Abstract
Legionella viability was monitored during heat shock treatment at 70°C by a flow cytometric assay (FCA). After 30 min of treatment, for 6 of the 12 strains tested, the FCA still detected 10 to 25% of cells that were viable but nonculturable (VBNC). These VBNC cells were able to produce ATP and to be resuscitated after culture on amoebae.
Legionellae are widespread in natural and manmade aquatic habitats. Sources of contamination are aerosols from showerheads, air-cooling towers, and other systems distributing water. To prevent outbreaks, surveillance of Legionella environmental contamination by use of culture methods has been set up for hot sanitary water systems in collective settings such as hospitals, hotels, and thermal spas (4). However, the findings of environmental surveillance do not always correlate with the occurrence of Legionnaires' disease, since the concentration of Legionella bacteria in environmental samples is largely underestimated when culture on GVPC medium (8, 11, 12), the reference method required by current norms (1, 16), is used. The existence of viable but nonculturable (VBNC) bacteria (13, 21, 24), which reduce the sensitivity of culture-based assays, has been demonstrated. Analysis of membrane integrity in order to distinguish between viable and dead cells in various bacterial species has been proposed (2, 14, 17, 19); some of these assays are based on double staining combining Syto 9 and propidium iodide (PI) (6, 9, 26). To date, only three studies dealing with the detection of Legionella cells by flow cytometry have been reported (15, 27, 29), and none of them analyzed the presence of VBNC cells, which was undertaken in this study.
The flow cytometric assay (FCA) was performed on a BD FACSCanto II flow cytometer (Becton Dickinson Biosciences, Le Pont-de-Claix, France). A threshold was applied on the FL1 channel to eliminate background noise, and analyses were performed at a low flow rate setting. The concentrations of the two dyes were adjusted for the optimal discrimination of green- and red-fluorescing bacteria. One microliter of 2.3 mM Syto 9 and 5 μl of 1-mg/ml PI (Invitrogen SARL, Cergy Pontoise, France) were used for cell staining. A cytogram was generated for each different Legionella strain; unstained cells and double staining of sterilized water were used to define the background noise (data not shown).
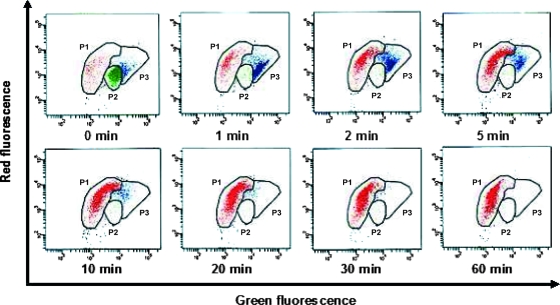
During a heat shock treatment from 0 to 60 min at 70°C, the concentration of dead cells increased proportionally with the duration of heat exposure. As shown for Legionella pneumophila serogroup 1 (sg 1) in Fig. 1, the bacteria could be segregated within three regions: dead cells stained with PI were located in the P1 region (Fig. 1, dot plot at 60 min); bacteria with intact membranes that were stained with Syto 9 were used to delineate the P2 region (Fig. 1, dot plot at 0 min); and a third population, located in the P3 region and appearing as early as 1 min after the beginning of the heat shock, represented an intermediate physiological state that could correspond to cells that were still alive but exhibited compromised membranes.
FIG. 1.
Kinetic analysis by FCA of Legionella extermination after heat shock treatment. A 3-day culture of L. pneumophila sg 1 on BCYE with 0.1% α-ketoglutarate was subjected to a heat shock at 70°C from 0 to 60 min. The bacteria were visualized on a dot plot of PI red fluorescence (FL3) versus Syto 9 green fluorescence (FL1). The P1 region corresponds to dead cells. Syto 9-stained bacteria with intact membranes were used to delineate the P2 region (viable, culturable cells). The P3 region (VBNC cells) corresponds to bacteria in an intermediate state with compromised membranes (see the text).
A total of 38 experiments using 12 clinical or environmental (isolated from contaminated water circuits) Legionella strains (L. pneumophila sg 1 [n = 7]; L. pneumophila sg 4, 6, and 13; Legionella anisa; and Legionella micdadei) were performed to compare flow cytometry and solid culture for cell quantification. The number of bacteria exhibiting intact membranes, as determined by FCA for the P2 region, was correlated with the colony counts determined either on buffered charcoal yeast extract agar (BCYE) medium (correlation coefficient [r]= 0.83; P < 0.0001) or on GVPC medium (r = 0.76; P < 0.0001). In terms of the ability to recover viable cells, FCA was found to be more sensitive than culture on either medium (P < 0.05 by Student's t test in both cases).
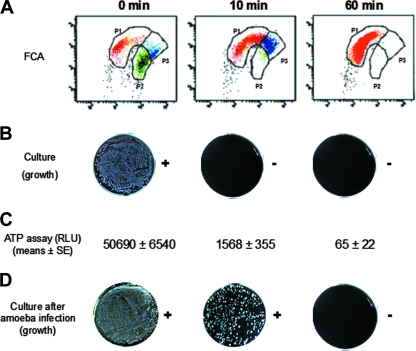
Figure 2 illustrates the production of VBNC cells during the shift of the P2 population to the P3 intermediate population when a suspension of L. pneumophila sg 1 was heated up to 70°C. The VBNC status of the cells was defined by their capacity (i) to produce ATP, as measured by the Profile-1 system (manufactured by New Horizons and purchased from Microbiodetection, Commercy, France), and (ii) to be resuscitated after passage on amoebae (13). In untreated suspensions (Fig. 2A, 0 min), most of the cells exhibiting intense green fluorescence and clustered in the P2 region were found to be culturable, infectious for amoebae, and able to produce high levels of ATP. After a heat shock of 60 min (Fig. 2A), all the cells clustered in the P1 region; no colonies were detected either after direct culture or after infection of amoebae, and no metabolic activity was detected. After a heat shock of 10 min (Fig. 2A), 70% of the bacteria clustered in the P3 region; they were found to be no longer culturable, but some of them were viable, as demonstrated by the detection of metabolic activity and by their ability to be resuscitated after intracellular multiplication in Acanthamoeba polyphaga (13). Under various stress conditions, many bacteria are able to enter a state where they are no longer culturable on standard media but still maintain metabolic activity (3, 5, 7, 18, 24). As previously shown for other bacteria (23), the present results demonstrate for the first time that FCA is a valuable tool for characterizing VBNC cells of the Legionella genus.
FIG. 2.
Evaluation of the characteristics of a suspension of L. pneumophila sg 1 PHB before (0 min) and 10 and 60 min after a heat shock treatment at 70°C. Cells were evaluated by an FCA (A), solid culture on BCYE with 0.1% α-ketoglutarate (B), ATP production (C), and solid culture on BCYE with 0.1% α-ketoglutarate after resuscitation on amoebae (D). Representative results (A, B, and D) or means ± standard errors (C) from four independent experiments are shown. RLU, relative light units; SE, standard errors.
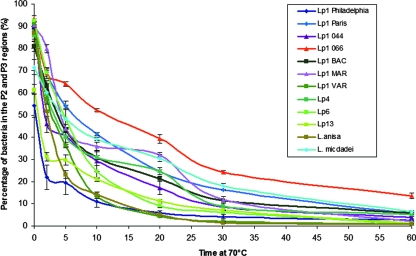
The 12 Legionella strains used in this study exhibited different heat inactivation curves as evaluated by FCA (Fig. 3). L. pneumophila sg 1 strain PHB was shown to be the most resistant, with more than 15% of cells exhibiting characteristics of viable bacteria after a heat shock exposure of 1 h at 70°C. At the opposite end, a few strains were totally killed by the heat shock in less than 30 min. Interestingly, for 6 of the 12 strains tested, 10 to 25% of the cells remained viable after a 30-min heat shock at 70°C (Fig. 3), the conditions recommended in France for the decontamination of water systems (10). These results call into question the use of plate culture methods for evaluating the efficacy of preventive or corrective actions against legionellae in the environment and may help to explain the rapid recontamination of some water systems (25, 28), despite the apparently negative results obtained by the reference cell culture protocol (1, 16), after heat shock or chlorination treatments.
FIG. 3.
Resistance patterns of various Legionella strains subjected to a heat shock treatment. After a 3-day culture on BCYE with 0.1% α-ketoglutarate, 12 Legionella strains were subjected to a heat shock treatment at 70°C from 0 to 60 min, and FCAs were performed. For each strain, the result at each time point shown is the percentage of bacteria detected in both the P2 (viable, culturable cells) and P3 (VBNC cells) regions and is expressed as the mean ± standard error from two to five independent experiments. Lp1, L. pneumophila sg 1.
In conclusion, flow cytometry opens new perspectives for environmental studies (22) of Legionella species. Further work is in progress to evaluate whether FCA could be used in association with an immunomagnetic separation assay for the specific detection of Legionella species in complex environmental samples. In combination with cell sorting (20), this assay could also help to better characterize the properties of the VBNC population, notably in terms of virulence.
Acknowledgments
This project was financially supported in part by the Agence Française de Sécurité Sanitaire de l'Environnement et du Travail (AFSSET) and by Microbiodetection SARL (Commercy, France).
We thank Charles Cervin, Claude Lambert, and Pascale Saby for valuable discussions and Philip Lawrence for revision of the English style. The Acanthamoeba polyphaga strain was a generous gift of D. Raoult (University of Marseille, Marseille, France).
Footnotes
Published ahead of print on 10 October 2008.
REFERENCES
- 1.AFNOR. 1993. Testing water-detection and enumeration of Legionella spp. and Legionella pneumophila—general method by direct culture and membrane filtration. French standard AFNOR NF T90-431. Association Française de Normalisation, Paris, France.
- 2.Alonso, J. L., S. Mascellaro, Y. Moreno, M. A. Ferrus, and J. Hernandez. 2002. Double-staining method for differentiation of morphological changes and membrane integrity of Campylobacter coli cells. Appl. Environ. Microbiol. 68:5151-5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armisen, T. G., and P. Servais. 2004. Combining direct viable count (DVC) and fluorescent in situ hybridisation (FISH) to enumerate viable E. coli in rivers and wastewaters. Water Sci. Technol. 50:271-275. [PubMed] [Google Scholar]
- 4.Atlas, R. M. 1999. Legionella: from environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1:283-293. [DOI] [PubMed] [Google Scholar]
- 5.Barer, M. R., and C. R. Harwood. 1999. Bacterial viability and culturability. Adv. Microb. Physiol. 41:93-137. [DOI] [PubMed] [Google Scholar]
- 6.Berney, M., F. Hammes, F. Bosshard, H. U. Weilenmann, and T. Egli. 2007. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight kit in combination with flow cytometry. Appl. Environ. Microbiol. 73:3283-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjergbaek, L. A., and P. Roslev. 2005. Formation of nonculturable Escherichia coli in drinking water. J. Appl. Microbiol. 99:1090-1098. [DOI] [PubMed] [Google Scholar]
- 8.Boccia, S., P. Laurenti, P. Borella, U. Moscato, G. Capalbo, A. Cambieri, R. Amore, G. Quaranta, F. Boninti, M. Orsini, G. Branca, G. Fadda, V. Romano-Spica, and G. Ricciardi. 2006. Prospective 3-year surveillance for nosocomial and environmental Legionella pneumophila: implications for infection control. Infect. Control Hosp. Epidemiol. 27:459-465. [DOI] [PubMed] [Google Scholar]
- 9.Boulos, L., M. Prevost, B. Barbeau, J. Coallier, and R. Desjardins. 1999. LIVE/DEAD BacLight: application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Methods 37:77-86. [DOI] [PubMed] [Google Scholar]
- 10.DGS. 22 April 2002. Circulaire DGS/SD7A/SD5C/DHOS/E4 no. 2002/243 du 22 avril 2002 relative à la prévention du risque lié aux légionelles dans les établissements de santé, p. 243. Direction Générale de la Santé, Paris, France.
- 11.Doleans, A., H. Aurell, M. Reyrolle, G. Lina, J. Freney, F. Vandenesch, J. Etienne, and S. Jarraud. 2004. Clinical and environmental distributions of Legionella strains in France are different. J. Clin. Microbiol. 42:458-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García, M. T., S. Jones, C. Pelaz, R. D. Millar, and Y. Abu Kwaik. 2007. Acanthamoeba polyphaga resuscitates viable non-culturable Legionella pneumophila after disinfection. Environ. Microbiol. 9:1267-1277. [DOI] [PubMed] [Google Scholar]
- 14.Hoefel, D., W. L. Grooby, P. T. Monis, S. Andrews, and C. P. Saint. 2003. Enumeration of water-borne bacteria using viability assays and flow cytometry: a comparison to culture-based techniques. J. Microbiol. Methods 55:585-597. [DOI] [PubMed] [Google Scholar]
- 15.Ingram, M., T. J. Cleary, B. J. Price, R. L. Price III, and A. Castro. 1982. Rapid detection of Legionella pneumophila by flow cytometry. Cytometry 3:134-137. [DOI] [PubMed] [Google Scholar]
- 16.ISO. 1998. Water quality-detection and enumeration of Legionella. International Organization for Standardization, Geneva, Switzerland.
- 17.Joux, F., and P. Lebaron. 2000. Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microbes Infect. 2:1523-1535. [DOI] [PubMed] [Google Scholar]
- 18.Lee, J., and R. A. Deininger. 2004. Detection of E. coli in beach water within 1 hour using immunomagnetic separation and ATP bioluminescence. Luminescence 19:31-36. [DOI] [PubMed] [Google Scholar]
- 19.Leuko, S., A. Legat, S. Fendrihan, and H. Stan-Lotter. 2004. Evaluation of the LIVE/DEAD BacLight kit for detection of extremophilic archaea and visualization of microorganisms in environmental hypersaline samples. Appl. Environ. Microbiol. 70:6884-6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nebe-von-Caron, G., P. J. Stephens, C. J. Hewitt, J. R. Powell, and R. A. Badley. 2000. Analysis of bacterial function by multi-colour fluorescence flow cytometry and single cell sorting. J. Microbiol. Methods 42:97-114. [DOI] [PubMed] [Google Scholar]
- 21.Oliver, J. D. 2005. The viable but nonculturable state in bacteria. J. Microbiol. 43(Spec. No.):93-100. [PubMed] [Google Scholar]
- 22.Phe, M. H., M. Dossot, H. Guilloteau, and J. C. Block. 2005. Nucleic acid fluorochromes and flow cytometry prove useful in assessing the effect of chlorination on drinking water bacteria. Water Res. 39:3618-3628. [DOI] [PubMed] [Google Scholar]
- 23.Sachidanandham, R., K. Y. Gin, and C. L. Poh. 2005. Monitoring of active but non-culturable bacterial cells by flow cytometry. Biotechnol. Bioeng. 89:24-31. [DOI] [PubMed] [Google Scholar]
- 24.Steinert, M., L. Emody, R. Amann, and J. Hacker. 1997. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl. Environ. Microbiol. 63:2047-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinert, M., G. Ockert, C. Luck, and J. Hacker. 1998. Regrowth of Legionella pneumophila in a heat-disinfected plumbing system. Zentralbl. Bakteriol. 288:331-342. [DOI] [PubMed] [Google Scholar]
- 26.Stocks, S. M. 2004. Mechanism and use of the commercially available viability stain, BacLight. Cytometry A 61:189-195. [DOI] [PubMed] [Google Scholar]
- 27.Temmerman, R., H. Vervaeren, B. Noseda, N. Boon, and W. Verstraete. 2006. Necrotrophic growth of Legionella pneumophila. Appl. Environ. Microbiol. 72:4323-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas, V., T. Bouchez, V. Nicolas, S. Robert, J. F. Loret, and Y. Levi. 2004. Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 97:950-963. [DOI] [PubMed] [Google Scholar]
- 29.Tyndall, R. L., R. E. Hand, Jr., R. C. Mann, C. Evans, and R. Jernigan. 1985. Application of flow cytometry to detection and characterization of Legionella spp. Appl. Environ. Microbiol. 49:852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]