Abstract
The sequencing of Aspergillus genomes has revealed that the products of a large number of secondary metabolism pathways have not yet been identified. This is probably because many secondary metabolite gene clusters are not expressed under normal laboratory culture conditions. It is, therefore, important to discover conditions or regulatory factors that can induce the expression of these genes. We report that the deletion of sumO, the gene that encodes the small ubiquitin-like protein SUMO in A. nidulans, caused a dramatic increase in the production of the secondary metabolite asperthecin and a decrease in the synthesis of austinol/dehydroaustinol and sterigmatocystin. The overproduction of asperthecin in the sumO deletion mutant has allowed us, through a series of targeted deletions, to identify the genes required for asperthecin synthesis. The asperthecin biosynthesis genes are clustered and include genes encoding an iterative type I polyketide synthase, a hydrolase, and a monooxygenase. The identification of these genes allows us to propose a biosynthetic pathway for asperthecin.
Secondary metabolites are a remarkably rich source of medically useful compounds. A survey of the literature on 1,500 secondary metabolites isolated and characterized between 1993 and 2001 revealed that over half of these compounds have antibacterial, antifungal, or antitumor activity (19). Fungal secondary metabolites, in particular, include a number of important compounds, such as penicillin, cephalosporin, the antihypercholesterolemic agent lovastatin and other statins, and immunosuppressants such as cyclosporine, as well as antifungals (reviewed in references 3, 13, 16, and 19). Other fungal secondary metabolites are important not for their benefits but rather for the problems they cause. For example, the carcinogenic toxins aflatoxin and sterigmatocystin are produced by members of the genus Aspergillus (see references 7, 28, and 29 and earlier references therein; reviewed additionally in references 1 and 26).
The sequencing of fungal genomes has revealed several important things about fungal secondary metabolism. First, as was suspected from the results of previous work (13), the genes of pathways that produce particular secondary metabolites are often clustered together (10, 14, 18). Second, fungi have many more secondary metabolism pathways than was previously thought. Analyses of the A. nidulans genome, for example, indicate that A. nidulans has 50 clusters that are predicted to synthesize secondary metabolites (27 polyketides, 14 nonribosomal peptides, 6 fatty acids, 1 terpene, and 2 indole alkaloids) (3, 18). Our own genomic analyses suggest that this number may be a slight overestimate (because more than one polyketide synthase [PKS] and/or nonribosomal peptide synthetase may be involved in a single pathway), but clearly A. nidulans has the ability to synthesize many secondary metabolites. Only a limited number of secondary metabolites in A. nidulans have been identified (aspyridones A and B [from a single pathway], aspoquinolones A to D [probably products of a single pathway], penicillin, asperlin, sterigmatocystin, triacetylfusarinine, ferricrocin, shamixanthone, variecoxanthone, terrequinone A, emericellin, dehydroaustinol, and the sterols, ergosterol, peroxiergosterol, and cerevisterol [3, 15, 20]). It follows that the majority of A. nidulans secondary metabolites remain to be identified.
These data raise the obvious question of why such a large fraction of A. nidulans secondary metabolites (and for that matter, those of other species of Aspergillus as well) remain undiscovered. A likely reason is that most secondary metabolism gene clusters are expressed at very low levels under standard lab growth conditions and that their products are, thus, at sufficiently low levels that they are difficult to detect or identify. It is thus of great importance to find conditions that lead to the expression of secondary metabolites. One strategy has been to use genetic and molecular genetic approaches to increase the expression of individual metabolites or several metabolites (3-5). In this regard, we were curious as to whether sumoylation might play a part in secondary metabolite regulation in A. nidulans. SUMO is a small ubiquitin-like protein that is added posttranslationally to a number of proteins in the cell. It plays a role in many types of regulation, including the regulation of transcription (reviewed in references 11, 12, and 24), and thus might be expected to play a role in the regulation of secondary metabolite production.
In A. nidulans, there is a single SUMO gene, sumO (designated AN1191.3 in the Broad Institute system; http://www.broad.mit.edu/annotation/genome/aspergillus_group/MultiHome.html). It is not essential, and the deletion of sumO causes only a slight inhibition of growth (27). We have deleted sumO and examined secondary metabolite production in deletion mutant and control strains. We found that the deletion of sumO dramatically altered the production of certain secondary metabolites. The production of austinol, dehydroaustinol, and sterigmatocystin decreased, while the production of a compound that we have now identified as asperthecin dramatically increased. The overproduction of asperthecin in a sumO deletion strain has allowed us, through a series of targeted deletions, to identify and characterize the asperthecin synthase gene cluster.
MATERIALS AND METHODS
Fungal strains, growth conditions, and molecular genetic manipulations.
A. nidulans strains used in this study are listed in Table 1. The construction of fusion PCR products, protoplast production, and transformation were carried out as described previously (23). For the construction of the fusion PCR fragments, two ∼1,000-bp fragments of genomic A. nidulans DNA, upstream and downstream of the targeted gene, were amplified by PCR. Primers used in this study are listed in Table S2 in the supplemental material. A fusion PCR was set up with the two amplified flanking sequences and the A. fumigatus pyrG selectable marker cassette as the template DNA. The three fragments were fused into a single molecule and amplified with two nested primers (see Table S2 in the supplemental material). sumO was deleted from the nkuAΔ strain TN02A7 (17), creating strain LO2059. The fact that LO2059 carried a correct sumO deletion and no additional integrations of transforming DNA was confirmed by Southern hybridization and diagnostic PCR (data not shown). Subsequent sumO knock-ins and deletions in LO2059 were made (Table 1). Diagnostic PCR analyses of the deletion mutant and wild-type (WT) strains were performed with the external primers used in the first round of PCR. The difference in the size between the gene replaced by the selective marker and the native gene allowed the determination of correct gene replacement. In cases in which the sizes of the WT and deletion mutant products were similar, the diagnostic PCR was performed using one of the external primers and the primer located inside the marker gene. In those cases, the deletion mutant gave the PCR product of the expected size while no product was present in non-deletion mutants.
TABLE 1.
A. nidulans strains used in this studya
| Fungal strain or transformant(s) | sumO and/or secondary metabolite mutation(s) | Genotype |
|---|---|---|
| TN02A7 | None (WT) | pyrG89 pyroA4 nkuA::argB riboB2 |
| LO2059 | sumOΔ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG |
| LO2083 to LO2087 | sumO_ki | pyrG89 wA::sumO+-AfriboB pyroA4 nkuA::argB riboB2 sumO::AfpyrG |
| LO2099 to LO2101 | sumOΔ AN0150Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN0150::AfpyroA |
| LO2104 to LO2106 | sumOΔ AN0523Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN0523::AfpyroA |
| LO2109 to LO2111 | sumOΔ AN1034Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN1034::AfpyroA |
| LO2114 to LO2116 | sumOΔ AN2032Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN2032::AfpyroA |
| LO2119 to LO2121 | sumOΔ AN3230Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN3230::AfpyroA |
| LO2124 to LO2126 | sumOΔ AN3386Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN3386::AfpyroA |
| LO2129 to LO2131 | sumOΔ AN6000Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN6000::AfpyroA |
| LO2134 to LO2136 | sumOΔ AN6448Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN6448::AfpyroA |
| LO2139 to LO2141 | sumOΔ AN7071Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN7071::AfpyroA |
| LO2144 to LO2146 | sumOΔ AN7909Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN7909::AfpyroA |
| LO2423 to LO2425 | sumOΔ AN5998Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN5998::AfpyroA |
| LO2428 to LO2430 | sumOΔ AN5999Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN5999::AfpyroA |
| LO2433 to LO2435 | sumOΔ AN6001Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN6001::AfpyroA |
| LO2438 to LO2440 | sumOΔ AN6002Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN6002::AfpyroA |
| LO2443 to LO2445 | sumOΔ AN6003Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN6003::AfpyroA |
| LO2448 to LO2450 | sumOΔ AN6004Δ | pyrG89 pyroA4 nkuA::argB riboB2 sumO::AfpyrG AN6004::AfpyroA |
Multiple (in principle identical) transformants were used in analyses of secondary metabolite production. Thus, for example, LO2083 to LO2087 are five transformants that have the same genotype. AfriboB, AfpyrG, and AfpyroA are A. fumigatus genes (17) used for the replacement of A. nidulans genes. All strains were produced in this study except for TN02A7 (17).
Fermentation and LC-MS analysis.
A. nidulans control and deletion strains were cultivated at 37°C on solid YAG (complete medium; 5 g of yeast extract/liter, 15 g of agar/liter, and 20 g of d-glucose/liter [9] supplemented with a 1.0-ml/liter trace element solution [25] and required supplements) at 22.5 × 106 spores per 15-cm plate (with ∼40 ml of medium per plate). After 5 days, agar was chopped into small pieces and the material was extracted with 50 ml of methanol (MeOH) followed by 50 ml of 1:1 CH2Cl2-MeOH, each with 1 h of sonication. The extract was evaporated in vacuo to yield a residue, which was suspended in H2O (50 ml), and this suspension was then partitioned with ethyl acetate (EtOAc) twice. The combined EtOAc layer was evaporated in vacuo, redissolved in MeOH (1 mg/ml), and injected with 10 μl for high-performance liquid chromatography (HPLC)-photodiode array detection-mass spectrometry (MS) analysis. liquid chromatography-MS (LC-MS) was carried out using a ThermoFinnigan LCQ advantage ion trap mass spectrometer with a reverse-phase C18 column (2.1 by 100 mm with a 3-μm particle size; Alltech Prevail) at a flow rate of 125 μl/min. The solvent gradient for HPLC was 95% acetonitrile (MeCN)-H2O (solvent B) in 5% MeCN-H2O (solvent A), both containing 0.05% formic acid, as follows: 0% solvent B from 0 to 5 min, 0 to 100% solvent B from 5 to 35 min, 100% solvent B from 35 to 40 min, 100 to 0% solvent B from 40 to 45 min, and reequilibration with 0% solvent B from 45 to 50 min. For quantification, negative-ion electrospray ionization (ESI) was used for the detection of the austinol, dehydroaustinol, asperthecin, and emericellamides. The positive mode was used for the detection of sterigmatocystin. Linear curves for each compound were generated using extract ion chromatograms (EIC) at the molecular weight of each corresponding parent ion. The extracts were prepared so that the concentration of each compound lay within the standard curves. Conditions for MS included a capillary voltage of 5.0 kV, a sheath gas flow rate at 60 arbitrary units, an auxiliary gas flow rate at 10 arbitrary units, and an ion transfer capillary temperature of 350°C.
Isolation and identification of secondary metabolites.
For structure elucidation, 20 YAG plates were inoculated with WT A. nidulans, the cultures were grown for 5 days at 37°C, and extract was obtained with EtOAc as described above. The crude extract was applied to a Merck Si gel column (230 to 400 mesh; ASTM) and eluted with 300-ml CHCl3-MeOH mixtures of increasing polarity (fraction A, 1:0; fraction B, 19:1; fraction C, 9:1; and fraction D, 7:3). Fraction B was further purified by normal-phase HPLC with a Phenomenex Luna column (250 by 10 mm with a 5-μm Si particle size) at a flow rate of 5.0 ml/min and measured by a UV detector at 254 nm using isocratic 1:1 EtOAc-hexane to afford austinol (16.2 mg; retention time [tR], 9.4 min) and dehydroaustinol (24.7 mg; tR = 8.5 min). Twenty YAG plates inoculated with A. nidulans sumOΔ mutants were used to purify asperthecin as described above. Fractions C and D, which contained asperthecin, were further purified by reverse-phase HPLC with a Phenomenex Luna C18 column (5-μm particle size; 250 by 21.2 mm) at a flow rate of 10.0 ml/min and measured by a UV detector at 254 nm. The gradient system was MeCN (solvent B) in 5% MeCN-H2O (solvent A), both containing 0.05% trifluoroacetic acid, as follows: 20 to 50% solvent B from 0 to 20 min, 50 to 100% solvent B from 20 to 21 min, 100% solvent B from 21 to 26 min, 100 to 20% solvent B from 26 to 27 min, and reequilibration with 20% solvent B from 27 to 34 min. Asperthecin (12.0 mg) was eluted at 18.0 min.
Compound identification.
Infrared (IR) spectra were recorded on a PerkinElmer 983G spectrophotometer. 1H and 13C nuclear magnetic resonance (NMR) spectra were collected on a Varian Mercury Plus 400 spectrometer.
Austinol.
Data for austinol were as follows: colorless solid; IR (ZnSe) spectrum, 3,368, 1,778, 1,726, 1,688, 1,538, 1,292, and 1,217 cm−1; for UV and ESI-MS spectra, see Fig. S1 in the supplemental material. 1H and 13C NMR data were in good agreement with published data (21).
Dehydroaustinol.
Data for dehydroaustinol were as follows: colorless solid; IR (ZnSe) spectrum, 3,400, 1,748, 1,711, 1,378, 1,275, 1,119, and 1,065 cm−1; for UV and ESI-MS spectra, see Fig. S1 in the supplemental material. 1H and 13C NMR data were in good agreement with published data (15).
Asperthecin.
Data for asperthecin were as follows: purple powder; IR (ZnSe) spectrum, 3,306, 1,605, 1,447, 1,266, 1,192, and 1,140 cm−1; for UV and ESI-MS spectra, see Fig. S1 in the supplemental material. For 1H and 13C NMR data, see Table S1 in the supplemental material.
RESULTS
Creation of a sumO deletion strain.
For accuracy of gene targeting, we wished to work with strains carrying a deletion of the nkuA gene (17). To determine if the deletion of nkuA alters the secondary metabolite profile of a strain, we compared the profile of a control nkuA+ strain with that of the nkuAΔ strain TN02A7. We detected no differences in the secondary metabolite profiles of the strains (see Fig. S6 in the supplemental material), and we have used TN02A7 as the parental strain for our studies.
A single gene, the sumO gene (27), encodes SUMO in A. nidulans. We deleted sumO by replacing it with the A. fumigatus pyrG gene. We used a fusion PCR approach to create the transforming fragment (23). The deletion of sumO (sumOΔ) was verified by diagnostic PCR and Southern hybridizations (Table 1 and data not shown).
Analysis of the metabolite profile of the sumOΔ strain.
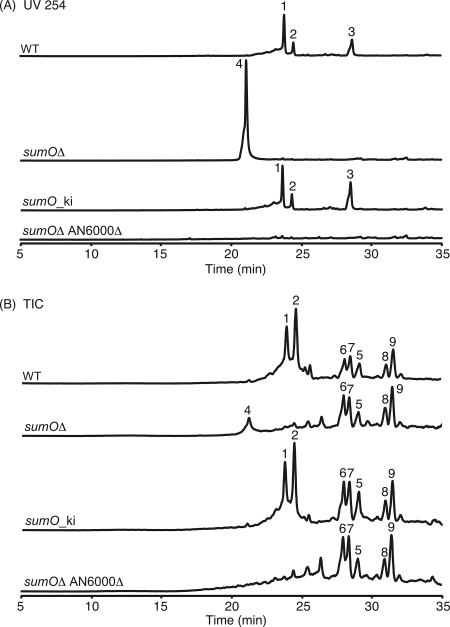
The secondary metabolite profiles of the organic extracts from a sumOΔ strain and a sumO+ control strain were analyzed by HPLC-photodiode array detection-MS. In the control strain, three UV-active peaks could be detected clearly at a UV wavelength of 254 nm (Fig. 1A). Compounds 1 and 2 were isolated and characterized by both one- and two-dimensional NMR (data not shown), UV-Vis, and mass spectrum analyses (see Fig. S1 in the supplemental material). These compounds were determined to be austinol (21) and dehydroaustinol (15), respectively (Fig. 2). Compound 3, which had a molecular weight of 324 (see Fig. S1 in the supplemental material), was identified as sterigmatocystin (Fig. 2), a known major metabolite of A. nidulans, and this identification was also confirmed by coinjection with a commercially available standard. Thus, the three compounds corresponded to austinol (tR = 23.5 min), dehydroaustinol (tR = 24.2 min), and sterigmatocystin (tR = 28.4 min). In the sumOΔ strain, the level of production of the three compounds detected in the WT strain decreased significantly, while another compound detectable at a UV wavelength of 254 nm was produced in copious quantities. The large amount of compound 4 produced in the sumOΔ strain resulted in the overloading of the column and slight shouldering in the compound peak. This result suggests that, directly or indirectly, sumoylation is necessary for the expression of the austinol/dehydroaustinol and the sterigmatocystin biosynthetic pathways and that the absence of SUMO leads to the repression of these two secondary metabolism pathways.
FIG. 1.
(A) HPLC profiles of extracts as detected by UV absorption at 254 nm. (B) Total ion chromatograms (TIC). Levels of production by the different strains were at the same order of magnitude. WT, sumO+ strain; sumOΔ, sumO deletion mutant; sumO_ki, sumOΔ strain complemented by the integration of a functional copy of sumO at the white (wA) locus; sumOΔ AN6000.3Δ, double deletion mutant.
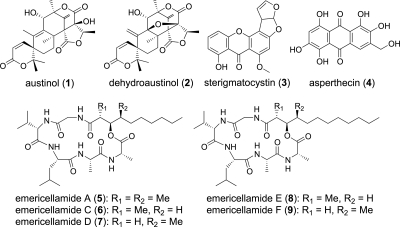
FIG. 2.
Structures of austinol (1), dehydroaustinol (2), sterigmatocystin (3), asperthecin (4), emericellamide A (5), emericellamide C (6), emericellamide D (7), emericellamide E (8), and emericellamide F (9).
Structural characterization of the upregulated compound was accomplished by extensive spectral analysis. 1H NMR (see Table S1 and Fig. S4 in the supplemental material) exhibited two methylene protons (δH 4.58, s, 2H), one D2O exchangeable hydroxyl proton (δH 5.43), two aromatic protons (δH 6.63 and 7.93), and five D2O exchangeable phenolic protons (δH 10.67, 11.75, 12.50, 12.54, and 13.69). The 13C NMR spectrum revealed 15 carbon resonances with chemical shifts consistent with a polyhydroxylated anthraquinone (see Table S1 and Fig. S5 in the supplemental material). The proton detected by heteronuclear multiple-quantum coherence and heteronuclear multiple-bond correlation (see Fig. S2 in the supplemental material) measurements allowed the structure to be fully elucidated as asperthecin (Fig. 2), a compound not known to be produced by A. nidulans. UV-Vis and mass spectra (see Fig. S1 in the supplemental material) also confirmed the assigned structure (6). Asperthecin was first detected in a different Aspergillus species (2), and because no NMR data could be found in the literature, the full structural characterization of asperthecin is presented in Table S1 in the supplemental material. For comparison, the amounts of each metabolite isolated from the WT control and sumOΔ strains were determined by using a standard solution of each compound and analyzing EIC by LC-MS (Table 2). Our analysis shows that asperthecin was produced by the WT control strain in microgram range quantities per 15-cm plate, providing an explanation as to why this compound has not been identified previously in A. nidulans. The asperthecin biosynthetic pathway is, thus, an example in which sumoylation normally functions, directly or indirectly, in the repression of production. In addition to compounds that are active at a UV wavelength of 254 nm, we examined compounds not active at the 254-nm UV wavelength in the total ion chromatogram trace (Fig. 1B). We observed that the emericellamide family of cyclopeptides (compounds 5 to 9) (Fig. 2) that we have recently described (8) is produced by the WT strain. Analysis of a sumOΔ strain showed that emericellamides were produced in amounts comparable to those in the WT strain (Table 2). Sumoylation is, thus, not a nonspecific regulator of all secondary metabolism but regulates specific secondary metabolism pathways.
TABLE 2.
Quantification of secondary metabolites on YAG plates
| Compound name | Mean concn ± SEM (μg/plate) for:
|
|
|---|---|---|
| WT control (TN02A7) | sumOΔ (LO2059) strain | |
| Austinol | 1,057.7 ± 97.0 | 50.4 ± 4.3 |
| Dehydroaustinol | 1,592.0 ± 101.5 | 157.7 ± 8.3 |
| Sterigmatocystin | 38.6 ± 13.0 | 0.7 ± 0.3 |
| Asperthecin | 4.2 ± 1.5a | 771.4 ± 151.0 |
| Emericellamide A | 9.2 ± 1.5 | 9.0 ± 1.6 |
| Emericellamide C | 16.6 ± 0.5 | 32.4 ± 4.8 |
| Emericellamide D | 33.6 ± 1.1 | 47.2 ± 5.6 |
| Emericellamide E | 9.1 ± 3.4 | 9.9 ± 2.9 |
| Emericellamide F | 63.1 ± 6.8 | 61.5 ± 6.2 |
The concentration was below the lowest concentration of standard used (0.08 μg/ml) and was estimated by the extrapolation of linear regression data.
To verify that the observed changes in secondary metabolite production were due specifically to sumOΔ, we crossed sumOΔ out into different genetic backgrounds and found that the changes in secondary metabolite production followed sumOΔ in the crosses. For further confirmation, we transformed the wA locus with a fragment carrying a functional sumO gene. As anticipated, the restoration of a functional sumO gene reversed the changes in secondary metabolite production seen in the sumOΔ strains (Fig. 1).
Identification of the asperthecin biosynthetic pathway.
The copious production of asperthecin in sumOΔ strains, coupled with the sequencing of the A. nidulans genome (10), allowed us to identify for the first time the asperthecin pathway. Examination of the A. nidulans genome reveals that it contains 27 secondary metabolite gene clusters with at least one PKS gene. The aromatic structure of asperthecin suggests that a nonreducing PKS (NR-PKS) is responsible for its biosynthesis. NR-PKS contains ketosynthase, acyltransferase, and acyl carrier protein domains. The domain structures of the 27 PKS proteins were examined, and 10 potential gene candidates (AN0150.3, AN0523.3, AN1034.3, AN2032.3, AN3230.3, AN3386.3, AN6000.3, AN6448.3, AN7071.3, and AN7909.3) were selected for deletion experiments. Using the gene-targeting procedures we have recently developed for A. nidulans (17, 23), we created deletions for each of the target genes in the sumOΔ strain background (Table 1). Extracts from the 10 PKS deletion strains were analyzed, and only the AN6000.3 deletion mutant failed to produce asperthecin, while the nine other PKS deletion strains still produced asperthecin. This result allowed us to identify AN6000.3 as the gene encoding the PKS responsible for the biosynthesis of asperthecin (Fig. 1 and 3 and data not shown). We designate this gene aptA. Broad Institute sequence data reveal that aptA is located on chromosome I, in contig 102 (http://www.broad.mit.edu/annotation/genome/aspergillus_group/MultiHome.html). The positioning of this contig relative to other contigs by John Clutterbuck reveals that this gene is not subtelomeric, as is the case for a number of secondary metabolite gene clusters, but is located on the left arm of chromosome I, closer to the centromere than the telomere (http://www.gla.ac.uk/ibls/molgen/aspergillus/icontigs.html).
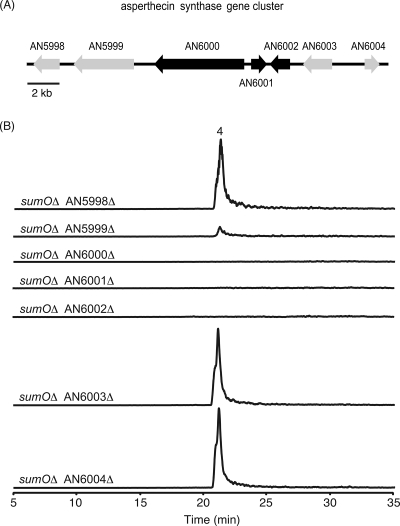
FIG. 3.
(A) Organization of the asperthecin synthase gene cluster in A. nidulans. Each arrow indicates the direction of transcription and the relative size of the open reading frame deduced from the analysis of the nucleotide sequence. Black arrows, representing the AN6000.3 (PKS), AN6001.3 (hydrolase), and AN6002.3 (monooxygenase) genes, are required for asperthecin synthesis. Gray arrows represent genes not involved in asperthecin biosynthesis according to our deletion analysis. (B) Analysis of the effects on asperthecin production of deletions of genes surrounding the asperthecin PKS gene. EIC were generated using the negative mode at m/z 317 for the sumOΔ AN5998.3Δ, sumOΔ AN5999.3Δ, sumOΔ AN6000.3Δ, sumOΔ AN6001.3Δ, sumOΔ AN6002.3Δ, sumOΔ AN6003.3Δ, and sumOΔ AN6004.3Δ strains. Levels of production by the different strains were at the same order of magnitude.
Since secondary metabolite genes in Aspergillus are usually clustered, we created deletions of six additional genes flanking aptA (AN5998.3, AN5999.3, AN6001.3, AN6002.3, AN6003.3, and AN6004.3) to determine which genes are involved in asperthecin biosynthesis (Fig. 3A). Metabolite analysis of the extracts from the deletion mutant strains revealed that two genes, AN6001.3 and AN6002.3, were required for asperthecin biosynthesis (Fig. 3; see also Fig. S3 in the supplemental material), and we designated these genes aptB and aptC, respectively. Mutants with deletions of AN5998.3, AN6003.3, and AN6004.3 still retained the ability to produce asperthecin at levels comparable to that produced by the sumOΔ strain. Interestingly, the AN5999.3 deletion mutant retained asperthecin production but at greatly reduced levels. Because asperthecin production was not eliminated, AN5999.3 is clearly not required for asperthecin biosynthesis. The reduced level of asperthecin production was accompanied by poor growth under conditions necessary for asperthecin production, which leads us to speculate that AN5999.3 is a critical gene involved in the primary metabolism of Aspergillus.
DISCUSSION
Analysis of the secondary metabolite profile of sumOΔ strains reveals that sumoylation is involved, directly or indirectly, in the regulation of the synthesis of a subset of secondary metabolites in A. nidulans. The deletion of sumO greatly decreased the expression of the austinol/dehydroaustinol and sterigmatocystin pathways. It dramatically increased the production of asperthecin, and it did not alter the production of emericellamides. SUMO regulation of protein activity is a complex field. The regulation of secondary metabolite production by sumoylation may occur at several levels. For example, sumoylation may affect the expression of secondary metabolite gene clusters at the chromatin level, the sumoylation of transcription factors involved in the regulation of secondary metabolite gene clusters may affect their activity, and some of the effects of sumOΔ may be secondary consequences of the effects on growth or primary metabolism. Determining the mechanisms by which sumoylation regulates secondary metabolite production is well beyond the scope of this study, but we have used the enhanced production of asperthecin in sumOΔ strains to identify, for the first time in any organism, the genes of the asperthecin biosynthetic gene cluster.
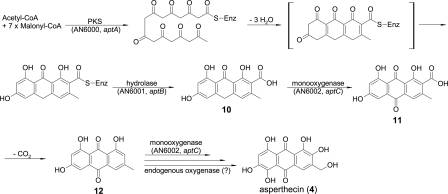
The asperthecin biosynthetic pathway has not been determined previously, and our identification of the genes required for asperthecin biosynthesis allows us to propose a likely pathway (Fig. 4). The asperthecin PKS (encoded by AN6000.3, or aptA) contains ketosynthase, acyltransferase, and acyl carrier protein domains, as is typical for an NR-PKS. BLAST analysis indicates that the AN6001.3 (aptB) protein is a lactamase or hydrolase and that the AN6002.3 (aptC) protein is a monooxygenase (see Table S3 in the supplemental material). We propose that the asperthecin PKS catalyzes the formation of the aromatic polyketide from acetyl coenzyme A and seven malonyl coenzyme A molecules. The polyketide is subsequently hydrolyzed from the PKS by the action of the hydrolase (encoded by AN6001.3, or aptB) into endocrocin-9-anthrone (compound 10). We propose that the single monooxygenase in the cluster, the AN6002.3 (aptC) protein, is responsible for the oxidation of endocrocin-9-anthrone into endocrocin (compound 11). Endocrocin is likely to decarboxylate spontaneously to form emodin (compound 12), because we have observed that amidated aklanonic acid WJ83Q2 decarboxylates spontaneously (30) in an NMR tube within 2 days. This finding also explains why there is no decarboxylase in the asperthecin biosynthesis cluster. Finally, the monoxygenase (encoded by aptC) or other endogenous oxygenase catalyzes additional oxidation steps to form asperthecin. Consistent with this proposed biosynthesis pathway, no aromatic intermediate of the pathway could be detected in the aptC deletion mutant (see Fig. S3 in the supplemental material) since the polyketide intermediate was still enzyme bound. The aptC deletion mutant did not produce any major compound detectable at a UV wavelength of 254 nm (see Fig. S3 in the supplemental material) since endocrocin-9-anthrone is known to be unstable in solution (22) and probably decomposed during the extraction process.
FIG. 4.
Proposed biosynthesis pathway for asperthecin (4) in A. nidulans. CoA, coenzyme A; compound 10, endocrocin-9-anthrone; compound 11, endocrocin; compound 12, emodin.
Supplementary Material
Acknowledgments
The project described herein was supported by grants PO1GM084077 and R01GM031837 from the National Institute of General Medical Sciences.
The content is the responsibility solely of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Published ahead of print on 31 October 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bhatnagar, D., J. Yu, and K. C. Ehrlich. 2002. Toxins of filamentous fungi. Chem. Immunol. 81:167-206. [DOI] [PubMed] [Google Scholar]
- 2.Birkinshaw, J. H., and R. Gourlay. 1961. Studies in the biochemistry of micro-organisms. 109. The structure of asperthecin. Biochem. J. 81:618-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bok, J. W., D. Hoffmeister, L. A. Maggio-Hall, R. Murillo, J. D. Glasner, and N. P. Keller. 2006. Genomic mining for Aspergillus natural products. Chem. Biol. 13:31-37. [DOI] [PubMed] [Google Scholar]
- 4.Bok, J. W., and N. P. Keller. 2004. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell 3:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bok, J. W., D. Noordermeer, S. P. Kale, and N. P. Keller. 2006. Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol. Microbiol. 61:1636-1645. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. W., and J. J. Salvo. 1994. Isolation and characterization of sexual spore pigments from Aspergillus nidulans. Appl. Environ. Microbiol. 60:979-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, D. W., J. H. Yu, H. S. Kelkar, M. Fernandes, T. C. Nesbitt, N. P. Keller, T. H. Adams, and T. J. Leonard. 1996. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 93:1418-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang, Y. M., E. Szewczyk, T. Nayak, A. D. Davidson, J. F. Sanchez, H. C. Lo, W. Y. Ho, H. Simityan, E. Kuo, A. Praseuth, K. Watanabe, B. R. Oakley, and C. C. Wang. 2008. Molecular genetic mining of the Aspergillus secondary metabolome: discovery of the emericellamide biosynthetic pathway. Chem. Biol. 15:527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cove, D. J. 1966. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 113:51-56. [DOI] [PubMed] [Google Scholar]
- 10.Galagan, J. E., S. E. Calvo, C. Cuomo, L. J. Ma, J. R. Wortman, S. Batzoglou, S. I. Lee, M. Basturkmen, C. C. Spevak, J. Clutterbuck, V. Kapitonov, J. Jurka, C. Scazzocchio, M. Farman, J. Butler, S. Purcell, S. Harris, G. H. Braus, O. Draht, S. Busch, C. D'Enfert, C. Bouchier, G. H. Goldman, D. Bell-Pedersen, S. Griffiths-Jones, J. H. Doonan, J. Yu, K. Vienken, A. Pain, M. Freitag, E. U. Selker, D. B. Archer, M. A. Penalva, B. R. Oakley, M. Momany, T. Tanaka, T. Kumagai, K. Asai, M. Machida, W. C. Nierman, D. W. Denning, M. Caddick, M. Hynes, M. Paoletti, R. Fischer, B. Miller, P. Dyer, M. S. Sachs, S. A. Osmani, and B. W. Birren. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105-1115. [DOI] [PubMed] [Google Scholar]
- 11.Gill, G. 2004. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 18:2046-2059. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, E. S. 2004. Protein modification by SUMO. Annu. Rev. Biochem. 73:355-382. [DOI] [PubMed] [Google Scholar]
- 13.Keller, N. P., G. Turner, and J. W. Bennett. 2005. Fungal secondary metabolism—from biochemistry to genomics. Nat. Rev. Microbiol. 3:937-947. [DOI] [PubMed] [Google Scholar]
- 14.Machida, M., K. Asai, M. Sano, T. Tanaka, T. Kumagai, G. Terai, K. Kusumoto, T. Arima, O. Akita, Y. Kashiwagi, K. Abe, K. Gomi, H. Horiuchi, K. Kitamoto, T. Kobayashi, M. Takeuchi, D. W. Denning, J. E. Galagan, W. C. Nierman, J. Yu, D. B. Archer, J. W. Bennett, D. Bhatnagar, T. E. Cleveland, N. D. Fedorova, O. Gotoh, H. Horikawa, A. Hosoyama, M. Ichinomiya, R. Igarashi, K. Iwashita, P. R. Juvvadi, M. Kato, Y. Kato, T. Kin, A. Kokubun, H. Maeda, N. Maeyama, J. Maruyama, H. Nagasaki, T. Nakajima, K. Oda, K. Okada, I. Paulsen, K. Sakamoto, T. Sawano, M. Takahashi, K. Takase, Y. Terabayashi, J. R. Wortman, O. Yamada, Y. Yamagata, H. Anazawa, Y. Hata, Y. Koide, T. Komori, Y. Koyama, T. Minetoki, S. Suharnan, A. Tanaka, K. Isono, S. Kuhara, N. Ogasawara, and H. Kikuchi. 2005. Genome sequencing and analysis of Aspergillus oryzae. Nature 438:1157-1161. [DOI] [PubMed] [Google Scholar]
- 15.Marquez-Fernandez, O., A. Trigos, J. L. Ramos-Balderas, G. Viniegra-Gonzalez, H. B. Deising, and J. Aguirre. 2007. Phosphopantetheinyl transferase CfwA/NpgA is required for Aspergillus nidulans secondary metabolism and asexual development. Eukaryot. Cell 6:710-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin, J. F. 1998. New aspects of genes and enzymes for beta-lactam antibiotic biosynthesis. Appl. Microbiol. Biotechnol. 50:1-15. [DOI] [PubMed] [Google Scholar]
- 17.Nayak, T., E. Szewczyk, C. E. Oakley, A. Osmani, L. Ukil, S. L. Murray, M. J. Hynes, S. A. Osmani, and B. R. Oakley. 2006. A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172:1557-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nierman, W. C., A. Pain, M. J. Anderson, J. R. Wortman, H. S. Kim, J. Arroyo, M. Berriman, K. Abe, D. B. Archer, C. Bermejo, J. Bennett, P. Bowyer, D. Chen, M. Collins, R. Coulsen, R. Davies, P. S. Dyer, M. Farman, N. Fedorova, N. Fedorova, T. V. Feldblyum, R. Fischer, N. Fosker, A. Fraser, J. L. Garcia, M. J. Garcia, A. Goble, G. H. Goldman, K. Gomi, S. Griffith-Jones, R. Gwilliam, B. Haas, H. Haas, D. Harris, H. Horiuchi, J. Huang, S. Humphray, J. Jimenez, N. Keller, H. Khouri, K. Kitamoto, T. Kobayashi, S. Konzack, R. Kulkarni, T. Kumagai, A. Lafon, J. P. Latge, W. Li, A. Lord, C. Lu, W. H. Majoros, G. S. May, B. L. Miller, Y. Mohamoud, M. Molina, M. Monod, I. Mouyna, S. Mulligan, L. Murphy, S. O'Neil, I. Paulsen, M. A. Penalva, M. Pertea, C. Price, B. L. Pritchard, M. A. Quail, E. Rabbinowitsch, N. Rawlins, M. A. Rajandream, U. Reichard, H. Renauld, G. D. Robson, S. Rodriguez de Cordoba, J. M. Rodriguez-Pena, C. M. Ronning, S. Rutter, S. L. Salzberg, M. Sanchez, J. C. Sanchez-Ferrero, D. Saunders, K. Seeger, R. Squares, S. Squares, M. Takeuchi, F. Tekaia, G. Turner, C. R. Vazquez de Aldana, J. Weidman, O. White, J. Woodward, J. H. Yu, C. Fraser, J. E. Galagan, K. Asai, M. Machida, N. Hall, B. Barrell, and D. W. Denning. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151-1156. [DOI] [PubMed] [Google Scholar]
- 19.Palaez, F. 2005. Biological activities of fungal metabolites, p. 49-92. In Z. An (ed.), Handbook of industrial microbiology. Marcel Dekker, New York.
- 20.Scherlach, K., and C. Hertweck. 2006. Discovery of aspoquinolones A-D, prenylated quinoline-2-one alkaloids from Aspergillus nidulans, motivated by genome mining. Org. Biomol. Chem. 4:3517-3520. [DOI] [PubMed] [Google Scholar]
- 21.Simpson, T. J., D. J. Stenzel, A. J. Bartlett, E. O'Brien, and J. S. E. Holker. 1982. 13C N.m.r. spectral and structural studies on austin and new related meroterpenoids from Aspergillus ustus, Aspergillus variecolor, and Penicillium diversum. J. Chem. Soc. Perkin Trans. 1 1982:2687-2692. [Google Scholar]
- 22.Steglich, W., and W. Reininger. 1970. A synthesis of endocrocin, endocrocin-9-anthrone, and related compounds. Chem. Commun. 1970:178. [Google Scholar]
- 23.Szewczyk, E., T. Nayak, C. E. Oakley, H. Edgerton, Y. Xiong, N. Taheri-Talesh, S. A. Osmani, and B. R. Oakley. 2006. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 1:3111-3120. [DOI] [PubMed] [Google Scholar]
- 24.Verger, A., J. Perdomo, and M. Crossley. 2003. Modification with SUMO. A role in transcriptional regulation. EMBO Rep. 4:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vishniac, W., and M. Santer. 1957. The thiobacilli. Bacteriol. Rev. 21:195-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walton, J. D. 1996. Host-selective toxins: agents of compatibility. Plant Cell 8:1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong, K. H., R. B. Todd, B. R. Oakley, C. E. Oakley, M. J. Hynes, and M. A. Davis. 2008. Sumoylation in Aspergillus nidulans: sumO inactivation, overexpression and live-cell imaging. Fungal Genet. Biol. 45:728-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu, J., D. Bhatnagar, and T. E. Cleveland. 2004. Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus. FEBS Lett. 564:126-130. [DOI] [PubMed] [Google Scholar]
- 29.Yu, J., P. K. Chang, K. C. Ehrlich, J. W. Cary, D. Bhatnagar, T. E. Cleveland, G. A. Payne, J. E. Linz, C. P. Woloshuk, and J. W. Bennett. 2004. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 70:1253-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, W., K. Watanabe, C. C. Wang, and Y. Tang. 2007. Investigation of early tailoring reactions in the oxytetracycline biosynthetic pathway. J. Biol. Chem. 282:25717-25725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.