Abstract
Background and purpose:
Traumatic brain injury (TBI) triggers a complex series of inflammatory responses that contribute to secondary tissue damage. The aim of this study was to investigate the effect of baicalein, a flavonoid possessing potent anti-inflammatory properties, on functional and histological outcomes and inflammatory cytokine expression, following TBI in rats.
Experimental approach:
Rats subjected to controlled cortical impact injury were injected with baicalein (30 mg kg−1) or vehicle immediately after injury or daily for 4 days. Neurological status was evaluated using the rotarod, adhesive removal, modified neurological severity scores and beam walk tests. Contusion volume and neuronal degeneration were measured using cresyl violet and FluoroJade B (FJB) histochemistry. Levels of tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) mRNA and protein were assessed by real-time quantitative reverse transcriptase-PCR, enzyme-linked immunosorbent assay and immunohistochemistry.
Key results:
Single-dose and multiple-dose treatment with baicalein significantly improved functional recovery and reduced contusion volumes up to day 28 post-injury, although multiple-dose baicalein was the more effective treatment. Single-dose baicalein also significantly reduced the number of degenerating neurons (31%) on post-injury day 1 as indicated by FJB staining. These changes were associated with significantly decreased levels, at the contusion site, of TNF-α, IL-1β and IL-6 mRNA at 6 h, and cytokine protein on day 1 post-injury.
Conclusions and implications:
Post-injury treatment with baicalein improved functional and histological outcomes and reduced induction of proinflammatory cytokines in rat TBI. The neuroprotective effect of baicalein may be related to a decreased inflammatory response following the injury.
Keywords: baicalein, inflammation, tumour necrosis factor-α, interleukin-1β, interleukin-6, cytokines, traumatic brain injury
Introduction
Traumatic brain injury (TBI) sets off a cascade of inflammatory responses that are believed to participate in the pathogenesis of secondary injury (Morganti-Kossmann et al., 2002). These inflammatory responses include the activation of glia and neurons, infiltration of neutrophils and macrophages as well as upregulation of adhesion molecules and cytokines (Holmin et al., 1997; Morganti-Kossmann et al., 2002). Tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) are major proinflammatory cytokines involved in the post-traumatic inflammatory responses (Shohami et al., 1996; Sanderson et al., 1999; Lu et al., 2005). The elevated levels of these cytokines have been detected in the CSF and serum of patients with severe head injuries (Goodman et al., 1990; Csuka et al., 1999; Hans et al., 1999a; Hayakata et al., 2004) and in brain parenchyma of animals with experimental brain injuries (Taupin et al., 1993; Shohami et al., 1994, 1996; Hans et al., 1999b). Although the potential pathophysiological role of these cytokines in human brain injury remains controversial, evidence from animal studies suggests that the presence of TNF-α, IL-1β or IL-6 in the initial post-injury period is harmful, and that suppressing acute surges of these cytokines may reduce brain oedema and tissue damage and improve functional outcome (Morganti-Kossmann et al., 2002; Lu et al., 2005).
Tumour necrosis factor-α and IL-1β may mediate secondary damage following TBI through several different mechanisms (Shohami et al., 1996; Sanderson et al., 1999; Lu et al., 2005). These two cytokines are known to affect the integrity of the blood–brain barrier (Megyeri et al., 1992; Touzani et al., 1999; Holmin and Mathiesen, 2000), and induce both post-traumatic apoptotic (Laster et al., 1988; Friedlander et al., 1996; Holmin and Mathiesen, 2000) and necrotic cell death (Laster et al., 1988; Sanderson et al., 1999). Moreover, TNF-α and IL-1β have been shown to participate in glutamate neurotoxicity by mechanisms involving glutamatergic receptors and nitric oxide (Chao and Hu, 1994; Chao et al., 1995). Similarly, IL-6 is an important inflammatory mediator triggered by TNF-α and IL-1β (Allan and Rothwell, 2001). Although IL-6 may display either beneficial or detrimental effects following TBI, accumulating data suggest that high IL-6 concentrations and extended production of this cytokine in the damaged brain may activate microglia/macrophages and trigger a series of events ultimately leading to neuronal death (Allan and Rothwell, 2001; Morganti-Kossmann et al., 2002). Taken together, the above evidence suggests that TNF-α, IL-1β and IL-6 may be ideal targets for therapeutic intervention in the acute post-traumatic period to reduce secondary brain injury.
Baicalein is a major flavonoid extracted from the root of Scutellaria baicalensis Georgire (also called Huanf Gui), a traditional oriental medicine widely used in treating allergic and inflammatory diseases. This flavonoid has been shown to exert potent anti-inflammatory effects in vitro (Huang et al., 2006; Woo et al., 2006) as well as in vivo (Hong et al., 2002; Huang et al., 2006). Antioxidant effects of baicalein are also well documented (Ishige et al., 2001; Li et al., 2005; Huang et al., 2006; Wu et al., 2006). In this regard, baicalein has been reported to scavenge free radicals (Gao et al., 2001; Li et al., 2005; Huang et al., 2006; Wu et al., 2006) and inhibit lipid peroxidation activities (Im et al., 2005; Li et al., 2005; Huang et al., 2006; Wu et al., 2006). Moreover, neuroprotective effects of baicalein were proposed in experimental cerebral ischaemia and Parkinson's disease (Hamada et al., 1993; Hwang et al., 2002; Wu et al., 2006; van Leyen et al., 2006; Lapchak et al., 2007). However, no information is available concerning the possible therapeutic efficacy of baicalein administered after acute TBI.
The aim of this study was to evaluate the effect of baicalein in TBI, based on the hypothesis that post-injury baicalein treatment would reduce functional deficits and extent of anatomical brain damage and that baicalein would attenuate levels of proinflammatory cytokines TNF-α, IL-1β and IL-6 after controlled cortical impact (CCI) injury. CCI is a readily reproducible model of TBI and displays many of the same pathophysiological hallmarks, such as oedema formation, motor and cognitive behavioural impairments and necrotic and apoptotic cell death (Morales et al., 2005). In this study, neurological status in animals suffering injuries from the CCI was evaluated using the rotarod, tactile adhesive removal, modified neurological severity scores (mNSS) and beam walk tests. Both mRNA expression and protein levels of the cytokines were assessed through the use of real time quantitative reverse transcriptase-PCR (RT-PCR), ELISA and immunohistochemistry. Our results suggest that post-injury bacalein can reduce cytokine upregulation and ameliorate the extent of injury associated with CCI.
Materials and methods
Preparation of baicalein
Baicalein was synthesized as described earlier (Huang et al., 2003). Briefly, the mixture of equimolar (20 mmol) trimethoxyphenol and cinnamoyl chloride was converted, through the Fries reaction in the presence of boron trifluoride-etherate, to the corresponding trimethoxychalcone. Further oxidation and cyclization of trimethoxylchalcone by catalytic iodine in dimethyl sulphoxide (Merck, Darmstadt, Germany) gave a crude trimethoxyflavone, followed by demethylation using hydrobromic acid and acetic acid. Final recrystallization was from hexane/ethyl acetate. The synthetic baicalein was further purified by column chromatography with silica gel in acetone/hexane (9/1). The purity was identified by HPLC through Shimadzu SPD-20A series instrument with a Purospher STAR RP-18e column (150 × 4.6 mm, 5 μm). The retention time of baicalein was at 2.146 min with a mobile phase of MeOH/water at isocratic elution at 1 mL min−1. The purity of synthetic baicalein is over 98.5%, compared with commercial baicalein.
Surgical procedures
All animals were treated in accordance with the International Guidelines for animal research, and the study design was approved by the animal ethics committee of Cheng Hsin Rehabilitation Medical Center. Animals were housed in groups in a temperature- (21–25 °C) and humidity (45–50%)-controlled room with a 12-h light/dark cycle and ad libitum access to pellet chow and water.
A previously described CCI injury procedure was utilized (Chen et al., 2003). Male Sprague–Dawley rats (250–300 g, body weight) were anaesthetized with sodium pentobarbital (i.p.; 50 mg kg−1; Rhone Merieux, Harlow, UK) and placed in a stereotaxic frame. A 5-mm craniotomy was performed over the left parietal cortex, centred on the coronal suture and 3 mm lateral to the sagittal suture. Considerable care was taken to avoid injury to the underlying dura. Injury was made using a pneumatic piston with a rounded metal tip (2.5 mm diameter) that was angled 22.5°C to the vertical so that the tip was perpendicular with the brain surface at the centre of the craniotomy. A velocity of 4 m s−1 and a deformation depth 2 mm below the dura were used. The bone flap was immediately replaced and sealed, and the scalp was closed with sutures. Body temperature was monitored throughout the surgery by a rectal probe; temperature was maintained at 37.0±0.5 °C using a heated pad. Rats were placed in a heated cage to maintain body temperature, while recovering from anaesthesia.
Sham-operated rats received a craniotomy as before but no CCI; the impact tip was placed lightly on the dura before sealing the wound. After the trauma or sham surgery, animals were housed under the same conditions, as described above.
Experimental protocol
Study 1
Baicalein (30 mg kg−1) dissolved in 10% dimethyl sulphoxide (0.25–0.3 mL) or a corresponding volume of vehicle (10% dimethyl sulphoxide) was administered i.p. immediately following injury or daily for 4 days (immediately, 25, 49 and 73 h) after injury. Behaviour testing using the rotarod, tactile adhesive removal, mNSS and beam walk tests was performed at 28 h as well as 4, 7, 14, 21 and 28 days post-injury. At 14 and 28 days, the animals were killed, perfused intravascularly and their brains were processed for cresyl violet staining to assess contusion volume (n=8 for each group). The dose of baicalein administration was selected based on pilot studies conducted in our laboratory in which 10 and 30 mg kg−1 were tested and 30 mg kg−1 was found to have neuroprotetcive effects in improving behavioural deficits (unpublished observations).
Study 2
The temporal profile of cytokine mRNA and protein levels was evaluated in a further group of injured and sham rats by RT-PCR and ELISA analysis. The purpose was to determine the optimal time point to examine the effect of baicalein on TNF-α, IL-1β and IL-6 mRNA and protein expression. Following the CCI procedure, rats were processed for RT-PCR at 3, 6, 18 and 24 h and ELISA at 3, 6, 24 and 96 h (all times post-injury; n=7 for each time point). Fourteen additional sham-operated rats were used for RT-PCR and ELISA analysis (n=7 for each group).
Study 3
Single-dose baicalein (30 mg kg−1) or a corresponding volume of vehicle was administered i.p. immediately following injury. The single-dose regimen was chosen from the results of study 1 (in which single-dose baicalein significantly improved neurological outcomes) and study 2 (in which cytokine expression peaked early post-injury; see Results). Testing after injury was as follows: (1) real-time quantitative RT-PCR analysis for TNF-α, IL-1β and IL-6 mRNA expression at 6 h post-injury (n=7 for each group); (2) FluoroJade B (FJB) staining, TNF-α, IL-1β and IL-6 immunohistochemistry, as well as ELISA at 24 h post-injury to assess degenerative neurons and to determine cytokine expression (n=7 for each group). Twenty-one additional sham-operated rats were used for RT-PCR, ELISA and histology analysis (n=7 for each group).
Neurological functional evaluation
Behaviour testing was performed before CCI and at 1, 4, 7, 14, 21 and 14 days after CCI by an observer who was unaware of the experimental treatments. The battery of tests consisted of the rotarod motor test, tactile adhesive-removal somatosensory test, mNSS and beam walk test. Animals were pretrained for 3 days for the rotarod, tactile adhesive-removal somatosensory and beam walk tests (see below).
Rotarod test
An accelerating rotarod was used to measure rat motor function and balance (Hamm, 2001). Each rat was placed on the rotarod cylinder, and the time for which the animal remained on the rotarod was measured. Speed was slowly increased from 4 to 20 r.p.m. within 5 min. A trial would be ended if the animal fell off the rungs or gripped the device and spun around for two consecutive revolutions without attempting to walk on the rungs. One hour before CCI, the mean duration on the device was recorded with three rotarod measurements as the preinjury baseline values. Post-injury latencies were expressed as a percentage of their respective baseline values to reduce inter-animal variability.
Tactile adhesive-removal test
For the tactile adhesive-removal somatosensory test, two small adhesive-backed paper dots (each 113.1 mm2) were used as bilateral tactile stimuli occupying the distal–radial region on the wrist of each forelimb (Chen et al., 2001). The time required for each rat to remove adhesive from the forelimb was recorded for five trials per day. Individual trials were separated by at least 5 min. One hour before CCI, mean latency (in seconds) over five trials to remove the contralateral (right) adhesive was recorded as the preinjury baseline value.
Modified neurological severity score
As shown in Table 1, the mNSS is a composite of motor, sensory, reflex and balance tests (Chen et al., 2001). One point was scored for the inability to perform the test or for the lack of a tested reflex; thus, the higher the score, the more severe the injury. Neurological function was graded on a scale of 0–18 (normal score, 0; maximal deficit score, 18).
Table 1.
Modified neurological severity scores (mNSS)
| Tests | Point |
|---|---|
| Motor tests | |
| Raising the rat by the tail | |
| Flexion of forelimb | 1 |
| Flexion of hindlimb | 1 |
| Head moving more than 10° (vertical axis) | 1 |
| Placing the rat on the floor | |
| Inability to walk straight | 1 |
| Circling towards the paretic side | 1 |
| Falling down to the paretic side | 1 |
| Sensory tests | |
| Visual and tactile placing | 1 |
| Proprioceptive test (deep sensory) | 1 |
| Beam balance tests | |
| Grasps side of the beam | 1 |
| Hugs the beam and one limb falls down from the beam | 2 |
| Hugs the beam and two limbs fall down from the beam or spins on beam (>60 s) | 3 |
| Attempts to balance on the beam but falls off (>40 s) | 4 |
| Attempts to balance on the beam but falls off (>20 s) | 5 |
| Falls off: no attempt to balance or hang on to the beam (<20 s) | 6 |
| Reflexes (blunt or sharp stimulation) absence of: | |
| Pinna reflex (a head shake when touching the auditory meatus) | 1 |
| Corneal reflex (an eye blink when lightly touching the cornea with cotton) | 1 |
| Startle reflex (a motor response to a brief loud paper noise) | 1 |
| Seizures, myoclonus, myodystony | 1 |
| Maximum points | 18 |
One point is awarded for the inability to perform the tasks or for the lack of a tested reflex.
Beam walk test
The beam walk test was utilized to evaluate fine motor coordination and function (Feeney et al., 1982). Rats escaped a bright light and loud white noise by walking along a narrowed wooden beam (2.5 × 122.0 cm) to enter a darkened goal box at the opposite end of the beam. The latency for the rat to reach the goal box (not to exceed 60 s) and hindlimb performance as he traversed the beam (based on a 1–7 rating scale) were recorded. A score of 7 was given when animals traversed the beam with two or less footslips; 6 was given when animals traversed the beam with less than 50% footslips; 5 was given for more than 50% but less than 100% footslips; 4 was given for 100% footslips; 3 was given for traversal with the affected limb extended and not reaching the surface of the beam; 2 was given when the animal was able to balance on the beam but not traverse it; 1 was given when the animal could not balance on the beam. Three trials were recorded 1 h before CCI (baseline), and each day after CCI. Mean values of latency and score for each day were computed.
Real-time quantitative RT-PCR
Brains from injured or sham animals were removed without fixation after cervical dislocation, 6 h following injury or sham surgery. A 3-mm coronal section was taken from the injured area over the parietal cortex, snap-frozen in liquid nitrogen, and stored at −70 °C until use. Approximately 50 mg tissue was collected from the ipsilateral (injured) hemisphere and processed for real-time quantitative RT-PCR. Total RNA was extracted from tissue samples using the RNeasy Mini Kits (Qiagen, Valencia, CA, USA). The purity and quality of extracted total RNA were confirmed by determining the ratio of absorbance at 260 nm to that at 280 nm and by ethidium bromide staining of 1 μg total RNA separated on a 1.5% agarose gel. Nondegraded RNA shows well-defined bands for both the 18S and 28S RNAs with no visible degradation. One microgram total RNA was first treated with DNase I, then subjected to reverse transcription using SUPERSCRIPT II RNase H Reverse Transcriptase, following the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA). Real-time quantitative RT-PCR analysis was performed with an ABI PRISM 7500 sequence detector (Applied Biosystems, Foster City, CA, USA). The primers and probe for TNF-α (TaqMan Gene Expression Assay ID Rn00562055_gl), IL-1β (TaqMan Gene Expression Assay ID Rn000580432_gl) and IL-6 (TaqMan Gene Expression Assay ID Rn99999011_ml) were obtained from Applied Biosystems. The endogenous control was β-actin (TaqMan Gene Expression Assay ID Rn00667869_m1). FAM (6-carboxyfluorescin) was used as the reporter dye, with TAMRA (6-carboxy-tetramethyl-rhodamine) used as the quencher dye. Thermal cycling was initiated with a 2-min incubation at 50 °C, followed by a first denaturation step of 10 min at 95 °C and then 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The ABI PRISM 7500 sequence detector measures fluorescence emission synchronized with the thermal cycler during each extension step. Each sample was run in triplicate. Relative standard curves for the candidate gene and β-actin ribosomal RNAs (rRNAs) were performed each time genes were analysed. The value of β-actin rRNA, which was consistent regardless of experimental condition, was used as an internal control. All PCR products were analysed in the geometric range of the exponential phase during PCR amplification. Relative quantities of the candidate genes and β-actin rRNA were calculated by the previously described comparative threshold cycle (Ct) method (Chen et al., 2007; Wen et al., 2007). In brief, the Ct value of TNF-α, IL-1β or IL-6 gene was subtracted from the Ct value of β-actin rRNA (expressed as ΔCt) as a standard for the amount of RNA template and efficiencies of reverse transcription. Then the ΔCt of samples from injured rats or sham-control rats was normalized to the sham-control sample with the lowest ΔCt values. The resulting change in ΔCt values (expressed as ΔΔCt) was converted to a linear form using 2(−ΔΔCt); and the transformed value was used in subsequent statistical analysis.
ELISA
Brains from injured or sham-control animals were removed without fixation after cervical dislocation 1 day following surgery. A 3-mm coronal section was taken from the injured area over the parietal cortex, snap-frozen in liquid nitrogen, and stored at −70 °C until use. Brain samples were homogenized in a buffer consisting of 0.05 M Tris–HCl, 0.15 M NaCl, 0.1% Nonidet 40, 0.5 M phenylmethylsuplhonyl fluoride, 50 μg mL−1 aprotinin, 10 μg mL−1 leupeptin, 50 μg mL−1 pepstatin, 4 mM sodium orthovanadate, 10 mM sodium fluoride and 10 mM sodium pyrophosphate. Homogenates were centrifuged at 4 °C and 12 000 × g for 15 min. Supernatants were removed and assayed in duplicate using R&D TNF-α, IL-1β and IL-6 assay kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer's guidelines. Tissue cytokine concentrations were expressed as picograms of antigen per milligram of protein.
Tissue processing and histology
Following terminal anaesthesia, rats were killed by transcardial perfusion first with phosphate-buffered saline (PBS) and then with 4% paraformaldehyde and their tissues were processed, day 1 (for FJB staining and cytokine immunohistochemistry), day 14 (for cresyl violet histology) and day 28 (for cresyl violet histology), post-injury. All solutions were maintained at pH 7.4 and 4 °C. Brains were removed and post-fixed in 4% paraformaldehyde overnight and transferred to PBS containing 30% sucrose and 0.1% sodium azide (Sigma Chemical Co., St Louis, MO, USA) for cryoprotection. Coronal sections were cut in a cryostat at 10 μm from the level of the olfactory bulbs to the visual cortex. Every 50th slice was used for cresyl violet histology, FJB staining or immunohistochemistry. The distance between similarly stained sections within each group was thus 500 μm, with approximately 20 sections per group. The following primary antibodies and dilutions were used: (1) goat polyclonal anti-TNF-α (E-20) (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:50); (2) rabbit polyclonal anti-IL-1β (BioSource International, Camarillo, CA, USA; 1:200); (3) goat polyclonal anti- IL-6 (Santa Cruz Biotechnology; 1:200); (4) mouse monoclonal anti-neuronal nuclei antigen (NeuN) (Chemicon, Temecula, CA, USA; 1:400); (5) mouse monoclonal anti-OX42 (Serotec, Raleigh, NC, USA; 1:200); (6) mouse monoclonal anti-glial fibrillary acidic protein (GFAP) (Dako, Carpenteria, CA, USA; 1:200).
Immunohistochemistry
All sections were dried, rehydrated in PBS, fixed in 4% paraformaldehyde for 20 min and rinsed in PBS. Sections were quenched in a solution of 10% methanol/10% hydrogen peroxide in distilled water for 5 min before washing three times in Trizma (Sigma)-buffered saline (TBS). Sections were blocked for 60 min in TXTBS (TBS containing 0.2% Triton X-100; Sigma) with 3% normal goat serum (NGS; Dako) and incubated overnight at 4 °C in the relevant primary antibody (goat polyclonal anti-TNF-α, rabbit polyclonal anti-IL-1β or goat polyclonal anti-IL-6) in TXTBS containing 1% NGS. After three washes in TBS, sections were left in the appropriate biotinylated secondary antibody (biotinylated anti-goat IgG or biotinylated anti-rabbit IgG; Vector, Burlingame, VT, USA) at a concentration of 1:200 in TBS with 1% NGS for 3 h, followed by three washes in TBS. The primary antibody was visualized with diaminobenzidine using a streptavidin-biotinylated horseradish peroxidase complex kit (Dako) in 1% NGS in TBS for 2 h followed by three washes in TBS and two washes in Trizma nonsaline. Sections were developed with diaminobenzidine in Trizma nonsaline containing 0.03% hydrogen peroxide, and excess stain was removed by washing in Trizma nonsaline three times. Nonspecific staining was investigated by omitting the primary antibody and was negative.
For the immunofluorescence double-labelling of cell-specific markers in cytokine-immunoreactive cells, mouse monoclonal anti-NeuN, mouse monoclonal anti-OX42 and mouse monoclonal anti-GFAP were used to identify the neurons, microglia/macrophages and astrocytes, respectively. Sections were incubated (overnight, 4 °C) with anti-TNFα, anti-IL 1β or anti-IL6 antibody plus one of the antibodies to a specific cellular marker and then serially incubated (2 h, room temperature) first with the appropriate biotinylated secondary antibody (biotinylated anti-goat IgG or biotinylated anti-rabbit IgG, 1:200; Vector), second with Alex-Fluor 488 labelled goat anti-mouse antibody (1:200; Molecular Probes, Eugene, OR, USA) or Alex-Fluor 488 labelled goat anti-rabbit antibody (1:200; Molecular Probes) and finally with streptavidin Alexa Fluor 594 (1:400; Molecular Probes). The slides were then washed and coverslipped with Vecta-shield mounting medium (Vector). All sections were observed and photographed under a fluorescence microscope (Olympus BX-51; Olympus, Tokyo, Japan).
FJB histochemistry
FluoroJade B is a polyanionic fluorescein derivative that sensitively and specifically binds to degenerating neurons, and staining was carried out using a published technique (Schmued and Hopkins, 2000), with some modification. Briefly, sections were first incubated in a solution of 1% NaOH in 80% ethanol for 5 min and then hydrated in graded ethanol (75, 50 and 25%; 5 min each) and distilled water. They were incubated in a solution of 0.06% potassium permanganate for 10 min, rinsed in distilled water for 2 min and incubated in a 0.0004% solution of FJB (Chemicon) for 30 min. The slides were washed and mounted on coverslips with Vecta-shield mounting medium (Vector). All sections were observed and photographed under a fluorescence microscope (Olympus BX-51) with blue (450–490 nm) excitation light.
Quantification of cytokine and FJB staining
Cytokine and FJB staining were quantified on TNF-α, IL-1β-, IL-6- and FJB-stained sections between Bregma level 0.2–0.6 mm. Three 10-μm sections per animal were randomized selected between the two levels and stained with FJB or cytokine stainings. The region of interest was defined and delineated under a 4X objective on each section as the TNF-α-, IL-1β-, IL-6- or FJB-positive cells in the contusion margin along the cortex. Using a × 20 objective, five randomly selected, nonoverlapping fields with an area of 690 μm width and 520 μm height for cytokine immunostainings or an area of 1350 μm width and 1060 μm height for FJB stainings were examined. The total number of TNF-α-, IL-1β-, IL-6- and FJB-positive cells were expressed as the mean number per field of view. Analysis was conducted by two experimenters who were unaware of all animal characteristics. Inter-rater reliability in cell counts was well within 10%.
Contusion volume
To measure contusion volumes, cresyl violet-stained sections were digitized and analysed using a × 1.5 objective and computer image analysis system (Scion Image, Beta Release 4.0.2; Scion Corp., Frederick, MD, USA). Contusion volume measurement was performed as previously described (Chen et al., 2003). Contusion area was calculated from all images of cresyl violet-stained sections that contained contused brain; volume measurement was computed by summation of areas multiplied by interslice distance (500 μm).
Statistical analyses
Data are presented as mean±s.e.mean. For ELISA measurement of cytokine levels, an overall difference between the groups were tested with one-way ANOVA and a post hoc (Bonferroni) test was used to determine individual group differences (n=7 for each group). Nonparametric Kruskall and Wallis rank analysis was used to evaluate the RT-PCR data with subsequent group comparisons using the Mann–Whitney U-test (n=7 for each group). For behaviour testing and contusion volume measurement, two-way ANOVA with repeated measurements followed by a post hoc (Bonferroni) test was used to determine significant differences (n=8 for each group). For TNF-α-, IL-1β-, IL-6- and FJB-positive cell counts, a Student t-test was used to determine significant differences (n=7 for each group). Differences between means were assessed at the probability level of P<0.05, 0.01, and 0.001.
Results
Body weight
All animals lost a small proportion of body weight (∼6%) in the first 24 h following CCI injury but regained baseline weight within 4 days. There was no significant difference between groups treated with baicalein or vehicle regarding body weight (P=0.8; data not shown).
Rotarod test
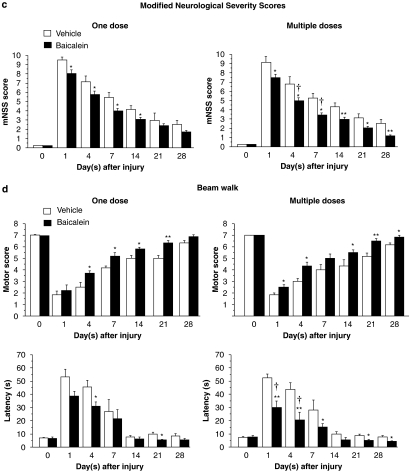
Motor function impairment caused by CCI was evident in the vehicle-treated group (Figure 1a). Performance on the rotarod test was significantly better for both single-dose and multiple-dose baicalein-treated rats than for vehicle-treated rats on test days 1–28 after injury (all P<0.05) (Figure 1a). Multiple-dose treatment was significantly more effective than single-dose treatment on days 1 (P<0.05), 4 (P<0.01), 7 (P<0.05) and 21 (P<0.05) after injury.
Figure 1.
Effects of single-dose and multiple-dose baicalein treatment on functional outcomes in rats with controlled cortical impact (CCI) injury, as evaluated by (a) rotarod, (b) tactile adhesive removal, (c) modified neurological severity score (mNSS) and (d) beam walk tests. CCI injury impaired performance on all tests in vehicle-treated and baicalein-treated injured rats. (a) Both the single-dose and multiple-dose baicalein-treated rats showed significantly better performance on the rotarod test than did vehicle-treated rats between 1 and 28 days after injury. Compared with single-dose baicalein, multiple-dose baicalein resulted in a significantly better rotarod performance on days 1, 4, 7 and 21. (b) Compared with vehicle-treated rats, both the single-dose and multiple-dose baicalein-treated rats had significantly lower adhesive-removal times at 1, 4 and 7 days. The adhesive removal time was significantly lower only on day 7 in rats treated with multiple doses of baicalein compared with rats treated with a single dose of baicalein. (c) The mNSS scores were significantly lower for the single-dose baicalein-treated group than vehicle-treated group on days 1–14 and significantly lower for the multiple-dose group than the vehicle-treated group on days 1–28. As compared with a single-dose baicalein treatment, multiple-dose treatment significantly reduced neurological deficits on mNSS on days 4 and 7. (d) The hindlimb motor scores were significantly decreased when compared to those of the vehicle group on days 4, 7, 14 and 21 for the single-dose baicalein-treated group and on days 1, 4, 14, 21 and 28 for the multiple-dose group. There were no significant differences in hindlimb motor scores between the single-dose and multiple-dose baicalein-treated groups on any testing day. Similarly, beam walk latencies were significantly shorter when compared to those of the vehicle group on days 4 and 21 for the single-dose baicalein group and on days 1, 4, 7, 21 and 28 for the multiple-dose group. The beam walk latencies were significantly shorter for the multiple-dose baicalein-treated group than the single-dose group on days 1 and 4. Values are means±s.e.m.; *P<0.05, **P<0.01, ***P<0.001 versus vehicle-treated injured rats. †P<0.05, ††P<0.01, single-dose versus multiple-dose baicalein-treated injured rats (n=8 for each group).
Tactile adhesive removal test
In both baicalein-treated and vehicle-treated groups of rats, unilateral contusion resulted in a delay in the time needed to remove the patch (Figure 1b). Both the single-dose and multiple-dose baicalein-treated rats had significantly lower adhesive-removal times at 1, 4 and 7 days than vehicle-treated rats (all P<0.05). Rats treated with multiple doses of baicalein had significantly lower adhesive-removal times only on day 7 after injury, as compared with those treated with single dose of baicalein (P<0.05).
Modified neurological severity score
Injury in the left hemispheric cortex resulted in neurological functional deficits as measured by mNSS (Figure 1c). The mNSS scores were significantly less for the single-dose baicalein-treated group than the corresponding vehicle-treated group on days 1–14 (all P<0.05) and significantly less for the multiple-dose group than the corresponding vehicle-treated group on days 1–28 (all P<0.05). As compared with single-dose treatment, multiple-dose treatment resulted in a significantly greater reduction of neurological deficit on days 4 and 7 after injury (both P<0.05).
Beam walk test
Marked impairment in beam walk performance was observed on the first day after surgery, regardless of treatment (Figure 1d). The decrease in hindlimb motor scores was significantly different between the single-dose group and vehicle group on days 4, 7, 14 and 21 (all P<0.05) and between the multiple-dose group and vehicle group on days 1, 4, 14, 21 and 28 (all P<0.05). However, differences in hindlimb motor scores between the single-dose and multiple-dose groups were not significant on any testing day (all P>0.05). Similarly, beam walk latencies were significantly shorter for the single-dose group than the vehicle group on days 4 and 21 (both P<0.05) and for the multiple-dose group than the vehicle group on days 1, 4, 7, 21 and 28 (all P<0.05). The beam walk latencies on days 1 and 4 were significantly shorter for the multiple-dose group than the single-dose group (both P<0.05).
Post-injury baicalein treatment reduces neuronal injury
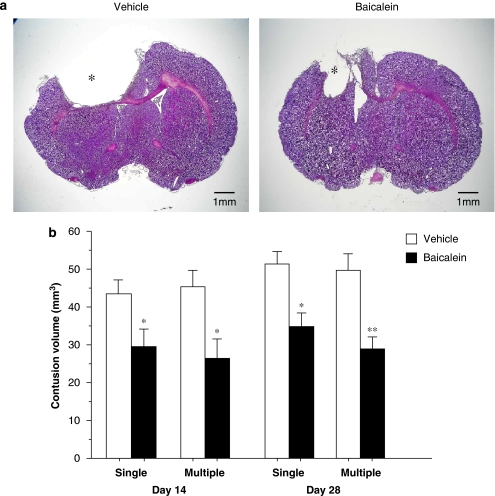
Controlled cortical impact injury resulted in a loss of cortical tissue in the ipsilateral parietal cortex, as reflected by gross reductions in cresyl violet staining intensity (Figure 2a). In contrast, the cytoarchitecture of the cortex remained normal in the contralateral hemisphere. At 14 days, the volume of contusion estimated from cresyl violet-stained sections after vehicle treatment was the same for single-dose and for multiple-dose treatment, and these values were significantly greater than the contusion volume after baicalein treatment, either after a single dose or after multiple doses (both P<0.05; Figure 2b). The contusion volume increased, up to 28 days post-injury, after single-dose and multiple-dose vehicle treatment (Figure 2b) and this volume was significantly reduced after single-dose (P<0.05) or multiple-dose bacalein treatment (P<0.01; Figure 2b). However, there was no significant difference in contusion volume between the single-dose and multiple-dose baicalein-treated groups on both testing days (both P>0.05). Overall, single and multiple doses of baicalein reduced contusion volume, respectively, by 32 and 42% at 14 days post-injury and by 34 and 42% at 28 days post-injury.
Figure 2.
Effect of single-dose and multiple-dose baicalein treatment on cortical contusion volume, as evaluated by cresyl violet staining, on 14 and 28 days post-injury. (a) Representative cresyl violet-stained brain sections of a vehicle- and single-dose baicalein-treated injured rat, 14 days post-injury showing hypointensive regions and an obvious cavitation immediately below the impact site (*) in the cortex. Scale bar is 1 mm. (b) Bar graphs demonstrating cortical contusion volumes in vehicle-treated and baicalein-treated injured rats on days 14 and 28. Both single-dose and multiple-dose baicalein-treated rats had a significant reduction in contusion volume relative to vehicle-treated rats on days 14 and 28. Values are means±s.e.m.; *P<0.05 versus vehicle-treated injured rats (n=8 for each group).
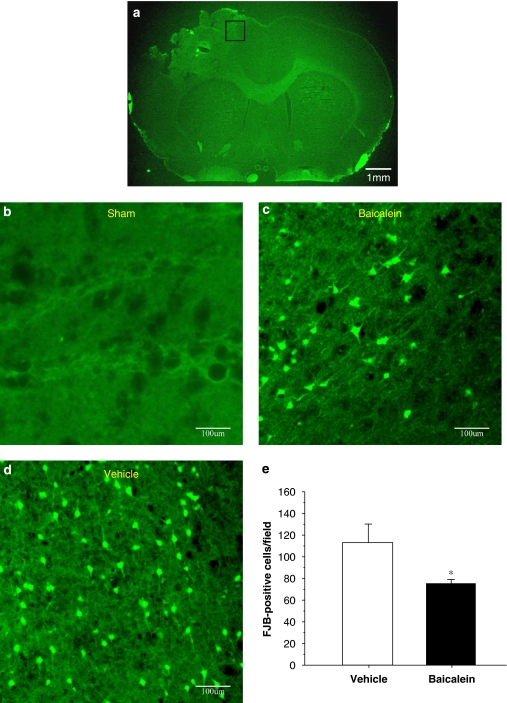
As FJB reactivity has been shown to be maximal 1 day after moderate CCI injury (Anderson et al., 2005), the time point of 1 day post-injury was chosen for FJB staining in our model. FJB-positive cells with neuronal morphology were evident 1 day after injury in the cortical contusion margin (Figure 3) and striatum in the ipsilateral, but not the contralateral hemisphere. Single-dose baicalein significantly reduced (by 31%) the number of FJB-positive cells compared with vehicle treatment (Figure 3; P<0.05).
Figure 3.
Effect of single-dose baicalein treatment on neuronal degeneration in rats with controlled cortical impact (CCI) injury, as evaluated by FluororJade B (FJB) staining 1 day post-injury. (a) A representative FJB-stained brain section of a vehicle treated, injured rat on day 1 post-injury. Scale bar is 1 mm. (b) High-power views of FJB-stained regions of interest on day 1 post-injury in (b) a sham-injured control rat, (c) a vehicle-treated brain-injured rat and (d) a baicalein-treated brain-injured rat. Note that there was a marked decrease in the number of FJB-positive cells after baicalein treatment. Scale bar is 100 μm. (e) Bar graphs of mean densities of FJB-positive cells in vehicle-treated and baicalein-treated injured rats in the cortical contusion margin 1 day after injury showing a significant decrease in the number of FJB-positive cells in the baicalein-treated group. The total number of FJB-positive cells was expressed as the mean number per field of view (1.43 mm2). Values are means±s.e.m.; **P<0.05 versus vehicle-treated injured rats (n=7 for each group).
Post-injury baicalein treatment downregulates proinflammatory cytokine mRNA expression
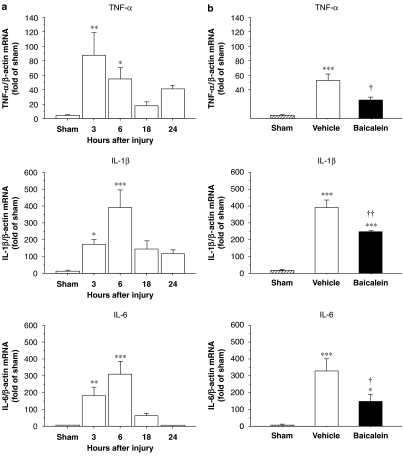
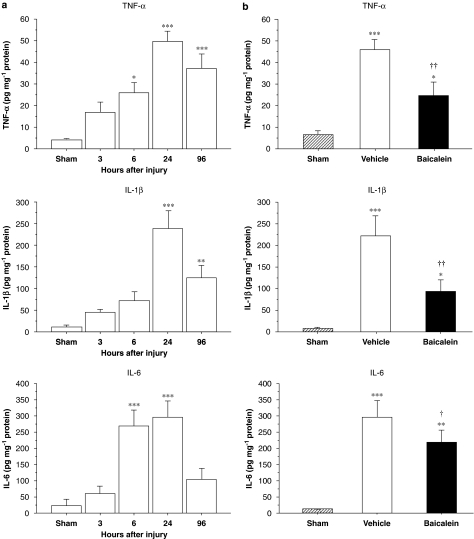
As single-dose baicalein treatment significantly improved neurological outcomes, we investigated whether this treatment paradigm reduced proinflammatory cytokine expression as hypothesized. Following injury, the mRNA expression increased significantly in the injured hemisphere for TNF-α, IL-1β and IL-6 compared with the corresponding region of sham controls at 3 and 6 h (P<0.05 for all values) (Figure 4a). The peak level of TNF-α, IL-1β and IL-6 mRNA was observed around 6 h, therefore, the time point of 6 h was selected for evaluating treatment effect on cytokine mRNA expression. Vehicle-treated injured rats had a 14-fold higher TNF-α level, a 33-fold higher IL-1β level and a 60-fold higher IL-6 level than sham controls (Figure 4b). The profound increase in proinflammatory cytokines (associated with trauma) was significantly attenuated by baicalein treatment; as there was significant reduction in TNF-α, IL-1β and IL-6 mRNA levels 6 h after injury. On average, TNF-α, IL-1β and IL-6 mRNA levels in brains of baicalein-treated injured rats were 49 % (P<0.05), 63 % (P<0.01) and 43.6% (P<0.05), respectively, of the levels found in vehicle-treated injured rats.
Figure 4.
Expression of inflammatory cytokine mRNAs after controlled cortical impact (CCI) injury, assessed by Taqman RT-PCR. (a) Expression of tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 mRNAs increased significantly in the injured hemisphere compared with the corresponding region of the sham-injured controls at 3 and 6 h. (b) Bar graphs demonstrating TNF-α, IL-1β and IL-6 mRNA expressions in sham-control, vehicle and single-dose baicalein-treated injured rats in ipsilateral cortices 6 h post-injury. Single-dose baicalein significantly reduced injury-induced TNF-α, IL-1β and IL-6 mRNA expressions in the ipsilateral hemisphere compared with vehicle-treated rats. Values are means±s.e.m.; *P<0.05, **P<0.01, ***P<0.001 versus sham-control and †P<0.05, ††P<0.01, baicalein versus vehicle-treated injured rats (n=7 for each group).
Post-injury baicalein treatment reduces cytokine protein expression
To examine the effect of baicalein on TNF-α, IL-1β and IL-6 protein expression, ELISA and immunohistochemistry were used to confirm gene translation 1 day after injury. Basal protein levels of TNF-α, IL-1β and IL-6 were low in the cortex of sham-injured animals. Following injury, TNF-α, IL-1β and IL-6 protein levels in the ipsilateral cortex increased significantly from 6 to 96 h and peaked at 1 day (Figure 5a). Therefore, day 1 post-injury was selected for evaluating the effect of treatment on cytokine protein expression. At this time, cytokine levels in the ipsilateral cortex of vehicle-treated, injured rats were significantly increased; TNF-α, by 7-fold (P<0.001); IL-1β, by 30-fold (P<0.001) and IL-6 also by 30-fold (P<0.001), compared with sham values (Figure 5b). Baicalein significantly reduced the injury-induced increase in cortical tissue levels of all three cytokines (Figure 5b). On average, TNF-α, IL-1β and IL-6 protein concentrations in brains of baicalein-treated rats were 54% (P<0.05), 42% (P<0.01) and 67% (P<0.01), respectively, of the concentrations seen in vehicle-treated rats.
Figure 5.
Concentrations of inflammatory cytokine proteins after controlled cortical impact (CCI) injury, assessed by ELISA. (a) tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 protein levels in untreated, injured rats significantly increased in the ipsilateral cortex from 6 to 96 h and peaked at 1 day. (b) Bar graphs of TNF-α, IL-1β and IL-6 protein concentrations in sham control, vehicle and single-dose baicalein-treated injured rats in ipsilateral cortices 1 day post-injury. Baicalein significantly reduced the injury-induced increases of TNF-α, IL-1β and IL-6 protein concentrations compared with vehicle-treated injured rats. Values are means±s.e.m.; *P<0.05, **P<0.01, ***P<0.001 versus sham-control and †P<0.05, ††P<0.01, baicalein versus vehicle-treated injured rats (n=7 for each group).
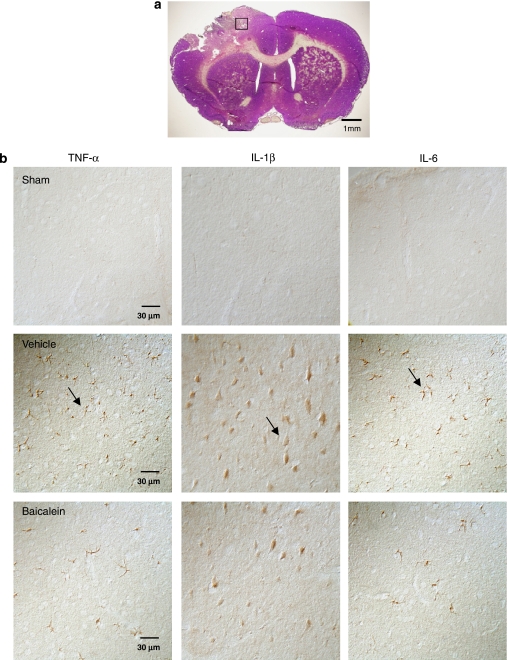
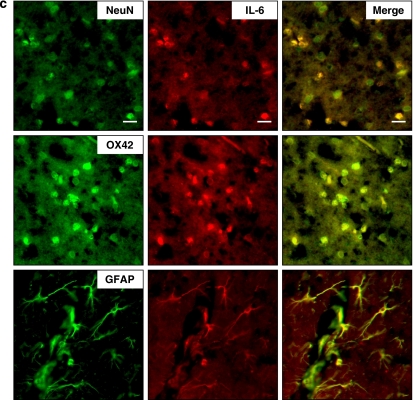
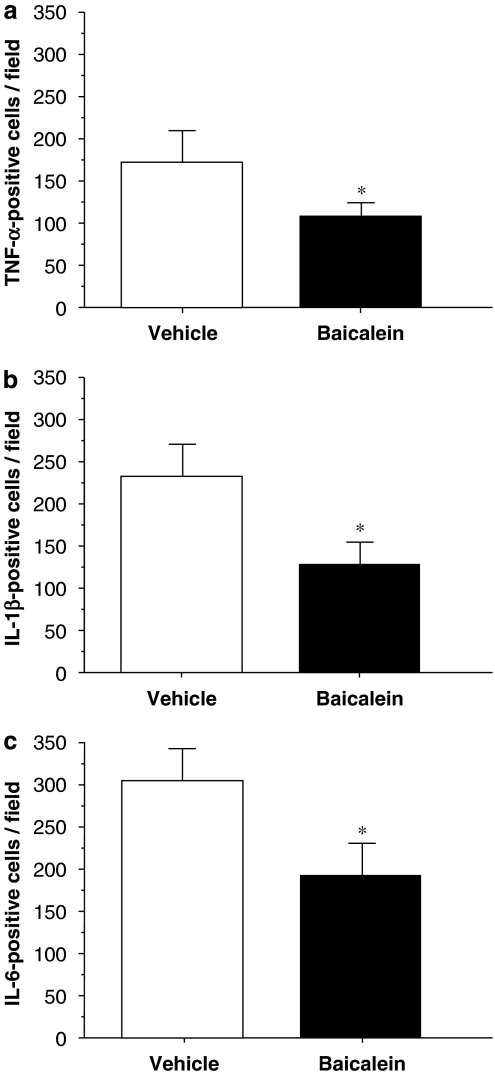
Cytokine immunohistochemistry indicated that neither normal brains nor contralateral hemispheres of injured brains displayed immunoreactivity for TNF-α, IL-1β or IL-6. However, TNF-α, IL-1β and IL-6 immunoreactivity was markedly increased in the injured hemisphere by 1 day post-injury in vehicle-treated rats (Figure 6). Most TNF-α-, IL-1β- and IL-6-positive cells were located within and adjacent to the contusion area in the cortex and striatum. To identify which cerebral cells expressed cytokine after brain injury, double immunofluorescence staining was performed. Although TNF-α and IL-6 were colocalized in neurons, microglia and astrocytes in the pericontusional area, IL-1β was present only in microglia and neurons (Figure 7). Baicalein significantly reduced the number of cells positive for TNF-α (P<0.01), IL-1β (P<0.05) and IL-6 (P<0.05) 1 day after trauma (Figures 6 and 8). Quantitative analysis revealed a 37% reduction in TNF-α-positive cells, a 40% reduction in IL-1β-positive cells and a 33% reduction in IL-6-positive cells, respectively, in cortical tissue adjacent to the contusion core in rats treated with baicalein relative to cell numbers in rats treated with vehicle (Figure 8).
Figure 6.
Effect of single-dose baicalein treatment on cytokine protein expression in rats with controlled cortical impact (CCI) injury, as evaluated by cytokine immunoreactivity 1 day post-injury. (a) A representative cresyl violet-stained brain section of a vehicle-treated injured rat 1 day post-injury showing the region of interest. Scale bar is 1 mm. (b) High-power photomicrographs of the regions of interest showing tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 immunoreactivity 1 day post-injury in the sham-injured control, vehicle and single-dose baicalein-treated injured animals. Note that there was a marked rise in the number of TNF-α-, IL-1β- and IL-6-positive cells (arrows) after injury. The number of TNF-α-positive, IL-1β- and IL-6-positive cells was significantly reduced in baicalein-treated animals compared with vehicle-treated animals. Scale bar is 30 μm for TNF-α, IL-1β and IL-6 immunostaining.
Figure 7.
Identification of (a) tumour necrosis factor (TNF)-α-, (b) interleukin (IL)-1β- and (c) IL-6-positive cells 1 day post-injury in the pericortical contusion margin by double immunofluorescence labelling. Cytokine immunoreactivity is shown in green, and immunolabeling of NeuN (a cell marker for neurons), OX-42 (a cell marker for microglia) or GFAP (a cell marker for astrocytes) is shown in red. Yellow labelling indicates colocalization. TNF-α and IL-6 were colocalized in neurons, microglia and astrocytes, whereas IL-1β was localized in neurons and microglia. Scale bar is 20 μm.
Figure 8.
Mean densities of immunopositive profiles displaying (a) tumour necrosis factor (TNF)-α, (b) interleukin (IL)-1β and (c) IL-6 immunoreactivity in vehicle-treated and single-dose baicalein-treated injured rats in the cortical contusion margin 1 day post-injury. There was a significant decrease in the number of both TNF-α-positive, IL-1β- and IL-6-positive cells in baicalein-treated rats. The total number of TNF-α-, IL-1β- and IL-6-positive cells was expressed as the mean number per field of view (2.56 mm2). Values are means±s.e.m.; *P<0.05 versus vehicle-treated injured rats (n=7 for each group).
Discussion
Our data show that post-injury administration of baicalein significantly reduced long-term neurological deficits and brain tissue damage following CCI. These effects correlate with a decrease of TNF-α, IL-β and IL-6 mRNA transcription and protein synthesis in the brain. The major novel finding in this study is that baicalein, given i.p., improved both functional and histological outcomes in a model of CCI, perhaps through modulation of inflammation. Although previous studies have shown that baicalein treatment can reduce neuronal damage in an experimental model of cerebral ischaemia (Hamada et al., 1993; Hwang et al., 2002; Lapchak et al., 2007), our study provides the first evidence that post-injury baicalein treatment can attenuate TBI-induced tissue damage and can improve functional recovery following TBI. As previously reported (Hunter et al., 1998), preclinical studies combining both histological and neurobehavioral evaluations may improve the predictive value of animal models for demonstrating clinical efficacy of novel neuroprotective agents. Our results suggest that baicalein may provide a potential therapy for TBI. Moreover, our findings suggest that the anti-inflammatory properties of baicalein contribute to its neuroprotective effect and provide further evidence that the post-traumatic inflammatory response contributes to the morphological and behavioural pathophysiology of TBI.
Functional deficits are common neurological sequelae in patients with brain injuries and in experimental models of TBI. Behavioural parameters are useful measures of functional deficits following experimental TBI, and the degree of sensorimotor dysfunction is an important indicator of severity of injury (Fujimoto et al., 2004). We demonstrated that baicalein significantly improved long-term sensori-motor outcomes assessed using a combination of neurobehavioral tests. The reduction in functional deficits seen in the baicalein-treated animals correlated with the histological findings in this study. Compared with vehicle-treated rats, baicalein-treated animals showed smaller contusion volumes and fewer degenerative neurons, as estimated by Nissl and FJB staining, respectively. Post-injury treatment with baicalein, therefore, protects some tissue that would otherwise be vulnerable to damage by trauma, resulting in amelioration of brain injury and improvement of functional outcome.
Post-traumatic inflammatory response is a major contributing factor to secondary injury of TBI and has been shown to be an important therapeutic target for reducing evolution of tissue damage following TBI (Allan and Rothwell, 2001; Morganti-Kossmann et al., 2002). We show for the first time that post-TBI treatment with baicalein significantly reduced cytokine expression in parallel with reduced brain damage and neurological deficits. Although we did not establish a direct causal relationship between cytokine reduction and histological and functional deficits, several lines of evidence suggest that TNF-α, IL-1β and IL-6 are detrimental in the acute post-traumatic period (Morganti-Kossmann et al., 2002; Lu et al., 2005) and that inhibiting cytokine activation or blocking cytokine receptors can have a neuroprotective effect (Shohami et al., 1996; Shohami et al., 1997; Knoblach et al., 1999). In fact, TNF-α and IL-1β are potent enhancers of inflammatory reactions by the activation of glia, capillary endothelial cells and blood elements (for example, macrophages and neutrophils) and through enhanced expression of multiple downstream inflammatory factors (Wang and Shuaib, 2002). These two cytokines also trigger the rise of IL-6 (Allan and Rothwell, 2001), which has been suggested to act in concert with TNF-α and IL-1β to mediate many of their biological effects.
We have showed that baicalein suppressed the induction of proinflammatory cytokines in the injured brain following TBI. This finding agrees with previous reports that baicalein inhibits several inflammatory processes known to be important during TBI. For example, upregulation of adhesion molecule expression has been documented in animal (Balabanov et al., 2001) and in human TBI (Pleines et al., 1998). Enhanced expression of adhesion molecules within the contusion area facilitates the migration of leukocytes through the brain parenchyma (Knoblach and Faden, 2002). Baicalein can inhibit the expression of endothelial leucocyte adhesion molecule-1 and intercellular adhesion molecule-1 in cultured endothelial cells (Kimura et al., 2001), thereby perhaps suppressing the influx of leukocytes. Baicalein also attenuates leucocyte adhesion in vitro, either by suppressing cell surface receptors and adhesion molecule expression or by antagonizing Ca2+ influx (Shen et al., 2003). Furthermore, inflammatory mediators such as cytokines and nitric oxide, which are produced by activated glial cells in the CNS, may have harmful effects on neurons. Baicalein inhibits such microglial activation, and reduces the production of nitric oxide and other proinflammatory cytokines including TNF-α, IL-6 and IL-8 in primary neuron-glia cultures (Suk et al., 2003; Li et al., 2005) and in human retinal pigment epithelial cell lines (Nakamura et al., 2003), while concomitantly reducing neuronal degeneration (Li et al., 2005). Finally, TBI can induce the activation of PLA2, which hydrolyses the membrane phospholipids resulting in the release of arachidonic acid for subsequent metabolism through the COX and lipoxygenase pathways, which are two important mediators of inflammation (Phillis and O'Regan, 2003). In vitro studies have further shown that baicalein inhibits the pathways of prostaglandin E2 production (Nakahata et al., 1998) and leukotriene biosynthesis (Tanno et al., 1988), both of which are downstream metabolites formed by COX and lipoxygenase. However, compared to the well-known effects of baicalein on peripheral inflammatory cells, little information is available about its effects on the inflammatory responses in the CNS.
We found that a single i.p. injection of baicalein, given immediately after the CCI procedure, had a protective effect for as long as 28 days (4 weeks) post-injury. A previous study of the pharmacokinetics and tissue distribution of baicalein in rats has shown that baicalein rapidly penetrates the blood–brain barrier by 20–30 min after administration and becomes homogenously distributed over various regions of the brain (Tsai et al., 2002). Accordingly, it is possible that early administration of this drug offers protection by interfering with the production of a harmful mediator. Indeed, TNF-α or IL-β may induce a cascade of other proinflammatory cytokines and chemokines during the very early post-injury stages, thus amplifying the inflammatory response (Allan and Rothwell, 2001; Morganti-Kossmann et al., 2002). Our present results are compatible with earlier reports on the effects of baicalein in animal models of focal cerebral ischaemia and Parkinson's disease. They demonstrate that prophylactic baicalein treatment can reduce neuronal death following cerebral ischaemia and inhibit myeloperoxidase activity, which is an index of neutrophil infiltration in the ischaemic brain tissue (Hamada et al., 1993; Hwang et al., 2002). In addition, preinjury baicalein can improve functional recovery in the Parkinson's disease model, with a decrease in lipid peroxidation level (Wu et al., 2006). Our results showing that post-injury baicalein also exerts a neuroprotective effect are particularly important because cerebral injuries could arise from different types of primary insults to result in diverse cellular vulnerability patterns as well as a spectrum of injury processes (Bramlett and Dietrich, 2004). Our data suggest that baicalein could have potential clinical applications in treating post-traumatic brain injuries as prophylactic treatment is not feasible, because of the unpredictability of TBI (Roberts et al., 1998). However, a question that requires further investigation is how long the neuroprotective effect of baicalein can last if administration of the drug is delayed after brain injury in rats. Clearly, a wider therapeutic window would make clinical use more feasible.
Our results suggest that the early initiation and prolonged baicalein treatment are viable treatment strategies in rats, not only for reducing neural death, but also for improving sensorimotor behaviour. As cytokine levels were raised as early as 1 h after the initial injury and lasted until 24 h post-injury in experimental models of TBI (Fan et al., 1996; Vitarbo et al., 2004; Kamm et al., 2006), our current study shows that the early effects of baicalein may be important. Baicalein-associated reduction in cytokines such as TNF-α, IL-β and IL-6 offers an attractive explanation for preferring the early treatment. However, our results also showed that 4 days of treatment was more effective than a single treatment, suggesting that reducing cytokine expression may be only one mechanism of minimizing brain injury. It is possible that other pathways might also contribute to the protective effect of baicalein against TBI. Other potential neuroprotective mechanisms might include reducing free radical damage (Hamada et al., 1993) or attenuating apoptotic cell death (Chen et al., 2006), which might explain why the longer duration of treatment is still beneficial. Further investigations are needed to clarify the mechanisms underlying the beneficial effects of baicalein.
In conclusion, our findings indicate that post-injury treatment with baicalein leads to improved functional and histological outcomes in a clinically relevant model of TBI. This improvement was associated with attenuated expression of TNF-α, IL-1β and IL-6 mRNA and protein, suggesting that the neuroprotective effect of baicalein following TBI may be, in part, mediated through modulation of the injury-induced proinflammatory cascades. Among the advantages of baicalein treatment are that chronic dosing is not required, that baicalein has low levels of toxicity and that it is easy to administer in emergency situations. Thus, baicalein offers great promise as a potential treatment for TBI.
Acknowledgments
We thank Mr Kuei-Han Lin and Mr Jay-How Yang for their expert technical assistance in the experiments. This study was supported in part by grants from the National Science Council of Taiwan, Republic of China. (NSC 94-2314-B-350 –001), National Defense Medical Center (DOD94-02-14 and DOD 95-02-18) and the Cheng Hsin General Hospital.
Abbreviations
- CCI
controlled cortical impact
- FJB
FluoroJade B
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- mNSS
modified neurological severity scores
- NGS
normal goat serum
- PBS
phosphate-buffered saline
- rRNA
ribosomal RNA
- RT-PCR
reverse transcriptase-PCR
- TBI
traumatic brain injury
- TBS
Trizma-buffered saline
- TNF-α
tumour necrosis factor-α
Conflict of interest
The authors state no conflict of interest.
References
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Anderson KJ, Miller KM, Fugaccia I, Scheff SW. Regional distribution of fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp Neuro. 2005;193:125–130. doi: 10.1016/j.expneurol.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Goldman H, Murphy S, Pellizon G, Owen C, Rafols J, et al. Endothelial cell activation following moderate traumatic brain injury. Neurol Res. 2001;23:175–182. doi: 10.1179/016164101101198514. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S. Tumor necrosis factor-alpha potentiates glutamate neurotoxicity in human fetal brain cell cultures. Dev Neurosci. 1994;16:172–179. doi: 10.1159/000112104. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen S, Pickard JD, Harris NG. Time course of cellular pathology after controlled cortical impact injury. Exp Neurol. 2003;182:87–102. doi: 10.1016/s0014-4886(03)00002-5. [DOI] [PubMed] [Google Scholar]
- Chen SF, Hung TH, Chen CC, Lin KH, Huang YN, Tsai HC, et al. Lovastatin improves histological and functional outcomes and reduces inflammation after experimental traumatic brain injury. Life Sci. 2007;81:288–298. doi: 10.1016/j.lfs.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Chen YC, Chow JM, Lin CW, Wu CY, Shen SC. Baicalein inhibition of oxidative-stress-induced apoptosis via modulation of ERKs activation and induction of HO-1 gene expression in rat glioma cells C6. Toxicol Appl Pharmacol. 2006;216:263–273. doi: 10.1016/j.taap.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Csuka E, Morganti-Kossmann MC, Lenzlinger PM, Joller H, Trentz O, Kossmann T. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J Neuroimmunol. 1999;101:211–221. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- Fan L, Young PR, Barone FC, Feuerstein GZ, Smith DH, McIntosh TK. Experimental brain injury induces differential expression of tumor necrosis factor-alpha mRNA in the CNS. Brain Res Mol Brain Res. 1996;36:287–291. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Friedlander RM, Gagliardini V, Rotello RJ, Yuan J. Functional role of interleukin 1 beta (IL-1 beta) in IL-1 beta-converting enzyme-mediated apoptosis. J Exp Med. 1996;184:717–724. doi: 10.1084/jem.184.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gao Z, Huang K, Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol Res. 2001;43:173–178. doi: 10.1006/phrs.2000.0761. [DOI] [PubMed] [Google Scholar]
- Goodman JC, Robertson CS, Grossman RG, Narayan RK. Elevation of tumor necrosis factor in head injury. J Neuroimmunol. 1990;30:213–217. doi: 10.1016/0165-5728(90)90105-v. [DOI] [PubMed] [Google Scholar]
- Hamada H, Hiramatsu M, Edamatsu R, Mori A. Free radical scavenging action of baicalein. Arch Biochem Biophys. 1993;306:261–266. doi: 10.1006/abbi.1993.1509. [DOI] [PubMed] [Google Scholar]
- Hamm RJ. Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures. J Neurotrauma. 2001;18:1207–1216. doi: 10.1089/089771501317095241. [DOI] [PubMed] [Google Scholar]
- Hans VH, Kossmann T, Joller H, Otto V, Morganti-Kossmann MC. Interleukin-6 and its soluble receptor in serum and cerebrospinal fluid after cerebral trauma. Neuroreport. 1999a;10:409–412. doi: 10.1097/00001756-199902050-00036. [DOI] [PubMed] [Google Scholar]
- Hans VH, Kossmann T, Lenzlinger PM, Probstmeier R, Imhof HG, Trentz O, et al. Experimental axonal injury triggers interleukin-6 mRNA, protein synthesis and release into cerebrospinal fluid. J Cereb Blood Flow Metab. 1999b;19:184–194. doi: 10.1097/00004647-199902000-00010. [DOI] [PubMed] [Google Scholar]
- Hayakata T, Shiozaki T, Tasaki O, Ikegawa H, Inoue Y, Toshiyuki F, et al. Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock. 2004;22:102–107. doi: 10.1097/01.shk.0000131193.80038.f1. [DOI] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T. Intracerebral administration of interleukin-1beta and induction of inflammation, apoptosis, and vasogenic edema. J Neurosurg. 2000;92:108–120. doi: 10.3171/jns.2000.92.1.0108. [DOI] [PubMed] [Google Scholar]
- Holmin S, Schalling M, Hojeberg B, Nordqvist AC, Skeftruna AK, Mathiesen T. Delayed cytokine expression in rat brain following experimental contusion. J Neurosurg. 1997;86:493–504. doi: 10.3171/jns.1997.86.3.0493. [DOI] [PubMed] [Google Scholar]
- Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268–271. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- Huang WH, Chien PY, Yang CH, Lee AR. Novel synthesis of flavonoids of Scutellaria baicalensis Georgi. Chem Pharm Bull (Tokyo) 2003;51:339–340. doi: 10.1248/cpb.51.339. [DOI] [PubMed] [Google Scholar]
- Huang WH, Lee AR, Yang CH. Antioxidative and anti-inflammatory activities of polyhydroxyflavonoids of Scutellaria baicalensis Georgi. Biosci Biotechnol Biochem. 2006;70:2371–2380. doi: 10.1271/bbb.50698. [DOI] [PubMed] [Google Scholar]
- Hunter AJ, Mackay KB, Rogers DC. To what extent have functional studies of ischaemia in animals been useful in the assessment of potential neuroprotective agents. Trends Pharmacol Sci. 1998;19:59–66. doi: 10.1016/s0165-6147(97)01157-7. [DOI] [PubMed] [Google Scholar]
- Hwang YS, Shin CY, Huh Y, Ryu JH. Hwangryun-Hae-Dok-tang (Huanglian-Jie-Du-Tang) extract and its constituents reduce ischemia-reperfusion brain injury and neutrophil infiltration in rats. Life Sci. 2002;71:2105–2117. doi: 10.1016/s0024-3205(02)01920-3. [DOI] [PubMed] [Google Scholar]
- Im HI, Joo WS, Nam E, Lee ES, Hwang YJ, Kim YS. Baicalein prevents 6-hydroxydopamine-induced dopaminergic dysfunction and lipid peroxidation in mice. J Pharmacol Sci. 2005;98:185–189. doi: 10.1254/jphs.sc0050014. [DOI] [PubMed] [Google Scholar]
- Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30:433–446. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- Kamm K, Vanderkolk W, Lawrence C, Jonker M, Davis AT. The effect of traumatic brain injury upon the concentration and expression of interleukin-1beta and interleukin-10 in the rat. J Trauma. 2006;60:152–157. doi: 10.1097/01.ta.0000196345.81169.a1. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Matsushita N, Yokoi-Hayashi K, Okuda H. Effects of baicalein isolated from Scutellaria baicalensis Radix on adhesion molecule expression induced by thrombin and thrombin receptor agonist peptide in cultured human umbilical vein endothelial cells. Planta Med. 2001;67:331–334. doi: 10.1055/s-2001-14328. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Administration of either anti-intercellular adhesion molecule-1 or a nonspecific control antibody improves recovery after traumatic brain injury in the rat. J Neurotrauma. 2002;19:1039–1050. doi: 10.1089/089771502760341956. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-alpha after experimental brain injury contributes to neurological impairment. J Neuroimmunol. 1999;95:115–125. doi: 10.1016/s0165-5728(98)00273-2. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Maher P, Schubert D, Zivin JA. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150:585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988;141:2629–2634. [PubMed] [Google Scholar]
- Li FQ, Wang T, Pei Z, Liu B, Hong JS. Inhibition of microglial activation by the herbal flavonoid baicalein attenuates inflammation-mediated degeneration of dopaminergic neurons. J Neural Transm. 2005;112:331–347. doi: 10.1007/s00702-004-0213-0. [DOI] [PubMed] [Google Scholar]
- Lu KT, Wang YW, Yang JT, Yang YL, Chen HI. Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J Neurotrauma. 2005;22:885–895. doi: 10.1089/neu.2005.22.885. [DOI] [PubMed] [Google Scholar]
- Megyeri P, Abraham CS, Temesvari P, Kovacs J, Vas T, Speer CP. Recombinant human tumor necrosis factor alpha constricts pial arterioles and increases blood–brain barrier permeability in newborn piglets. Neurosci Lett. 1992;148:137–140. doi: 10.1016/0304-3940(92)90823-p. [DOI] [PubMed] [Google Scholar]
- Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, Maxwell WL, et al. Experimental models of traumatic brain injury: do we really need to build a better mousetrap. Neuroscience. 2005;136:971–989. doi: 10.1016/j.neuroscience.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Nakahata N, Kutsuwa M, Kyo R, Kubo M, Hayashi K, Ohizumi Y. Analysis of inhibitory effects of scutellariae radix and baicalein on prostaglandin E2 production in rat C6 glioma cells. Am J Chin Med. 1998;26:311–323. doi: 10.1142/S0192415X9800035X. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Hayasaka S, Zhang XY, Nagaki Y, Matsumoto M, Hayasaka Y, et al. Effects of baicalin, baicalein, and wogonin on interleukin-6 and interleukin-8 expression, and nuclear factor-kappab binding activities induced by interleukin-1beta in human retinal pigment epithelial cell line. Exp Eye Res. 2003;77:195–202. doi: 10.1016/s0014-4835(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Phillis JW, O'Regan MH. The role of phospholipases, cyclooxygenases, and lipoxygenases in cerebral ischemic/traumatic injuries. Crit Rev Neurobiol. 2003;15:61–90. doi: 10.1615/critrevneurobiol.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- Pleines UE, Stover JF, Kossmann T, Trentz O, Morganti-Kossmann MC. Soluble ICAM-1 in CSF coincides with the extent of cerebral damage in patients with severe traumatic brain injury. J Neurotrauma. 1998;15:399–409. doi: 10.1089/neu.1998.15.399. [DOI] [PubMed] [Google Scholar]
- Roberts I, Schierhout G, Alderson P. Absence of evidence for the effectiveness of five interventions routinely used in the intensive care management of severe head injury: a systematic review. J Neurol Neurosurg Psychiatry. 1998;65:729–733. doi: 10.1136/jnnp.65.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson KL, Raghupathi R, Saatman KE, Martin D, Miller G, McIntosh TK. Interleukin-1 receptor antagonist attenuates regional neuronal cell death and cognitive dysfunction after experimental brain injury. J Cereb Blood Flow Metab. 1999;19:1118–1125. doi: 10.1097/00004647-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Shen YC, Chiou WF, Chou YC, Chen CF. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur J Pharmacol. 2003;465:171–181. doi: 10.1016/s0014-2999(03)01378-5. [DOI] [PubMed] [Google Scholar]
- Shohami E, Bass R, Wallach D, Yamin A, Gallily R. Inhibition of tumor necrosis factor alpha (TNFalpha) activity in rat brain is associated with cerebroprotection after closed head injury. J Cereb Blood Flow Metab. 1996;16:378–384. doi: 10.1097/00004647-199605000-00004. [DOI] [PubMed] [Google Scholar]
- Shohami E, Gallily R, Mechoulam R, Bass R, Ben Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J Neuroimmunol. 1997;72:169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994;14:615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- Suk K, Lee H, Kang SS, Cho GJ, Choi WS. Flavonoid baicalein attenuates activation-induced cell death of brain microglia. J Pharmacol Exp Ther. 2003;305:638–645. doi: 10.1124/jpet.102.047373. [DOI] [PubMed] [Google Scholar]
- Tanno Y, Kakuta Y, Aikawa T, Shindoh Y, Ohno I, Takishima T. Effects of qing-fei-tang (seihai-to) and baicalein, its main component flavonoid, on lucigenin-dependent chemiluminescence and leukotriene B4 synthesis of human alveolar macrophages. Am J Chin Med. 1988;16:145–154. doi: 10.1142/S0192415X88000212. [DOI] [PubMed] [Google Scholar]
- Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol. 1993;42:177–185. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999;100:203–215. doi: 10.1016/s0165-5728(99)00202-7. [DOI] [PubMed] [Google Scholar]
- Tsai TH, Liu SC, Tsai PL, Ho LK, Shum AY, Chen CF. The effects of the cyclosporin A, a P-glycoprotein inhibitor, on the pharmacokinetics of baicalein in the rat: a microdialysis study. Br J Pharmacol. 2002;137:1314–1320. doi: 10.1038/sj.bjp.0704959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD. Tumor necrosis factor alpha expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. Neurosurgery. 2004;55:416–424. doi: 10.1227/01.neu.0000130036.52521.2c. [DOI] [PubMed] [Google Scholar]
- Wang CX, Shuaib A. Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol. 2002;67:161–172. doi: 10.1016/s0301-0082(02)00010-2. [DOI] [PubMed] [Google Scholar]
- Wen LL, Chiu CT, Huang YN, Chang CF, Wang JY. Rapid glia expression and release of proinflammatory cytokines in experimental Klebsiella pneumoniae meningoencephalitis. Exp Neurol. 2007;205:270–278. doi: 10.1016/j.expneurol.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Woo KJ, Lim JH, Suh SI, Kwon YK, Shin SW, Kim SC, et al. Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPbeta DNA-binding activity. Immunobiology. 2006;211:359–368. doi: 10.1016/j.imbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Wu PH, Shen YC, Wang YH, Chi CW, Yen JC. Baicalein attenuates methamphetamine-induced loss of dopamine transporter in mouse striatum. Toxicology. 2006;226:238–245. doi: 10.1016/j.tox.2006.06.015. [DOI] [PubMed] [Google Scholar]