Abstract
Disulfide bonds are important for the correct folding, structural integrity, and activity of many biotechnologically relevant proteins. For synthesis and subsequent secretion of these proteins in bacteria, such as the well-known “cell factory” Bacillus subtilis, it is often the correct formation of disulfide bonds that is the greatest bottleneck. Degradation of inefficiently or incorrectly oxidized proteins and the requirement for costly and time-consuming reduction and oxidation steps in the downstream processing of the proteins still are major limitations for full exploitation of B. subtilis for biopharmaceutical production. Therefore, the present study was aimed at developing a novel in vivo strategy for improved production of secreted disulfide-bond-containing proteins. Three approaches were tested: depletion of the major cytoplasmic reductase TrxA; introduction of the heterologous oxidase DsbA from Staphylococcus carnosus; and addition of redox-active compounds to the growth medium. As shown using the disulfide-bond-containing molecule Escherichia coli PhoA as a model protein, combined use of these three approaches resulted in secretion of amounts of active PhoA that were ∼3.5-fold larger than the amounts secreted by the parental strain B. subtilis 168. Our findings indicate that Bacillus strains with improved oxidizing properties can be engineered for biotechnological production of heterologous high-value proteins containing disulfide bonds.
Disulfide bonds play pivotal roles in the folding, structural integrity, and activity of numerous proteins found in nature. Without the correct thiol oxidation that links their cysteines to disulfide bonds, these proteins are neither fully stable nor active (6, 32). Importantly, many eukaryotic proteins of biopharmaceutical interest contain multiple disulfide bonds. As the demand for such proteins is growing, there is a clear need for cost-effective and high-quality production platforms. Bacterial cell factories, such as Bacillus subtilis, can fulfill these criteria very well, but so far their use in biopharmaceutical production has been limited by their relatively poor performance in the production of proteins with disulfide bonds (5, 33, 47).
The formation of disulfide bonds can occur spontaneously, but this process is slow and nonspecific (2). For this reason, so-called thiol-disulfide oxidoreductases (TDORs) that catalyze the formation (oxidation) of disulfide bonds in vivo have evolved. Notably, the TDORs also include enzymes that break (reduce) or isomerize disulfide bonds. Cytoplasmic TDORs generally function as reductases, while the extracytoplasmic equivalents are oxidases or isomerases (9, 32, 39). The enzyme-dependent formation of disulfide bonds is a prime reason why proteins containing such bonds are still difficult to produce in bacterial cell factories. Slow and/or nonspecific oxidation of overproduced proteins often results in slow and incorrect folding of these proteins, making them vulnerable to proteolytic degradation (33). For this reason, we addressed the question of how to increase the oxidative power of Bacillus species during protein production in order to produce disulfide-bond-containing proteins more efficiently.
The gram-positive bacterium B. subtilis is a preferred organism for secretory protein production, because proteins transported across the cytoplasmic membrane are directly released into the growth medium (42, 43). Other advantages of B. subtilis are its high genetic amenability and the fact that it is generally recognized as safe (5, 16, 21, 50). Previous studies on disulfide bond formation in B. subtilis have shown that this organism contains at least four TDORs with presumed oxidase activity. These proteins were designated Bdb (bacillus disulfide bond) proteins and annotated BdbA to BdbD (7, 8, 25). Especially BdbC and BdbD have major roles in the folding of a secreted heterologous model protein by B. subtilis, the PhoA alkaline phosphatase of Escherichia coli (4, 7, 18, 25). This is related to the fact that E. coli PhoA contains two disulfide bonds that are indispensable for both the enzymatic activity and stability of the protein (38). BdbC and BdbD seem to cooperate as a redox pair in an oxidation pathway in B. subtilis (33), similar to the DsbA-DsbB redox pair of E. coli (15, 30, 31). It has been proposed that BdbD is the major oxidase that facilitates the formation of disulfide bonds in secretory proteins. Upon oxidation of a substrate, reduced BdbD is reoxidized by the quinone reductase homologue BdbC. To become reoxidized for the next catalytic reaction, BdbC donates its electrons to quinones in the electron transport chain.
Despite the presence of BdbC and BdbD, the total oxidative power of B. subtilis is rather limited (33). To increase the thiol-oxidizing capacity, attempts to overexpress individual Bdb proteins or combinations of several Bdb proteins were made. However, this did not result in significantly improved production of proteins with disulfide bonds (7, 8, 25; our unpublished observations). Therefore, in the present study we searched for alternative strategies that could increase the thiol-oxidizing power of B. subtilis. Decreasing the levels of a cytoplasmic TDOR with a reductase, TrxA, resulted in increased yields of secreted E. coli PhoA. The yields of this protein could be further improved by introduction of staphylococcal DsbA, which is known to be one of the strongest bacterial thiol oxidases (10). Additional improvement was obtained by including redox-active compounds in the growth medium of DsbA-producing strains. Together, our observations provide proof of principle that Bacillus strains with optimized oxidative properties can be engineered for the production proteins with disulfide bonds.
MATERIALS AND METHODS
Sequence comparisons and predictions.
Thioredoxin amino acid sequences of B. subtilis or E. coli were used for a BLASTP search of the SubtiList B. subtilis sequence database (http://genolist.pasteur.fr/SubtiList/) with the algorithms described by Altschul et al. (1). An arbitrary E value less than or equal to 10−3 was used to limit the number of sequences for further analysis. After the first run, using either the B. subtilis TrxA or E. coli TrxA or TrxC sequence as the query sequence, the sequences found were used in turn as query sequences for a BLASTP search of the SubtiList database. Multiple alignments were constructed using ClustalX 1.81 (41). Various protein weight matrices were used with pairwise and multiple alignment parameters. The best alignment, defined by the lowest score, was obtained with the PAM350 matrix (gap opening, 10) for both pairwise (gap extension, 0.1) and multiple (gap extension, 0.2) alignment parameters. The presence of possible signal peptidase I cleavage sites was analyzed using the algorithms described by Nielsen et al. (28). The presence of possible transmembrane segments was analyzed using the algorithms described by Krogh et al. (20).
Bacterial strains, plasmids, and growth media.
Bacterial strains and plasmids used in this study are listed in Table 1. Strains were grown with agitation at 37°C in Luria-Bertani (LB) medium consisting of 1% tryptone, 0.5% yeast extract, and 1% NaCl (pH 7.4). When necessary, media were supplemented with antibiotics at the following concentrations: ampicillin, 100 μg/ml (E. coli); chloramphenicol, 5 μg/ml (B. subtilis); erythromycin, 50 μg/ml (E. coli) or 2 μg/ml (B. subtilis); kanamycin, 20 μg/ml (E. coli and B. subtilis); spectinomycin, 100 μg/ml (E. coli and B. subtilis); and tetracycline, 10 μg/ml (B. subtilis). To visualize α-amylase activity (encoded by the amyE gene), LB medium plates were supplemented with 1% starch. When required, cystine or cysteine was added to the growth media at a concentration of 100 μg/ml.
TABLE 1.
Plasmids and strains used in this study
| Plasmid or strain | Relevant properties | Reference or source |
|---|---|---|
| Plasmids | ||
| pMutin2mcs | pBR322-based integration vector for B. subtilis; contains a multiple cloning site downstream of the Pspac promoter and a promoterless lacZ gene; Apr Emr | 44 |
| pUC18 | Apr | 45 |
| pDG1727 | pMTL23 derivative; Apr Spr | 12 |
| pTOPO | pCR-Blunt II-TOPO vector; Kmr | Invitrogen Life Technologies |
| pXTC | pX derivative containing a tetracycline resistance marker instead of a chloramphenicol resistance marker; Apr Tcr | 7 |
| pXTC-ScdsbA | pXTC carrying dsbA of S. carnosus fused to the signal sequence and ribosome binding site of mntA of B. subtilis under transcriptional control of the xylA promoter; Apr Tcr | This study |
| pXTC-SadsbA | pXTC carrying dsbA of S. aureus fused to the signal sequence and ribosome binding site of mntA of B. subtilis under transcriptional control of the xylA promoter; Apr Tcr | 18 |
| pPSPhoA5 | Plasmid carrying the E. coli phoA gene fused to the pre-pro region of the lipase gene from S. hyicus; Cmr | 7 |
| pKTH10 | Encodes AmyQ of B. amyloliquefaciens; Kmr | 29 |
| B. subtilis strains | ||
| 168 | trpC2 | 21 |
| ItrxA | Derivative of 168; contains an integrated copy of plasmid pMutin2 in the trxA gene; trxA-lacZ Emr Pspac-trxA; IPTG dependent | 34 |
| ybdE | Derivative of 168; contains an integrated copy of plasmid pMutin2 in the ybdE gene; ybdE-lacZ | 16 |
| ydfQ | Derivative of 168; contains an integrated copy of plasmid pMutin2 in the ydfQ gene; ydfQ-lacZ | 16 |
| ydbP | Derivative of 168; contains an integrated copy of plasmid pMutin2 in the ydbP gene; ydbP-lacZ | 16 |
| ykuV | trpC2 ykuV::Spr (MunI-NgoMIV integration) | This study |
| ytpP | trpC2 ytpP::Spr (BsaAI integration) | This study |
| yusE | trpC2 yusE::Spr (BclI-BclI integration) | This study |
| stoA (ykvV) | Derivative of 168; contains an integrated copy of plasmid pMutin2 in the stoA gene; stoA-lacZ | 16 |
| yneN | Derivative of 168; contains an integrated copy of plasmid pMutin2 in the yneN gene; yneN-lacZ | 16 |
| SPβ | trpC2; ΔSPβ; sublancin 168 sensitive; laboratory name, CBB312 | 8 |
| bdbC | trpC2 bdbC::Kmr | 8 |
| ItrxA bdbC | trpC2 trxA::pMutin2 bdbC::Kmr; IPTG dependent | This study |
| X-ScdsbA | trpC2; amyE::XTC-ScdsbA | This study |
| ItrxA X-ScdsbA | trpC2; Pspac-trxA; amyE::XTC-ScdsbA | This study |
| X-SadsbA | trpC2; amyE::XTC-SadsbA; also known as XdsbA | 18 |
| ItrxA X-SadsbA | trpC2; Pspac-trxA; amyE::XTC-SadsbA | This study |
| S. carnosus TM300 | Source of dsbA gene used in the present study | 35 |
| S. aureus RN4220 | Restriction-deficient derivative of NCTC 8325, cured of all known prophages | 19 |
| E. coli DH5α | supE44 hsdR17 recA1 gyrA96 thi-1 relA1 | 13 |
Construction of mutant strains.
DNA techniques were performed as previously described (18). All TDOR mutant strains were based on B. subtilis 168. Strain ItrxA bdbC was obtained by transformation of B. subtilis ItrxA with chromosomal DNA of a bdbC::Kmr mutant and subsequent selection of isopropyl-β-d-thiogalactopyranoside (IPTG)-dependent and kanamycin-resistant transformants. It should be noted that trxA is an essential gene (34). TrxA can be depleted in ItrxA mutant strains because the trxA gene of these strains is under transcriptional control of the IPTG-inducible Pspac promoter (37).
The B. subtilis yusE strain was constructed as follows. A 1,384-bp DNA fragment starting in the yusG gene and ending in the yusD gene was amplified using primers GGGGAATTCATAAGACAGCCGATGTGGTC and GGGGGATCCGTAGAATAGCTCGGCGAATG, which contain EcoRI and BamHI restriction sites, respectively. This fragment was subsequently cleaved with EcoRI and BamHI and ligated to EcoRI-BamHI-cleaved pUC18. The spectinomycin resistance cassette from plasmid pDG1727 was excised with BamHI and XhoII and used to replace an internal BclI fragment of the pUC18-borne copy of yusE. The resulting plasmid, pUSE-Spec, was used to transform B. subtilis 168. As shown by PCR, the yusE gene of all spectinomycin-resistant transformants tested (B. subtilis yusE) was disrupted with the spectinomycin resistance cassette of pUSE-Spec as a result of a double-crossover recombination event.
To construct the B. subtilis ykuV strain, a 1,516-bp DNA fragment starting in the ykuU gene and ending in the rok gene was amplified using primers GGGGGATCCCGGCAAAGTAAGTCTTGAGG and GGGGTCGACATTGTTCTAACCGCAAGCGC, which contain BamHI and SalI restriction sites, respectively. The amplified fragment was cleaved with BamHI and SalI and ligated to BamHI-SalI-cleaved pUC18. The spectinomycin resistance cassette from pDG1727 was excised using EcoRI and BsaWI and used to replace an internal MunI-NgoMIV fragment of the pUC18-borne copy of ykuV. The resulting plasmid, pKUV-Spec, was used to transform B. subtilis 168. As shown by PCR, the ykuV gene of all spectinomycin-resistant transformants tested (B. subtilis ykuV) was disrupted with the spectinomycin resistance cassette of pKUV-Spec as a result of a double-crossover recombination event.
The B. subtilis ytpP strain was constructed as follows. A 1,386-bp DNA fragment starting in the ytoP gene and ending in the ytpQ gene was amplified using primers GGGGGTACCCATTGCCGTGTTCCACTGTT and GGGCTGCAGGGCAACCGTATCCTCTTTGA, which contain KpnI and PstI restriction sites, respectively. The amplified fragment was cleaved with KpnI and PstI and ligated to KpnI-PstI-cleaved pUC18. The spectinomycin resistance cassette from pDG1727 was excised with HincII and StuI and cloned in the unique BsaAI restriction site in the middle of the ytpP gene. The resulting plasmid, pTPP-Spec, was used to transform B. subtilis 168. As shown by PCR, the ytpP gene of all spectinomycin-resistant transformants tested (B. subtilis ytpP) was disrupted with the spectinomycin resistance cassette of pTPP-Spec as a result of a double-crossover recombination event.
Single ybdE, ydbP, ydfQ, stoA (ykvV), yneN, and yusE mutants and a strain that lacks the SPβ prophage carrying the bdbA and yosR genes were obtained from the BSFA or JAFAN strain collections (16). The correct chromosomal integration of plasmids or antibiotic resistance markers was verified by PCR.
To overexpress the oxidative TDOR DsbA from Staphylococcus carnosus, the pXTC expression system was used (7, 18). First, dsbA of S. carnosus was fused by PCR to the ribosome binding site and signal sequence of mntA of B. subtilis. For this purpose, a 92-bp fragment containing the ribosome binding site and signal sequence of mntA of B. subtilis was amplified using primers pXTC_MntA_F (GGGGGACTAGTAAGAGGAGGAGAAATATGAGACAA) and pXTC_MntA_Scar_R (TTTTTGTGAGCATCCCGTTAAAGCAAAGGTCGC). A second PCR fragment, which was 566 bp long, resulted from amplification of the dsbA gene of S. carnosus using primers pXTC_Scar_F (TTAACGGGATGCTCACAAAAAGACCCTGATTTA) and pXTC_Scar_R (GGGGGGGATCCTTATTTTTCTAGTAAATCTTTATATTCTT). The two resulting PCR products had a 21-nucleotide overlap. Using this overlap, the two different fragments could be fused using 10 PCR cycles without added primers. Next, the fused product was PCR amplified with primers pXTC_MntA_F and pXTC_Scar_R using 20 additional PCR cycles. The resulting 637-bp product was cloned into pTOPO (Invitrogen Life Technologies). After sequence verification, the fused dsbA gene was excised from this plasmid with BamHI and SpeI and ligated into the same restriction sites of plasmid pXTC downstream of PxylA, resulting in plasmid pXTC-ScdsbA.
Plasmid pXTC-ScdsbA was used to integrate the PxylA ScdsbA cassette together with the tetracycline resistance marker of pXTC (referred to below as the XTC-ScdsbA cassette) into the amyE locus of B. subtilis 168 and B. subtilis ItrxA by double-crossover recombination. Selection for tetracycline resistance and screening for an AmyE-negative phenotype on starch-containing plates enabled us to obtain B. subtilis strains X-ScdsbA and ItrxA X-ScdsbA, respectively.
Plasmid pXTC-SadsbA (also designated pXTC-dsbA) was obtained from previous studies (18). This plasmid carries the Staphylococcus aureus dsbA gene fused to the ribosome binding site and signal sequence of mntA of B. subtilis under transcriptional control of the xylA promoter (PxylA). pXTC-SadsbA was used to integrate the XTC-SadsbA cassette into the amyE loci of B. subtilis 168 and B. subtilis ItrxA by double-crossover recombination. Selection for tetracycline resistance and screening for an AmyE-negative phenotype on starch-containing plates yielded B. subtilis strains X-SadsbA and ItrxA X-SadsbA, respectively.
Alkaline phosphatase activity assay.
The alkaline phosphatase assay was performed with growth medium samples as previously described (7). PhoA activity was expressed in U/ml/unit of optical density at 600 nm. All experiments were repeated at least three times.
SDS-PAGE and Western blotting.
The presence of TrxA, PhoA, AmyQ, BdbD, and DsbA in growth medium samples and/or cell lysates was detected by Western blotting. Cellular or secreted proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (using precast NuPAGE gels from Invitrogen), and proteins were then semidry blotted (1.25 h at 100 mA per gel) onto a nitrocellulose membrane (Protran; Schleicher & Schuell). Subsequently, the TrxA, PhoA, AmyQ, BdbD, and DsbA proteins were detected with specific polyclonal antibodies raised in rabbits (Eurogentec). These antibodies were detected either with horseradish peroxidase-conjugated immunoglobulin G secondary antibodies and the Super Signal West Dura extended duration substrate (Pierce) in combination with the ChemiGenius XE bioimaging system (Syngene) or with fluorescent immunoglobulin G secondary antibodies (IRDye 800 CW goat anti-rabbit antibodies from LiCor Biosciences) in combination with the Odyssey infrared imaging system (LiCor Biosciences). In the latter case, fluorescence at 800 nm was recorded. Densitometric image analysis to quantify relative amounts of proteins as detected by SDS-PAGE and Western blotting was performed with the program ImageJ (http://rsbweb.nih.gov/ij/).
AMS labeling of reduced proteins.
The cellular levels of oxidized and reduced BdbD were monitored by labeling proteins with 4-acetamido-4′-maleimidyl-stilbene-2,2′-disulfonate (AMS) (Molecular Probes), essentially as described by Kobayashi et al. (17). Cells were grown in LB medium, collected by centrifugation, and resuspended in 25 mM Tris-HCl, 10 mM EDTA, 0.5 M glycerol, 0.25 mg/ml lysozyme (pH 8.0), 15 mM AMS. After 30 min of incubation at 37°C, the samples were mixed with a loading buffer for SDS-PAGE that lacked reducing agents such as β-mercaptoethanol or 1,4-dithiotreitol. Interestingly, when we tested various protocols for trichloroacetic acid precipitation, which was used by Kobayashi et al. (17) to quench the redox state of DsbA of E. coli, we determined that in the case of B. subtilis this step was not needed. Colabeling during cell lysis was sufficient to distinguish between the different redox states of BdbD. Notably, AMS binds covalently only to free thiol groups in reduced BdbD molecules. After boiling of the samples for 7 min, BdbD molecules with or without bound AMS were separated by nonreducing SDS-PAGE. Finally, the different BdbD species were visualized by Western blotting and immunodetection with specific polyclonal antibodies. The relative amounts of BdbD species with or without bound AMS were determined using the ChemiGenius XE2 bioimaging system and the GeneTools Analysis software package (Synoptics). All experiments were repeated at least four times.
RESULTS
Cellular levels of TrxA determine the level of PhoA secretion.
In the first attempt to increase the oxidative power of B. subtilis for more efficient secretion of disulfide-bond-containing proteins, we focused on potentially reductive systems of this organism. Our assumption was that deletion or decreased expression of the corresponding genes would make B. subtilis less reductive. In turn, this might improve the folding of proteins with disulfide bonds. For this purpose, three possible systems from other organisms could be excluded a priori. First, B. subtilis lacks homologues of the enzymes that are required for the synthesis of glutathione in gram-negative bacteria and eukaryotes; second, B. subtilis lacks the reducing agent mycothiol, which can be found in the cytoplasm of Streptomyces species and fungi (27); and third, B. subtilis lacks the proteins involved in isomerization pathways, like the pathway found in E. coli. Thus, we focused on thioredoxins and thioredoxin-like proteins. BLASTP searches revealed that the B. subtilis 168 genome encodes 12 thioredoxin(-like) proteins (data not shown). These proteins include four membrane proteins (BdbA, ResA, StoA/SpoIVH, and YneN) and eight predicted cytoplasmic proteins (TrxA, YbdE, YdbP, YdfQ, YkuV, YosR, YtpP, and YusE), which are very similar to known thioredoxins and/or to ResA (11, 51).
To test possible effects of the (potential) TDORs mentioned above on the secretion of a disulfide-bond-containing protein, trxA, ybdE, ydbP, ydfQ, ykuV, stoA (ykvV or spoIVH), yneN, ytpP, and yusE single mutants and a strain that lacks the SPβ prophage carrying the bdbA and yosR genes were transformed with plasmid pPSPhoA5. This plasmid specifies a fusion between the pre-pro region of a lipase from Staphylococcus hyicus and the mature PhoA protein (7). E. coli PhoA is a sensitive reporter for TDOR activity in B. subtilis, because this protein with two disulfide bonds requires oxidative TDORs for folding into a protease-resistant conformation (4, 25). Especially in the absence of BdbC and/or BdbD, unfolded PhoA is readily degraded in the highly proteolytic environments of the B. subtilis cell wall and growth medium (33). This basically provides an in vivo protease protection assay for probing the folding efficiency of secreted PhoA in relation to oxidative TDOR activity. Interestingly, none of the strains lacking an intact bdbA, stoA, ybdE, ydbP, ydfQ, ykuV, yneN, yosR, ytpP, or yusE gene were significantly affected in terms of secretion of active PhoA of E. coli (data not shown). Unexpectedly, however, depletion of TrxA resulted in increased levels of secretion of PhoA (Fig. 1A). It should be noted that this result could be obtained only with a conditional trxA mutant strain (ItrxA), because TrxA is essential for the growth and viability of B. subtilis (16, 34). In the ItrxA strain, the trxA promoter region (PtrxA) is replaced by the IPTG-dependent Pspac promoter. The growth of B. subtilis ItrxA on plates or in broth is strictly IPTG dependent, unlike the growth of parental strain 168. When cells of B. subtilis ItrxA are grown in LB broth, wild-type growth rates are observed with an IPTG concentration of 25 μM or higher (37).
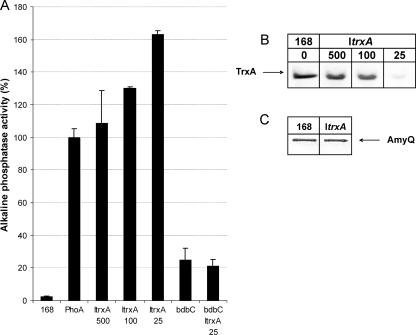
FIG. 1.
BdbC-dependent secretion of PhoA by B. subtilis ItrxA. (A) Alkaline phosphatase activity was assayed using the growth media of B. subtilis 168(pPSPhoA5) (PhoA), B. subtilis ItrxA(pPSPhoA5) grown in the presence of 500, 100, or 25 μM IPTG (ItrxA 500, ItrxA 100, and ItrxA 25, respectively), B. subtilis bdbC(pPSPhoA5) (bdbC), and B. subtilis ItrxA bdbC(pPSPhoA5) grown in the presence of 25 μM IPTG (bdbC ItrxA 25). B. subtilis 168 (168) was used as a negative control. All strains were grown overnight in LB medium at 37°C. (B) Western blot analysis of TrxA production in B. subtilis ItrxA and parental strain 168. Cells were grown overnight at 37°C in LB medium. Cellular proteins were separated by SDS-PAGE, and TrxA was visualized by Western blotting. The IPTG concentrations in the medium (0, 25, 100, and 500 μM) are indicated above the blots. (C) Secretion of AmyQ by B. subtilis ItrxA. Cells of B. subtilis ItrxA(pKTH10) (ItrxA) and B. subtilis 168(pKTH10) (168) were grown in LB medium at 37°C in the presence of 25 μM IPTG. The presence of AmyQ in growth medium fractions was analyzed by SDS-PAGE and Western blotting.
To investigate the importance of the cytoplasmic TrxA level for secretion of active PhoA, the ItrxA mutant strain transformed with plasmid pPSPhoA5 was analyzed in detail. The level of secretion of PhoA into the growth medium was determined by alkaline phosphatase activity assays. For this purpose, cells were grown in LB medium supplemented with 25, 100, or 500 μM IPTG. Interestingly, the results showed that, compared to parental strain 168, the ItrxA strain secreted at least 1.5-fold more active PhoA when it was grown in the presence of 25 μM IPTG (Fig. 1A). Under these conditions, cellular TrxA was barely detectable by Western blotting (Fig. 1B). The level of secreted PhoA was similar to the levels observed for the parental strain when B. subtilis ItrxA was grown in the presence of 500 μM IPTG (Fig. 1A), which resulted in wild-type levels of cellular TrxA (Fig. 1B). In contrast, PhoA secretion by the parental strain 168 was independent of the IPTG concentration in the growth medium, and the presence of IPTG (25 to 500 μM) in the growth medium had no detectable influence on the TrxA levels in this strain (not shown). These observations imply that, for the range of IPTG concentrations tested, the amount of active PhoA secreted by the ItrxA mutant is inversely proportional to the amount of TrxA in the cells. Notably, TrxA depletion in the ItrxA strain did not influence the yields of the secreted α-amylase AmyQ of Bacillus amyloliquefaciens, which lacks disulfide bonds. This could be demonstrated by transformation of B. subtilis ItrxA with plasmid pKTH10, which encodes AmyQ. Densitometric image analysis of the secreted AmyQ protein bands shown in Fig. 1C revealed that the growth media of the ItrxA mutant (25 μM IPTG) and parental strain 168 contained comparable amounts of AmyQ. These findings suggest that the improved PhoA secretion by the ItrxA mutant strain is not due to generally improved synthesis or secretion of proteins but rather to improved posttranslocational folding resulting in protease resistance of the mature PhoA protein. This view was confirmed by the observation that the extracellular proteome of the ItrxA mutant strain grown in the presence of 25 μM IPTG was indistinguishable from the extracellular proteome of parental strain 168 (H. Antelmann and J. Y. Dubois, data not shown).
The observed increase in active PhoA secretion by the ItrxA mutant strain grown in the presence of 25 μM IPTG raised the question of whether this increase required the activity of BdbC. To answer this question, a pPSPhoA5-containing ItrxA bdbC double-mutant strain was constructed. Importantly, this double mutant displayed IPTG-dependent growth, showing that the bdbC mutation did not suppress the ItrxA mutation. As shown in Fig. 1A, PhoA secretion remained strongly BdbC dependent irrespective of the presence of the ItrxA mutation. Furthermore, the secretion of PhoA by the ItrxA bdbC mutant did not vary when different amounts of IPTG were present in the growth medium (data not shown). These findings suggest that the BdbCD-dependent thiol oxidiation pathway (18) is also required for PhoA folding under TrxA depletion conditions.
Cellular levels of TrxA and BdbC influence the redox state of BdbD.
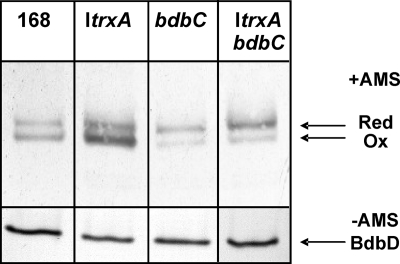
To test whether the presence of TrxA can have an impact on the activity of the BdbCD thiol oxidation pathway, we verified the redox state of BdbD with the thiol-specific cross-linking reagent AMS. Due to the molecular mass of AMS, cross-linking of this reagent to reduced cysteine residues in a protein results in a significant reduction in the mobility of the protein during SDS-PAGE (17). In this respect, it is important to note that BdbD contains only two cysteine residues, which are part of the CXXC active site. To study the redox state of BdbD, overnight cultures were used to prepare fresh lysates from the ItrxA strain (25 μM IPTG), the bdbC single mutant, the ItrxA bdbC double mutant, or parental strain 168. Importantly, the lysates were prepared either in the presence or in the absence of 15 mM AMS. Next, AMS binding to BdbD was analyzed by SDS-PAGE and Western blotting using BdbD-specific antibodies. As shown in Fig. 2, somewhat less than 50% of the BdbD molecules of parental strain 168 were labeled with AMS. By contrast, the ratio of reduced BdbD molecules (i.e., molecules labeled with AMS) to oxidized BdbD molecules (i.e., molecules not labeled with AMS) was significantly shifted toward the oxidized form in the ItrxA strain, whereas a significant shift toward the reduced BdbD species was observed in the bdbC and ItrxA bdbC mutant strains. These differences were observed with both exponentially and postexponentially growing cells of B. subtilis 168 and the ItrxA and bdbC strains (Fig. 2 and results not shown). These findings indicate that significantly more BdbD molecules were oxidized in the ItrxA strain than in the parental strain and, conversely, that the bdbC mutation resulted in lower numbers of oxidized BdbD molecules. Furthermore, the control experiments performed with lysates of the strains prepared in the absence of AMS showed that BdbD migrated as a single band (Fig. 2). Together, these findings indicate that the cytoplasmic TrxA levels impact the redox state of BdbD in a BdbC-dependent manner.
FIG. 2.
Redox state of BdbD. Cells of the B. subtilis ItrxA, bdbC, or ItrxA bdbC strain or parental strain 168 were grown overnight in LB medium at 37°C in the presence of 25 μM IPTG. Cellular proteins were separated by SDS-PAGE, and BdbD was visualized by Western blotting. (Upper panel) Cell extracts prepared in the presence of 15 mM AMS (+AMS). (Lower panel) Cell extracts prepared in the absence of AMS (−AMS). The positions of reduced (Red) and oxidized (Ox) BdbD are indicated on the right.
Expression of DsbA increases PhoA secretion.
An potential alternative approach for increasing the capacity of B. subtilis for thiol oxidation is the overproduction of known thiol oxidases. However, our attempts to increase the levels of BdbC and BdbD have not been successful thus far (data not shown). Therefore, we investigated the possibility of expressing heterologous oxidases. For this purpose, we capitalized on our previous studies with the major oxidase DsbA of Staphylococcus aureus (referred to below as SaDsbA) (18). This homologue of B. subtilis BdbD was able to complement the loss of both BdbC and BdbD for secretion of active PhoA. Moreover, when SaDsbA was expressed in parental strain 168, an approximately 1.5- to 2.0-fold increase in the secretion of active E. coli PhoA was observed, which was similar to the increase observed when TrxA was depleted, as described above. However, since S. aureus is known to be a dangerous pathogen (24, 36), the potential use of SaDsbA for biotechnological purposes is limited. For this reason, we searched for a DsbA protein in a nonpathogenic close relative of S. aureus. The best source for a dsbA gene turned out to be S. carnosus, which is well known because of its use as a starter in the fermentation of cheese and dry sausage. Accordingly, this organism has the generally recognized as safe status. Therefore, B. subtilis strains containing genes that originated from this staphylococcal species should be more generally acceptable for industrial applications.
An S. carnosus homologue of S. aureus DsbA (referred to below as ScDsbA) was identified by BLASTP searches (51% identical amino acids) using the sequenced genome of S. carnosus strain TM300 (R. Rosenstein and F. Götz, personal communication). Like SaDsbA, the ScDsbA protein is homologous to B. subtilis BdbD (36% identical amino acids overall). In order to express S. carnosus ScDsbA in B. subtilis, the same xylose-inducible pXTC expression system that was used to express S. aureus SaDsbA (18) was used. For this purpose, we fused the sequence encoding the mature ScDsbA lipoprotein to the ribosome binding site and signal sequence of the B. subtilis mntA gene, which codes for an abundantly expressed lipoprotein in this organism (3). Upon integration of the XTC-ScdsbA cassette containing the hybrid ScdsbA gene into the B. subtilis 168 chromosome, xylose-inducible expression of cell-associated ScDsbA was obtained (data not shown) (below the strains containing this cassette are referred to as X-ScdsbA strains). For immunodetection of ScDsbA we used antibodies against SaDsbA that also showed cross-reactivity with ScDsbA (Fig. 3). As expected, the cellular levels of ScDsbA depended on the amount of xylose added to the growth medium. The highest levels of cellular ScDsbA were observed when the X-ScdsbA cells were induced with a concentration of xylose of 1.0% or higher, whereas no ScDsbA was detectable when cells were grown in the absence of xylose (data not shown).
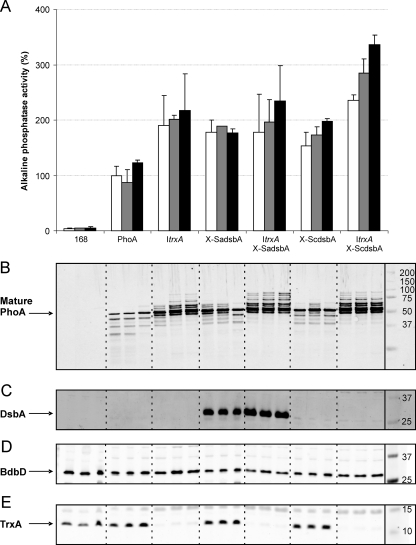
FIG. 3.
Increased production of E. coli PhoA by engineered B. subtilis strains. The B. subtilis ItrxA, X-SadsbA, ItrxA X-SadsbA, X-ScdsbA, and ItrxA X-SadsbA strains and parental strain 168 (PhoA) were transformed with pPSPhoA5 for E. coli PhoA production. All mutant strains and parental strain 168 (no PhoA production) were grown overnight in LB medium containing 0.5% xylose and 25 μM IPTG (open bars) and additional 100 μg/ml cystine (gray bars) or cysteine (black bars). Next, growth medium samples were removed and used for alkaline phosphatase activity assays (A), as well as SDS-PAGE and Western blotting with specific antibodies against PhoA (B). (C to E) Lysates of cells from cultures corresponding to the samples used for panels A and B were analyzed by Western blotting with specific antibodies against S. aureus DsbA (C), BdbD (D), or TrxA (E). PhoA activity was expressed in U/ml/unit of optical density at 600 nm relative to the PhoA activity of the parental strain expressing PhoA, which was defined as 100% (6.0 U/ml/unit of optical density at 600 nm). The arrow in panel B indicates the position of mature PhoA. Bands with higher mobility on SDS-PAGE gels are breakdown products of PhoA, and bands with lower mobility are unprocessed forms of proPhoA. The arrows in panels C, D, and E indicate the positions of DsbA, BdbD, and TrxA, respectively. Note that the antibody against S. aureus DsbA is cross-reactive with S. carnosus DsbA. The positions of molecular weight markers are indicated on the right.
To assess the effect of ScDsbA expression on the extracellular accumulation of active E. coli PhoA, B. subtilis strain X-ScdsbA was transformed with plasmid pPSPhoA5. For comparison, PhoA production by the single-mutant strains X-SadsbA and ItrxA and the double-mutant strains X-SadsbA ItrxA and X-ScdsbA ItrxA was assayed in parallel (all these strains were transformed with plasmid pPSPhoA5; ItrxA strains were grown in the presence of 25 μM IPTG). The results indicate that expression of ScDsbA indeed led to increased secretion of active PhoA (Fig. 3A) compared to the secretion by the parental strain. The extent to which ScDsbA expression increased the level of extracellular PhoA was comparable to the extent to which SaDsbA expression increased the level of extracellular PhoA, indicating that these proteins are functionally interchangeable when they are expressed in B. subtilis. Furthermore, the expression of ScDsbA or SaDsbA and the depletion of TrxA resulted in comparable increases in the extracellular levels of active PhoA (approximately 1.5- to 2.0-fold increases). When TrxA depletion was combined with ScDsbA or SaDsbA expression, even higher levels of active extracellular PhoA were observed, especially with the X-ScdsbA ItrxA strain (Fig. 3A).
Importantly, the levels of PhoA activity in the different growth medium samples correlated well with the levels of mature PhoA protein detected in the corresponding samples, as shown by Western blotting (Fig. 3B). Interestingly, compared to parental strain 168 expressing PhoA, significantly smaller amounts of extracellular breakdown products of mature PhoA were observed for all mutant strains tested. The smallest amounts of degradation products of mature PhoA were observed for ItrxA strains with TrxA depleted. Furthermore, multiple PhoA products with lower SDS-PAGE mobility than mature PhoA, probably representing intermediate processing products of pro-PhoA, were detectable in growth media of strains containing the ItrxA mutation, and to a lesser extent, this was also the case for the X-SadsbA and X-ScdsbA strains. The largest amounts of PhoA protein were observed in medium fractions of the X-ScdsbA ItrxA strain in which TrxA was depleted. Taken together, these results show that modulation of cytoplasmic TrxA and/or extracytoplasmic TDOR levels can result in increased levels of secreted active PhoA.
Optimized levels of secreted PhoA facilitated by redox-active medium compounds.
Recently, we reported that the activity of staphylococcal DsbA depends on redox-active compounds in the growth medium. This was shown by growing DsbA-producing B. subtilis in synthetic media with or without cysteine/cystine and subsequently measuring the extracellular accumulation of active E. coli PhoA (18). Therefore, we investigated whether this DsbA activity could also be enhanced by addition of excess redox-active compounds, such as cysteine or cystine, to the rich LB growth medium. For this purpose, we grew all TrxA depletion and/or DsbA-expressing strains in parallel cultures in the presence of 100 μg/ml added cystine (i.e., oxidized cysteine) or cysteine. The results show that addition of either cystine or cysteine to the ItrxA X-ScdsbA strain resulted in strongly increased levels of active extracellular PhoA (Fig. 3A). The increases in extracellular PhoA activity were most pronounced (about 3.5-fold) when cysteine was added. Quantification of the Western blot data in Fig. 3B indicated that the amount of mature PhoA was about fourfold larger in the ItrxA X-ScdsbA strain when it was grown in the presence of cysteine. Furthermore, this positive trend was also observed for the X-ScdsbA strain, but the degree of stimulation was lower than that in the ItrxA X-ScdsbA strain. For the other strains addition of cysteine or cystine to the growth medium did not result in statistically significant increased extracellular PhoA levels under the conditions tested. The PhoA activity data were confirmed by Western blotting, which showed that all observed increases in activity correlated with increased PhoA protein levels (Fig. 3B). Moreover, especially the addition of cysteine seemed to result in reduced extracellular accumulation of degradation products of mature PhoA, even in parental strain 168. To investigate whether cystine or cysteine in the growth medium had an influence on known TDORs that impact the secretion of active PhoA, we investigated the cellular levels of DsbA, BdbD, and TrxA. As shown in Fig. 3C to E, the levels of these proteins were not affected by the presence of added cystine or cysteine.
Taken together, our study aimed at increasing the thiol-oxidizing power of Bacillus yielded three approaches leading to stepwise improvements in the yields of secreted active E. coli PhoA.
DISCUSSION
In the present study we developed novel concepts for optimized production of disulfide-bond-containing proteins in Bacillus. In essence, this goal can be achieved by (i) depletion of the major cytoplasmic disulfide bond reductase TrxA; (ii) coexpression of a staphylococcal thiol oxidase (DsbA) with the disulfide-bond-containing protein that is to be produced; (iii) use of growth medium supplemented with a redox-active compound, such as cysteine; and (iv) a combination of these three approaches. Proof of principle was obtained by combined use of these three approaches for optimized secretion of the disulfide-bond-containing protein PhoA from E. coli. This resulted in approximately 3.5-fold-larger amounts of active PhoA protein in the growth medium. Our findings underpin the fact that significant amounts of high-value proteins are degraded after heterologous production in Bacillus species and that significant amounts of such “lost proteins” can be rescued by cell factory engineering.
In previous studies we investigated the roles of membrane-associated TDORs in the secretion of E. coli PhoA and showed that BdbC and BdbD were of prime importance for preventing the degradation of PhoA that was translocated across the membrane (4, 25). Our studies also revealed that, despite the PhoA folding activity of BdbCD, substantial amounts of translocated PhoA were degraded (7, 18). This was likely to be due to the known limited capacity for disulfide bond formation of B. subtilis (33). At the time, we did not include cytoplasmic TDORs in our studies, because these enzymes are generally believed to function as thiol reductases rather than as thiol oxidases that might facilitate the folding of PhoA. In the present study we investigated how the thiol-oxidizing power of B. subtilis could be increased. For this purpose, the cytoplasmic TDORs were interesting, because lowering their cellular levels would decrease the thiol-reducing power and concomitantly increase the oxidative power. Of the 10 thioredoxinlike proteins of B. subtilis tested, only TrxA impacted the secretion of PhoA. Our results indicate that TrxA counteracts the production of secreted active PhoA, most likely due to its general thiol reductase function in the cytoplasm. At present, we do not know why removing the remaining thioredoxin-like proteins had no effect on PhoA secretion, but these proteins either may have very specific functions, as was shown previously for StoA (11), or may not be expressed under the conditions tested.
Thioredoxins are small, heat-stable, ubiquitous TDORs that are involved in a large variety of processes, ranging from enzyme activation to mitochondrion-dependent apoptosis (40). During catalysis, the cysteine residues of their CXXC active site undergo a reversible oxidation-reduction reaction. In the bacterial cytoplasm, thioredoxin is usually present in a reduced state in order to prevent the formation of disulfide bonds in cytoplasmic proteins. Interestingly, depletion of TrxA in B. subtilis resulted in specific approximately 1.5- to 2-fold increases in the extracellular levels of E. coli PhoA. The increased PhoA levels coincided with the disappearance of PhoA degradation products and the appearance of incompletely processed pro-PhoA in the medium. Notably, the results of our previous DNA array analyses showed that the expression of none of the known genes for major secretion machinery components or proteases of B. subtilis is affected by TrxA depletion (37). Taken together, these findings imply that TrxA influences the activity (but not the amounts) of secretion machinery components that are specifically involved in PhoA secretion. Indeed, TrxA was shown to have a significant impact on the redox state of the extracytoplasmic TDOR BdbD since TrxA depletion resulted in increased levels of oxidized BdbD molecules. Our observations indicate, therefore, that diminished reductive power of the cytoplasm as a consequence of TrxA depletion results in increased levels of oxidized BdbD molecules. Thus, TrxA depletion has an effect that is opposite the effect of a bdbC mutation, which results in strongly reduced folding of PhoA (4) and, at the same time, significantly increased levels of reduced BdbD. Notably, the improved PhoA secretion by cells with TrxA depleted still depends on the presence of BdbC. Therefore, it seems that the increased levels of oxidized BdbD molecules in cells with TrxA depleted increase the cellular capacity to oxidize exported proteins, such as PhoA. Thus, our data indicate that the improved secretion of PhoA by cells with TrxA depleted can be attributed to improved posttranslocational disulfide bond formation in PhoA rather than to improved pre-PhoA translocation across the membrane.
The observation that addition of cysteine or cystine to the media of strains expressing S. carnosus DsbA leads to increased extracellular levels of active PhoA is in agreement with the recently documented dependence of S. aureus DsbA on cysteine for activity (18). Most likely, this is related to the fact that all (sequenced) staphylococci lack BdbC-like quinone reductases for DsbA reoxidation during catalysis (18). This requirement for redox-active medium components for DsbA activity is not immediately obvious when cells are grown in LB medium, because this growth medium contains such components. However, our present findings with S. carnosus DsbA indicate that limiting amounts of redox-active components are present for optimal activity of this thiol oxidase. It should be noted that addition of cysteine or cystine to the media had positive effects on the yields of extracellular active PhoA from S. carnosus DsbA-expressing cells. Most likely, this was due to the fact that cysteine is readily oxidized to cystine in the presence of molecular oxygen. In fact, addition of cysteine seems to be more effective than addition of cystine, which can be explained at least in part by the poor solubility of cystine. Furthermore, recent studies by Smits et al. suggested that cells with TrxA depleted are auxotrophic for cysteine as growth inhibition of cells with TrxA severely depleted could be reversed by adding cysteine to the growth medium (37). Under these conditions cysteine may actually protect various cytoplasmic proteins against irreversible thiol oxidation (14, 22). Addition of cysteine is therefore preferred over addition of cystine in order to increase the oxidative potential of strains expressing S. carnosus DsbA.
A clear outcome of the present study is that the highest extracellular levels of active E. coli PhoA are produced when TrxA is depleted and S. carnosus DsbA is expressed in a strain growing in medium that is supplemented with cysteine. Specifically, these conditions resulted in a 3.5-fold increase in production of active extracellular PhoA or an estimated increase in the concentration of extracellular mature PhoA protein from ∼90 to ∼350 mg/liter (7, 46, 48; data not shown). These findings suggest that more than 70% of the PhoA synthesized in parental B. subtilis strain 168 is subject to degradation and that the degraded PhoA can be rescued in appropriately modified strains. To date, it is not clear whether further increases can be achieved (for example, by deletion of particular protease genes), but this seems to be a promising approach on the basis of previous studies (23, 26, 47, 49). In this context, it is relevant to note that the membrane-cell wall interface of B. subtilis is a highly proteolytic environment in which heterologous translocated proteins, including even α-amylases from other bacilli, are readily degraded due to suboptimal conditions for their folding (33). Clearly, even in the cysteine-supplemented medium of our best-producing strain, PhoA degradation products were still observed. We are therefore convinced that the B. subtilis cell factory provides an opportunity for further improvement. This improvement may be achieved by constructing strains with optimized properties for protein folding after translocation across the membrane or reduced proteolytic activity and by using optimized growth protocols. In turn, such strains should make downstream processing of protein products (with or without specific affinity tags) considerably easier. Even though further improvements are conceivable, we are convinced that our present study represents a major step toward the generation of optimized Bacillus strains for production of high-value proteins with disulfide bonds.
Acknowledgments
We thank Friedrich Götz and Ralf Rosenstein for providing the dsbA sequence of S. carnosus prior to publication, Haike Antelmann for performing the proteomic analysis of the ItrxA strain, Patty Mulder and Annemieke van der Goot for providing technical assistance, and other members of the Groningen and European Bacillus Secretion Groups for stimulating discussions.
T.R.H.M.K, J.-Y.F.D, R.F, W.J.Q, and J.M.V.D. were supported in part by European Union grants LSHG-CT-2004-503468, LSHG-CT-2004-005257, LSHM-CT-2006-019064, and LSHG-CT-2006-037469, by the transnational SysMO initiative through project BACELL SysMO, and by grant 04-EScope 01-011 from the Research Council for Earth and Life Sciences (ALW), The Netherlands Organization for Scientific Research (NWO).
Footnotes
Published ahead of print on 24 October 2008.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anfinsen, C. B. 1973. Principles that govern the folding of protein chains. Science 181:223-230. [DOI] [PubMed] [Google Scholar]
- 3.Antelmann, H., H. Tjalsma, B. Voigt, S. Ohlmeier, S. Bron, J. M. van Dijl, and M. Hecker. 2001. A proteomic view on genome-based signal peptide predictions. Genome Res. 11:1484-1502. [DOI] [PubMed] [Google Scholar]
- 4.Bolhuis, A., G. Venema, W. J. Quax, S. Bron, and J. M. van Dijl. 1999. Functional analysis of paralogous thiol-disulfide oxidoreductases in Bacillus subtilis. J. Biol. Chem. 274:24531-24538. [DOI] [PubMed] [Google Scholar]
- 5.Braun, P., G. Gerritse, J. M. van Dijl, and W. J. Quax. 1999. Improving protein secretion by engineering components of the bacterial translocation machinery. Curr. Opin. Biotechnol. 10:376-381. [DOI] [PubMed] [Google Scholar]
- 6.Collet, J. F., and J. C. Bardwell. 2002. Oxidative protein folding in bacteria. Mol. Microbiol. 44:1-8. [DOI] [PubMed] [Google Scholar]
- 7.Darmon, E., R. Dorenbos, J. Meens, R. Freudl, H. Antelmann, M. Hecker, O. P. Kuipers, S. Bron, W. J. Quax, J. Y. Dubois, and J. M. van Dijl. 2006. A disulfide bond-containing alkaline phosphatase triggers a BdbC-dependent secretion stress response in Bacillus subtilis. Appl. Environ. Microbiol. 72:6876-6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorenbos, R., T. Stein, J. Kabel, C. Bruand, A. Bolhuis, S. Bron, W. J. Quax, and J. M. van Dijl. 2002. Thiol-disulfide oxidoreductases are essential for the production of the lantibiotic sublancin 168. J. Biol. Chem. 277:16682-16688. [DOI] [PubMed] [Google Scholar]
- 9.Dorenbos, R., J. M. van Dijl, and W. J. Quax. 2005. Thiol disulfide oxidoreductases in bacteria, p. 237-269. In S. G. Pandalai (ed.), Recent research developments in microbiology 9. Research Signpost, Kerala, India.
- 10.Dumoulin, A., U. Grauschopf, M. Bischoff, L. Thony-Meyer, and B. Berger-Bachi. 2005. Staphylococcus aureus DsbA is a membrane-bound lipoprotein with thiol-disulfide oxidoreductase activity. Arch. Microbiol. 184:117-128. [DOI] [PubMed] [Google Scholar]
- 11.Erlendsson, L. S., M. Moller, and L. Hederstedt. 2004. Bacillus subtilis StoA is a thiol-disulfide oxidoreductase important for spore cortex synthesis. J. Bacteriol. 186:6230-6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 14.Hochgrafe, F., J. Mostertz, D. C. Pother, D. Becher, J. D. Helmann, and M. Hecker. 2007. S-Cysteinylation is a general mechanism for thiol protection of Bacillus subtilis proteins after oxidative stress. J. Biol. Chem. 282:25981-25985. [DOI] [PubMed] [Google Scholar]
- 15.Inaba, K., S. Murakami, M. Suzuki, A. Nakagawa, E. Yamashita, K. Okada, and K. Ito. 2006. Crystal structure of the DsbB-DsbA complex reveals a mechanism of disulfide bond generation. Cell 127:789-801. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, K., S. D. Ehrlich, A. Albertini, G. Amati, K. K. Andersen, M. Arnaud, K. Asai, S. Ashikaga, S. Aymerich, P. Bessieres, F. Boland, S. C. Brignell, S. Bron, K. Bunai, J. Chapuis, L. C. Christiansen, A. Danchin, M. Debarbouille, E. Dervyn, E. Deuerling, K. Devine, S. K. Devine, O. Dreesen, J. Errington, S. Fillinger, S. J. Foster, Y. Fujita, A. Galizzi, R. Gardan, C. Eschevins, T. Fukushima, K. Haga, C. R. Harwood, M. Hecker, D. Hosoya, M. F. Hullo, H. Kakeshita, D. Karamata, Y. Kasahara, F. Kawamura, K. Koga, P. Koski, R. Kuwana, D. Imamura, M. Ishimaru, S. Ishikawa, I. Ishio, D. Le Coq, A. Masson, C. Mauel, R. Meima, R. P. Mellado, A. Moir, S. Moriya, E. Nagakawa, H. Nanamiya, S. Nakai, P. Nygaard, M. Ogura, T. Ohanan, M. O'Reilly, M. O'Rourke, Z. Pragai, H. M. Pooley, G. Rapoport, J. P. Rawlins, L. A. Rivas, C. Rivolta, A. Sadaie, Y. Sadaie, M. Sarvas, T. Sato, H. H. Saxild, E. Scanlan, W. Schumann, J. F. Seegers, J. Sekiguchi, A. Sekowska, S. J. Seror, M. Simon, P. Stragier, R. Studer, H. Takamatsu, T. Tanaka, M. Takeuchi, H. B. Thomaides, V. Vagner, J. M. van Dijl, K. Watabe, A. Wipat, H. Yamamoto, M. Yamamoto, Y. Yamamoto, K. Yamane, K. Yata, K. Yoshida, H. Yoshikawa, U. Zuber, and N. Ogasawara. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 100:4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, T., S. Kishigami, M. Sone, H. Inokuchi, T. Mogi, and K. Ito. 1997. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc. Natl. Acad. Sci. USA 94:11857-11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kouwen, T. R., A. van der Goot, R. Dorenbos, T. Winter, H. Antelmann, M. C. Plaisier, W. J. Quax, J. M. van Dijl, and J. Y. Dubois. 2007. Thiol-disulphide oxidoreductase modules in the low-GC Gram-positive bacteria. Mol. Microbiol. 64:984-999. [DOI] [PubMed] [Google Scholar]
- 19.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 20.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 21.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 22.Lee, J. W., S. Soonsanga, and J. D. Helmann. 2007. A complex thiolate switch regulates the Bacillus subtilis organic peroxide sensor OhrR. Proc. Natl. Acad. Sci. USA 104:8743-8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, W., X. Zhou, and P. Lu. 2004. Bottlenecks in the expression and secretion of heterologous proteins in Bacillus subtilis. Res. Microbiol. 155:605-610. [DOI] [PubMed] [Google Scholar]
- 24.Massey, R. C., M. J. Horsburgh, G. Lina, M. Hook, and M. Recker. 2006. The evolution and maintenance of virulence in Staphylococcus aureus: a role for host-to-host transmission? Nat. Rev. Microbiol. 4:953-958. [DOI] [PubMed] [Google Scholar]
- 25.Meima, R., C. Eschevins, S. Fillinger, A. Bolhuis, L. W. Hamoen, R. Dorenbos, W. J. Quax, J. M. van Dijl, R. Provvedi, I. Chen, D. Dubnau, and S. Bron. 2002. The bdbDC operon of Bacillus subtilis encodes thiol-disulfide oxidoreductases required for competence development. J. Biol. Chem. 277:6994-7001. [DOI] [PubMed] [Google Scholar]
- 26.Murashima, K., C. L. Chen, A. Kosugi, Y. Tamaru, R. H. Doi, and S. L. Wong. 2002. Heterologous production of Clostridium cellulovorans engB, using protease-deficient Bacillus subtilis, and preparation of active recombinant cellulosomes. J. Bacteriol. 184:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton, G. L., K. Arnold, M. S. Price, C. Sherrill, S. B. Delcardayre, Y. Aharonowitz, G. Cohen, J. Davies, R. C. Fahey, and C. Davis. 1996. Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 178:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen, H., J. Engelbrecht, S. Brunak, and H. G. von. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581-599. [DOI] [PubMed] [Google Scholar]
- 29.Palva, I. 1982. Molecular cloning of alpha-amylase gene from Bacillus amyloliquefaciens and its expression in B. subtilis. Gene 19:81-87. [DOI] [PubMed] [Google Scholar]
- 30.Regeimbal, J., and J. C. Bardwell. 2002. DsbB catalyzes disulfide bond formation de novo. J. Biol. Chem. 277:32706-32713. [DOI] [PubMed] [Google Scholar]
- 31.Rietsch, A., and J. Beckwith. 1998. The genetics of disulfide bond metabolism. Annu. Rev. Genet. 32:163-184. [DOI] [PubMed] [Google Scholar]
- 32.Ritz, D., and J. Beckwith. 2001. Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol. 55:21-48. [DOI] [PubMed] [Google Scholar]
- 33.Sarvas, M., C. R. Harwood, S. Bron, and J. M. van Dijl. 2004. Post-translocational folding of secretory proteins in Gram-positive bacteria. Biochim. Biophys. Acta 1694:311-327. [DOI] [PubMed] [Google Scholar]
- 34.Scharf, C., S. Riethdorf, H. Ernst, S. Engelmann, U. Volker, and M. Hecker. 1998. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J. Bacteriol. 180:1869-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schleifer, K.-H., and U. Fischer. 1982. Description of a new species of the genus Staphylococcus: Staphylococcus carnosus. Int. J. Syst. Bacteriol. 32:153-156. [Google Scholar]
- 36.Sibbald, M. J., A. K. Ziebandt, S. Engelmann, M. Hecker, A. de Jong, H. J. Harmsen, G. C. Raangs, I. Stokroos, J. P. Arends, J. Y. Dubois, and J. M. van Dijl. 2006. Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol. Mol. Biol. Rev. 70:755-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smits, W. K., J. Y. Dubois, S. Bron, J. M. van Dijl, and O. P. Kuipers. 2005. Tricksy business: transcriptome analysis reveals the involvement of thioredoxin A in redox homeostasis, oxidative stress, sulfur metabolism, and cellular differentiation in Bacillus subtilis. J. Bacteriol. 187:3921-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sone, M., S. Kishigami, T. Yoshihisa, and K. Ito. 1997. Roles of disulfide bonds in bacterial alkaline phosphatase. J. Biol. Chem. 272:6174-6178. [DOI] [PubMed] [Google Scholar]
- 39.Tan, J. T., and J. C. Bardwell. 2004. Key players involved in bacterial disulfide-bond formation. Chembiochem 5:1479-1487. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka, T., F. Hosoi, Y. Yamaguchi-Iwai, H. Nakamura, H. Masutani, S. Ueda, A. Nishiyama, S. Takeda, H. Wada, G. Spyrou, and J. Yodoi. 2002. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 21:1695-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tjalsma, H., H. Antelmann, J. D. Jongbloed, P. G. Braun, E. Darmon, R. Dorenbos, J. Y. Dubois, H. Westers, G. Zanen, W. J. Quax, O. P. Kuipers, S. Bron, M. Hecker, and J. M. van Dijl. 2004. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68:207-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tjalsma, H., A. Bolhuis, J. D. Jongbloed, S. Bron, and J. M. van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 45.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 46.Westers, L., D. S. Dijkstra, H. Westers, J. M. van Dijl, and W. J. Quax. 2006. Secretion of functional human interleukin-3 from Bacillus subtilis. J. Biotechnol. 123:211-224. [DOI] [PubMed] [Google Scholar]
- 47.Westers, L., H. Westers, and W. J. Quax. 2004. Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochim. Biophys. Acta 1694:299-310. [DOI] [PubMed] [Google Scholar]
- 48.Westers, L., H. Westers, G. Zanen, H. Antelmann, M. Hecker, D. Noone, K. M. Devine, J. M. van Dijl, and W. J. Quax. 2008. Genetic or chemical protease inhibition causes significant changes in the Bacillus subtilis exoproteome. Proteomics 8:2704-2713. [DOI] [PubMed] [Google Scholar]
- 49.Wu, X. C., W. Lee, L. Tran, and S. L. Wong. 1991. Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J. Bacteriol. 173:4952-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeigler, D. R., and J. B. Perkins. 2008. The genus Bacillus, p. 309-338. In E. Goldman and L. Green (ed.), Practical handbook of microbiology. CRC Press, Boca Raton, FL.
- 51.Zhang, X., Y. Hu, X. Guo, E. Lescop, Y. Li, B. Xia, and C. Jin. 2006. The Bacillus subtilis YkuV is a thiol:disulfide oxidoreductase revealed by its redox structures and activity. J. Biol. Chem. 281:8296-8304. [DOI] [PubMed] [Google Scholar]