Abstract
Proteins that are produced for commercial purposes in Bacillus subtilis are commonly secreted via the Sec pathway. Despite its high secretion capacity, the secretion of heterologous proteins via the Sec pathway is often unsuccessful. Alternative secretion routes, like the Tat pathway, are therefore of interest. Two parallel Tat pathways with distinct specificities have previously been discovered in B. subtilis. To explore the application potential of these Tat pathways, several commercially relevant or heterologous model proteins were fused to the signal peptides of the known B. subtilis Tat substrates YwbN and PhoD. Remarkably, the YwbN signal peptide directed secretion of active subtilisin, a typical Sec substrate, via the B. subtilis TatAyCy route. In contrast, the same signal peptide directed Tat-independent secretion of the Bacillus licheniformis α-amylase (AmyL). Moreover, the YwbN signal peptide directed secretion of SufI, an Escherichia coli Tat substrate, in a Tat-independent manner, most likely via Sec. Our results suggest that cytoplasmic protein folding prior to translocation is probably a major determinant of Tat-dependent protein secretion in B. subtilis, as is the case with E. coli. We conclude that future applications for the Tat system of B. subtilis will most likely involve commercially interesting proteins that are Sec incompatible.
Bacillus subtilis is an industrially important bacterium that is commonly used as a host for the commercial production of proteins, especially enzymes, such as proteases, amylases, and lipases (15, 19, 58, 71). One of the major advantages of B. subtilis over many other bacteria is its ability to secrete large amounts of proteins into the growth medium, allowing efficient recovery of these protein products. Unfortunately, the secretion of heterologous proteins is often inefficient or not successful at all, and this applies in particular to proteins from gram-negative bacteria or eukaryotes (20). Various potential bottlenecks in the secretion of heterologous proteins have been identified. Many of the encountered problems relate to the particular properties of the secreted protein, the secretion pathway, or both (7, 67). Notably, in most documented cases the general secretion (Sec) pathway was used, which is known for its high capacity to transport proteins from the cytoplasm to the growth medium. Importantly, however, B. subtilis contains several alternative routes for protein export and secretion, one of which is known as the twin-arginine translocation (Tat) pathway (26, 27, 64, 65). This pathway may offer an alternative secretion route for Sec-incompatible proteins in bacilli.
The Tat pathway is present in many bacteria and chloroplasts (6, 39, 47, 48, 53, 55). It is distinguishable from the Sec pathway by two major features. First, preproteins are directed to the Tat pathway by signal peptides that bear a characteristic sequence motif: the twin-arginine (RR) motif, which is essential for signal peptide recognition by the Tat translocation machinery (2, 3, 21). Second, proteins can be exported via Tat in a folded state, whereas the Sec pathway exclusively transports proteins in an unfolded state (10, 22, 37, 49, 62). To date, active Tat pathways have been identified in many gram-negative and gram-positive bacteria. Interestingly, these two classes of bacteria seem to contain Tat translocases of similar but not identical subunit composition. Minimal Tat translocases have been identified in gram-positive bacteria, such as B. subtilis. These translocases consist of two subunits, named TatA and TatC (26, 44). In contrast, the Tat translocases from gram-negative bacteria, mycobacteria, and streptomycetes contain an additional third subunit, named TatB. To date, the Escherichia coli TatABC system is the best characterized. The TatA, TatB, and TatC subunits are indispensable for translocase activity (3, 17, 40, 54). Notably, a fourth Tat subunit from E. coli, TatE, is a paralogue of TatA, and this subunit can complement for the absence of TatA (56). In minimal TatAC translocases, the TatA protein is thought to be bifunctional (28), performing the functions of both the TatA and TatB proteins in the TatABC translocases. Notably, two minimal TatAC systems with distinct substrate specificities have been reported for B. subtilis (26, 29, 44). These systems have been termed TatAdCd and TatAyCy. The view that the B. subtilis TatA subunits are bifunctional is supported by the recent observation that the TatAd subunit can complement for the absence of the E. coli TatA or TatB protein (1).
The genes encoding the two B. subtilis TatAC systems are located at distinct genomic positions (27). The tatCd and tatCy genes are preceded by the cognate tatAd and tatAy genes, respectively. A third tatA gene, denoted tatAc, is not genetically linked to another tat gene, and no function has been identified for the TatAc protein. The TatAyCy translocase is essential for secretion of YwbN, a protein belonging to the family of heme-containing DyP-type peroxidases (26). In contrast, the TatAdCd translocase is essential for secretion of the PhoD phosphodiesterase, which is produced exclusively upon phosphate starvation (26, 27). The phoD gene is located upstream of the tatAdCd genes, whereas the ywbN gene is not genetically linked to the tatAyCy genes.
A search for RR-signal peptides encoded by the genome of B. subtilis revealed several proteins that could potentially use the Tat pathway for secretion (25, 65). However, until now, strictly Tat-dependent secretion in B. subtilis has been demonstrated only for PhoD and YwbN. Recently it was shown that two additional RR-signal peptides, which were derived from the B. subtilis QcrA and YkuE proteins, can direct Tat-dependent secretion of agarase in Streptomyces lividans. This indicates that QcrA and YkuE are also transported by the B. subtilis Tat machinery (70). So far, there are few published data available concerning the secretion of heterologous proteins via the Tat pathway of B. subtilis. The E. coli phytase AppA was shown to be exported TatAdCd dependently when fused to the RR-signal peptide of PhoD (16), and also Tat-dependent secretion of the green fluorescent protein (GFP) was reported, albeit in an inactive form (37). Furthermore, when expressed in E. coli, the TatAdCd translocase was able to translocate the E. coli trimethylamine N-oxide reductase (TorA) or GFP fused to the DmsA or TorA RR-signal peptide (1, 38). These findings indicate that the B. subtilis Tat machinery is capable of translocating heterologous and tightly folded proteins. In the present studies, we have further explored the capabilities for transport of homologous and heterologous proteins via the Tat pathways of B. subtilis. For this purpose, fusions were made between reporter proteins that are normally secreted via the Sec system of Bacillus species or the Tat system of E. coli and the RR-signal peptides of the B. subtilis Tat substrate PhoD or YwbN.
MATERIALS AND METHODS
Plasmids, bacterial strains, and media.
Two E. coli strains were used: E. coli DH5α [supE44 ΔlacU169 (Φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] and E. coli TOP10 (F− mcrA Δ(mrr-hsdRMS-mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG; Invitrogen). All B. subtilis strains that were used are derivatives of the sequenced B. subtilis strain 168 (32). Plasmids used in this study are listed in Table 1. E. coli strains were grown on 2× TY medium (Bacto tryptone, 16 g/liter; yeast extract, 10 g/liter; and NaCl, 5 g/liter). B. subtilis strains were grown in tryptone soya broth (30 g/liter; Oxoid) or on heart infusion agar (40 g/liter; Difco). When appropriate, media were supplemented with skim milk (1.5%), starch (0.2%), spectinomycin (100 μg/ml), kanamycin (20 μg/ml), erythromycin (2 μg/ml for B. subtilis or 100 μg/ml for E. coli), chloramphenicol (5 μg/ml), and/or tetracycline (10 μg/ml). Starch was stained with Lugol's iodine solution (1 g crystalline iodine, 2 g KI, and 300 ml H2O).
TABLE 1.
Plasmids
| Plasmid | Properties | Source/reference |
|---|---|---|
| pASA | B. subtilis integration plasmid; replicates in E. coli (ColE1 ori); Tcr; see Fig. 1 | This study |
| pASA3 | pASA plasmid encoding proAprE without a signal peptide | This study |
| pASA4 | pASA plasmid encoding SPYwbN-proAprE | This study |
| pASA5 | pASA plasmid encoding SPAprE-proAprE | This study |
| pASA6 | pASA plasmid encoding SPPhoD-proAprE | This study |
| pASA4AmyL | pASA plasmid encoding SPYwbN-AmyL | This study |
| pASA3SufI | pASA plasmid encoding E. coli SufI without a signal peptide | This study |
| pASA4SufI | pASA plasmid encoding SPYwbN-SufI | This study |
| pASA5SufI | pASA plasmid encoding SPAprE-SufI | This study |
| pRACd2 | pUC21 derivative for the replacement of tatAd-tatCd; 5.7 kb; Apr Kmr | Jongbloed et al., 2002 (29) |
| pRACy3 | pUC21 derivative for the replacement of tatAy-tatCy; 5.8 kb; Apr Spr | Jongbloed et al., 2002 (29) |
| pHB201 | Shuttle vector for E. coli and B. subtilis with a p59 promoter; Emr Cmr | Bron et al., 1998 (8) |
| pHB-AyCy | pHB201 plasmid encoding the tatAy-tatCy operon | This study |
DNA techniques.
Plasmid DNA was isolated using the QIAprep spin miniprep kit (Qiagen) according to the instructions of the supplier. Procedures for DNA restriction, ligation, agarose gel electrophoresis, and transformation of E. coli were carried out as described by Sambrook et al. (50). Restriction enzymes and T4 DNA ligase were purchased from Invitrogen. PCR was carried out with High Fidelity Platinum Taq polymerase (Invitrogen). Transformation of competent B. subtilis was performed as described previously (9). Protease-deficient B. subtilis Δtat, ΔtatAdCd, and ΔtatAyCy strains were obtained by transformation of protease mutant strains with linearized pRACd2 and/or pRACy3 (25, 26). Transformants were selected for spectinomycin and/or kanamycin resistance, and tat mutations were verified by PCR.
Construction of pASA plasmids.
The plasmid pASA and its derivatives (Table 1) were constructed by ligation of various DNA fragments obtained through PCR. Part of the plasmid pCR2.1-TOPO (Invitrogen), including the ColE1 origin, was used as a basis for pASA construction. The tetracycline resistance cassette in pASA was amplified from pDG1515 (18). The aprE promoter region and aprE gene were amplified from B. subtilis 168 chromosomal DNA. In the resulting pASA plasmid (Fig. 1), signal sequences can be cloned directly downstream of the aprE ribosome binding site as XbaI-NheI fragments, without affecting the integrity of this ribosome binding site. Genes for secretory reporter proteins can be cloned using the restriction sites SstII-SpeI or SstII-NsiI. By using the NsiI site, it is possible to fuse the cloned secretory gene in-frame to a (C-terminal) His tag sequence, making it possible to detect and/or purify the target protein. The amino acid sequence ASAA (Ala-Ser-Ala-Ala) is present as the standard signal peptidase I cleavage site, linking the signal peptide and the reporter protein. Finally, pASA contains the aprE transcription terminator downstream of the target genes.
FIG. 1.
Schematic representation of plasmid pASA. pASA contains the ColE1 ori for replication in E. coli, a tetracycline resistance gene for selection in E. coli and B. subtilis, a 500-bp fragment from the B. subtilis chromosome containing the aprE promoter in front of the cloning sites for signal peptide sequences (pre) and target genes (pro-mature) and the transcription terminator of the aprE gene. Restriction sites for cloning are shown in the plasmid map, and the fusion sites for cloned signal sequences and target genes are shown underneath the plasmid map.
The signal sequences of ywbN (SPYwbN), aprE (SPAprE), and phoD (SPPhoD) were amplified from the chromosome of B. subtilis 168 and fused to the aprE gene, resulting in the plasmids pASA4, pASA5, and pASA6, respectively. Accordingly, pASA4 encoded the YwbN signal peptide (MSDEQKKPEQIHRRDILKWGAMAGAAVAIGASGLGGLAPLVASA/A); pASA5 encoded the AprE signal peptide (MRSKKLWISLLFALTLIFTMAFSNMSASA/A); and pASA6 encoded the PhoD signal peptide (MAYDSRFDEWVQKLKEESFQNNTFDRRKFIQGAGKIAGLSLGLTIAQSASA/A) (the signal peptidase I recognition sequence ASA and the +1 Ala residue of the mature protein are indicated in bold). Furthermore, pASA3 was constructed as a negative control containing the aprE gene without a signal sequence. Next, the aprE gene in pASA4 was replaced with the amyL gene from Bacillus licheniformis or the sufI gene from E. coli K12. This resulted in the plasmids pASA4AmyL and pASA4SufI, respectively. Furthermore, the copies of the aprE gene in pASA3 and pASA5 were replaced with the sufI gene, respectively, resulting in the plasmids pASA3SufI and pASA5SufI. All pASA plasmids were obtained upon transformation of E. coli TOP10 and selection for tetracycline resistance. The correct construction and absence of PCR mistakes were verified by sequencing (ServiceXS, Leiden, The Netherlands). Next, competent B. subtilis cells (aprE nprE epr ispA bpr amyE) were transformed with the different pASA plasmids. Since the pASA plasmids lack replication functions for B. subtilis, all tetracycline-resistant transformants contained a pASA copy that was integrated into the chromosomal aprE promoter region by a single-crossover recombination event. To induce expression of the signal sequence-reporter gene fusions on the chromosomally integrated pASA plasmids, the degU32(Hy) mutation was introduced into the different strains by transformation with DNA from B. subtilis MD300 and subsequent selection for tetracycline- and kanamycin-resistant transformants (35).
Construction of the pHB-AyCy plasmid.
To construct the plasmid pHB-AyCy, the B. subtilis 168 tatAy-tatCy genes were amplified by PCR as described by Jongbloed et al. (26). Next, the amplified fragment was cloned in the SmaI and EcoRI sites of pHB201 (8), resulting in pHB-AyCy. Correct amplification of the tatAy and tatCy genes was verified by sequencing.
SDS-PAGE and Western blot analysis.
sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out as described by Laemmli (33). After separation by SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane (Roche Diagnostics). Immunoblotting was performed with antibodies against SufI (kindly provided by Tracy Palmer). Bound antibodies were detected with alkaline phosphatase-labeled conjugate and the BM chromogenic Western blotting kit (Roche Diagnostics) according to the instructions of the manufacturer.
RESULTS
Tat-dependent secretion of subtilisin directed by the YwbN signal peptide.
The Tat pathway stands out in its ability to transport folded proteins across membranes, while the Sec pathway transports proteins in an unfolded state. Nevertheless, a few examples of rerouting of normally Sec-dependently secreted proteins into the Tat pathway have been described for E. coli (4, 13, 37, 66). These include the maltose-binding protein MalE and the alkaline phosphatase PhoA. However, Tat-dependent transport of these proteins to the periplasm requires their folding prior to translocation. To investigate whether a commercially highly relevant Sec-dependent protein can be rerouted into the Tat pathway of B. subtilis, we used the B. subtilis subtilisin, encoded by the aprE gene, as a reporter. To direct subtilisin toward the B. subtilis Tat machinery, this protein was fused to the genuine RR-signal peptides of B. subtilis PhoD or YwbN. The respective hybrid genes were integrated into the chromosomal aprE promoter region of a Tat-proficient (Tat+) or completely Tat-deficient (ΔTat) B. subtilis strain. Both strains contained the degU32(Hy) mutation for high-level expression of the fusion proteins and lacked five major proteases (AprE, NprE, Epr, IspA, and Bpr), allowing the detection of secreted subtilisin on skim milk plates. As shown in Fig. 2A, fusion of the YwbN signal peptide (SPYwbN) to subtilisin resulted in complete Tat-dependent secretion of subtilisin: no zone of subtilisin activity was detectable around colonies of the Tat-deficient strain producing the SPYwbN-subtilisin fusion protein, while a clear zone of subtilisin activity was detectable around colonies of the Tat-proficient strain producing this fusion protein. A completely different result was obtained when subtilisin was fused to the PhoD signal peptide (SPPhoD). In this case, active subtilisin was secreted by both the Tat-proficient strain and the ΔTat strain. Thus, the PhoD signal peptide directed the Tat-independent export of subtilisin, as was also observed for subtilisin secretion directed by its authentic signal peptide (SPAprE) (Fig. 2A). As expected, no active subtilisin was detected in the extracellular environment of cells that produced this protein without a signal peptide (Fig. 2A).
FIG. 2.
Tat-dependent secretion of subtilisin. (A) The secretion of active subtilisin by Tat-proficient (Tat+) or Tat-deficient (ΔTat) derivatives of B. subtilis aprE nprE epr ispA bpr amyE was monitored by halo formation on skim milk plates. Prosubtilisin was fused to the PhoD signal peptide (SPPhoD), the YwbN signal peptide (SPYwbN), or the signal peptide of subtilisin (SPAprE). Alternatively, prosubtilisin was produced without a signal peptide (No SP). (B) Secretion of active subtilisin directed by SPYwbN was monitored in B. subtilis tatAyCy (ΔTatAyCy), B. subtilis tatAdCd (ΔTatAdCd), B. subtilis tatAyCy with the empty vector pHB201 (ΔTatAyCy pHB201), or the complemented B. subtilis tatAyCy strain containing pHB-AyCy (ΔTatAyCy pHB-AyCy) as described for panel A.
Secretion of subtilisin with the YwbN signal peptide is TatAyCy dependent.
It was previously shown that the TatAyCy translocase is specifically required for the secretion of YwbN (26). To examine whether the subtilisin secretion directed by the YwbN signal peptide would also be TatAyCy dependent, we expressed the SPYwbN-subtilisin fusion protein in a tatAyCy or tatAdCd mutant strain. Secretion of active subtilisin was again assayed by halo formation on skim milk plates (Fig. 2B). The results showed that the tatAdCd mutant was still capable of subtilisin secretion, since clear halos were formed around colonies producing SPYwbN-subtilisin. In contrast, no halos were formed around colonies of the tatAyCy mutant expressing the SPYwbN-subtilisin fusion protein. This indicated that subtilisin secretion directed by the YwbN signal peptide is TatAyCy dependent. To confirm this idea, we investigated whether subtilisin secretion directed by the YwbN signal peptide in a tatAyCy mutant could be restored by introduction of a plasmid expressing the tatAyCy genes. This was indeed the case (Fig. 2B), showing that the YwbN signal peptide directs subtilisin secretion in a TatAyCy-dependent manner.
Tat-independent secretion of the Bacillus licheniformis α-amylase AmyL directed by the YwbN signal peptide.
To investigate whether the signal peptide of YwbN would also direct the secretion of Sec-dependent proteins other than subtilisin, we fused this signal peptide to the mature part of the Bacillus licheniformis α-amylase (AmyL). Next, secretion of AmyL directed by the YwbN signal peptide was tested in B. subtilis strains lacking either tatAyCy or tatAdCd. The secretion of active amylase by these tat mutant strains or the Tat-proficient control strain was monitored by growth on starch-containing plates and subsequent staining of the plates with iodine (Fig. 3). Unexpectedly, both tat mutant strains and the wild-type control showed similar levels of YwbN-directed secretion of the AmyL amylase. Furthermore, expression of SPYwbN-AmyL in a strain lacking both the TatAdCd and TatAyCy translocases resulted in the secretion of active α-amylase at a similar level to that observed for the Tat-proficient control strain (data not shown). This shows that the YwbN signal peptide does not direct Tat-dependent AmyL secretion in B. subtilis.
FIG. 3.
Tat-independent secretion of B. licheniformis α-amylase with the signal peptide of YwbN. The secretion of active AmyL by Tat-proficient (Tat+), TatAdCd-deficient (ΔTatAdCd), or TatAyCy-deficient (ΔTatAyCy) derivatives of B. subtilis aprE nprE epr ispA bpr amyE was visualized by halo formation on a heart infusion agar plate containing 0.2% starch. AmyL was fused to the YwbN signal peptide (SPYwbN). Alternatively, AmyL was produced without a signal peptide (No SP). After 24 h of growth at 37°C, the plates were stained with iodine.
Tat-independent secretion of E. coli SufI with the YwbN signal peptide.
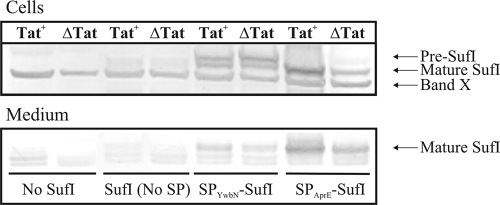
The results reported above show that the YwbN signal peptide was capable of directing both Tat-dependent and Tat-independent export of different reporter proteins that are normally secreted via the Sec pathway. This raised the question of whether the YwbN signal peptide would be capable of directing a heterologous Tat-dependent reporter protein into the B. subtilis Tat pathway. To answer this question, we employed the SufI protein from E. coli, which is targeted to the periplasm via the Tat pathway (59). SufI was fused to the YwbN signal peptide, and the secretion of SufI in B. subtilis Tat-deficient or Tat-proficient strains was analyzed by SDS-PAGE and Western blotting using cellular and growth medium fractions. As shown in Fig. 4, the YwbN signal peptide directed Tat-independent secretion of SufI in B. subtilis. Furthermore, a significant amount of the SufI produced remained cell associated. These findings suggested that the YwbN signal peptide was capable of directing at least some SufI secretion via the Sec pathway of B. subtilis, despite the fact that this protein is a Sec-incompatible Tat substrate in E. coli. To investigate whether SufI secretion in B. subtilis could be directed with a Sec-specific signal peptide, a hybrid SufI precursor was constructed using the signal peptide of subtilisin (SPAprE). This signal peptide also directed SufI secretion in a Tat-independent manner, but much more effectively than the YwbN signal peptide (Fig. 4). This was evident from the ratio of cellular SufI over secreted SufI, which was much higher for the strain expressing the SPYwbN-SufI hybrid precursor than for cells expressing the SPAprE-SufI precursor. Furthermore, the SPYwbN-SufI precursor was detectable in the cellular fraction, while the SPAprE-SufI precursor could not be detected. Nevertheless, mature SufI was clearly detectable in the cellular fraction of the SPAprE-SufI-producing Tat-proficient and Tat-deficient strains. Finally, when SufI was expressed without a signal peptide, barely any SufI protein was detectable, suggesting that it was not efficiently synthesized without a signal peptide or rapidly degraded.
FIG. 4.
Tat-independent secretion of E. coli SufI. The secretion of SufI by a Tat-proficient (Tat+) or Tat-deficient (ΔTat) derivative of B. subtilis aprE nprE epr ispA bpr amyE was analyzed by Western blotting. SufI was either not expressed (No SufI) or expressed without a signal peptide [SufI (No SP)], with the signal peptide of YwbN (SPYwbN-SufI), or with the signal peptide of subtilisin (SPAprE-SufI), as indicated. Samples were taken from cultures in stationary phase. After centrifugation, proteins in the cellular and growth medium fractions were separated by SDS-PAGE. Western blotting and immunodetection were performed with specific anti-SufI antibodies. An unidentified cross-reacting band is marked as band X.
DISCUSSION
The B. subtilis Sec system has a massive capacity for exporting a wide range of proteins into the extracellular milieu (20, 71). This pathway is particularly effective in secreting homologous proteins and proteins from closely related gram-positive species, like other bacilli. Notably, proteins transported via the Sec pathway pass the membrane translocation channel in an unfolded state, which makes these proteins vulnerable to posttranslocational degradation (64, 65, 69). Indeed, posttranslocational folding defects set important limits on the yields of heterologous proteins secreted via the Sec pathway of B. subtilis (12, 20, 31, 57). This prompted us to investigate the capabilities of the Tat pathway using Bacillus enzymes of major commercial significance as reporter proteins. Posttranslocational folding of AmyL from B. licheniformis in B. subtilis is known to be relatively inefficient, and consequently, significant amounts of translocated protein are lost due to proteolysis (60, 61). In such a case, folding in the cytoplasm and translocation via the Tat pathway might be an interesting alternative route for secretion.
Our present results show that subtilisin can indeed be retargeted from the Sec pathway to the Tat pathway, but this requires the twin-arginine signal peptide of YwbN. Consistent with the function of this signal peptide in the translocation of YwbN via the TatAyCy translocase, subtilisin was secreted via this particular Tat translocase of B. subtilis when provided with the YwbN signal peptide. The TatAdCd translocase appeared not to be involved in this process. Surprisingly, the fusion of subtilisin to the twin-arginine signal peptide of PhoD did not result in secretion via Tat. Nevertheless, subtilisin was secreted with the aid of SPPhoD, most likely via the Sec pathway. This suggests that SPPhoD does not include an effective Sec avoidance signal. Such a Sec avoidance signal function was previously shown for positively charged residues at the interface of the h and c regions of twin-arginine signal peptides (4, 5, 11, 24). It thus seems that the Lys residue located 14 residues N-terminal to the signal peptidase I recognition sequence of SPPhoD in our constructs does not efficiently serve this role. Although no exclusive TatAdCd-dependent secretion of subtilisin could be observed when this protein was fused to the PhoD signal peptide, we cannot rule out the possibility that some subtilisin was secreted via TatAdCd in parallel to secretion via Sec. These observations have interesting implications regarding subtilisin folding. The generally accepted view is that the Tat pathway translocates mainly folded proteins (52, 68), but some exceptions to this general rule appear to exist, as evidenced by the translocation of unfolded dihydrofolate reductase via the thylakoidal Tat system (22) and the Tat-dependent translocation of unstructured, small, hydrophilic proteins in E. coli (46). Folding of subtilisin is known to be coordinated by the propeptide of this protein, which acts as a folding catalyst. Thus, it is conceivable that prosubtilisin folds in the cytoplasm prior to translocation via TatAyCy if this protein is provided with the YwbN signal peptide. If so, SPYwbN might increase the folding kinetics of subtilisin. However, it is difficult to assess whether pretranslocational folding of SPYwbN-subtilisin did indeed occur or whether this precursor was translocated via TatAyCy in an unfolded state. It is not clear what happens in the case of the SPPhoD-subtilisin precursor, but three alternative explanations are conceivable. First, the TatAdCd translocase is predominantly synthesized under conditions of phosphate starvation (27), although some activity is detectable in rich media (37). Thus, the availability of TatAdCd may have been limiting under the conditions tested, which might have favored translocation via Sec. Second, the SPPhoD-subtilisin precursor may not be able to fold in the cytoplasm, resulting in rejection by TatAdCd but allowing acceptance by Sec. This would be the case, for example, if SPPhoD could not effectively increase the folding kinetics of subtilisin to allow Tat-dependent transport. Thirdly, the TatAdCd translocase may be less capable of handling proteins that do not fold effectively in the cytoplasm. Notably, these possibilities are not mutually exclusive, which makes it hard to pinpoint the reason for the observed differences. Clearly, cytoplasmic folding of proteins rerouted from Sec to Tat is a strict requirement in E. coli, as was shown for GFP (14, 51, 63), the E. coli alkaline phosphatase PhoA (13), and the E. coli maltose binding protein Mbp (4, 66). It seems therefore likely that the same will apply in B. subtilis. If this view is correct, the observed Tat incompatibility of AmyL will probably relate to an inability of this protein to fold in the cytoplasm. An important difference here is that AmyL requires extracytoplasmic folding catalysts, like the lipoprotein PrsA (30), whereas subtilisin has its own intramolecular folding catalyst, the propeptide (23).
It has been proposed that the E. coli SufI protein requires translocation via Tat, not because it is a cofactor-containing protein but rather because it folds too rapidly in the cytoplasm (48, 59). This raised the expectation that it would be fairly easy to translocate SufI via the Tat pathway of B. subtilis if this protein were provided with an appropriate Bacillus twin-arginine signal peptide. It was anticipated especially that the YwbN signal peptide might perform well for SufI translocation because this signal peptide directs strictly Tat-dependent secretion of subtilisin. Much to our surprise, however, only Tat-independent secretion of SufI was observed in B. subtilis, and substantial amounts of the SPYwbN-SufI precursor remained detectable in the cell. This protein was in fact even far better secreted when fused to the subtilisin signal peptide (SPAprE), which is a genuine Sec signal peptide. These findings suggest that SufI does not fold rapidly in the milieu of the B. subtilis cytoplasm. If so, this could be due to the following: (i) absence of an as yet unidentified folding catalyst, (ii) inhibition of SufI folding by an unidentified cytoplasmic factor, or (iii) slow cytoplasmic folding of SufI caused by the AprE and YwbN signal peptides. The observed Tat-independent secretion of SufI also raises the question of whether “Tat proofreading” occurs in B. subtilis, as reported for Tat-dependent protein transport in E. coli (36, 43, 45). This would require dedicated chaperones, such as DmsD or TorD of E. coli (34, 41, 42). Furthermore, the Tat-independent secretion of SufI directed by SPYwbN suggests that this twin-arginine signal peptide of B. subtilis, like SPPhoD, does not contain an effective Sec avoidance signal. The latter view is supported by the observed Tat-independent secretion of AmyL fused to SPYwbN and by the fact that this signal peptide completely lacks positively charged residues between the h and c regions.
Taken together, our studies with subtilisin show that a commercially important Sec-dependent protein can be rerouted into the Tat pathway of B. subtilis if it is provided with the right twin-arginine signal peptide. Nevertheless, the capability of the Tat pathway of B. subtilis to transport heterologous or rerouted “cargo” proteins or even heterologous Tat-dependent proteins like SufI seems to be fairly limited. For example, we tested GFP, tobacco etch virus protease, the Bowman-Birk inhibitor, E. coli AppA, and human trypsin without observing efficient or strictly Tat-dependent secretion (data not shown). On the other hand, inactive GFP is secreted via Tat of B. subtilis, and secretion of the E. coli phytase AppA seems possible with the signal peptide of PhoD (16). Our results suggest that cytoplasmic folding is probably a major issue for Tat-dependent protein secretion in B. subtilis, as is the case in E. coli. Ironically, rerouting from Sec to Tat also “reroutes” the folding problem from the posttranslocational stage to the pretranslocational stage. However, this may turn out to be an advantage, because it is probably easier to express appropriate heterologous folding catalysts in the cytoplasm than at the extracytoplasmic membrane surface of B. subtilis. Thus, despite the various examples of Tat incompatibility reported in our present article, we do foresee possible interesting applications for the Tat system of B. subtilis, for example, in the production of commercially relevant Sec-incompatible proteins.
Acknowledgments
We thank Tracy Palmer for providing SufI antibodies, Jan Jongbloed for the plasmids pRACd2 and pRACy3, and Roopa Ghirnikar for critical reading of the manuscript.
This work was supported in part by grants QLK3-CT-1999-00917 and LSHG-CT-2004-005257 from the European Union.
Footnotes
Published ahead of print on 17 October 2008.
REFERENCES
- 1.Barnett, J. P., R. T. Eijlander, O. P. Kuipers, and C. Robinson. 2008. A minimal Tat system from a gram-positive organism: a bifunctional TatA subunit participates in discrete TatAC and TatA complexes. J. Biol. Chem. 283:2534-2542. [DOI] [PubMed] [Google Scholar]
- 2.Berks, B. 1996. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22:12. [DOI] [PubMed] [Google Scholar]
- 3.Berks, B. C., F. Sargent, and T. Palmer. 2000. The Tat protein export pathway. Mol. Microbiol. 35:260-274. [DOI] [PubMed] [Google Scholar]
- 4.Blaudeck, N., P. Kreutzenbeck, R. Freudl, and G. A. Sprenger. 2003. Genetic analysis of pathway specificity during posttranslational protein translocation across the Escherichia coli plasma membrane. J. Bacteriol. 185:2811-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogsch, E., S. Brink, and C. Robinson. 1997. Pathway specificity for a delta pH-dependent precursor thylakoid lumen protein is governed by a ‘Sec-avoidance’ motif in the transfer peptide and a ‘Sec-incompatible’ mature protein. EMBO J. 16:3851-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogsch, E. G., F. Sargent, N. R. Stanley, B. C. Berks, C. Robinson, and T. Palmer. 1998. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J. Biol. Chem. 273:18003-18006. [DOI] [PubMed] [Google Scholar]
- 7.Bolhuis, A., H. Tjalsma, H. E. Smith, A. de Jong, R. Meima, G. Venema, S. Bron, and J. M. van Dijl. 1999. Evaluation of bottlenecks in the late stages of protein secretion in Bacillus subtilis. Appl. Environ. Microbiol. 65:2934-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bron, S., A. Bolhuis, H. Tjalsma, S. Holsappel, G. Venema, and J. M. van Dijl. 1998. Protein secretion and possible roles for multiple signal peptidases for precursor processing in bacilli. J. Biotechnol. 64:3-13. [DOI] [PubMed] [Google Scholar]
- 9.Bron, S., and G. Venema. 1972. Ultraviolet inactivation and excision-repair in Bacillus subtilis. I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet-sensitive derivatives. Mutat. Res. 15:1-10. [DOI] [PubMed] [Google Scholar]
- 10.Creighton, A. M., A. Hulford, A. Mant, D. Robinson, and C. Robinson. 1995. A monomeric, tightly folded stromal intermediate on the delta pH-dependent thylakoidal protein transport pathway. J. Biol. Chem. 270:1663-1669. [DOI] [PubMed] [Google Scholar]
- 11.Cristobal, S., J. W. de Gier, H. Nielsen, and G. von Heijne. 1999. Competition between Sec- and TAT-dependent protein translocation in Escherichia coli. EMBO J. 18:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darmon, E., R. Dorenbos, J. Meens, R. Freudl, H. Antelmann, M. Hecker, O. P. Kuipers, S. Bron, W. J. Quax, J. Y. Dubois, and J. M. van Dijl. 2006. A disulfide bond-containing alkaline phosphatase triggers a BdbC-dependent secretion stress response in Bacillus subtilis. Appl. Environ. Microbiol. 72:6876-6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLisa, M. P., D. Tullman, and G. Georgiou. 2003. Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc. Natl. Acad. Sci. USA 100:6115-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feilmeier, B. J., G. Iseminger, D. Schroeder, H. Webber, and G. J. Phillips. 2000. Green fluorescent protein functions as a reporter for protein localization in Escherichia coli. J. Bacteriol. 182:4068-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari, E., A. S. Jarnagin, and B. F. Schmidt. 1993. Commercial production of extracellular enzymes, p. 917-937. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria. American Society for Microbiology, Washington, DC.
- 16.Gerlach, R., O. Pop, and J. P. Muller. 2004. Tat dependent export of E. coli phytase AppA by using the PhoD-specific transport system of Bacillus subtilis. J. Basic Microbiol. 44:351-359. [DOI] [PubMed] [Google Scholar]
- 17.Gohlke, U., L. Pullan, C. A. McDevitt, I. Porcelli, E. de Leeuw, T. Palmer, H. R. Saibil, and B. C. Berks. 2005. The TatA component of the twin-arginine protein transport system forms channel complexes of variable diameter. Proc. Natl. Acad. Sci. USA 102:10482-10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 19.Harwood, C. R. 1992. Bacillus subtilis and its relatives: molecular biological and industrial workhorses. Trends Biotechnol. 10:247-256. [DOI] [PubMed] [Google Scholar]
- 20.Harwood, C. R., and R. Cranenburgh. 2008. Bacillus protein secretion: an unfolding story. Trends Microbiol. 16:73-79. [DOI] [PubMed] [Google Scholar]
- 21.Hinsley, A. P., N. R. Stanley, T. Palmer, and B. C. Berks. 2001. A naturally occurring bacterial Tat signal peptide lacking one of the ‘invariant’ arginine residues of the consensus targeting motif. FEBS Lett. 497:45-49. [DOI] [PubMed] [Google Scholar]
- 22.Hynds, P. J., D. Robinson, and C. Robinson. 1998. The sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J. Biol. Chem. 273:34868-34874. [DOI] [PubMed] [Google Scholar]
- 23.Inouye, M. 1991. Intramolecular chaperone: the role of the pro-peptide in protein folding. Enzyme 45:314-321. [DOI] [PubMed] [Google Scholar]
- 24.Ize, B., F. Gerard, and L. F. Wu. 2002. In vivo assessment of the Tat signal peptide specificity in Escherichia coli. Arch. Microbiol. 178:548-553. [DOI] [PubMed] [Google Scholar]
- 25.Jongbloed, J. D., H. Antelmann, M. Hecker, R. Nijland, S. Bron, U. Airaksinen, F. Pries, W. J. Quax, J. M. van Dijl, and P. G. Braun. 2002. Selective contribution of the twin-arginine translocation pathway to protein secretion in Bacillus subtilis. J. Biol. Chem. 277:44068-44078. [DOI] [PubMed] [Google Scholar]
- 26.Jongbloed, J. D., U. Grieger, H. Antelmann, M. Hecker, R. Nijland, S. Bron, and J. M. van Dijl. 2004. Two minimal Tat translocases in Bacillus. Mol. Microbiol. 54:1319-1325. [DOI] [PubMed] [Google Scholar]
- 27.Jongbloed, J. D., U. Martin, H. Antelmann, M. Hecker, H. Tjalsma, G. Venema, S. Bron, J. M. van Dijl, and J. Muller. 2000. TatC is a specificity determinant for protein secretion via the twin-arginine translocation pathway. J. Biol. Chem. 275:41350-41357. [DOI] [PubMed] [Google Scholar]
- 28.Jongbloed, J. D., R. van der Ploeg, and J. M. van Dijl. 2006. Bifunctional TatA subunits in minimal Tat protein translocases. Trends Microbiol. 14:2-4. [DOI] [PubMed] [Google Scholar]
- 29.Jongbloed, J. D. H. 2002. Specificity determinants for protein secretion in Bacillus subtilis. Ph.D. thesis. University of Groningen, Groningen, The Netherlands.
- 30.Kontinen, V. P., and M. Sarvas. 1993. The PrsA lipoprotein is essential for protein secretion in Bacillus subtilis and sets a limit for high-level secretion. Mol. Microbiol. 8:727-737. [DOI] [PubMed] [Google Scholar]
- 31.Kouwen, T. R., A. van der Goot, R. Dorenbos, T. Winter, H. Antelmann, M. C. Plaisier, W. J. Quax, J. M. van Dijl, and J. Y. Dubois. 2007. Thiol-disulphide oxidoreductase modules in the low-GC Gram-positive bacteria. Mol. Microbiol. 64:984-999. [DOI] [PubMed] [Google Scholar]
- 32.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 34.Li, S. Y., B. Y. Chang, and S. C. Lin. 2006. Coexpression of TorD enhances the transport of GFP via the TAT pathway. J. Biotechnol. 122:412-421. [DOI] [PubMed] [Google Scholar]
- 35.Mader, U., H. Antelmann, T. Buder, M. K. Dahl, M. Hecker, and G. Homuth. 2002. Bacillus subtilis functional genomics: genome-wide analysis of the DegS-DegU regulon by transcriptomics and proteomics. Mol. Genet. Genomics 268:455-467. [DOI] [PubMed] [Google Scholar]
- 36.Maillard, J., C. A. Spronk, G. Buchanan, V. Lyall, D. J. Richardson, T. Palmer, G. W. Vuister, and F. Sargent. 2007. Structural diversity in twin-arginine signal peptide-binding proteins. Proc. Natl. Acad. Sci. USA 104:15641-15646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meissner, D., A. Vollstedt, J. M. van Dijl, and R. Freudl. 2007. Comparative analysis of twin-arginine (Tat)-dependent protein secretion of a heterologous model protein (GFP) in three different Gram-positive bacteria. Appl. Microbiol. Biotechnol. 76:633-642. [DOI] [PubMed] [Google Scholar]
- 38.Mendel, S., A. McCarthy, J. P. Barnett, R. T. Eijlander, O. P. Kuipers, and C. Robinson. 2008. The Escherichia coli TatABC system and a Bacillus subtilis TatAC-type system recognise three distinct targeting determinants in twin-arginine signal peptides. J. Mol. Biol. 375:661-672. [DOI] [PubMed] [Google Scholar]
- 39.Natale, P., T. Bruser, and A. J. Driessen. 2008. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane—distinct translocases and mechanisms. Biochim. Biophys. Acta 1778:1735-1756. [DOI] [PubMed] [Google Scholar]
- 40.Oates, J., C. M. Barrett, J. P. Barnett, K. G. Byrne, A. Bolhuis, and C. Robinson. 2005. The Escherichia coli twin-arginine translocation apparatus incorporates a distinct form of TatABC complex, spectrum of modular TatA complexes and minor TatAB complex. J. Mol. Biol. 346:295-305. [DOI] [PubMed] [Google Scholar]
- 41.Oresnik, I. J., C. L. Ladner, and R. J. Turner. 2001. Identification of a twin-arginine leader-binding protein. Mol. Microbiol. 40:323-331. [DOI] [PubMed] [Google Scholar]
- 42.Papish, A. L., C. L. Ladner, and R. J. Turner. 2003. The twin-arginine leader-binding protein, DmsD, interacts with the TatB and TatC subunits of the Escherichia coli twin-arginine translocase. J. Biol. Chem. 278:32501-32506. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Rodriguez, R., A. C. Fisher, J. D. Perlmutter, M. G. Hicks, A. Chanal, C. L. Santini, L. F. Wu, T. Palmer, and M. P. DeLisa. 2007. An essential role for the DnaK molecular chaperone in stabilizing over-expressed substrate proteins of the bacterial twin-arginine translocation pathway. J. Mol. Biol. 367:715-730. [DOI] [PubMed] [Google Scholar]
- 44.Pop, O., U. Martin, C. Abel, and J. P. Muller. 2002. The twin-arginine signal peptide of PhoD and the TatAd/Cd proteins of Bacillus subtilis form an autonomous Tat translocation system. J. Biol. Chem. 277:3268-3273. [DOI] [PubMed] [Google Scholar]
- 45.Qiu, Y., R. Zhang, T. A. Binkowski, V. Tereshko, A. Joachimiak, and A. Kossiakoff. 2008. The 1.38 A crystal structure of DmsD protein from Salmonella typhimurium, a proofreading chaperone on the Tat pathway. Proteins 71:525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter, S., U. Lindenstrauss, C. Lucke, R. Bayliss, and T. Bruser. 2007. Functional tat transport of unstructured, small, hydrophilic proteins. J. Biol. Chem. 282:33257-33264. [DOI] [PubMed] [Google Scholar]
- 47.Robinson, C. 2000. The twin-arginine translocation system: a novel means of transporting folded proteins in chloroplasts and bacteria. Biol. Chem. 381:89-93. [DOI] [PubMed] [Google Scholar]
- 48.Robinson, C., and A. Bolhuis. 2001. Protein targeting by the twin-arginine translocation pathway. Nat. Rev. Mol. Cell Biol. 2:350-356. [DOI] [PubMed] [Google Scholar]
- 49.Robinson, C., and A. Bolhuis. 2004. Tat-dependent protein targeting in prokaryotes and chloroplasts. Biochim. Biophys. Acta 1694:135-147. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 51.Santini, C. L., A. Bernadac, M. Zhang, A. Chanal, B. Ize, C. Blanco, and L. F. Wu. 2001. Translocation of jellyfish green fluorescent protein via the Tat system of Escherichia coli and change of its periplasmic localization in response to osmotic up-shock. J. Biol. Chem. 276:8159-8164. [DOI] [PubMed] [Google Scholar]
- 52.Santini, C. L., B. Ize, A. Chanal, M. Muller, G. Giordano, and L. F. Wu. 1998. A novel sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 17:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sargent, F. 2007. The twin-arginine transport system: moving folded proteins across membranes. Biochem. Soc Trans. 35:835-847. [DOI] [PubMed] [Google Scholar]
- 54.Sargent, F., B. C. Berks, and T. Palmer. 2002. Assembly of membrane-bound respiratory complexes by the Tat protein-transport system. Arch. Microbiol. 178:77-84. [DOI] [PubMed] [Google Scholar]
- 55.Sargent, F., B. C. Berks, and T. Palmer. 2006. Pathfinders and trailblazers: a prokaryotic targeting system for transport of folded proteins. FEMS Microbiol. Lett. 254:198-207. [DOI] [PubMed] [Google Scholar]
- 56.Sargent, F., E. G. Bogsch, N. R. Stanley, M. Wexler, C. Robinson, B. C. Berks, and T. Palmer. 1998. Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J. 17:3640-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarvas, M., C. R. Harwood, S. Bron, and J. M. van Dijl. 2004. Post-translocational folding of secretory proteins in Gram-positive bacteria. Biochim. Biophys. Acta 1694:311-327. [DOI] [PubMed] [Google Scholar]
- 58.Schallmey, M., A. Singh, and O. P. Ward. 2004. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 50:1-17. [DOI] [PubMed] [Google Scholar]
- 59.Stanley, N. R., T. Palmer, and B. C. Berks. 2000. The twin arginine consensus motif of Tat signal peptides is involved in Sec-independent protein targeting in Escherichia coli. J. Biol. Chem. 275:11591-11596. [DOI] [PubMed] [Google Scholar]
- 60.Stephenson, K., N. M. Carter, C. R. Harwood, M. F. Petit-Glatron, and R. Chambert. 1998. The influence of protein folding on late stages of the secretion of alpha-amylases from Bacillus subtilis. FEBS Lett. 430:385-389. [DOI] [PubMed] [Google Scholar]
- 61.Stephenson, K., C. L. Jensen, S. T. Jorgensen, J. H. Lakey, and C. R. Harwood. 2000. The influence of secretory-protein charge on late stages of secretion from the Gram-positive bacterium Bacillus subtilis. Biochem. J. 350:31-39. [PMC free article] [PubMed] [Google Scholar]
- 62.Teter, S. A., and D. J. Klionsky. 1999. How to get a folded protein across a membrane. Trends Cell Biol. 9:428-431. [DOI] [PubMed] [Google Scholar]
- 63.Thomas, J. D., R. A. Daniel, J. Errington, and C. Robinson. 2001. Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol. 39:47-53. [DOI] [PubMed] [Google Scholar]
- 64.Tjalsma, H., H. Antelmann, J. D. Jongbloed, P. G. Braun, E. Darmon, R. Dorenbos, J. Y. Dubois, H. Westers, G. Zanen, W. J. Quax, O. P. Kuipers, S. Bron, M. Hecker, and J. M. van Dijl. 2004. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68:207-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tjalsma, H., A. Bolhuis, J. D. Jongbloed, S. Bron, and J. M. van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tullman-Ercek, D., M. P. DeLisa, Y. Kawarasaki, P. Iranpour, B. Ribnicky, T. Palmer, and G. Georgiou. 2007. Export pathway selectivity of Escherichia coli twin arginine translocation signal peptides. J. Biol. Chem. 282:8309-8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Dijl, J. M., P. G. Braun, C. Robinson, W. J. Quax, H. Antelmann, M. Hecker, J. Muller, H. Tjalsma, S. Bron, and J. D. Jongbloed. 2002. Functional genomic analysis of the Bacillus subtilis Tat pathway for protein secretion. J. Biotechnol. 98:243-254. [DOI] [PubMed] [Google Scholar]
- 68.Weiner, J. H., P. T. Bilous, G. M. Shaw, S. P. Lubitz, L. Frost, G. H. Thomas, J. A. Cole, and R. J. Turner. 1998. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell 93:93-101. [DOI] [PubMed] [Google Scholar]
- 69.Westers, L., H. Westers, G. Zanen, H. Antelmann, M. Hecker, D. Noone, K. M. Devine, J. M. van Dijl, and W. J. Quax. 2008. Genetic or chemical protease inhibition causes significant changes in the Bacillus subtilis exoproteome. Proteomics 8:2704-2713. [DOI] [PubMed] [Google Scholar]
- 70.Widdick, D. A., R. T. Eijlander, J. M. van Dijl, O. P. Kuipers, and T. Palmer. 2008. A facile reporter system for the experimental identification of twin-arginine translocation (Tat) signal peptides from all kingdoms of life. J. Mol. Biol. 375:595-603. [DOI] [PubMed] [Google Scholar]
- 71.Zeigler, D. R., and J. B. Perkins. 2008. Bacillus, 2nd ed. CRC Press, Boca Raton, FL.