Abstract
For a long time, it has been assumed that the mode of action of Cry2A toxins was unique and different from that of other three-domain Cry toxins due to their apparent nonspecific and unsaturable binding to an unlimited number of receptors. However, based on the homology of the tertiary structure among three-domain Cry toxins, similar modes of action for all of them are expected. To confirm this hypothesis, binding assays were carried out with 125I-labeled Cry2Ab. Saturation assays showed that Cry2Ab binds in a specific and saturable manner to brush border membrane vesicles (BBMVs) of Helicoverpa armigera. Homologous-competition assays with 125I-Cry2Ab demonstrated that this toxin binds with high affinity to binding sites in H. armigera and Helicoverpa zea midgut. Heterologous-competition assays showed a common binding site for three toxins belonging to the Cry2A family (Cry2Aa, Cry2Ab, and Cry2Ae), which is not shared by Cry1Ac. Estimation of Kd (dissociation constant) values revealed that Cry2Ab had around 35-fold less affinity than Cry1Ac for BBMV binding sites in both insect species. Only minor differences were found regarding Rt (concentration of binding sites) values. This study questions previous interpretations from other authors performing binding assays with Cry2A toxins and establishes the basis for the mode of action of Cry2A toxins.
Insecticidal proteins from Bacillus thuringiensis constitute the active ingredient in many biological insecticides and biotech crops expressing B. thuringiensis genes (Bt crops). For the control of lepidopteran pests, B. thuringiensis Cry1 and Cry2 class proteins are being used either in sprayable products or in transgenic plants (16, 40). Most extensively planted Bt crops express Cry1Ab (maize) or Cry1Ac (cotton), but second-generation products include the combination of Cry1Ac and Cry2Ab in cotton (16). In all cases, B. thuringiensis proteins act by ingestion, and their mode of action is so specific that they are harmless to most nontarget organisms, beneficial insects, and vertebrates (38).
The mode of action of Cry proteins has been studied for over 20 years, using a small number of selected Cry proteins belonging mainly to the Cry1 family, and a general model has been accepted (21, 40). After ingestion of the Cry protein, the solubilized protoxin is proteolytically processed to render a stable active fragment which crosses the peritrophic membrane and binds to specific sites in the brush border membrane. Specific binding is the crucial step in the mode of action for the exquisite specificity of Cry proteins (46), and binding site alteration on midgut cells is the main mechanism conferring high levels of resistance to Cry proteins in insect populations (15, 16). There is strong evidence that, after binding, Cry1A proteins form oligomers which insert into the midgut epithelium, causing cell lysis (8, 42), though the involvement of a signaling pathway has also been proposed (48, 49).
The structures of the three-domain Cry proteins Cry1Aa, Cry2Aa, Cry3Aa, Cry3Bb, Cry4Aa, and Cry4Bb have been determined by X-ray crystallography, and all these proteins share a striking three-dimensional similarity, indicating similar functions of their three domains (reviewed in references 10 and 37). Saturable binding to specific receptors has been shown amply with Cry1 proteins (reviewed in reference 37) and more limitedly with the coleopteran-active Cry3A (6, 30, 34, 41) and the dipteran-specific Cry4 and Cry11 class proteins (1, 11, 31, 35). In contrast, an early study of the mode of action of Cry2Aa proposed a unique mode of action for this protein in that binding was nonsaturable (13). The authors concluded that Cry2Aa “might bind to an essentially unlimited number of relatively low affinity sites” (13). The nonsaturable nature of Cry2A binding has been assumed or reiterated in subsequent reports (22, 23, 24). However, a close inspection of the published results reveals a contradiction with the assumption of nonsaturable binding (3, 23, 24, 25, 28).
Taking into account the species-specific toxicity of Cry2A proteins (25, 26), the above-referenced published data on Cry2Aa binding analyses, and the strikingly similar three-dimensional structures of this protein and the other three-domain Cry proteins, we hypothesized that Cry2A proteins would likely also bind saturably and with high affinity to specific sites in the midgut epithelium. The goals of the present study were to show that Cry2Ab binds saturably to specific sites in Helicoverpa armigera and Helicoverpa zea, two important cotton pests which are targets of the second-generation cotton expressing B. thuringiensis Cry1Ac and Cry2Ab, and to determine whether such sites are shared with other Cry proteins, such as Cry1Ac, Cry2Aa, and Cry2Ae.
MATERIALS AND METHODS
Toxin purification and activation of Cry1Ac and Cry2Ab toxins.
B. thuringiensis strain HD73 from the Bacillus Genetic Stock Collection (Columbus, OH) expressing Cry1Ac was grown in casein hydrolysate-yeast extract medium (44) at 28.5°C with continuous shaking and air supplementation for 48 h. The pelleted insoluble fraction was washed twice with 1 M NaCl, 10 mM EDTA and once with 10 mM KCl. Cry1Ac crystals were solubilized in freshly prepared carbonate buffer (50 mM Na2CO3-NaHCO3, 10 mM dithiothreitol, pH 10.5) and incubated at room temperature with shaking at 150 rpm for 2.5 h. Insoluble debris was discarded by centrifugation at 25,000 × g for 10 min at 4°C. The solubilized Cry1Ac protoxin was activated by incubation with trypsin (Sigma T-8642) with a trypsin-to-protein ratio of 1:10 (wt/wt) at 37°C for 2 h. After centrifugation at 25,000 × g for 10 min at 4°C, the supernatant was dialyzed in buffer A (20 mM Tris-HCl, pH 8.65) and filtered prior to anion-exchange purification in a MonoQ 5/5 column using an ÄKTA chromatography system (GE Healthcare, United Kingdom). A continuous gradient of buffer B (20 mM Tris-HCl, 1 M NaCl, pH 8.65), up to 60%, was used to elute the Cry1Ac-activated toxin.
Strain ECE126 from the Bacillus Genetic Stock Collection (Columbus, OH) expressing Cry2Aa was grown and activated as Cry1Ac except that solubilization was performed in NEE buffer (50 mM Na2CO3, 5 mM EDTA, 10 mM EGTA, pH 12.1). The activated Cry2Aa protein showed an apparent molecular mass between 55 and 60 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
The recombinant B. thuringiensis strain IPS78/11 expressing Cry2Ab2 was grown in C2 medium (12) containing 6 μg/ml chloramphenicol at 28°C with shaking at 80 rpm for 47 h. Following two wash steps in phosphate-buffered saline (PBS; 8 mM Na2HPO4, 2 mM KH2PO4, 150 mM NaCl, pH 7.4) to which 250 mM NaCl was added, the cell pellet was solubilized in NEE buffer. Trypsin was added to a final concentration of 0.3 mg/ml out of freshly prepared 25 mg trypsin/ml, and the mixture was incubated at 37°C for 75 min and centrifuged. The activated Cry2Ab protein showed an apparent molecular mass slightly less than 50 kDa by SDS-PAGE. The Cry2Ab toxin solution was precipitated with ammonium sulfate, and the resulting pellet was dissolved in TEE buffer (50 mM Tris-HCl, 5 mM EDTA, 10 mM EGTA, pH 8.6).
The recombinant B. thuringiensis subsp. berliner 1715 Cry− mutant (Institut Pasteur, Paris, France) harboring plasmid pGA32 expressing Cry2Ae was grown in C2 medium containing erythromycin at 20 μg/ml at 28°C with shaking at 80 rpm for 144 h. Following two wash steps in PBS plus 250 mM NaCl, the cell pellet was solubilized in alkaline buffer (0.1 M CAPS [3-(cyclohexylamino)-1-propanesulfonic acid], 10 mM EGTA, 5 mM EDTA, pH 12.0) and incubated for 1 h at 37°C. Trypsin was added (at a 3:1 [wt/wt] ratio) out of freshly prepared 7.5 mg trypsin/ml, and the mixture was incubated overnight at 37°C and centrifuged. The activated Cry2Ae protein showed an apparent molecular mass of about 60 kDa by SDS-PAGE. The Cry2Ae toxin solution was dialyzed against PBS.
Bioassays.
Seven different concentrations of activated Cry1Ac and Cry2Ab proteins were tested using 16 neonate larvae of H. armigera for each concentration. A volume of 50 μl of the sample dilutions was applied on the surface of the artificial diet dispensed in multiple-well plates. One larva was placed in each well. The plates were incubated at 25 ± 2°C, with a relative humidity of 65% ± 5% and a photoperiod of 16 h:8 h (light/dark). Mortality was evaluated after 7 days. Toxicity data were analyzed using the POLO-PC probit analysis program (LeOra Software, Berkeley, CA).
Midgut isolation and BBMV preparation.
Last-instar larvae of H. armigera (ANGR strain; CSIRO Entomology, Australia) and H. zea (USDA-ARS, Stoneville, MS) were dissected, and the midguts were preserved at −80°C until required. Brush border membrane vesicles (BBMVs) were prepared by the differential magnesium precipitation method (47), frozen in liquid nitrogen, and stored at −80°C. The protein concentration in the BBMV preparations was determined by the Bradford method (7) using bovine serum albumin (BSA) as standard.
Radiolabeling of Cry toxins.
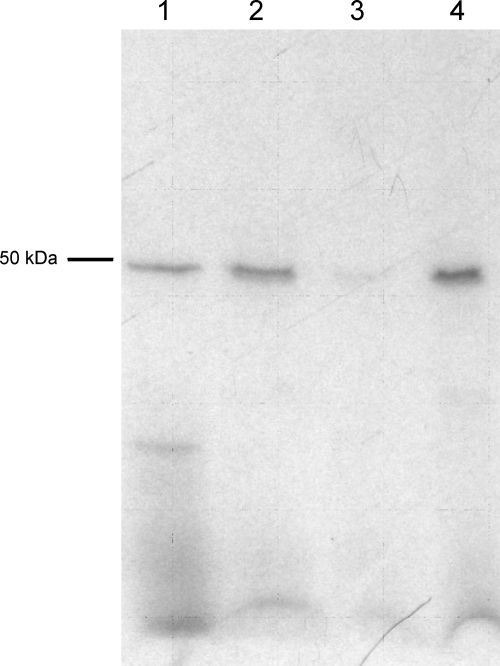
Cry1Ac and Cry2Ab toxins were labeled using the chloramine T method. Na125I (0.5 mCi) (PerkinElmer, Boston, MA) was added to 25 μg of Cry toxin in the presence of 1/3 (vol/vol) of 18 mM of chloramine T in PBS. After incubation for 45 s, the reaction was stopped by the addition of 1/4 (vol/vol) of 23 mM potassium metabisulfite in H2O. Finally, 1/4 (vol/vol) of 1 M NaI was added, and the mixture was loaded onto a PD10 desalting column (GE HealthCare, United Kingdom) equilibrated with column buffer (20 mM Tris-HCl, 150 mM NaCl, 0.1% BSA). The purity of the labeled protein was checked by analyzing the elution fractions by SDS-PAGE with further exposure of the dry gel to an X-ray film. The specific activity of the labeled toxin was calculated based on the input toxin, the radioactivity eluting in the protein peak, and the percentage of radioactivity in the toxin band versus that in minor bands as revealed by SDS-PAGE (Fig. 1). The estimated specific activities of 125I-Cry1Ac and 125I-Cry2Ab toxins were 3 mCi/mg and 2.4 mCi/mg, respectively.
FIG. 1.
Autoradiography of 125I-Cry2Ab binding to BBMVs from H. armigera. 125I-Cry2Ab was incubated with BBMVs in the absence or presence of an excess of competitor, and the pellet produced from centrifuging the binding reaction mixture was subjected to SDS-PAGE and exposed to an X-ray film for a week. Lane 1, 125I-Cry2Ab toxin; lane 2, 125I-Cry2Ab incubated with BBMVs in the absence of competitor; lane 3, homologous competition (excess of unlabeled Cry2Ab); lane 4, heterologous competition with Cry1Ac.
Binding assays with 125I-labeled Cry1Ac and Cry2Ab.
Prior to being used, BBMVs were centrifuged for 10 min at 16,000 × g and resuspended in binding buffer (8 mM Na2HPO4, 2 mM KH2PO4, 150 mM NaCl, pH 7.4, 0.1% BSA). Saturation experiments were carried out by incubating 20 μg of BBMVs from H. armigera with increasing amounts of 125I-Cry2Ab in binding buffer for 1 h at 25°C. After incubation, samples were centrifuged at 16,000 × g for 10 min and the pellet was washed twice with 500 μl of cold binding buffer. The radioactivity retained in the pellet was measured in an LKB 1282 Compugamma CS gamma counter. Nonspecific binding was determined by adding an excess of unlabeled Cry2Ab (1 μM) to the reaction mixture. Specific binding was calculated by subtracting the nonspecific binding from the total binding.
To determine the optimal concentration of BBMVs for use in competition experiments, increasing amounts of BBMVs were incubated with 0.4 nM and 1.2 nM of labeled Cry1Ac and Cry2Ab, respectively, in a final volume of 0.1 ml of binding buffer for 1 h at 25°C. An excess of unlabeled toxin was used to calculate the nonspecific binding.
Competition experiments were done by incubating either 20 μg of BBMVs and 1 nM 125I-Cry2Ab or 5 μg of BBMVs and 0.6 nM 125I-Cry1Ac for 1 h at 25°C in the presence of increasing amounts of unlabeled toxins. For quantitative assays, the fraction of labeled toxin bound to BBMVs was determined in a gamma counter. The dissociation constant (Kd) and concentration of binding sites (Rt) were calculated using the LIGAND program (33). For qualitative assays, pellets were boiled for 10 min in loading buffer and run in SDS-PAGE. The labeled toxin retained in the pellet was detected by autoradiography after a 1-week exposure.
RESULTS
Toxicity of Cry1Ac and Cry2Ab proteins to H. armigera.
The toxicity of the Cry1Ac and Cry2Ab preparations was tested. Activated Cry1Ac and Cry2Ab proteins were toxic to H. armigera larvae, and the 50% lethal concentrations are shown in Table 1.
TABLE 1.
Toxicity of Cry1Ac- and Cry2Ab-activated proteins to neonate larvae of H. armigera (measured after 7 days)a
| Protein | LC50 (ng/cm2) | 95% FL | Slope ± SE |
|---|---|---|---|
| Cry1Ac | 123 | 23-257 | 1.5 ± 0.3 |
| Cry2Ab | 314 | 112-515 | 2.6 ± 0.6 |
LC50, 50% lethal concentration; FL, fiducial limits at the 95% level.
Specific binding of 125I-Cry2Ab to H. armigera BBMVs.
As a first approach, specific binding of Cry2Ab was tested by incubating BBMVs from H. armigera with radiolabeled Cry2Ab (Fig. 1). Although other labeled contaminants were present in the 125I-Cry2Ab preparation, only the band corresponding to Cry2Ab bound to the BBMVs. An excess of unlabeled Cry2Ab drastically reduced the binding of 125I-Cry2Ab, indicating that most of this binding was specific. In contrast, an excess of unlabeled Cry1Ac did not decrease the binding of labeled Cry2Ab, indicating that Cry2Ab binding sites are not recognized by Cry1Ac.
Saturation of 125I-Cry2Ab binding to H. armigera BBMVs.
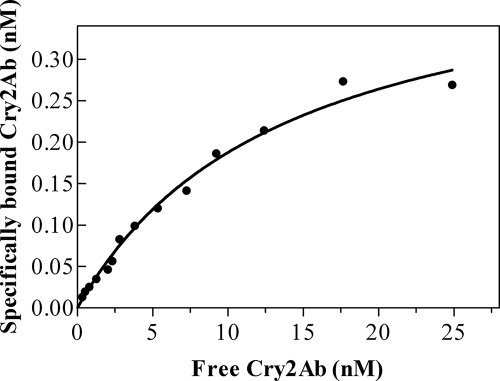
Binding of Cry2Ab was shown to be saturable by incubating H. armigera BBMVs with increasing concentrations of labeled Cry2Ab. Under the conditions used (0.2 mg BBMV protein/ml), the curve deviated from linearity starting approximately at 5 nM 125I-Cry2Ab and reached a maximum at approximately 20 nM (Fig. 2).
FIG. 2.
Saturation of 125I-Cry2Ab-specific binding to H. armigera BBMVs. A fixed amount of BBMVs (20 μg of vesicle proteins) was incubated with increasing amounts of 125I-Cry2Ab for 1 h. The binding reaction was stopped by centrifugation, and the radioactivity retained in the pellet was measured. Nonspecific binding was calculated by incubating with an excess of unlabeled Cry2Ab and subtracted from the total binding.
Competition experiments with H. armigera BBMVs.
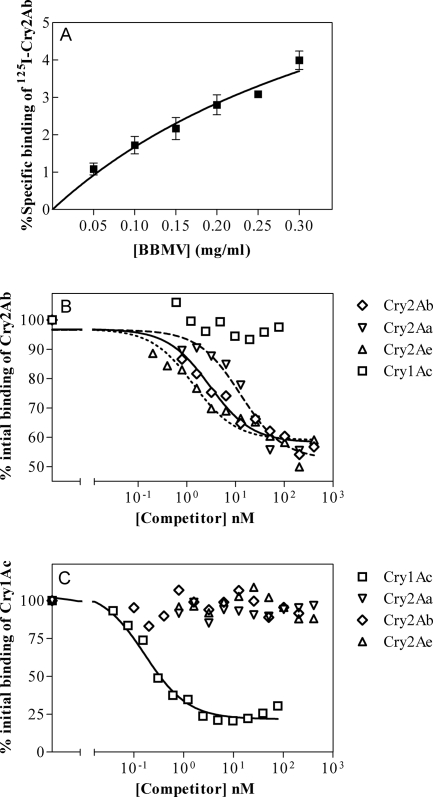
To find out the optimal concentration of BBMVs for competition binding experiments, a fixed concentration of 125I-Cry2Ab was incubated with increasing concentrations of H. armigera BBMVs. As expected, after subtraction of the nonspecific binding, an increase in specific binding was observed corresponding to the increase in binding sites (Fig. 3A). A concentration of 0.2 mg/ml of BBMVs was chosen to perform competition binding experiments. A similar experiment carried out with 125I-Cry1Ac indicated an optimal BBMV concentration of 0.05 mg/ml for competition experiments (data not shown).
FIG. 3.
Binding of 125I-Cry2Ab to H. armigera BBMVs. (A) Specific binding of 125I-Cry2Ab to increasing concentrations of BBMVs. Nonspecific binding was calculated in the presence of an excess of unlabeled Cry2Ab. Data points correspond to the mean of five replicates using two independent 125I-Cry2Ab batches. (B) Competition experiments with 125I-Cry2Ab. (C) Competition experiments with 125I-Cry1Ac. In competition assays, each data point represents the mean of at least two independent replicates.
The 125I-Cry2Ab displacement observed with unlabeled Cry2Ab protein in homologous-competition assays confirmed that binding of Cry2Ab in this insect is specific and saturable (Fig. 3B). Under our experimental conditions, a high level of nonspecific binding was observed. Competition binding assays using Cry2Aa and Cry2Ae as heterologous competitors showed that these Cry proteins readily competed with 125I-Cry2Ab (Fig. 3B). In contrast, unlabeled Cry1Ac was unable to compete for 125I-Cry2Ab binding in the range of concentrations tested (higher concentrations of Cry1Ac than those shown in Fig. 3B resulted in precipitation of 125I-Cry2Ab). These results indicate that Cry2Ab binding sites are shared by the other two Cry2 toxins but not by Cry1Ac.
Competition assays were also carried out with 125I-Cry1Ac using Cry1Ac, Cry2Ab, Cry2Aa, and Cry2Ae as competitors (Fig. 3C). None of the Cry2A proteins competed for binding with 125I-Cry1Ac, confirming the occurrence of different binding sites for Cry1Ac and these Cry2A proteins.
Binding parameters, Kd and Rt, were calculated for Cry2Ab and Cry1Ac from homologous-competition curves (Table 2). In both cases, homologous-competition data fit a single-site model equation. As shown in Table 2, Cry2Ab has slightly more specific binding sites than Cry1Ac in H. armigera, but the affinity of Cry2Ab is lower than the affinity of Cry1Ac for their respective binding sites.
TABLE 2.
Kd and Rt calculated from the homologous-competition assays with BBMVs from H. armigera and H. zea
| Toxin | Mean ± SEM fora:
|
|||
|---|---|---|---|---|
|
H. armigera
|
H. zea
|
|||
| Kd (nM) | Rt (pmol/mg)b | Kd (nM) | Rt (pmol/mg)b | |
| Cry1Ac | 0.08 ± 0.02 | 0.96 ± 0.10 | 0.17 ± 0.04 | 10 ± 2 |
| Cry2Ab | 3.1 ± 0.5 | 2.5 ± 0.3 | 5.9 ± 1.5 | 2.7 ± 0.5 |
Values for Cry1Ac were obtained from two replicates, and values for Cry2Ab were obtained from at least four replicates.
Values are expressed in picomoles per milligram of BBMV protein.
Radiolabeling of Cry2Ab and Cry1Ac was done twice independently, and all the experiments described above were repeated with the second preparation of radiolabeled Cry1Ac and Cry2Ab. Results obtained with the second set of radiolabeled toxins were similar to those described above.
Specific binding of Cry2Ab to H. zea BBMVs.
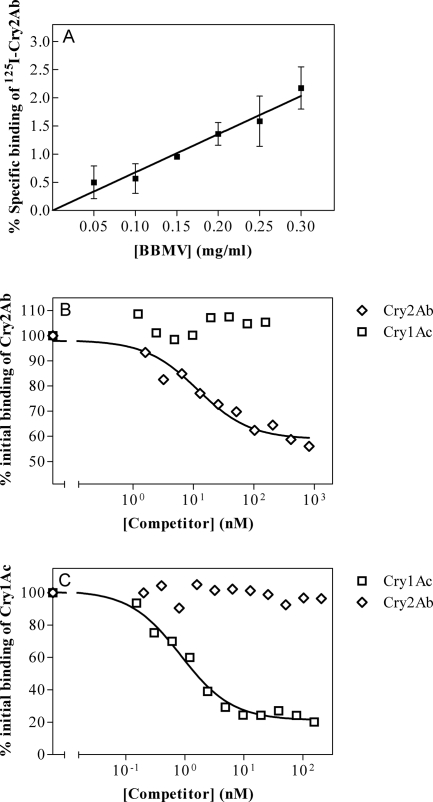
To confirm the model of binding sites obtained from experiments for H. armigera, binding assays were also performed for H. zea. In this insect, specific binding was also observed when increasing amounts of BBMVs were incubated with 125I-Cry2Ab. The percentage of bound toxin in H. zea was lower than that in H. armigera when the same range of BBMV concentrations was used (compare Fig. 4A and 3A).
FIG. 4.
Binding of 125I-Cry2Ab to H. zea BBMVs. (A) Specific binding of 125I-Cry2Ab to increasing concentrations of BBMVs. Nonspecific binding was calculated in the presence of an excess of unlabeled Cry2Ab. (B) Competition experiments with 125I-Cry2Ab. (C) Competition experiments with 125I-Cry1Ac. Each data point represents the mean of at least two independent replicates.
125I-Cry2Ab was used to perform homologous- and heterologous-competition assays for H. zea. As in H. armigera, unlabeled Cry2Ab competed for 125I-Cry2Ab binding sites (Fig. 4B). The homologous competition indirectly demonstrated saturable binding of Cry2Ab in this insect, since the curve reflects a limited number of binding sites in the concentration range of unlabeled competitor. As in H. armigera, about half of the total binding corresponded to nonspecific binding. In the heterologous-competition assay, Cry1Ac did not compete with 125I-Cry2Ab.
Experiments with 125I-Cry1Ac showed no competition of Cry2Ab for Cry1Ac binding sites (Fig. 4C), demonstrating the existence of different binding sites for Cry2Ab and Cry1Ac also in H. zea.
DISCUSSION
The first and only in-depth study so far carried out on the mode of action of Cry2A proteins was done by English et al. (13), using Cry2Aa and H. zea BBMVs. In that study, English et al. proposed that Cry2Aa binding was nonsaturable, suggesting that this toxin binds to an unlimited number of low-affinity sites and setting forward the idea that this was a unique mode of action among δ-endotoxins. However, we hypothesized that the mode of action of Cry2A toxins, and in particular the binding step, would in fact be quite similar to that of other Cry toxins. First, like other Cry toxins, Cry2A crystal inclusions can be solubilized to inactive protoxins that become active toxins upon proteolytic cleavage (5). Second, the global tertiary structure of Cry2Aa is strikingly similar to those of Cry1Aa, Cry3Aa, Cry3Bb, Cry4Aa, and Cry4Bb (10). In that sense, the three-domain organization shared by Cry proteins is also present in Cry2A toxins, suggesting that Cry2A proteins may display functional properties similar to those of other Cry proteins. The N-terminal domain of Cry2A proteins contains a bundle of α helices that likely act as a transmembrane region (27, 32). This feature is related to the final step in the mode of action of Cry toxins: the insertion of the toxin into the microvilli of apical membranes that leads to the formation of lytic pores (4, 9). A putative receptor binding epitope in domain II of Cry2A has been predicted (32). This domain is implicated in binding to specific sites in Cry1A toxins (9). Third, Cry2A proteins also display a restricted spectrum of toxicity, which appears difficult to reconcile with nonspecific binding. Based on these observations, we wanted to verify if Cry2A proteins do indeed bind nonsaturably, as proposed originally by English et al. (13) and later by other authors as well.
Saturation binding experiments carried out with Cry2Ab showed that there are a limited number of specific receptors in the membrane of H. armigera midgut cells. An analogous saturation assay with labeled Cry1Ac revealed that, with the same concentration of BBMVs, saturation of binding sites was achieved using four times less toxin than that in the Cry2Ab assay (data not shown). Our results are in contrast with previous results from Cry2Aa saturation binding assays which suggested nonsaturable binding (13, 22). There are a number of potential explanations for the observation of a more- or less-linear curve in those Cry2Aa saturation experiments. First, these authors used labeled Cry2Aa protoxins rather than activated toxins. Moreover, in one study, the labeling was performed with denatured Cry2Aa protein, whereas it was not verified that Cry2Aa can withstand a cycle of denaturation and renaturation under the conditions used (13). Second, buffers were used at a pH value at which the Cry2A protoxins are not soluble (43). In saturation experiments, the low solubility and tendency to aggregate of Cry2A proteins (36) may lead to a linear increase of recovered radioactivity due to precipitation. Third, the concentration range of Cry2Aa toxin used in saturation experiments might not have been sufficient to saturate binding sites. The range used was the same as that for Cry1Ac toxin, but due to the lower affinity of Cry2A toxins, a higher concentration of Cry2Aa toxin may have been required to show saturation of the binding sites. Fourth, the concentration of BBMVs used might have been inadequate to distinguish specific binding. High levels of nonspecific binding of Cry2A toxins (to vesicle components and/or vials or filters) might have masked specific binding if the concentration of binding sites was not high enough.
In some studies with 125I-Cry2A toxins, the BBMV binding ability assay (i.e., a fixed concentration of labeled toxin and increasing amounts of BBMVs) has been presented as a 125I-Cry2A saturation assay (i.e., fixed BBMV concentration and increasing amounts of labeled toxin) (23, 24, 28). Saturability of binding sites cannot be determined if the concentration of binding sites is changing along the experiment. Therefore, the classification of Cry2A binding as saturable (28) or nonsaturable (23, 24) was based on the wrong type of experiment in these studies. The present study is the first report describing the saturable binding of a Cry2 toxin based on the proper saturation experiment.
Homologous-competition assays with Cry2Ab in H. armigera and H. zea in the present study confirm the existence of a limited number of binding sites. Indeed, if Cry2A binding was nonsaturable, there would be an essentially infinite number of binding sites available for binding, and competition of the unlabeled toxin with the labeled toxin for these sites would not be practically possible. In fact, indirect evidence of saturable binding of Cry2A toxins, even if the original interpretation of the results was different, has been found in homologous-competition experiments reported for different insect species (3, 23, 24, 25, 28). Nevertheless, in agreement with some of these studies, a large percentage of Cry2Ab binding in our experiments seems to be nonspecific and, therefore, nonsaturable. It is very likely, though, that most of the radioactivity catalogued as nonspecific binding is actually radioactivity coming from precipitated labeled Cry2Ab. Under our experimental conditions, these two alternatives cannot be distinguished.
The analysis of binding parameters from homologous-competition assays gave Kd values that were around 35 times higher for Cry2Ab toxin than for Cry1Ac in both Helicoverpa species, indicating a lower binding affinity of Cry2Ab. However, differences between toxins in terms of concentration of binding sites were much lower (around threefold). The Kd values for Cry2Ab and Cry1Ac calculated from competition assays were similar to those calculated from saturation experiments in H. armigera (data not shown). Mandal et al. (29) suggested that the low receptor binding affinity of Cry2A toxin reported by some authors is attributed to the presence of an extra N-terminal region in this toxin.
To our knowledge, this is the first report on binding competition assays among Cry2A proteins. Heterologous-competition experiments with labeled Cry2Ab and unlabeled Cry2Aa and Cry2Ae toxins showed that these proteins share common binding sites in H. armigera. In addition, high-affinity competition between Cry1Ac and Cry2A proteins in H. armigera and H. zea was not found, in agreement with results previously reported (14, 24, 28). In these insects, high-affinity competition between Cry2A toxins and other toxins belonging to the Cry1A family was also not found (14, 24, 28). Based on all these results, we propose for these two Helicoverpa species a model with independent binding sites for Cry1A and Cry2A toxins. This model may be extended to other insect species for which heterologous-competition experiments have shown the absence of competition between Cry1A and Cry2A toxins (3, 18, 22, 23, 24, 25, 39). In these studies, it was reported that competition either did not occur or occurred only at very high concentrations of competitor, suggesting that in some cases, the binding sites of Cry1A toxins may function as low-affinity binding sites of Cry2A toxins and vice versa.
The model of a common binding site for Cry2A toxins that is not shared by the common binding site of Cry1A toxins is supported by cross-resistance studies. No relevant cross-resistance has been found between toxins from these two Cry families in most of the species tested (2, 20, 28, 45). Cross-resistance between Cry1Ac and Cry2Aa has been described only in Heliothis virescens (17, 19, 22). However, in these studies, alteration of a common receptor for Cry1A and Cry2A was found not to be responsible for resistance, and therefore, a common mechanism in some other step in the mode of action of both toxins must be responsible for the observed cross-resistance.
In summary, our results, in agreement with some indirect evidence from the other authors discussed above, clearly show that Cry2A proteins bind saturably and with high affinity to specific sites in H. armigera and H. zea BBMVs. In addition, the high-affinity binding sites are shared among Cry2Aa, Cry2Ab, and Cry2Ae but not with Cry1Ac. This situation is similar to that of Cry1A proteins, for which at least one common high-affinity binding site has been reported for Cry1Aa, Cry1Ab, and Cry1Ac in all insect species tested. The demonstration that Cry2A proteins have a high-affinity binding site different from that of Cry1Ac, and most likely from all Cry1A proteins, has important implications for insect resistance management: it offers a biochemical explanation of why cross-resistance between Cry1A and Cry2A proteins is so rare and provides solid support for the resistance management strategy of combining cry1A and cry2A genes in the same crop.
Footnotes
Published ahead of print on 17 October 2008.
REFERENCES
- 1.Abdullah, M. A., A. P. Valaitis, and D. H. Dean. 2006. Identification of a Bacillus thuringiensis Cry11Ba toxin-binding aminopeptidase from the mosquito, Anopheles quadrimaculatus. BMC Biochem. 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhurst, R. J., W. James, L. J. Bird, and C. Beard. 2003. Resistance to the Cry1Ac delta-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 96:1290-1299. [DOI] [PubMed] [Google Scholar]
- 3.Alcantara, E. P., R. M. Aguda, A. Curtiss, D. H. Dean, and M. B. Cohen. 2004. Bacillus thuringiensis delta-endotoxin binding to brush border membrane vesicles of rice stem borers. Arch. Insect Biochem. Physiol. 55:169-177. [DOI] [PubMed] [Google Scholar]
- 4.Aronson, A. I., and Y. Shai. 2001. Why Bacillus thuringiensis insecticidal toxins are so effective: unique features of their mode of action. FEMS Microbiol. Lett. 195:1-8. [DOI] [PubMed] [Google Scholar]
- 5.Audtho, M., A. P. Valaitis, O. Alzate, and D. H. Dean. 1999. Production of chymotrypsin-resistant Bacillus thuringiensis Cry2Aa1 δ-endotoxin by protein engineering. Appl. Environ. Microbiol. 65:4601-4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belfiore, C. J., R. K. Vadlamudi, Y. A. Osman, and L. A. Bulla, Jr. 1994. A specific binding protein from Tenebrio molitor for the insecticidal toxin of Bacillus thuringiensis subsp. tenebrionis. Biochem. Biophys. Res. Commun. 200:359-364. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. J. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.Bravo, A., I. Gómez, J. Conde, C. Muñoz-Garay, J. Sánchez, R. Miranda, M. Zhuang, S. S. Gill, and M. Soberón. 2004. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta 1667:38-46. [DOI] [PubMed] [Google Scholar]
- 9.Bravo, A., S. S. Gill, and M. Soberón. 2005. Bacillus thuringiensis mechanisms and use, p. 175-206. In Comprehensive molecular insect science. Elsevier B.V., St. Louis, MO.
- 10.Bravo, A., S. Gill, and M. Soberón. 2007. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Barros Moreira Beltrão, H., and M. H. Silva-Filha. 2007. Interaction of Bacillus thuringiensis svar. israelensis Cry toxins with binding sites from Aedes aegypti (Diptera: Culicidae) larvae midgut. FEMS Microbiol. Lett. 266:163-169. [DOI] [PubMed] [Google Scholar]
- 12.Donovan, W. P., C. C. Dankocsik, M. P. Gilbert, M. C. Gawron-Burke, R. G. Groat, and B. C. Carlton. 1988. Amino acid sequence and entomocidal activity of the P2 crystal protein: an insect toxin from Bacillus thuringiensis var. kurstaki. J. Biol. Chem. 263:561-567. [PubMed] [Google Scholar]
- 13.English, L., H. L. Robbins, M. A. Von Tersch, C. A. Kulesza, D. Ave, D. Coyle, C. S. Jany, and S. L. Slatin. 1994. Mode of action of CryIIA: a Bacillus thuringiensis delta-endotoxin. Insect Biochem. Mol. Biol. 24:1025-1035. [Google Scholar]
- 14.Estela, A., B. Escriche, and J. Ferré. 2004. Interaction of Bacillus thuringiensis with larval midgut binding sites of Helicoverpa armigera (Lepidoptera: Noctunidae). Appl. Environ. Microbiol. 70:1378-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferré, J., and J. Van Rie. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47:501-533. [DOI] [PubMed] [Google Scholar]
- 16.Ferré, J., J. Van Rie, and S. C. MacIntosh. 2008. Insecticidal genetically modified crops and insect resistance management (IRM), p. 41-85. In J. Romeis, A. M. Shelton, and G. G. Kennedy (ed.), Integration of insect-resistant genetically modified crops within IPM programs. Springer Science and Business Media, Dordrecht, The Netherlands.
- 17.Gahan, L. J., Y. T. Ma, M. L. Coble, F. Gould, W. J. Moar, and D. G. Heckel. 2005. Genetic basis of resistance to Cry1Ac and Cry2Aa in Heliothis virescens (Lepidoptera: Noctuidae). J. Econ. Entomol. 98:1357-1368. [DOI] [PubMed] [Google Scholar]
- 18.González-Cabrera, J., B. Escriche, B. E. Tabashnik, and J. Ferré. 2003. Binding of Bacillus thuringiensis toxins in resistant and susceptible strains of pink bollworm (Pectinophora gosypiella). Insect Biochem. Mol. Biol. 33:929-935. [DOI] [PubMed] [Google Scholar]
- 19.Gould, F., Martínez-Ramírez, A. Anderson, J. Ferré, F. J. Silva, and W. J. Moar. 1992. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc. Natl. Acad. Sci. USA 89:7986-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould, F., A. Anderson, A. Reynolds, L. Bumgarner, and W. Moar. 1995. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high level of resistance to Bacillus thuringiensis toxins. J. Econ. Entomol. 88:1545-1559. [Google Scholar]
- 21.Höfte, H., and H. R. Whiteley. 1989. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 53:242-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jurat-Fuentes, J. L., F. L. Gould, and M. J. Adang. 2003. Dual resistance to Bacillus thuringiensis Cry1Ac and Cry2Aa toxins in Heliothis virescens suggests multiple mechanisms of resistance. Appl. Environ. Microbiol. 69:5898-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karim, S., and D. H. Dean. 2000. Toxicity and receptor binding properties of Bacillus thuringiensis delta-endotoxins to the midgut brush border membrane vesicles of the rice leaf folders, Cnaphalocrocis medinalis and Marasmia patnalis. Curr. Microbiol. 41:276-283. [DOI] [PubMed] [Google Scholar]
- 24.Karim, S., S. Riazuddin, F. Gould, and D. H. Dean. 2000. Determination of receptor binding properties of Bacillus thuringiensis delta-endotoxins to cotton bollworm (Helicoverpa zea) and pink bollworm (Pectinophora gossypiella) midgut brush border membrane vesicles. Pestic. Biochem. Physiol. 67:198-216. [Google Scholar]
- 25.Lee, M. K., R. M. Aguda, M. B. Cohen, F. L. Gould, and D. H. Dean. 1997. Determination of binding of Bacillus thuringiensis δ-endotoxin receptors to rice stem borer midguts. Appl. Environ. Microbiol. 63:1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao, C., D. Heckel, and R. Akhurst. 2002. Toxicity of Bacillus thuringiensis insecticidal proteins for Helicoverpa armigera and Helicoverpa punctigera (Lepidoptera: Noctuidae), major pests of cotton. J. Invertebr. Pathol. 80:55-63. [DOI] [PubMed] [Google Scholar]
- 27.Lin, Y., G. Fang, and F. Cai. 2008. The insecticidal crystal protein Cry2Ab10 from Bacillus thuringiensis: cloning, expression, and structure simulation. Biotechnol. Lett. 30:513-519. [DOI] [PubMed] [Google Scholar]
- 28.Luo, S., K. Wu, Y. Tian, G. Liang, X. Feng, J. Zhang, and Y. Guo. 2007. Cross-resistance studies of Cry1Ac-resistant strains of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry2Ab. J. Econ. Entomol. 100:909-915. [DOI] [PubMed] [Google Scholar]
- 29.Mandal, C. C., S. Gayen, A. Basu, K. S. Ghosh, S. Dasgupta, M. K. Maiti, and S. K. Sen. 2007. Prediction-based protein engineering of domain I of Cry2A entomocidal toxin of Bacillus thuringiensis for the enhancement of toxicity against lepidopteran insects. Protein Eng. Des. Sel. 20:599-606. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Ramírez, A., and M. D. Real. 1996. Proteolytic processing of Bacillus thuringiensis CryIIIA toxin and specific binding to brush-border membrane vesicles of Leptinotarsa decemlineata (Colorado potato beetle). Pestic. Biochem. Physiol. 54:115-122. [Google Scholar]
- 31.Moonsom, S., U. Chaisri, W. Kasinrerk, and C. Angsuthanasombat. 2007. Binding characteristics to mosquito-larval midgut proteins of the cloned domain II-III fragment from the Bacillus thuringiensis Cry4Ba toxin. J. Biochem. Mol. Biol. 40:783-790. [DOI] [PubMed] [Google Scholar]
- 32.Morse, R. J., T. Yamamoto, and R. M. Stroud. 2001. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure 9:409-417. [DOI] [PubMed] [Google Scholar]
- 33.Munson, P., and D. Rodbard. 1980. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 107:220-239. [DOI] [PubMed] [Google Scholar]
- 34.Ochoa-Campuzano, C., M. D. Real, A. C. Martínez-Ramírez, A. Bravo, and C. Rausell. 2007. An ADAM metalloprotease is a Cry3Aa Bacillus thuringiensis toxin receptor. Biochem. Biophys. Res. Commun. 362:437-442. [DOI] [PubMed] [Google Scholar]
- 35.Pérez, C., L. E. Fernández, J. Sun, J. L. Folch, S. S. Gill, M. Soberón, and A. Bravo. 2005. Bacillus thuringiensis subsp. israelensis Cyt1Aa synergizes Cry11Aa toxin by functioning as a membrane-bound receptor. Proc. Natl. Acad. Sci. 102:18303-18308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perlak, F. J., M. Oppenhuizen, K. Gustafson, R. Voth, S. Sivasupramaniam, D. Heering, B. Carey, R. A. Ihrig, and J. K. Roberts. 2001. Development and commercial use of Bollgard cotton in the USA—early promises versus today's reality. Plant J. 27:489-501. [DOI] [PubMed] [Google Scholar]
- 37.Pigott, C. R., and D. J. Ellar. 2007. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 71:255-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romeis, J., M. Meissle, and F. Biegler. 2006. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat. Biotechnol. 24:63-71. [DOI] [PubMed] [Google Scholar]
- 39.Ruíz de Escudero, I., A. Estela, B. Escriche, and P. Caballero. 2007. Potential of the Bacillus thuringiensis toxin reservoir for the control of Lobesia botrana (Lepidoptera: Tortricidae), a major pest of grape plants. Appl. Environ. Microbiol. 73:337-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slaney, A. C., H. L. Robbins, and L. English. 1992. Mode of action of Bacillus thuringiensis toxin CryIIIA: an analysis of toxicity in Leptinotarsa decemlineata (Say) and Diabrotica undecimpunctata howardi Barber. J. Insect Biochem. Mol. Biol. 22:9-18. [Google Scholar]
- 42.Soberón, M., L. E. Fernández, C. Pérez, S. S. Gill, and A. Bravo. 2007. Mode of action of mosquitocidal Bacillus thuringiensis toxins. Toxicon 49:597-600. [DOI] [PubMed] [Google Scholar]
- 43.Staples, N., D. Ellar, and N. Crickmore. 2001. Cellular localization and characterization of the Bacillus thuringiensis Orf2 crystallization factor. Curr. Microbiol. 42:388-392. [DOI] [PubMed] [Google Scholar]
- 44.Stewart, G. S., K. Johnstone, E. Hagelberg, and D. J. Ellar. 1981. Commitment of bacterial spores to germinate. Biochem. J. 198:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabashnik, B. E., T. J. Dennehy, M. A. Sims, K. Larkin, G. P. Head, W. J. Moar, and Y. Carrière. 2002. Control of resistant pink bollworm (Pectinophora gossypiella) by transgenic cotton that produces Bacillus thuringiensis toxin Cry2Ab. Appl. Environ. Microbiol. 68:3790-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Rie, J., S. Jansens, H. Höfte, D. Degheele, and H. Van Mellaert. 1990. Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis δ-endotoxins. Appl. Environ. Microbiol. 56:1378-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfersberger, M. G., P. Lüthy, P. Maurer, P. Parenti, V. F. Sacchi, B. Giordana, and G. M. Hanozet. 1987. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae). Comp. Biochem. Physiol. 86:301-308. [Google Scholar]
- 48.Zhang, X., M. Candas, N. B. Griko, L. Rose-Young, and L. A. Bulla, Jr. 2005. Cytotoxicity of Bacillus thuringiensis Cry1Ab toxin depends on specific binding of the toxin to the cadherin receptor BT-R(1) expressed in insect cells. Cell Death Differ. 12:1407-1416. [DOI] [PubMed] [Google Scholar]
- 49.Zhang, X., M. Candas, N. B. Ginko, R. Taussig, and L. A. Bulla, Jr. 2006. A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by Cry1Ab toxin of Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 103:9897-9902. [DOI] [PMC free article] [PubMed] [Google Scholar]