditions, maximum cus expression was reached at lower copper concentrations than those required under aerobic conditions and maximum cueO expression was reached at higher copper concentrations, while copA expression was largely unaltered (15).
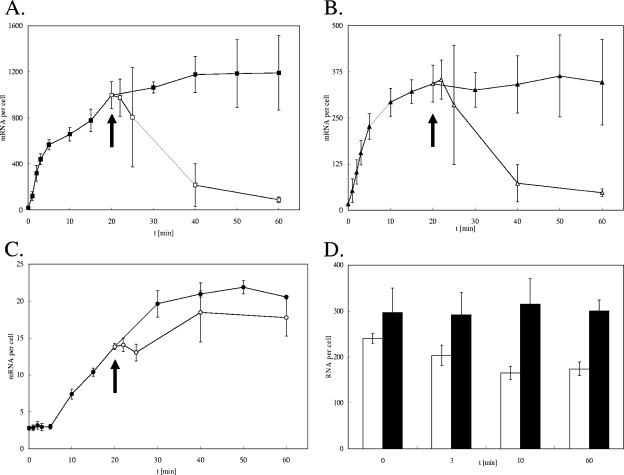
Time course experiments for cus, cueO, and copA expression under anaerobic conditions resulted in expression patterns completely different from those observed when oxygen was present (Fig. 4). The expression of all three target genes increased continuously and reached a maximum after 40 min (copA) (Fig. 4A), 20 min (cueO) (Fig. 4B), or 50 min (cus) (Fig. 4C). The transcription of copA and cueO started immediately after copper addition, whereas that of cus was a few minutes delayed, similar to what was observed in the presence of oxygen (Fig. 2 and 3). In stark contrast to the results under aerobic conditions, no decline in the transcription of any copper homeostasis gene within the time course of 60 min was observed. This finding may reflect the different toxicities of Cu(I) and Cu(II) for the cell. It was suggested earlier that copper as Cu(I) enters the cytoplasm nonspecifically by way of sodium or potassium channels (15). Probably, this mechanism leads to an extension of the time compared to that for Cu(II) before a new stable copper concentration within the cell is established. This pattern also indicates that the steady-state cytoplasmic copper concentration during anaerobiosis is higher than that during aerobiosis.
FIG. 4.
Profile of the expression of copper homeostasis genes in response to copper under anaerobic growth conditions and upon reoxygenation. E. coli strain W3110 grown anaerobically to mid-log phase in Hungate tubes was challenged with 1 mM CuCl2 (at time [t] zero). Cultures were transferred into Erlenmeyer flasks (at 20 min; arrows) and shaken aerobically. (A, B, and C) The concentrations of mRNA transcripts of the genes copA (A), cueO (B), and cusA (C) (expressed as signal-giving units per cell) in samples at several time points before and after the aerobic shift (arrows) were determined by SBSH. (D) As a control, an analysis of the expression of a copper-independent housekeeping gene, rpoZ, under aerobic (black columns) and anaerobic (white columns) conditions was also performed. Shown are averages with standard deviations (error bars) of results for at least three independent cultures with three SBSH replicates each.
To explore the possibility that Cu(I) cannot be successfully prevented from entering the cell, copper homeostasis gene expression following a shift from anaerobic to aerobic growth conditions was investigated. As expected, when cultures were reoxygenated after 20 min of copper exposure under anaerobic conditions, the expression of copA and cueO decreased by 90 and 87%, respectively, within the next 40 min (Fig. 4A and B). In contrast, the expression of cusA continued at a level similar to that under anaerobic growth conditions and showed only a mild but fast 14% decrease (Fig. 4C). This result may be best explained if the mechanisms of the respective regulators of the independent cue and cus systems are considered. CueR regulates copA and cueO expression in response to changes in cytoplasmic copper concentrations (16, 25). The expression of cus is governed by a sensor within CusS that is most likely located on the periplasmic side of the cytoplasmic membrane (12). In our study, the transcription of cueO responded immediately to a shift to aerobiosis by downregulation. This finding suggests that the copper concentration within the cytoplasm had reached a nontoxic level through the action of CopA, making further expression of the cue operon dispensable.
As a control to monitor the degree to which copper stress altered general gene expression under the conditions tested, the expression of a constitutively expressed housekeeping gene, rpoZ, was also investigated. Figure 4D clearly shows that the expression of rpoZ remained relatively constant upon aerobic copper stress. Under anaerobic conditions, a slight decrease in rpoZ transcript abundance during the first 10 min after copper shock was observed. This result may reflect the higher toxicity of copper during anaerobiosis than during aerobiosis, which is also corroborated by the overall increase in the expression of all copper detoxification systems. Both copA and cueO, as well as cus to a lower degree, were well-expressed after the first 10 min post-copper stress.
For a deeper understanding of copper toxicity in E. coli, the differences in the gene induction patterns in aerobiosis and anaerobiosis should be further addressed in future studies. It would be especially interesting to investigate the molecular background of the downregulation of copA and cueO in the presence of oxygen but not under anaerobic copper stress conditions.
Acknowledgments
This work was supported by the DFG graduate college “Stress” program and European Union structural funds within the “Bioworld I” project.
Footnotes
Published ahead of print on 24 October 2008.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Barken, K. B., M. Gabig-Ciminska, A. Holmgren, and S. Molin. 2004. Effect of unlabeled helper probes on detection of an RNA target by bead-based sandwich hybridization. BioTechniques 36:124-132. [DOI] [PubMed] [Google Scholar]
- 3.Beswick, P. H., G. H. Hall, A. J. Hook, K. Little, D. C. McBrien, and K. A. Lott. 1976. Copper toxicity: evidence for the conversion of cupric to cuprous copper in vivo under anaerobic conditions. Chem.-Biol. Interact. 14:347-356. [DOI] [PubMed] [Google Scholar]
- 4.Franke, S., G. Grass, and D. H. Nies. 2001. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology 147:965-972. [DOI] [PubMed] [Google Scholar]
- 5.Franke, S., G. Grass, C. Rensing, and D. H. Nies. 2003. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J. Bacteriol. 185:3804-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grass, G., and C. Rensing. 2001. Genes involved in copper homeostasis in Escherichia coli. J. Bacteriol. 183:2145-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Huhtamella, S., M. Leinonen, T. Nieminen, B. Fahnert, L. Myllykoski, A. Breitenstein, and P. Neubauer. 2007. RNA-based sandwich hybridisation method for detection of lactic acid bacteria in brewery samples. J. Microbiol. Methods. 68:543-553. [DOI] [PubMed] [Google Scholar]
- 9.Kennell, D. 1968. Titration of the gene sites on DNA by DNA-RNA hybridization. II. The Escherichia coli chromosome. J. Mol. Biol. 34:85-103. [DOI] [PubMed] [Google Scholar]
- 10.Reference deleted.
- 11.Leskelä, T., A. Tilsala-Timisjärvi, J. Kusnetsov, P. Neubauer, and A. Breitenstein. 2005. Sensitive genus-specific detection of Legionella by a 16S rRNA based sandwich hybridization assay. J. Microbiol. Methods 62:167-179. [DOI] [PubMed] [Google Scholar]
- 12.Munson, G. P., D. L. Lam, F. W. Outten, and T. V. O'Halloran. 2000. Identification of a copper-responsive two-component system on the chromosome of Escherichia coli K-12. J. Bacteriol. 182:5864-5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neubauer, A., J. Soini, M. Bollok, M. Zenker, J. Sandqvist, J. Myllyharju, and P. Neubauer. 2007. Fermentation process for tetrameric human collagen prolyl 4-hydroxylase in Escherichia coli: improvement by gene optimisation of the PDI/beta subunit and repeated addition of the inducer anhydrotetracycline. J. Biotechnol. 128:308-321. [DOI] [PubMed] [Google Scholar]
- 14.Nieminen, T., J. Pakarinen, I. Tsitko, M. Salkinoja-Salonen, A. Breitenstein, T. Ali-Vehmas, and P. Neubauer. 2006. 16S rRNA targeted sandwich hybridization method for direct quantification of mycobacteria in soils. J. Microbiol. Methods 67:44-55. [DOI] [PubMed] [Google Scholar]
- 15.Outten, F. W., D. L. Huffman, J. A. Hale, and T. V. O'Halloran. 2001. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 276:30670-30677. [DOI] [PubMed] [Google Scholar]
- 16.Outten, F. W., C. E. Outten, J. Hale, and T. V. O'Halloran. 2000. Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, cueR. J. Biol. Chem. 275:31024-31029. [DOI] [PubMed] [Google Scholar]
- 17.Pioch, D., B. Jürgen, S. Evers, K. H. Maurer, M. Hecker, and T. Schweder. 2008. Improved sandwich-hybridization assay for an electrical DNA-chip-based monitoring of bioprocess-relevant marker genes. Appl. Microbiol. Biotechnol. 78:719-728. [DOI] [PubMed] [Google Scholar]
- 18.Piovant, M., and C. Lazdunski. 1975. Different cyclic adenosine 3′,5′-monophosphate requirements for induction of beta-galactosidase and tryptophanase. Effect of osmotic pressure on intracellular cyclic adenosine 3,5-monophosphate concentrations. Biochemistry 14:1821-1825. [DOI] [PubMed] [Google Scholar]
- 19.Rautio, J., K. B. Barken, J. Lahdenperä, A. Breitenstein, S. Molin, and P. Neubauer. 2003. Sandwich hybridisation assay for quantitative detection of yeast RNAs in crude cell lysates. Microb. Cell Fact. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rensing, C., B. Fan, R. Sharma, B. Mitra, and B. P. Rosen. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. USA 97:652-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resina, D., M. Bollok, N. K. Khatri, F. Valero, P. Neubauer, and P. Ferrer. 2007. Transcriptional response of P. pastoris in fed-batch cultivations to Rhizopus oryzae lipase production reveals UPR induction. Microb. Cell Fact. 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruottinen, M., M. Bollok, M. Kögler, A. Neubauer, M. Krause, E. R. Hämäläinen, J. Myllyharju, A. Vasala, and P. Neubauer. 2008. Improved production of human type II procollagen in the yeast Pichia pastoris in shake flasks by a wireless-controlled fed-batch system. BMC Biotechnol. 8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selinger, D. W., R. M. Saxena, K. J. Cheung, G. M. Church, and C. Rosenow. 2003. Global RNA half-life analysis in Escherichia coli reveals positional patterns of transcript degradation. Genome Res. 13:216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh, S. K., G. Grass, C. Rensing, and W. R. Montfort. 2004. Cuprous oxidase activity of CueO from Escherichia coli. J. Bacteriol. 186:7815-7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoyanov, J. V., J. L. Hobman, and N. L. Brown. 2001. CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Mol. Microbiol. 39:502-511. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto, K., and A. Ishihama. 2005. Transcriptional response of Escherichia coli to external copper. Mol. Microbiol. 56:215-227. [DOI] [PubMed] [Google Scholar]