Abstract
The aim was to investigate (i) the occurrence of sublethal injury in Listeria monocytogenes, Escherichia coli, and Saccharomyces cerevisiae after high hydrostatic pressure (HHP) treatment as a function of the treatment medium pH and composition and (ii) the relationship between the occurrence of sublethal injury and the inactivating effect of a combination of HHP and two antimicrobial compounds, tert-butyl hydroquinone (TBHQ) and citral. The three microorganisms showed a high proportion of sublethally injured cells (up to 99.99% of the surviving population) after HHP. In E. coli and L. monocytogenes, the extent of inactivation and sublethal injury depended on the pH and the composition of the treatment medium, whereas in S. cerevisiae, inactivation and sublethal injury were independent of medium pH or composition under the conditions tested. TBHQ alone was not lethal to E. coli or L. monocytogenes but acted synergistically with HHP and 24-h refrigeration, resulting in a viability decrease of >5 log10 cycles of both organisms. The antimicrobial effect of citral depended on the microorganism and the treatment medium pH. Acting alone for 24 h under refrigeration, 1,000 ppm of citral caused a reduction of 5 log10 cycles of E. coli at pH 7.0 and almost 3 log10 cycles of L. monocytogenes at pH 4.0. The combination of citral and HHP also showed a synergistic effect. Our results have confirmed that the detection of sublethal injury after HHP may contribute to the identification of those treatment conditions under which HHP may act synergistically with other preserving processes.
High hydrostatic pressure (HHP) has attracted much interest as an alternative to heat for food preservative technology. This nonthermal processing technique is an effective method for increasing food safety and shelf life while preserving the organoleptic properties of food products (19, 23, 48).
Loss of cytoplasmic membrane integrity is believed to be one of the critical events leading to the death of pressure-treated microorganisms (38, 59). Several authors have inferred injury to the cytoplasmic membrane after pressure treatment based on loss of intracellular material, loss of osmotic responsiveness, or uptake of vital dyes (2, 4, 5, 38, 49, 56). However, partial loss of cytoplasmic membrane functionality or damage to the gram-negative outer membrane may not necessarily lead to cell death, but surviving cells may demonstrate enhanced sensitivity to inhibitors such as sodium chloride, bile salts, and ingredients of selective media (26). Many published reports (1, 7, 20, 33, 44, 46) have demonstrated the occurrence of sublethal injury after HHP treatment using a differential plating technique.
Because membrane damage caused by pressurization may enhance sensitivity to antimicrobial agents, it could allow the design of combined processes that increase the effectiveness of pressure processing (1, 18, 31). Published data have demonstrated that the application of specific additional hurdles such as antimicrobial peptides, the lactoperoxidase system, and phenolic compounds in combination with HHP treatments have synergistic inactivation effects (8, 16, 17, 18, 21, 22, 30, 31, 34, 55, 58). One of the most effective phenolic compounds is tert-butyl hydroquinone (TBHQ), which sensitizes some barotolerant Listeria monocytogenes strains to HHP (8, 58).
Nowadays, people demand more healthy foods. In this vein, natural sources of antimicrobial compounds, such as plant essential oils, have been evaluated as substitutes for chemical preservatives (11). Citral is one of the essential oil compounds for which antimicrobial action against some important pathogenic microorganisms (Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, Salmonella enterica, and Staphylococcus aureus) has been demonstrated (11, 36). Citral is an acyclic unsaturated monoterpene aldehyde found naturally in the volatile oils of citrus fruits, lemongrass, and other herbs and spices. It consists of a mixture of two isomers, geranial and neral, and is used for flavoring citrus-based beverages. Its antimicrobial properties and pleasant fruity scent could make citral a suitable antimicrobial ingredient for wider use in the food industry.
The aim of this work was to investigate the occurrence of sublethal injury after HHP treatment as a function of the treatment medium pH and composition in a gram-positive (Listeria monocytogenes) and a gram-negative (Escherichia coli) bacterial species and a yeast (Saccharomyces cerevisiae). Also, this work investigated the relation of sublethal injury to the inactivating effect of the combination of pressure treatments and two antimicrobials, TBHQ and citral.
MATERIALS AND METHODS
Microorganisms and growth conditions.
E. coli J1, an acid-resistant commensal strain, was kindly provided by Ian Booth (University of Aberdeen, Aberdeen, United Kingdom). The strains of L. monocytogenes NCTC 11994 and S. cerevisiae STCC 11034 were supplied by culture collections. The strains were maintained in frozen cultures at −80°C. Bacterial broth subcultures were prepared by inoculating a test tube containing 10 ml of tryptone soya broth (TSB; Oxoid, Basingstoke, United Kingdom) with a single colony from a plate and incubating the resulting culture at 37°C for 6 h in a shaking incubator. For S. cerevisiae, Sabouraud broth (Oxoid) was used instead of TSB and tubes were incubated at 30°C for 24 h. Next, 250-ml Erlenmeyer flasks containing 50 ml of TSB or Sabouraud broth (in the case of S. cerevisiae) were inoculated with 0.1 ml of culture. The flasks were incubated with agitation at 130 rpm at 37°C (E. coli and L. monocytogenes) or 30°C (S. cerevisiae) until the stationary growth phase was reached.
HHP treatment.
Cells were centrifuged at 3,000 × g for 20 min at 4°C, and pellets were resuspended in different treatment media, phosphate-buffered saline (PBS, pH 7.2; Oxoid) or citrate-phosphate buffer (McIlvaine's buffer), at either pH 7.0 or pH 4.0. Where indicated, 100 ppm of TBHQ or 1,000 ppm of citral was added to the buffer. These concentrations were chosen from those previously described in published literature (8, 11, 58). Cell suspensions (2 ml each) were then placed in sterile plastic pouches that were heat sealed and kept on ice before pressurization. Samples were pressure treated in a 300-ml pressure vessel (model S-FL-850-9-W; Stansted Fluid Power, Stansted, United Kingdom) at 18°C. The pressure-transmitting fluid was monopropylene glycol-water (30:70). Cells were exposed to pressures from 200 to 400 MPa for different times (0.5 to 20 min). The maximum temperature reached during pressurization was 28°C. After decompression, the pouches were removed from the unit and placed on ice until viable counts were evaluated.
Measurement of sensitivity to subsequent holding in the HHP treatment medium.
High-pressure-treated cells were held in the treatment medium (McIlvaine's buffer at pH 7.0 and pH 4.0, with or without 100 ppm of TBHQ or 1,000 ppm of citral) at 5°C for 24 h. For a comparison, untreated native cells were also held under the same conditions. Samples were taken at preset intervals, and survivors were evaluated.
Counts of viable cells.
Samples were adequately diluted in maximum recovery diluent (Oxoid), and 0.02 ml was spread on tryptone soya agar supplemented with 0.3% yeast extract (TSA-YE) (Oxoid) in the case of E. coli and L. monocytogenes or on potato dextrose agar (Oxoid) for S. cerevisiae (PDA). Both media were supplemented with 0.1% sodium pyruvate. Plates were incubated at 37°C for 24 h (E. coli and L. monocytogenes) or 30°C for 48 h (S. cerevisiae). Previous experiments showed that longer incubation times did not influence the amount of surviving cells. The error bars in the figures indicate the standard deviations of the means for data obtained from at least three independent experiments.
Detection of sublethal injury.
In order to determine microbial cell injury, treated samples were also plated onto TSA-YE containing 2.5% NaCl (E. coli) or 5% NaCl (L. monocytogenes) or onto PDA with 7% NaCl (S. cerevisiae) (TSAYE-SC and PDA-SC; Fisher Scientific, Loughborough, United Kingdom). These were the maximum concentrations of sodium chloride that caused no reduction in colony counts of unstressed cells. Plates containing selective medium were incubated for 24 h more than those containing nonselective medium. Previous experiments showed that longer incubation times did not influence survival counts.
The extent of sublethal injury in a population of pressure-treated cells was expressed as the difference between the log count (CFU) on nonselective medium (TSA-YE or PDA) and the log count on selective medium (TSAYE-SC or PDA-SC). According to this representation, “2 logs of injury” means a 2-log difference in the count on selective and nonselective media or that 99% of survivors were sublethally injured.
RESULTS
Effect of the treatment medium composition on microbial inactivation and on the occurrence of sublethal injury after HHP treatments.
In previous work investigating the effect of pH on sublethal injury caused by nonthermal preservation treatments, McIlvaine's citrate-phosphate buffer was used as a suspending medium because of its wide buffering range (14, 37). However, because PBS has often been used in high-pressure work (28, 38, 39), the effect of these two buffers on survival and sublethal injury was examined.
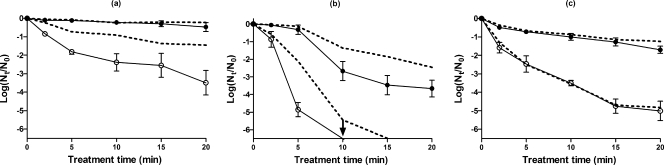
Figure 1 shows the influence of buffer composition on pressure resistance and sublethal injury in the three microorganisms. While the HHP resistance of E. coli and S. cerevisiae was unaffected (P > 0.05) by composition of the treatment medium, the degree of inactivation of L. monocytogenes cells was higher in PBS than in McIlvaine's buffer (>1 log10 cycle). HHP treatment in PBS caused an increase in the extent of sublethal injury in surviving populations compared with that in McIlvaine's buffer in E. coli and L. monocytogenes but not in S. cerevisiae. Regarding S. cerevisiae, there was no statistically significant difference (P > 0.05) in cell inactivation or sublethal injury between the two buffers. Since in the case of E. coli and L. monocytogenes McIlvaine's buffer was more protective than PBS, the former was chosen for use in subsequent experiments.
FIG. 1.
Survival fractions of E. coli at 400 MPa (a), L. monocytogenes at 300 MPa (b), and S. cerevisiae at 200 MPa (c) treated in PBS (solid line) and in McIlvaine's buffer at pH 7.0 (dashed line). Survivors recovered on the nonselective (filled circles) and selective (open circles) media are shown. Results are means of three observations ± standard deviations (error bars).
Occurrence of sublethal injury in different microorganisms after HHP treatment: effect of the pH of the treatment medium.
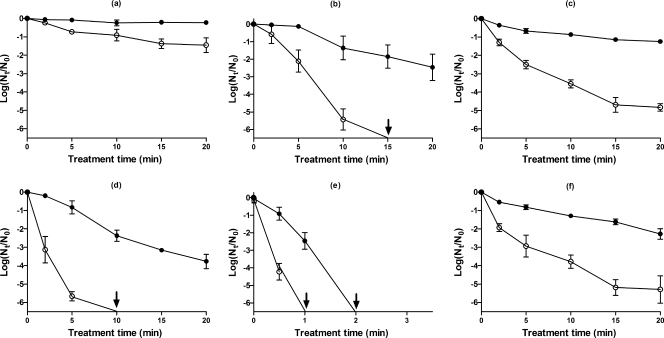
Figure 2 shows the surviving fractions of E. coli, L. monocytogenes, and S. cerevisiae cells in McIlvaine's buffer of pH 7.0 or 4.0 after HHP treatments at 400 MPa, 300 MPa, or 200 MPa, respectively, with recovery on nonselective and selective media. These treatment conditions were selected from preliminary work (data not shown) to identify pressure intensities and treatment times that would yield reasonably similar degrees of inactivation and injury in the three test organisms. The screening showed that both bacteria were more pressure resistant than S. cerevisiae and that E. coli J1 was more resistant than L. monocytogenes NCTC 11994.
FIG. 2.
Shown are survival fractions of E. coli at 400 MPa (a and d), L. monocytogenes at 300 MPa (b and e), and S. cerevisiae at 200 MPa (c and f) treated in McIlvaine's buffer at pH 7.0 (a, b, and c) and pH 4.0 (d, e, and f). Survivors recovered on the nonselective (filled circles) and the selective (open circles) media are shown. Results are means of three observations ± standard deviations (error bars). Arrows indicate that the viable count was below the limit of detection (500 CFU/ml).
By comparing data obtained with the nonselective medium, E. coli and L. monocytogenes were more resistant at pH 7.0 than at pH 4.0. On the contrary, in the case of S. cerevisiae, no statistically significant difference (P < 0.05) was observed between survival curves at either pH value. Listeria monocytogenes showed the greatest extent of sensitization at pH 4.0, where >6 log10 cycles of inactivation were achieved after 3 min at 300 MPa compared with <3 log10 cycles when the bacterium was pressurized at pH 7.0 for 20 min.
A high proportion of sublethally injured cells was seen after all treatments except with E. coli at pH 7.0. The maximum proportion of sublethally injured cells (>99.99% of the survivors, equivalent to a 4-log difference in count on selective and nonselective media) was observed when L. monocytogenes was pressurized at pH 7.0 for 15 min at 300 MPa or when E. coli and L. monocytogenes were pressurized at pH 4.0 for 5 and 0.5 min at 400 and 300 MPa, respectively. In general, the extent of injury remained constant or slightly decreased during longer treatment times. In the case of S. cerevisiae, the extent of injury did not vary as a function of the treatment medium pH. The effect of pressure combined with TBHQ or citral at different pH values was investigated for the two bacterial species, which were more pressure resistant than the yeast.
Microbial inactivation by TBHQ or citral.
Native cells of E. coli and L. monocytogenes at a concentration of 109 CFU/ml were insensitive to incubation in McIlvaine's buffer at pH 7.0 or pH 4.0 for 24 h at 5°C (Tables 1 and 2). The populations of both organisms remained constant (P > 0.05) on both nonselective and selective recovery media. The addition of 100 ppm TBHQ did not cause inactivation or injury to E. coli cells at either pH (Table 1). Regarding L. monocytogenes, the presence of 100 ppm TBHQ also caused no inactivation but resulted in >1 log10 cycle of injury when suspended at a pH of 7.0 and approximately 0.4 log10 cycles of injury when suspended at a pH of 4.0.
TABLE 1.
Log10 cycles of inactivation of E. coli after HHP treatment at 400 MPa for 5 min (at pH 4.0) and for 10 min (at pH 7.0) at time zero and after a storage of 24 h at refrigeration temperaturesa
| Type of cell | pH | Medium | Mean (± SD) at:
|
|||||
|---|---|---|---|---|---|---|---|---|
| 0 h
|
24 h
|
|||||||
| McIlvaine's buffer | TBHQ 100 ppm | Citral 1,000 ppm | McIlvaine's buffer | TBHQ 100 ppm | Citral 1,000 ppm | |||
| Native | 7.0 | NS | NE | NE | NE | NE | NE | 4.91 (0.18) |
| S | NE | NE | NE | NE | NE | >6.3 | ||
| 4.0 | NS | NE | NE | NE | NE | NE | NE | |
| S | NE | NE | NE | NE | NE | NE | ||
| Pressurized | 7.0 | NS | 0.08 (0.06) | 1.15 (0.26) | 0.30 (0.26) | 0.01 (0.09) | >6.3 | 6.06 (0.71) |
| S | 0.96 (0.37) | 2.58 (0.31) | 4.17 (0.43) | 0.55 (0.35) | >6.3 | >6.3 | ||
| 4.0 | NS | 0.75 (0.15) | 0.96 (0.10) | 1.60 (0.16) | 0.79 (0.08) | >6.3 | 5.47 (0.66) | |
| S | 4.93 (0.28) | >6.3 | >6.3 | 5.93 (0.17) | >6.3 | >6.3 | ||
NS, nonselective medium; S, selective medium; NE, no effect was found at this condition.
TABLE 2.
Log10 cycles of inactivation of L. monocytogenes after an HHP treatment of 300 MPa for 0.5 min (at pH 4.0) and for 10 min (at pH 7.0) at time zero and after a storage of 24 h at refrigeration temperaturesa
| Type of cell | pH | Medium | Mean (±SD) at:
|
|||||
|---|---|---|---|---|---|---|---|---|
| 0 h
|
24 h
|
|||||||
| McIlvaine's buffer | TBHQ (100 ppm) | Citral (1,000 ppm) | McIlvaine's buffer | TBHQ (100 ppm) | Citral (1,000 ppm) | |||
| Native | 7.0 | NS | NE | NE | NE | NE | NE | 0.74 (1.04) |
| S | NE | NE | NE | NE | 1.19 (0.88) | 0.95 (0.26) | ||
| 4.0 | NS | NE | NE | NE | NE | NE | 2.67 (0.88) | |
| S | NE | NE | NE | NE | 0.41 (0.30) | 4.53 (0.80) | ||
| Pressurized | 7.0 | NS | 1.03 (0.38) | >6.3 | 1.75 (0.67) | 0.80 (0.19) | >6.3 | 3.31 (0.23) |
| S | 6.26 (0.74) | >6.3 | >6.3 | 4.43 (0.58) | >6.3 | >6.3 | ||
| 4.0 | NS | 0.56 (0.16) | 1.11 (0.40) | 1.15 (0.49) | 3.46 (1.01) | >6.3 | 4.92 (0.51) | |
| S | 3.01 (0.60) | >6.3 | 4.89 (0.62) | >6.3 | >6.3 | >6.3 | ||
NS, nonselective medium; S, selective medium; NE, no effect was found at this condition.
During refrigerated holding in the presence of 1,000 ppm citral, no inactivation or sublethal injury was detected in E. coli at pH 4.0, but viable numbers of bacteria that were decreased by almost 5 log10 cell cycles and more than one extra log10 cycle were injured at pH 7.0. Under the same experimental conditions, the behavior of native cells of L. monocytogenes was the opposite, with inactivation and sublethal injury being greater at pH 4.0 than at pH 7.0.
Microbial inactivation by combining TBHQ or citral and HHP.
Cells were pressure treated in McIlvaine's buffer at pH 4.0 or pH 7.0, either alone or in combination with 100 ppm of TBHQ or 1,000 ppm of citral. Escherichia coli cells were treated at 400 MPa for 5 min (pH 4.0) or 10 min (pH 7.0). Listeria monocytogenes cells were treated at 300 MPa for 0.5 min (pH 4.0) or 10 min (pH 7.0). These pressure treatment conditions were chosen from the preliminary studies because of their capacity to cause the maximum proportion of sublethally injured cells among survivors.
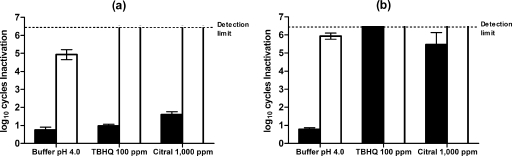
Figure 3 shows the number of log10 cycles of inactivation of E. coli cells suspended in McIlvaine's buffer at pH 4.0, or in buffer containing TBHQ or citral, after HHP and recovery on nonselective and selective media. Survivors were evaluated immediately after pressure treatment (Fig. 3a) and after 24-h storage at 5°C (Fig. 3b). Treatment in buffer alone resulted in a reduction in viable numbers of slightly <1 log10 cell cycle and a further 4 log10 cell cycles of sublethally injured cells. The presence of TBHQ or citral during the treatment caused a further decrease in viability of between 0.25 and 1 log cycle, respectively, while counts on the selective medium were below the detection limit, indicating >4 to 5 logs of injury.
FIG. 3.
Log10 of cycles of inactivation of E. coli after a HHP treatment at 400 MPa for 5 min in McIlvaine's buffer at pH 4.0 or in the same buffer with 100 ppm of TBHQ or with 1,000 ppm of citral added at time zero (a) and after a storage of 24 h (b) at refrigeration temperatures. Survivors recovered on the nonselective (black bars) and selective (white bars) media are shown. Results are means of three observations ± standard deviations (error bars).
During subsequent holding of pressure-treated E. coli cells in McIlvaine's buffer at pH 4.0, there was no reduction in viable numbers, but the number of injured cells increased by 1 log10 cycle. However, holding for 24 h in the presence of TBHQ or citral caused the inactivation of >5 log10 cycles. The survival counts obtained on selective and nonselective recovery media were very similar, indicating that most HHP-damaged cells were sensitive to the presence of TBHQ or citral. The final degree of inactivation of E. coli cells due to the combined process (approximately 6 log10 cycles) was much higher than that obtained by adding the effects of HHP treatment and incubation with TBHQ or citral acting separately (<1 log10 cycle). Therefore, at pH 4.0, treatment with citral or TBHQ and HHP was synergistic when these antimicrobials were simultaneously applied.
At pH 7.0, E. coli cells showed considerable HHP resistance when suspended in McIlvaine's buffer without any antimicrobial added (Table 1). The presence of TBHQ or citral increased both inactivation and injury when assessed immediately after HHP treatment, particularly with citral, which resulted in 4 log10 cycles of injury. Again, the final degree of inactivation of E. coli cells at a pH of 7.0, due to the combined process of pressure plus TBHQ (6 log10 cycles), was much higher after holding for 24 h than that obtained by adding together the separate effects of HHP treatment and incubation with TBHQ (<1 log10 cycle), showing a synergistic effect. The combined pressure treatment with citral did not exert a significant synergistic effect, because citral acting alone affected the whole population of native cells after 24 h of refrigeration (Table 1).
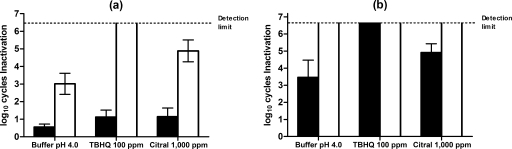
When L. monocytogenes was pressure treated at a pH of 4.0, the extent of sublethal injury assessed immediately after HHP treatment was increased by the presence of TBHQ or citral (Table 2; Fig. 4a). Pressure-treated cells of L. monocytogenes were sensitive to subsequent incubation for 24 h, even in the absence of added antimicrobials: a further reduction of 2 log10 cell cycles occurred during holding at a pH of 4.0 for 24 h with three extra log10 cell cycles of injury. Incubation of HHP-treated cells for 24 h in the presence of TBHQ caused the inactivation of most sublethally HHP-injured cells, achieving >6 log10 cycles of inactivation (Fig. 4b). Therefore, at a pH of 4.0, the combination of TBHQ and HHP treatment also acted synergistically when simultaneously applied to L. monocytogenes cells suspended at a pH of 4.0. Sublethally injured cells also died in the presence of citral during the 24-h incubation at 5°C, achieving approximately 5 log10 cycles of inactivation. Nevertheless, the final degree of inactivation was similar to that obtained by the incubation for 24 h in the presence of citral and the HHP treatment acting separately, and thus, the effect was additive rather than synergistic.
FIG. 4.
Log10 cycles of inactivation of L. monocytogenes after an HHP treatment at 300 MPa for 0.5 min in McIlvaine's buffer at pH 4.0 or in the same buffer with 100 ppm of TBHQ or 1,000 ppm of citral added at time zero (a) and after a storage of 24 h (b) at refrigeration temperatures. Survivors recovered on the nonselective (black bars) and selective (white bars) media are shown. Results are means of three observations ± standard deviations (error bars).
Inactivation of L. monocytogenes cells at pH 7.0 showed different behavior (Table 2). The combination of HHP and TBHQ showed a very large lethal effect such that the whole cell population was inactivated. A comparison of survival after combined and individual treatments showed this to be a synergistic effect. In contrast, the presence of citral scarcely increased the efficacy of the HHP treatment. After 24 h of incubation at pH 7.0 in the absence of additives, pressure-treated cells showed no decrease in viability, while some repair of injury occurred as shown by a 2-log decrease in the difference between counts on selective and nonselective media. Since the whole cell population had been inactivated by the presence of TBHQ during the HHP treatment, it was not possible to assess whether the subsequent incubation with TBHQ caused any extra inactivation. For cells pressure treated with citral, incubation for 24 h at pH 7.0 caused only a small further reduction in viability of about 3 log10 cycles, but the extent of injury remained large.
DISCUSSION
Saccharomyces cerevisiae was less pressure resistant than either E. coli or L. monocytogenes, in line with the general observation that vegetative eukaryotic cells are less resistant to HHP treatments than prokaryotic microorganisms (19, 51). Both bacterial species were more sensitive to pressure at acidic pHs, as expected, but there was no difference in the resistance of S. cerevisiae when the yeast was pressurized at pH 7.0 or pH 4.0. This might be regarded as unexpected, though survival of S. cerevisiae during pulsed electric field (PEF) treatment was similarly unaffected by pH (54). The extent of sublethal injury after HHP treatment was also greater at pH 4.0 than at pH 7.0 in E. coli and L. monocytogenes, whereas in S. cerevisiae, injury did not depend on the treatment medium pH. As with pressure, the occurrence of sublethal injury in yeast after PEF has been shown to be independent of the treatment medium pH (54). The similarity in the effect of pH on inactivation and injury by HHP and PEF may perhaps be related to the fact that the cytoplasmic membrane is a major target for damage in both processes (40).
Many others (1, 7, 20, 33, 44, 46) have demonstrated the occurrence of sublethally injured bacterial cells after HHP treatment using a selective medium plating technique, but to the best of our knowledge, only Pandya et al. (42) have demonstrated a kind of pressure-induced injury of yeast by plate count differential between PDA and PDA supplemented with glucose. Sublethal injury measured in this study by using NaCl as a selective compound in the recovery medium is presumably a consequence of the loss of osmoregulatory functions associated with the cytoplasmic membrane (35). The detection of sublethally injured cells after HHP treatment confirms that microbial inactivation by HHP is not an all-or-nothing event and that restoration of membrane function is an important event either in bacterial or yeast recovery after HHP.
The two bacterial species showed a higher HHP resistance and less sublethal injury in McIlvaine's buffer than in PBS, whereas S. cerevisiae cells showed the same pressure resistance in both. This suggests that the critical targets for pressure inactivation in bacteria and yeasts are affected differently by environmental factors (such as medium composition and pH). The reason for the greater bacterial sensitivity in PBS is not known but might be related to the presence of NaCl in the PBS but not in McIlvaine's buffer. The concentration in PBS (8.0 g/liter), though not high, might be sufficient to sensitize cells if present during the pressure treatment (20). Alternatively, the higher concentration of phosphate in McIlvaine's buffer (0.18 M) than in PBS buffer (0.01 M) might have a protective effect. It is well known that pressure induces changes in the pH of some buffers, but the effect of this on survival and the specific effects of buffer composition on survival have seldom been studied (32).
As noted by Hauben et al. (18), the detection of sublethal injury may help clarify the environmental circumstances under which other processes for food preservation in combination with HHP treatments may increase their bactericidal efficiency. The effect of combined treatments was studied further in the two bacterial species, which were more resistant to HHP than yeast.
Synergistic inactivation effects have been described when HHP treatments were combined with low pHs (15, 25) or with different antimicrobial substances, such as lysozyme (16, 18, 30), pediocin (17, 21, 22), nisin (8, 17, 18, 21, 30, 55), and lacticin (34). Several workers have reported the lethal effect of HHP in combination with phenolic antioxidants, such as TBHQ or butylated hydroxyanisole (8, 27, 58), or with plant essential oils, such as citral (41).
Many food-grade phenolic antioxidants, including TBHQ, have antimicrobial properties (13, 45, 60). TBHQ and HHP treatment also act synergistically when simultaneously applied to L. monocytogenes at pH 7.0 (8, 58). We found that 100 ppm of TBHQ acting alone at pH 7.0 did not affect the survival of L. monocytogenes, in agreement with Vurma et al. (58), but caused extensive sublethal injury following refrigerated storage for 24 h. This sensitizing effect might explain the efficacy of the combined treatment of HHP and TBHQ in L. monocytogenes, which allowed the inactivation of >6 log10 cycles at pH 7.0. Conversely, E. coli cells were not sensitized by the presence of TBHQ alone, but a synergistic killing effect of HHP and TBHQ was observed after incubation for 24 h at refrigeration temperatures, also allowing the inactivation of >6 log10 cycles of E. coli cells. To the best of our knowledge, the combined effect of HHP and TBHQ on a gram-negative bacterial cell has not previously been investigated. Several authors have suggested that phenolic compounds might affect the cell envelopes (50, 57, 58). The synergistic effect might result from the same component of the microbial cell, the cytoplasmic membrane, being targeted simultaneously by both HHP and TBHQ agents.
The demand by consumers for natural, healthy foods has stimulated the use of plant essential oils in the development of new combined processes. Many plant essential oil compounds have antimicrobial action (9, 24, 29, 52). Moreover, a majority of essential oils are classified as being generally recognized as safe. Citral is the major constituent of the essential oil fraction of lemongrass (43) and is one of the most commonly used flavor compounds worldwide (53). Combining citral with pressurization resulted in a significantly greater reduction in viable counts of Colletotrichum gloeosporioides in papaya than that caused by HHP alone (41). To the best of our knowledge, there have been no previous attempts to evaluate the combined effect of HHP and citral against bacteria.
Exposure to citral alone at refrigeration temperatures caused inactivation and sublethal injury in both L. monocytogenes and E. coli, whereas in L. monocytogenes, the effect was greater at pH 4.0 than at pH 7.0, and the opposite was true for E. coli. Friedman et al. (11) observed the bactericidal effect of citral by using a microplate assay, but only at pH 7.0. Our results confirm its bactericidal effect also at pH 4.0 against the gram-positive bacterium investigated. The mechanism of action of citral is unknown, but the enhanced sensitivity to salt suggests impairment of osmotic homeostasis, possibly involving membrane perturbations caused by the lipophilic citral. The different responses to citral in L. monocytogenes and E. coli could thus arise from differences in envelope composition, but this would require further investigation.
The effect of citral combined with HHP also varied depending on the treatment medium pH and the microorganism investigated. The combined treatment showed a synergistic effect at pH 7.0 in both microorganisms. However, at pH 4.0, the effect was synergistic in E. coli but only additive in L. monocytogenes. Most sublethally injured cells detected immediately after HHP in the presence of citral were inactivated following holding for 24 h in the same treatment medium under refrigeration conditions. With the exception of L. monocytogenes suspended in McIlvaine's buffer at pH 7.0, the combined treatment caused a reduction in viability of at least 5 log10 cycles.
Citral is used as a flavoring in a variety of foods, including juices, beverages, certain baked goods, confectionary foods, and ice cream. It is added as an essential oil concentrate at concentrations of up to 800 ppm or as a pure compound at concentrations of up to 170 ppm (3, 47). The average daily intake of citral in humans is estimated to be 5 mg/kg (Council of Europe). Because of its fruity odor, citral is used mainly with products where a “green” or fruit flavor is required. It has been particularly recommended as an antimicrobial additive for soft drinks, orange juice, and apple juice (3, 10, 12). In these fruit-flavored products, the use of citral at relatively high concentrations where its odor is obvious is likely to be acceptable.
In summary, we have demonstrated that the extent of sublethal injury after pressurization treatments depended on the type of microorganism, the pH, and the composition of the treatment medium. It is generally accepted that essential oils are most effective at acidic pHs (6), but as shown here, this did not apply to citral. The generalization that gram-positive organisms are more sensitive to essential oils than gram-negative ones was also shown to depend on pH. Despite the differences observed between microorganisms and the effects of treatment medium pH, the use of TBHQ in combination with HHP showed a synergistic effect, guaranteeing a 5-log reduction of L. monocytogenes and E. coli under any treatment condition investigated. The synergistic effect was sometimes observed immediately after the HHP treatment, as occurred when L. monocytogenes was treated at pH 7.0, but otherwise the effect was observed after a 24-h incubation under refrigeration conditions. The combination of citral and HHP also showed a synergistic effect, allowing the achievement of either a higher degree of inactivation or a higher proportion of sublethally injured cells. Our results have also confirmed that the detection of sublethal injury by the selective medium plating technique after HHP treatments may contribute to the identification of those treatment conditions under which HHP may act synergistically with other preserving processes.
Acknowledgments
This work was supported by Ministerio Español de Educación, Cultura, y Deporte, which provided M.S. with a grant to carry out this investigation.
Footnotes
Published ahead of print on 24 October 2008.
REFERENCES
- 1.Alpas, H., N. Kalchayanand, F. Bozoglu, and B. Ray. 2000. Interactions of high hydrostatic pressure, pressurization temperature and pH on death and injury of pressure-resistant and pressure-sensitive strains of foodborne pathogens. Int. J. Food Microbiol. 60:33-42. [DOI] [PubMed] [Google Scholar]
- 2.Ananta, E., V. Heinz, and D. Knorr. 2004. Assessment of high pressure induced damage on Lactobacillus rhamnosus GG by flow cytometry. Food Microbiol. 21:567-577. [Google Scholar]
- 3.Belletti, N., S. S. Kamdem, F. Patrignani R. Lanciotti, A. Covelli, and F. Gardin. 2007. Antimicrobial activity of aroma compounds against Saccharomyces cerevisiae and improvement of microbiological stability of soft drinks as assessed by logistic regression. Appl. Environ. Microbiol. 73:5580-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benito, A., G. Ventoura, M. Casadei, T. Robinson, and B. Mackey. 1999. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl. Environ. Microbiol. 65:1564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brul, S., A. J. M. Rommens, and C. T. Verrips. 2000. Mechanistic studies on the inactivation of Saccharomyces cerevisiae by high pressure. Innov. Food Sci. Emerg. Technol. 1:99-108. [Google Scholar]
- 6.Burt, S. 2004. Essential oils: their antibacterial properties and potential. Int. J. Food Microbiol. 94:223-253. [DOI] [PubMed] [Google Scholar]
- 7.Chilton, P., N. S. Isaacs, P. Mañas, and B. M. Mackey. 2001. Biosynthetic requirements for the repair of membrane damage in pressure-treated Escherichia coli. Int. J. Food Microbiol. 71:101-104. [DOI] [PubMed] [Google Scholar]
- 8.Chung, Y. K., M. Vurma, E. J. Turek, G. W. Chism, and A. E. Yousef. 2005. Inactivation of barotolerant Listeria monocytogenes in sausage by combination of high-pressure processing and food-grade additives. J. Food Prot. 68:744-750. [DOI] [PubMed] [Google Scholar]
- 9.Cowan, M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrante, S., S. Guerrero, and S. M. Alzamorat. 2007. Combined use of ultrasound and natural antimicrobials to inactivate Listeria monocytogenes in orange juice. J. Food Prot. 70:1850-1856. [DOI] [PubMed] [Google Scholar]
- 11.Friedman, M., P. R. Henika, and R. E. Mandrell. 2002. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes and Salmonella enterica. J. Food Prot. 65:1545-1560. [DOI] [PubMed] [Google Scholar]
- 12.Friedman, M., P. R. Henika, C. E. Levin, and R. E. Mandrell. 2004. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 52:6042-6048. [DOI] [PubMed] [Google Scholar]
- 13.Fung, D. Y., C. C. Lin, and M. B. Gailani. 1985. Effect of phenolic antioxidants on microbial growth. Crit. Rev. Microbiol. 12:153-183. [DOI] [PubMed] [Google Scholar]
- 14.García, D., N. Gómez, P. Mañas, S. Condón, J. Raso, and R. Pagán. 2005. Occurrence of sublethal injury after pulsed electric fields depending on the microorganism, the treatment medium pH and the intensity of the treatment investigated. J. Appl. Microbiol. 99:94-104. [DOI] [PubMed] [Google Scholar]
- 15.García-Graells, V., K. J. A. Hauben, and C. W. Michiels. 1998. High pressure inactivation and sublethal injury of pressure-resistant Escherichia coli mutants in fruit juices. Appl. Environ. Microbiol. 64:1566-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Graells, C., I. Van Opstal, S. C. M. Vanmuysen, and C. W. Michiels. 2003. The lactoperoxidase system increases efficacy of high-pressure inactivation of foodborne bacteria. Int. J. Food Microbiol. 81:211-221. [DOI] [PubMed] [Google Scholar]
- 17.Garriga, M., M. T. Aymerich, S. Costa, J. M. Monfort, and M. Hugas. 2002. Bactericidal synergism through bacteriocins and high pressure in a meat model system during storage. Food Microbiol. 19:509-518. [Google Scholar]
- 18.Hauben, K. J. A., E. Y. Wuytack, C. C. F. Soontjens, and C. W. Michiels. 1996. High-pressure transient sensitization of Escherichia coli to lysozyme and nisin by disruption of outer-membrane permeability. J. Food Prot. 59:350-355. [DOI] [PubMed] [Google Scholar]
- 19.Hoover, D. G., C. Metrick, A. M. Papineau, D. F. Farkas, and D. Knorr. 1989. Biological effects of high hydrostatic pressure on food microorganisms. Food Technol. 43:99-107. [Google Scholar]
- 20.Jordan, S. L., C. Pascual, E. Bracey, and B. M. Mackey. 2001. Inactivation and injury of pressure-resistant strains of Escherichia coli O157 and Listeria monocytogenes in fruit juices. J. Appl. Microbiol. 91:463-469. [DOI] [PubMed] [Google Scholar]
- 21.Kalchayanand, N., T. Sikes, C. P. Dunne, and B. Ray. 1994. Hydrostatic pressure and electroporation have increased bactericidal efficiency in combination with bacteriocins. Appl. Environ. Microbiol. 60:4174-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalchayanand, N., A. Sikes, C. P. Dunne, and B. Ray. 1998. Interaction of hydrostatic pressure, time and temperature of pressurization and pediocin AcH on inactivation of foodborne bacteria. J. Food Prot. 61:425-431. [DOI] [PubMed] [Google Scholar]
- 23.Knorr, D. 1993. Effects of high hydrostatic pressure on food safety and quality. Food Technol. 46:156-161. [Google Scholar]
- 24.Lachowicz, K. J., G. P. Jones, D. R. Briggs, F. E. Bienvenu, J. Wan, A. Wilcock, and M. J. Coventry. 1998. The synergistic preservative effects of the essential oils of sweet basil (Ocimum basilicum L.) against acid-tolerant food microflora. Lett. Appl. Microbiol. 26:209-214. [DOI] [PubMed] [Google Scholar]
- 25.Linton, M., J. M. J. McClements, and M. F. Patterson. 1999. Survival of Escherichia coli O157:H7 during storage in pressure-treated orange juice. J. Food Prot. 62:1038-1040. [DOI] [PubMed] [Google Scholar]
- 26.Mackey, B. M. 2000. Injured bacteria, p. 315-341. In M. Lund, T. C. Baird-Parker, and G. W. Gould (ed.), The microbiological safety and quality of food. Aspen Publisher, Inc., Gaithersburg, MD.
- 27.Mackey, B. M., K. Forestière, and N. Isaacs. 1995. Factors affecting the resistance of Listeria monocytogenes to high hydrostatic pressure. Food Biotechnol. 9:1-11. [Google Scholar]
- 28.Mañas, P., and B. M. Mackey. 2004. Morphological and physiological changes induced by high hydrostatic pressure in exponential- and stationary-phase cells of Escherichia coli: relationship with cell death. Appl. Environ. Microbiol. 70:1545-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangena, T., and N. Y. O. Muyima. 1999. Comparative evaluation of the antimicrobial activities of essential oils of Artemisia afra, Pteronia incana and Rosmarinus officinalis on selected bacteria and yeast strains. Lett. Appl. Microbiol. 28:291-296. [DOI] [PubMed] [Google Scholar]
- 30.Masschalck, B., C. García-Graells, E. van Haver, and C. W. Michiels. 2000. Inactivation of high pressure resistant Escherichia coli by lysozyme and nisin under high pressure. Innov. Food Sci. Emerg. Technol. 1:39-47. [Google Scholar]
- 31.Masschalck, B., D. Deckers, and C. W. Michiels. 2003. Sensitization of outer-membrane mutants of Salmonella typhimurium and Pseudomonas aeruginosa to antimicrobial peptides under high pressure. J. Food Prot. 66:1360-1367. [DOI] [PubMed] [Google Scholar]
- 32.Mathys, A., R. Kallmeyer, V. Heinz, and D. Knorr. 2008. Impact of dissociation equilibrium shift on bacterial spore inactivation by heat and pressure. Food Control 19:1165-1173. [Google Scholar]
- 33.Metrick, C., D. G. Hoover, and D. F. Farkas. 1989. Effects of hydrostatic pressure on heat-resistance and heat-sensitive strains of Salmonella. J. Food Sci. 54:1547-1549. [Google Scholar]
- 34.Morgan, S. M., R. P. Ross, T. Beresford, and C. Hill. 2000. Combination of hydrostatic pressure and lacticin 3147 causes increased killing of Staphylococcus and Listeria. J. Appl. Microbiol. 88:414-420. [DOI] [PubMed] [Google Scholar]
- 35.O'Byrne, C., and I. R. Booth. 2002. Osmoregulation and its importance to food-borne microorganisms. Int. J. Food Microbiol. 74:203-216. [DOI] [PubMed] [Google Scholar]
- 36.Onawunmi, G. O. 1989. Evaluation of the antimicrobial activity of citral. Lett. Appl. Microbiol. 9:105-108. [Google Scholar]
- 37.Pagán, R., P. Mañas, I. Alvarez, and S. Condón. 1999. Resistance of Listeria monocytogenes to ultrasonic waves under pressure at sublethal (manosonication) and lethal (manothermosonication) temperatures. Food Microbiol. 16:139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagán, R., and B. M. Mackey. 2000. Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential- and stationary-phase cells and variation among strains. Appl. Environ. Microbiol. 66:2829-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagán, R., S. Jordan, A. Benito, and B. M. Mackey. 2001. Enhanced acid sensitivity of pressure-damaged Escherichia coli O157 cells. Appl. Environ. Microbiol. 67:1983-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagán, R., and P. Mañas. 2006. Fundamental aspects of microbial membrane electroporation, p. 73-94. In J. Raso and V. Heinz (ed.), Pulsed electric fields technology for the food industry. Springer Science & Business Media, LLC, New York, NY.
- 41.Palhano, F. L., T. T. B. Vilches, R. B. Santos, M. T. D. Orlando, J. A. Ventura, and P. M. B. Fernandes. 2004. Inactivation of Colletotrichum gloeosporioides spores by high hydrostatic pressure combined with citral or lemongrass essential oil. Int. J. Food Microbiol. 95:61-66. [DOI] [PubMed] [Google Scholar]
- 42.Pandya, Y., F. F. Jewett, and D. G. Hoover. 1995. Concurrent effects of high hydrostatic pressure, acidity and heat on the destruction and injury of yeasts. J. Food Prot. 58:301-304. [DOI] [PubMed] [Google Scholar]
- 43.Paranagama, P. A., K. H. T. Abeysekera, K. Abeywickrama, and L. Nugaliyadde. 2003. Fungicidal and anti-aflatoxigenic effects of the essential oil of Cymbopogon citratus (DC.) Stapf. (lemongrass) against Aspergillus flavus Link. isolated from stored rice. Lett. Appl. Microbiol. 37:86-90. [DOI] [PubMed] [Google Scholar]
- 44.Patterson, M. F., M. Quinn, R. Simpson, and A. Gilmour. 1995. Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods. J. Food Prot. 58:524-529. [DOI] [PubMed] [Google Scholar]
- 45.Payne, K. D., E. Rico-Munoz, and P. M. Davidson. 1989. The antimicrobial activity of phenolic compounds against L. monocytogenes and their effectiveness in a model milk system. J. Food Prot. 52:151-153. [DOI] [PubMed] [Google Scholar]
- 46.Ponce, E., R. Pla, E. Sendra, B. Guamis, and M. Mor-Mur. 1999. Destruction of Salmonella enteritidis inoculated in liquid whole egg by high hydrostatic pressure: comparative study in selective and non-selective media. Food Microbiol. 16:357-365. [Google Scholar]
- 47.Ress, N. B., J. R. Hailey, R. R. Maronpot, J. R. Bucher, G. S. Travlos, J. K. Haseman, D. P. Orzech, J. D. Johnson, and M. R. Hejtmancik. 2003. Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol. Sci. 71:198-206. [DOI] [PubMed] [Google Scholar]
- 48.San Martín, M. F., G. V. Barbosa-Cánovas, and B. G. Swanson. 2002. Food processing by high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 42:627-645. [DOI] [PubMed] [Google Scholar]
- 49.Shigehisa, T., T. Ohmori, A. Saito, S. Taji, and R. Hayashi. 1991. Effects of high hydrostatic pressure on characteristics of pork slurries and inactivation of microorganisms associated with meat and meat products. Int. J. Food Microbiol. 12:207-216. [DOI] [PubMed] [Google Scholar]
- 50.Sikkema, J., J. A. M. de Bont, and B. Poolman. 1994. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 269:8022-8028. [PubMed] [Google Scholar]
- 51.Smelt, J. P. P. M. 1998. Recent advances in the microbiology of high pressure processing. Trends Food Sci. Technol. 9:152-158. [Google Scholar]
- 52.Smith-Palmer, A., J. Stewart, and L. Fyfe. 1998. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett. Appl. Microbiol. 26:118-122. [DOI] [PubMed] [Google Scholar]
- 53.Somogyi, L. P. 1996. The flavour and fragrance industry: serving a global market. Chem. Ind. (London) March:170-173. [Google Scholar]
- 54.Somolinos, M., D. García, S. Condón, P. Mañas, and R. Pagán. 2007. Relationship between sublethal injury and inactivation of yeast cells by the combination of sorbic acid and pulsed electric fields. Appl. Environ. Microbiol. 73:3814-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ter Steeg, P. F., J. C. Hellemonds, and A. E. Kok. 1999. Synergistic actions of nisin, sublethal ultrahigh pressure, and reduced temperature on bacteria and yeast. Appl. Environ. Microbiol. 65:4148-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulmer, H. M., M. G. Ganzle, and R. F. Vogel. 2000. Effects of high pressure on survival and metabolic activity of Lactobacillus plantarum TMW1.460. Appl. Environ. Microbiol. 66:3966-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ultee, A., L. G. M. Gorris, and E. J. Smid. 1998. Bactericidal activity of carvacrol towards the food-borne pathogen Bacillus cereus. J. Appl. Microbiol. 85:211-218. [DOI] [PubMed] [Google Scholar]
- 58.Vurma, M., Y.-K. Chung, T. H. Shellhammer, E. J. Turek, and A. E. Yousef. 2006. Use of phenolic compounds for sensitizing Listeria monocytogenes to high-pressure processing. Int. J. Food Microbiol. 106:263-269. [DOI] [PubMed] [Google Scholar]
- 59.Wouters, P. C., E. Glaasker, and J. P. P. M. Smelt. 1998. Effects of high pressure on inactivation kinetics and events related to proton efflux in Lactobacillus plantarum. Appl. Environ. Microbiol. 64:509-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yousef, A. E., R. J. Gajewski II, and E. H. Marth. 1991. Kinetics of growth and inhibition of Listeria monocytogenes in the presence of antioxidant food additives. J. Food Sci. 56:10-13. [Google Scholar]