Abstract
Adhesion and residence-time-dependent desorption of two Staphylococcus aureus strains with and without fibronectin (Fn) binding proteins (FnBPs) on Fn-coated glass were compared under flow conditions. To obtain a better understanding of the role of Fn-FnBP binding, the adsorption enthalpies of Fn with staphylococcal cell surfaces were determined using isothermal titration calorimetry (ITC). Interaction forces between staphylococci and Fn coatings were measured using atomic force microscopy (AFM). The strain with FnBPs adhered faster and initially stronger to an Fn coating than the strain without FnBPs, and its Fn adsorption enthalpies were higher. The initial desorption was high for both strains but decreased substantially within 2 s. These time scales of staphylococcal bond ageing were confirmed by AFM adhesion force measurement. After exposure of either Fn coating or staphylococcal cell surfaces to bovine serum albumin (BSA), the adhesion of both strains to Fn coatings was reduced, suggesting that BSA suppresses not only nonspecific but also specific Fn-FnBP interactions. Adhesion forces and adsorption enthalpies were only slightly affected by BSA adsorption. This implies that under the mild contact conditions of convective diffusion in a flow chamber, adsorbed BSA prevents specific interactions but does allow forced Fn-FnBP binding during AFM or stirring in ITC. The bond strength energies calculated from retraction force-distance curves from AFM were orders of magnitude higher than those calculated from desorption data, confirming that a penetrating Fn-coated AFM tip probes multiple adhesins in the outermost cell surface that remain hidden during mild landing of an organism on an Fn-coated substratum, like that during convective diffusional flow.
Staphylococcus aureus is a versatile pathogen which can adhere to epithelial cells, endothelial cells, and fibroblasts, as well as to plasma-exposed biomaterial implant surfaces in the human body (12), causing potentially persistent infections. The best-described mechanism of S. aureus adhesion to eukaryotic cells and other fibronectin (Fn)-coated surfaces involves Fn binding proteins (FnBPs) A and B on the surface of S. aureus (8, 9). Peacock et al. (18) demonstrated the significant role played by the FnBPs by comparing the adhesion of different isogenic S. aureus strains to human endothelial cells. Moreover, in vitro adhesion of S. aureus strain Wood 46 to Fn-coated surfaces was demonstrated to be inhibited in a dose-dependent manner by anti-Fn antibodies (24, 25).
At constant temperature and pressure, bacterial adhesion to surfaces is accompanied by a decrease in the Gibbs energy (ΔG). ΔG is composed of a change in enthalpy (ΔH) and a change in entropy (ΔS) according to the following equation:
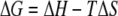 |
(1) |
where T is the temperature (in Kelvin). The enthalpy tends to reach a minimum value, whereas the entropy strives for a maximum value. The change in enthalpy can be determined by determining the heat exchange between a system and its environment, while direct determination of the entropy is practically impossible. Many biological processes are characterized by strong enthalpy-entropy compensation (10); that is, they occur spontaneously by virtue of an increase in entropy that compensates for an unfavorable enthalpy effect or vice versa. The enthalpy of the interaction between bacterial cell surfaces and proteins can be assessed using isothermal titration calorimetry (ITC). Xu et al. (28) determined the adsorption enthalpies of salivary proteins with Streptococcus mutans and found that S. mutans LT11 with antigen I/II, a cell surface binding protein involved in bacterial adhesion to extracellular matrix proteins, had a much higher, exothermic adsorption enthalpy when it was mixed with saliva at pH 6.8 than S. mutans IB03987, lacking surface antigen I/II, had. Thus, it was inferred that antigen I/II at the surface of S. mutans LT11 specifically binds different proteins with different affinities in the large pool of proteins present in whole saliva. Furthermore, Busscher et al. (4) used ITC to evaluate the adsorption of a single protein, laminin, to streptococcal cell surfaces and found that enthalpy is released upon adsorption of laminin to the surface of parent strain LT11 but not upon adsorption to IB03987. Whereas ITC operates at a macroscopic level, atomic force microscopy (AFM) operates at the nanometer level and allows workers to determine the force between a sharp probe attached to a flexible cantilever and a cell surface, and thus it can distinguish between different functional surface proteins (1). Using AFM, differences in interaction forces between protein-coated AFM probes and streptococcal strains with and without antigen I/II have been measured. Generally, upon retraction of streptococci from saliva- or laminin-coated probes, stronger forces were observed when the streptococcal strain possessed antigen I/II than when it did not.
Initial microbial adhesion is reversible, but over time the bond strength may increase and adhesion becomes less reversible. By registering the time of arrival and the time of detachment of an adhering microorganism in systems by using in situ observation and real-time image analysis, desorption can be measured as a function of the residence time of an adhering organism (7, 20, 23). Dabros and van de Ven (7) proposed that the desorption rate coefficient [β(t − τ)] of a particle adsorbed at time τ and desorbing at time t (i.e., after it resides on the surface for a time [t − τ]) changes exponentially from an initial value (β0) to a final value (β∞) during ageing of the bond with a relaxation time (1/δ) according to the following equation:
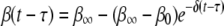 |
(2) |
The desorption rate for Streptococcus thermophilus B during nonspecific adhesion on glass decreased according to equation 2 from an initially high β0 value (2.5 × 10−3 s−1) to an almost negligible β∞ value (0.01 × 10−3 s−1) over an approximately 50-s period (13). Many years later, AFM was used directly to measure the strengthening of the adhesion force between S. thermophilus B and a silicon nitride (Si3Ni4) AFM tip, and bond strengthening by a factor of 2 to 3 was found to occur over a similar time as residence-time-dependent desorption (22). However, S. thermophilus B adheres to glass utilizing nonspecific adhesion mechanisms, which are very different from the specific mechanisms used by S. aureus strains during their adhesion to Fn films.
Therefore, the aim of this study was to analyze the role of FnBPs on the surfaces of S. aureus cells (S. aureus wild-type strain 8325-4 and the isogenic mutant DU5883 lacking FnBPs) in interactions with (adsorbed) Fn using ITC and AFM and in particular in relation to adhesion and residence-time-dependent desorption on Fn-coated surfaces under flow conditions.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. aureus strain 8325-4 and an isogenic mutant lacking FnBPs, DU5883 (kindly provided by T. J. Foster, Moyne Institute of Preventive Medicine, Dublin, Ireland), were used in this study. The bacteria were maintained at −80°C in tryptone soy broth (TSB) (Oxoid, Basingstoke, United Kingdom) containing 7% dimethyl sulfoxide (Merck, Germany). For culturing, both strains were incubated on TSB agar plates overnight at 37°C. Subsequently, bacterial colonies were precultured in 10-ml TSB batch cultures overnight with constant rotation. Each preculture was used to inoculate a main culture in 190 ml TSB. After approximately 2 h (growth to early stationary phase, corresponding to peak expression of FnBPs in S. aureus 8325-4 [19]), bacteria were harvested by centrifugation at 6,500 × g for 5 min at 10°C and washed twice with demineralized water. Bacterial aggregates were broken apart by mild sonication three times on ice for 10 s at 30 W (model 375 Vibra Cell; Sonics and Materials Inc., Danbury, CT). Then bacteria were resuspended in phosphate-buffered saline (PBS) (10 mM potassium phosphate, 0.15 M NaCl; pH 7) to obtain concentrations of 3 × 108 and 5 × 109 cells per ml for adhesion experiments and ITC, respectively, as determined with a Bürker-Türk counting chamber. In order to block FnBPs on the staphylococcal cell surfaces, staphylococci were also incubated for 60 min at 37°C in PBS supplemented with 1% bovine serum albumin (BSA).
Bacterial deposition on an Fn film in a parallel plate flow chamber.
The deposition experiments were carried out using a parallel plate flow chamber (inside length, 175 mm; inside width, 17 mm; inside height, 0.75 mm) equipped with image analysis options (3). The bottom glass plate (76 by 26 mm) of the flow chamber was first cleaned by sonication for 3 min in a surfactant solution (2% RBS 35 detergent in water; Omniclean), rinsed thoroughly with tap water, and then washed with methanol, thoroughly rinsed with tap water, and finally rinsed with demineralized water. Subsequently, as 0.05-ml droplet of Fn (25 μg ml−1 human Fn; Sigma-Aldrich BV, Zwijndrecht, The Netherlands) was placed in the center of the glass plate for 2 h at room temperature to create a circular Fn-coated region with a diameter of approximately 1 cm on which staphylococcal adhesion was monitored. In addition, glass plates were prepared on which nonspecific adhesion sites were blocked by immersing each entire glass plate, including the Fn-coated region, for 1 min in PBS containing 1% BSA. Glass plates were rinsed after protein coating with demineralized water. Bacterial adhesion was monitored with a phase-contrast microscope (Olympus BH-2) equipped with a ×40 ultra-long-working-distance lens (Olympus ULWD-CD plan 40 PL) and coupled to a Firewire charge-coupled device camera (Basler AG, Germany).
The flow rate during the experiments was adjusted to 1.4 ml min−1 under the influence of hydrostatic pressure, yielding a shear rate of 15 s−1. During flow experiments, 15 images (1,392 by 1,040 pixels) were grabbed every second. The 15 frames were averaged in order to distinguish between adhered bacteria and in-focus moving bacteria. The average frame was stored in a computer for subsequent offline analysis using proprietary software based on the Matlab image-processing toolkit (The Mathworks, Massachusetts). Further analysis consisted of locating the staphylococci on the substratum surface and comparing the positions of the staphylococci in a current image with their positions in previous images to determine the total number of adhering bacteria as a function of time during a 4-h period, as well as their residence times. The affinity of an organism for an Fn-coated glass surface was expressed as the initial deposition rate, which represented the initial increase in the total number of adhering bacteria with time. Note that since the initial deposition rate was derived using only the first adhering bacteria, it represented the affinity of the organisms for the adsorbed Fn coatings without the intervening influences of interactions between adhering bacteria, like the interactions which occur due to crowding at the surface (e.g., after 4 h) (3). Finally, the staphylococcal desorption rate coefficient as a function of residence time was calculated as follows (13):
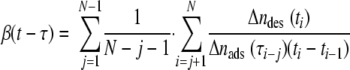 |
(3) |
where the summation runs over the number of images taken, Δndes(ti) is the number of bacteria desorbing between time ti − 1 and time ti and adsorbing between time τi − j − 1 and time τi − j, and Δnads(ti − j) is the total number of adsorbed bacteria between time τi − j − 1 and τi − j. The residence-time-dependent desorption rates calculated were fitted to equation 2 to obtain β0, β∞, and 1/δ.
All adhesion and desorption experiments in the parallel plate flow chamber were performed in quadruplicate with separate bacterial cultures.
AFM.
For AFM, the negatively charged bacteria were attached by electrostatic interaction to a glass slide that was positively charged by preadsorption of poly-l-lysine, as described previously (5). AFM tips (DNP; Veeco, Woodbury, NY) were coated with an Fn film by immersion for 30 min in an Fn solution (25 μg ml−1 in PBS, pH 7) with the aid of a micromanipulator. All glass slides with immobilized bacteria and Fn-coated AFM tips were used immediately after preparation. To block nonspecific binding sites on the bacterial cell surfaces, the glass slides with attached bacteria were also immersed for 1 min in PBS containing 1% BSA and rinsed with demineralized water.
AFM measurements were obtained at room temperature in PBS using a Dimension 3100 system (Nanoscope IV; Digital Instruments, Woodbury, NY). Nanoscope imaging software (version 6.13r1; Veeco) was used to analyze the resulting images. All AFM cantilevers were calibrated using resonant frequency measurements (6), and the slopes of the retraction force curves in the region where the probe and sample were in contact were used to translate the voltage into cantilever deflection. Force-distance curves were generated, and approach curves were analyzed to determine the repulsive force at contact. The tip was retracted from the bacterial surface immediately and after 2 s of contact between the AFM tip and the staphylococcal cell surface to demonstrate strengthening of the adhesion force. Retraction curves were integrated to obtain the bond strength energy for the two surface delay times evaluated.
Three different bacterial cells from different cultures were examined at 10 locations in each case with separately prepared Fn-coated AFM tips, yielding 30 force-distance curves. This resulted in a nonparametric distribution from which median, mode, and range values were derived. We regularly checked whether interactions of our Fn-coated tips with clean glass yielded the same force values, which was always the case in one series of experiments.
ITC.
The enthalpy of adsorption of Fn to the bacterial cell surfaces was measured with a twin-type, isothermal microcalorimeter (TAM 2277; Thermometric, Sweden). The calorimeter was placed in a temperature-controlled environment (20 ± 0.1°C), which allowed baseline stability of ±0.1 μW over a 24-h period (17). The instrument had electrical calibration with precision better than 1%, and proper calibration was regularly checked by measuring the dilution enthalpy of concentrated sucrose solutions (26). Experiments were performed isothermally at 25°C in 4-ml stainless steel ampoules. Four ampoules, connected with separate titration systems, were used inside the microcalorimeter. The use of a twin-type microcalorimeter allowed measurement of the heat flowing from the reaction ampoule compared with a reference ampoule. The output signal was collected as power versus time and was integrated to evaluate the isobaric heat exchange (change in enthalpy) during adsorption, using the dedicated Digitam 4.1 software (Thermometric, Sweden). Notably, the measured heat effect should have been corrected for the heat of dilution of the proteins to obtain the net adsorption enthalpy (2).
Typically, all four reaction ampoules, including the reference ampoule, were filled with 1.5 ml of a bacterial suspension (5 × 109 bacteria per ml) in PBS with constant stirring (90 rpm) using a specially designed two-blade stirrer. The ampoules were lowered gradually into the microcalorimeter and left in the measuring position to reach thermal equilibrium before data collection started. After equilibration, a stable baseline was obtained, and Fn was titrated into the reaction ampoules. Titration was done at a controlled rate of 2 μl s−1 using a stainless steel cannula connected to a syringe. In order to study possible saturation of adsorption sites, an Fn solution (25 μg ml−1) was added by using four consecutive injections of 60 μl into the ampoule at 40-min intervals. All calorimetric experiments were done in quadruplicate.
Statistical analysis.
Data were analyzed with the statistical package for the social sciences (version 11.0; SPSS, Chicago, IL). Median values for the repulsive force at the time of contact upon approach, the adhesion force upon retraction, and the bond strength energy were analyzed using the Wilcoxon signed-rank test for the median. A Student t test was used to determine significant differences in the initial deposition rates, the numbers of adhered bacteria after 4 h, β0, β∞, and 1/δ, as well as significant differences in interaction enthalpies. The level of significance used was P < 0.05.
RESULTS
Adhesion and residence-time-dependent desorption of S. aureus from Fn films.
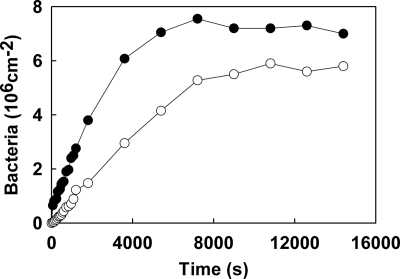
Figure 1 shows examples of the adhesion kinetics of S. aureus 8325-4 and DU5883 with Fn coatings in a parallel plate flow chamber in PBS at pH 7. The adhesion kinetics of both S. aureus strains were linear for approximately 4,000 to 5,000 s before they leveled off at stationary numbers, and the linear trajectories yielded the initial deposition rates, as summarized in Table 1. The initial deposition rate of S. aureus 8325-4 was about twice as high as that of FnBP-deficient strain DU5883, which indicates the relatively high affinity of strain 8325-4 for Fn coatings. Also, after 4 h of deposition, higher numbers of strain 8325-4 cells than of FnBP-deficient DU5883 cells adhered, but the difference was not twofold like the difference in the initial deposition rates. After exposure of either the Fn coating or the staphylococci to BSA, the initial deposition rates and the numbers of bacteria adhering after 4 h decreased significantly for both strains.
FIG. 1.
Representative examples of the kinetics of adhesion of S. aureus 8325-4 (•) and DU5883 (○) to Fn films in PBS.
TABLE 1.
Mean values for the initial deposition rate, the numbers of cells adhering after 4 h, β0, β∞, and 1/δ for S. aureus 8325-4 with FnBPs and isogenic mutant DU5883 without FnBPs with Fn coatings
| Substratum | Initial deposition rate (cm−2 s−1)b
|
No. of cells adhering after 4 h (106 cm−2)
|
β0 (10−3 s−1)
|
β∞ (10−3 s−1)
|
1/δ (s)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8325-4 | DU5883 | 8325-4 | DU5883 | 8325-4 | DU5883 | 8325-4 | DU5883 | 8325-4 | DU5883 | |
| Fn-coated glass | 2,438 | 1,290 | 7.0 | 5.2 | 307 | 463 | 1.0 | 1.2 | 0.9 | 0.9 |
| Fn- and BSA-coated glass | 815 | 678 | 5.2 | 4.4 | 200 | 170 | 0.6 | 0.4 | 1.0 | 1.2 |
| Fn-coated glassa | 704 | 527 | 3.9 | 3.5 | 334 | 504 | 0.6 | 1.8 | 0.9 | 0.9 |
The experiments were carried out with staphylococci exposed to 1% BSA prior to the experiments.
The average standard deviations (n = 4) were as follows: for the initial deposition rate, ±110 cm−2 s−1; for the number of cells adhering after 4 h, ±0.5 × 106 cm−2; for β0, ±124 × 10−3 s−1; for β∞, ±0.3 × 10−3 s−1; and for 1/δ, ±0.3 s.
Table 1 also summarizes desorption characteristics of the two staphylococcal strains. Exposure of either the Fn-coated surface or the bacterial cell surface to BSA had only a minor, if any, effect on β0, which suggests that the desorbing bacteria left mainly nonspecific binding sites. The desorption rates decreased with increasing residence times for both strains, regardless of the presence of a BSA coating on the surfaces, and the 1/δ for bond ageing were less than 2 s. The β∞ were similar for the two strains; there was not a significant influence of bacterial exposure to BSA, and there was a slight reduction in the final desorption rates after exposure of the Fn-coated surface to BSA.
Strengthening of the bond between Fn coatings and S. aureus cell surfaces.
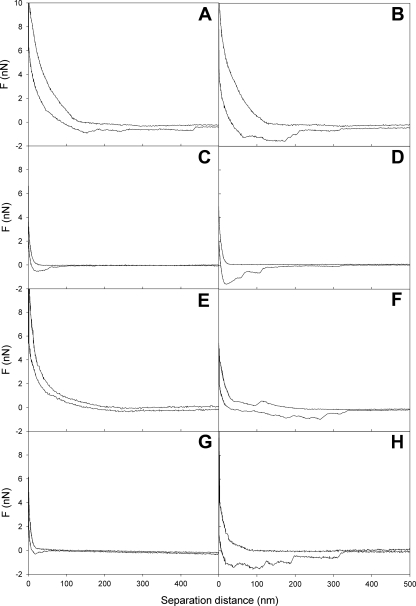
The median values for the interaction forces measured using AFM are summarized in Table 2. The repulsive force at contact was significantly (P < 0.05) stronger for S. aureus 8325-4 with FnBPs than for S. aureus DU5883. Blocking of nonspecific binding sites on the staphylococcal cell surfaces had little (S. aureus DU5883) or no (S. aureus 8325-4) influence on the repulsive force upon approach. However, upon retraction, the median adhesion forces were significantly stronger after a 2-s surface delay than when they were measured immediately. There was no significant difference in adhesion forces between the two strains. Interestingly, the distance over which the adhesion forces were operative varied considerably under the different conditions used (Fig. 2), which translated into significant differences in bond strength energies. The initial bond strength energies for S. aureus 8325-4 with FnBPs were significantly (P < 0.05) higher than those for S. aureus DU5883 without FnBPs, regardless of exposure of the staphylococci to a 1% BSA solution. Both strains showed significant increases in bond strength energy when the surface delay time was increased from 0 to 2 s; this energy increased by a factor 2 to 3 for S. aureus 8325-4 and even more (by a factor 4 to 5) for S. aureus DU5883 (Table 2). Furthermore, after a surface delay, the effects of BSA exposure of the staphylococci on bond strength energy disappeared.
TABLE 2.
Median values for the repulsive forces at contact upon approach, the adhesion force upon retraction, and the associated bond strength energy for the interaction between Fn-coated AFM tips and S. aureus 8325-4 or an isogenic mutant without FnBPs (DU5883) prior to and after exposure of bacteria to 1% BSAa
| Delay time (s) | Treatment | Repulsive force at contact upon approach (nN)
|
Adhesion force upon retraction (nN)
|
Bond strength energy (10−16 J)
|
|||
|---|---|---|---|---|---|---|---|
| 8325-4 | DU5883 | 8325-4 | DU5883 | 8325-4 | DU5883 | ||
| 0 | No BSA | 9.1 | 6.0 | −0.7 | −0.6 | −98 | −32 |
| 1% BSA | 9.0 | 3.9 | −0.6 | −0.5 | −64 | −21 | |
| 2 | No BSA | 9.1 | 6.0 | −1.5 | −1.7 | −187 | −181 |
| 1% BSA | 9.0 | 3.9 | −1.1 | −1.1 | −176 | −149 | |
Distribution functions were obtained using a class width of 0.1 nN. The data for the repulsive force at contact comprise repulsive forces at contact measured in experiments with and without a surface delay and thus refer to 30 force-distance curves.
FIG. 2.
Representative examples of force-distance curves for an Fn-coated AFM tip and staphylococcal cell surfaces. (A) S. aureus 8325-4 after no surface delay. (B) S. aureus 8325-4 after a 2-s surface delay. (C) S. aureus DU5883 after no surface delay. (D) S. aureus DU5883 after a 2-s surface delay. (E) S. aureus 8325-4 coated with BSA after no surface delay. (F) S. aureus 8325-4 coated with BSA after a 2-s surface delay. (G) S. aureus DU5883 coated with BSA after no surface delay. (H) S. aureus DU5883 coated with BSA after a 2-s surface delay.
Enthalpies of adsorption of Fn to the S. aureus cell surface.
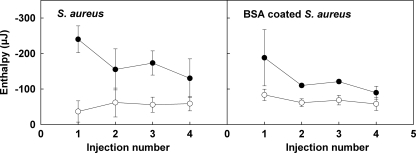
Measurement of the enthalpies of adsorption of Fn to the S. aureus cell surface required correction for the heat of dilution of the proteins in PBS. Four consecutive injections of 60 μl of a 25-μg ml−1 Fn solution into 1.5 ml of PBS resulted in heat effects of −55, −56, −37, and −37 × 10−9 μJ. Figure 3 summarizes the adsorption enthalpies after consecutive injections of Fn into the staphylococcal suspensions, after correction for protein dilution. For the parent strain 8325-4 with FnBPs, the adsorption enthalpies decreased with the number of injections, but no saturation of adsorption sites was observed with four injections. For strain DU5883 the enthalpy effects essentially did not vary with the number of injections. The cumulative adsorption enthalpies after four injections are shown in Table 3 and are expressed per bacterium and per m2 of bacterial cell surface. Fn adsorption to the bacterial cell surfaces was an exothermic process in all cases; i.e., enthalpy was released upon adsorption. Adsorption of Fn to S. aureus 8325-4 with FnBPs was enthalpically significantly more favorable than adsorption of Fn to S. aureus DU5883. After exposure of S. aureus 8325-4 to BSA, the adsorption enthalpy decreased significantly, but it remained greater than that for S. aureus DU5883. No significant effect of exposure to BSA on the adsorption enthalpy of S. aureus DU5883 lacking FnBPs was seen.
FIG. 3.
Adsorption enthalpies, after correction for dilution effects, for Fn with S. aureus cell surfaces after consecutive injections of 60 μl of an Fn solution (25 μg/ml) into 1.5-ml suspensions of S. aureus 8325-4 (•) and S. aureus DU5883 (○) in PBS. The data in the right panel are data for staphylococci that were first exposed to 1% BSA. The error bars indicate standard deviations based on four independent measurements.
TABLE 3.
Cumulative adsorption enthalpies per bacterium and per m2 of bacterial cell surfacea
| Expt | Cumulative adsorption enthalpy per bacterium (10−9 μJ)b
|
Cumulative adsorption enthalpy per m2 (mJ m−2)b
|
||
|---|---|---|---|---|
| 8325-4 | DU5883 | 8325-4 | DU5883 | |
| No BSA | −140 | −43 | −44 | −14 |
| 1% BSA | −102 | −54 | −32 | −17 |
For calculation of the adsorption enthalpy per m2, it was assumed that the bacterial cell radius was 0.5 μm.
The average standard deviations (n = 4) were ±25 × 10−9 μJ per bacterium and ±8 mJ m−2.
DISCUSSION
In this study, we compared the interactions mediating adhesion to Fn-coated surfaces, as well as desorption, of S. aureus strains with and without FnBPs, using three entirely different techniques. Adhesion and residence-time-dependent desorption were determined using a parallel plate flow chamber under convective diffusion conditions. In addition, the forces of adhesion to Fn-coated AFM tips were measured, as were the enthalpies of adsorption of Fn to staphylococcal cell surfaces. In general, the adhesion to Fn-coated substrata was faster and the numbers of cells were higher for the strain with FnBPs than for the strain lacking FnBPs, and, consistent with these findings, the adhesive bonds were stronger and the adsorption enthalpies were higher for the former strain. Adsorption of BSA to either the Fn-coated substrata or the staphylococcal cell surfaces not only blocked nonspecific adhesion/adsorption sites but also reduced the accessibility of the FnBPs on strain 8325-4 during convective-diffusional mass transport, as in this case the level of adhesion approached that of FnBP-deficient strain DU5883. The effects of a BSA coating on interaction forces and adsorption enthalpies were far less significant, however. Strengthening of the bond was evident from the residence-time-dependent desorption of both strains, as measured in a parallel plate flow chamber, as well as by a comparison of the adhesion forces and adhesive bond strength energies measured after 0- and 2-s surface delays in AFM.
Adsorption enthalpies.
The enthalpy changes associated with the interaction between Fn and the S. aureus cells were all exothermic and were markedly different for the two strains. For the FnBP-deficient DU5883 strain, the enthalpy change was essentially the same for each injection step, which was indicative of nonspecific adsorption in the subsaturation range. Assuming that the staphylococcal cell diameter is 1 μm, it was calculated that in the ITC ampoule there was 23.6 × 10−3 m2 of bacterial surface area available for adsorption. Since each Fn injection added 1.5 × 10−3 mg Fn, the maximal cell surface coverage by Fn after four consecutive injections was 0.25 mg Fn per m2 bacterial cell surface, which is far below the saturation limit for nonspecifically adsorbed Fn, which is at least a few mg per m2 (21). Assuming that all Fn added was adsorbed, the cumulative enthalpy effect measured (−14 mJ m−2) corresponded to −13.8 × 103 kJ per mol Fn (which corresponded to about 5,600 kT per molecule of Fn at 25°C), as calculated using a molecular mass of 250 kDa. Taking into account the high molecular mass of Fn, this value is quite reasonable compared with enthalpy effects reported for nonspecific adsorption of various proteins to different surfaces (11). The enthalpy effects measured for the FnBP-containing strain 8325-4 were more exothermic than those measured for the FnBP-deficient strain DU5883. This indicates that there was involvement of enthalpically favorable specific Fn-binding sites. The downward trend in the interaction enthalpy with consecutive injection steps shown in Fig. 3 (left panel) suggests that not all specific binding sites were equally favorable or, alternatively, that they became gradually saturated. Assuming that all Fn added during the first injection bound to FnBPs, the measured value (−250 μJ) corresponds to −41.7 × 103 kJ per mol Fn. This is about 300-fold higher than the enthalpy of the biotin-streptavidin interaction (16). However, it should be realized that the much larger Fn molecule may interact through a number of binding sites that is greater than the number of sites involved in a single biotin-streptavidin interaction.
Exposure of the bacteria to a BSA solution hardly influenced the enthalpy of the interaction between Fn and the FnBP-deficient strain DU5883. In contrast, exposure of the FnBP-containing strain 8325-4 to BSA significantly suppressed the enthalpy of the interaction with Fn, but not nearly at the level of a nonspecific interaction. However, the downward trend in enthalpy for BSA-coated strain 8325-4 (Fig. 3, right panel) seems to indicate that for the later Fn injections, smaller fractions of the Fn added found FnBPs compared to the non-BSA-coated cells. This is completely in line with the lack of effects of BSA coating on adhesion forces observed by AFM and attests to the forceful contact established during AFM or stirring in the microcalorimeter compared with the spontaneous and relatively mild nature of the cell-surface interaction during convective diffusion in the parallel plate flow chamber.
Interaction forces.
The AFM adhesion forces with Fn-coated surfaces were similar for the two S. aureus strains, despite their different abilities to adsorb Fn (19). Adsorption of Fn is a process that occurs at the outermost cell surface. However, an Fn-coated AFM tip penetrates a cell. It is clear that upon penetration, for both S. aureus strains FnBPs or other adhesins were encountered in the cell wall; this was true even for strain DU5883, which is generally considered to be devoid of FnBPs (19). However, the spatial distribution of adhesins in strain 8325-4 must be different than that in strain DU5883, as its adhesion forces reach out further; consequently, strain 8325-4 has a higher Fn bond strength energy than strain DU5883 (Table 2). It can be envisaged that under the convective diffusion conditions in the parallel plate flow chamber, bacteria landed mildly at the substratum surface and therefore allowed interactions only with the outermost cell surface. In contrast, the penetrating AFM tip sensed similar bond strength energies with no influence of an adsorbed BSA film on the cell surface. Mendez-Vilas et al. (14, 15) suggested that a penetrating AFM tip may cause irreversible damage to the inner cell surface, which they concluded from saw tooth patterns in the force-distance curves at close approach. As we observed no such patterns in our force-distance curves (Fig. 2), we think that it is unlikely that the AFM tip caused such cell surface damage.
Residence-time-dependent desorption.
The desorption rate coefficients from an Fn-coated surface decreased for both S. aureus strains within 2 s by a factor of about 300 to 400. The desorption rates and their residence time dependence were not sensitive to whether specific Fn-FnBP interactions were involved in the adhesion. Apparently, the cells of strain 8325-4 that desorbed belonged to the fraction of the population that was not able to adhere through strong specific bonds. Accordingly, exposure of either the bacterial cells or the Fn-coated surface to BSA, which blocked specific Fn-FnBP interactions, had no or little effect on the desorption kinetics.
Assuming that for a given condition, all bacteria adhered with the same bond strength, a staphylococcal bond strength energy could also be calculated from the desorption rate coefficients measured in the parallel plate flow chamber by using
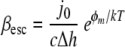 |
(4) |
where βesc is the desorption rate coefficient, j0 is the initial deposition rate, c is the bacterial cell concentration at the entrance of the flow chamber, Δh is the width of the energy minimum, φm is the depth of the energy minimum, and kT is the energy of thermal motion (13, 27). The initial bond strength energies for the binding of our staphylococcal strains to an Fn film in the absence of a BSA coating could be calculated from β0 and ranged from 2.2 to 3.3 kT, which seems quite reasonable for nonspecific binding. After bond ageing, use of β∝ yielded higher bond strength energies that were between 8.2 and 9.0 kT. Yet these bond strength energies are orders of magnitude lower than those derived from AFM, and conversion of the bond strength energies from Table 2 to a thermal energy scale yielded values of around 106 kT. This huge number attests to the fact that the penetrating, Fn-coated AFM tip must have encountered numerous adhesins in the cell surface.
Conclusions.
The use of a parallel plate flow chamber, AFM, and ITC together yielded new insights into the mechanisms of the interaction between adsorbed Fn films and two S. aureus strains, one of which has FnBPs on its cell surface and one of which is generally considered to be devoid of FnBPs. Differences between these two strains with respect to (i) the initial deposition rate for an Fn-coated surface, (ii) the strength of binding to an Fn-coated AFM tip, and (iii) the enthalpy of the interaction with Fn indicate that for 8325-4 additional attractive forces are involved, which are ascribed to a specific FnBP-Fn interaction. Exposure of either Fn coatings or staphylococcal cell surfaces to BSA strongly reduces staphylococcal adhesion under convective diffusion conditions, but the enthalpy of Fn adsorption and the adhesion force upon retraction of an Fn-coated tip from the staphylococcal cell surface are influenced less, if at all, by a BSA coating. This suggests that AFM and calorimetry probe not only interactions at the outermost surfaces of the interacting species but also interactions occurring underneath the BSA coating. Residence-time-dependent desorption data and AFM measurements revealed considerable bond strengthening within a few seconds after contact for both strains. The bond strength energies calculated from the retraction force-distance curves were orders of magnitude higher than those calculated from desorption rate coefficients, confirming that the penetrating Fn-coated AFM tip probed multiple receptor sites in the cell surface in both S. aureus strains. Apparently, mild landing of an organism on an Fn-coated substratum, like that during convective diffusion in the parallel plate flow chamber, does not result in specific interactions with adhesins located deeper.
Acknowledgments
We thank ZON-MW for grant 91105005, which enabled purchase of the Digital Instruments Nanoscope IV.
Footnotes
Published ahead of print on 24 October 2008.
REFERENCES
- 1.Binnig, G., C. F. Quate, and C. Gerber. 1986. Atomic force microscope. Phys. Rev. Lett. 56:930-933. [DOI] [PubMed] [Google Scholar]
- 2.Briggner, L. E., and I. Wadsö. 1991. Test and calibration processes for microcalorimeters, with special reference to heat conduction instruments used with aqueous systems. J. Biochem. Biophys. Methods 22:101-118. [DOI] [PubMed] [Google Scholar]
- 3.Busscher, H. J., and H. C. van der Mei. 2006. Microbial adhesion in flow displacement systems. Clin. Microbiol. Rev. 19:127-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busscher, H. J., B. van de Belt-Gritter, R. J. B. Dijkstra, W. Norde, F. C. Petersen, A. A. Scheie, and H. C. van der Mei. 2007. Intermolecular forces and enthalpies in the adhesion of Streptococcus mutans and an antigen I/II-deficient mutant to laminin films. J. Bacteriol. 189:2988-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camesano, T. A., M. J. Natan, and B. E. Logan. 2000. Observation of changes in bacterial cell morphology using tapping mode atomic force microscopy. Langmuir 16:4563-4572. [Google Scholar]
- 6.Cleveland, J. P., S. Manne, D. Bocek, and P. K. Hansma. 1993. A nondestructive method for determining the spring constant of cantilevers for scanning force microscopy. Rev. Sci. Instrum. 64:403-405. [Google Scholar]
- 7.Dabros, T., and T. G. M. van de Ven. 1982. Kinetics of coating by colloidal particles. J. Colloid Interface Sci. 89:232-244. [Google Scholar]
- 8.Fowler, T., E. R. Wann, D. Joh, S. Johansson, T. J. Foster, and M. Hook. 2000. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMS and host cell β1 integrins. Eur. J. Cell Biol. 79:672-679. [DOI] [PubMed] [Google Scholar]
- 9.Greene, C., D. McDevitt, P. Francois, P. E. Vaudaux, D. P. Lew, and T. J. Foster. 1995. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol. Microbiol. 17:1143-1152. [DOI] [PubMed] [Google Scholar]
- 10.Haynes, C. A., and W. Norde. 1995. Structures and stabilities of adsorbed proteins. J. Colloid Interface Sci. 169:313-328. [Google Scholar]
- 11.Haynes, C. A., and W. Norde. 1994. Globular proteins at solid/liquid interfaces. Colloids Surf. B 2:517-566. [Google Scholar]
- 12.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 13.Meinders, J. M., H. C. van der Mei, and H. J. Busscher. 1994. Physicochemical aspects of deposition of Streptococcus thermophilus B to hydrophobic and hydrophilic substrata in a parallel plate flow chamber. J. Colloid Interface Sci. 164:355-363. [Google Scholar]
- 14.Mendez-Vilas, A., A. M. Gallardo-Moreno, and M. L. González-Martín. 2006. Nano-mechanical exploration of the surface and sub-surface of hydrated cells of Staphylococcus epidermidis. Antonie van Leeuwenhoek 89:373-386. [DOI] [PubMed] [Google Scholar]
- 15.Mendez-Vilas, A., A. M. Gallardo-Moreno, R. Calzado-Montero, and M. L. Gonzalez-Martin. 2007. AFM probing in aqueous environment of Staphylococcus epidermidis cells naturally immobilized on glass: physico-chemistry behind successful immobilization. Colloids Surf. B 63:101-109. [DOI] [PubMed] [Google Scholar]
- 16.Moy, V. T., E. L. Florin, and H. E. Gaub. 1994. Intermolecular forces and energies between ligand and receptors. Science 266:257-259. [DOI] [PubMed] [Google Scholar]
- 17.Nordmark, M. G., J. Laynez, A. Schön, J. Suurkuusk, and I. Wadsö. 1984. Design and testing of a new microcalorimetric vessel for use with living cellular systems and in titration experiments. J. Biochem. Biophys. Methods 10:187-202. [DOI] [PubMed] [Google Scholar]
- 18.Peacock, S. J., T. J. Foster, B. J. Cameron, and A. R. Berendt. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477-3486. [DOI] [PubMed] [Google Scholar]
- 19.Saravia-Otten, P., H. P. Muller, and S. Arvidson. 1997. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J. Bacteriol. 179:5259-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjollema, J., H. C. van der Mei, H. M. Uyen, and H. J. Busscher. 1990. Direct observations of cooperative effects in oral streptococcal adhesion to glass by analysis of the spatial arrangement of adhering bacteria. FEMS Microbiol. Lett. 69:263-269. [DOI] [PubMed] [Google Scholar]
- 21.Sousa, S. R., M. Manuela Brás, P. Moradas-Ferreira, and M. A. Barbosa. 2007. Dynamics of fibronectin adsorption on TiO2 surfaces. Langmuir 23:7046-7054. [DOI] [PubMed] [Google Scholar]
- 22.Vadillo-Rodríguez, V., H. J. Busscher, W. Norde, J. de Vries, and H. C. van der Mei. 2004. Atomic force microscopic corroboration of bond aging for adhesion of Streptococcus thermophilus to solid substrata. J. Colloid Interface Sci. 278:251-254. [DOI] [PubMed] [Google Scholar]
- 23.van de Ven, T. G. M. 1989. Effects of electrolytes, polymers and polyelectrolytes on particle deposition and detachment. Colloids Surf. 39:107-126. [Google Scholar]
- 24.Vaudaux, P., R. Suzuki, F. A. Waldvogel, J. J. Morgenthaler, and U. E. Nydegger. 1984. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J. Infect. Dis. 150:546-553. [DOI] [PubMed] [Google Scholar]
- 25.Vaudaux, P. E., F. A. Waldvogel, J. J. Morgenthaler, and U. E. Nydegger. 1984. Adsorption of fibronectin onto polymethyl-methacylate and promotion of Staphylococcus aureus adherence. Infect. Immunol. 45:768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu, C. F., W. Y. Chen, and J. F. Lee. 1996. Microcalorimetric studies of the interactions of imidazole with immobilized Cu(II): effects of pH value and salt concentration. J. Colloid Interface Sci. 183:236-242. [DOI] [PubMed] [Google Scholar]
- 27.Xia, Z., H. L. Goldsmith, and T. G. van de Ven. 1994. Flow-induced detachment of red blood cells adhering to surfaces by specific antigen-antibody bonds. Biophys. J. 66:1222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu, C. P., B. van de Belt-Gritter, H. J. Busscher, H. C. van der Mei, and W. Norde. 2007. Calorimetric comparison of the interactions between salivary proteins and Streptococcus mutans with and without antigen I/II. Colloids Surf. B 54:193-199. [DOI] [PubMed] [Google Scholar]