Abstract
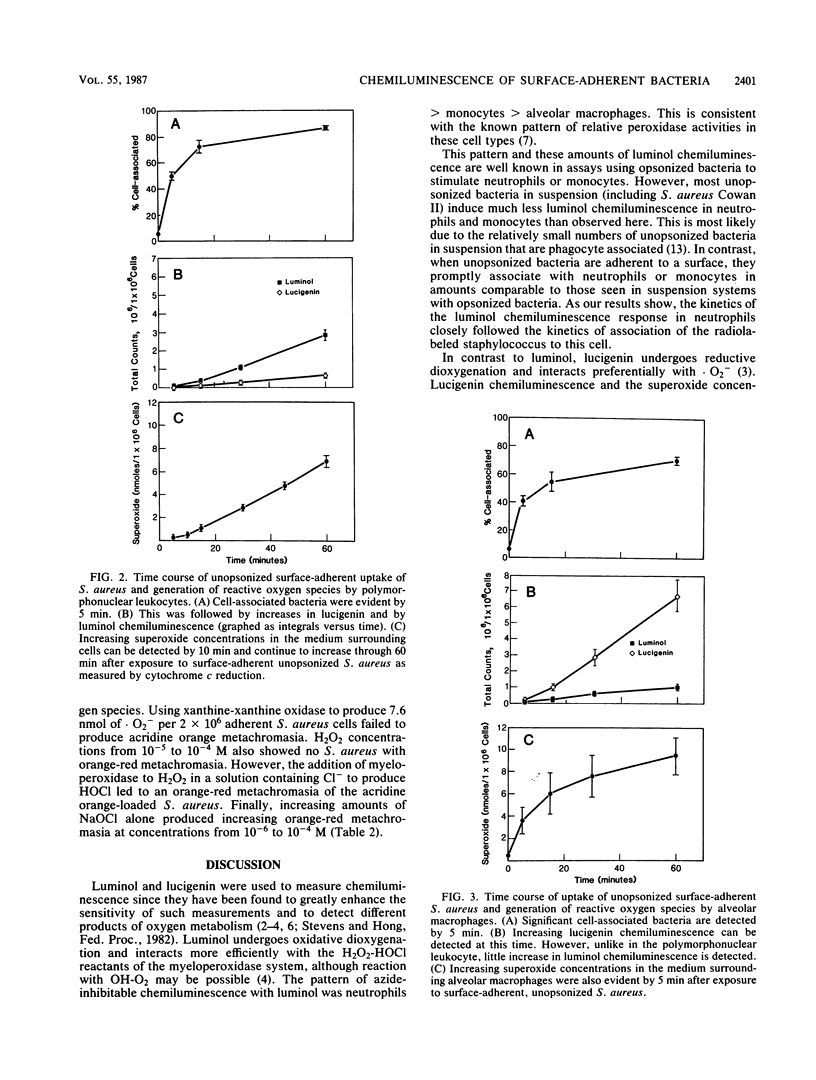
In contrast to results with bacterial suspensions, phagocytosis of unopsonized bacteria readily occurs when bacteria are adhered to glass or plastic surfaces. However, in contrast to neutrophils, alveolar macrophages produced much less DNA denaturation as measured by acridine orange metachromasia of phagocytized Staphylococcus aureus. We have studied the phagocytosis of unopsonized surface-adherent S. aureus and the subsequent production of reactive oxygen species by peripheral blood neutrophils, monocytes, and alveolar macrophages. Phagocyte-free systems were then used to show the relationship of the reactive oxygen species produced by neutrophils and alveolar macrophages and the denaturation of unopsonized S. aureus DNA with acridine orange. Peripheral blood neutrophils, monocytes, and alveolar macrophages from normal human volunteers were added to vials with adherent S. aureus without opsonin. Bacterial uptake and luminol- and lucigenin-dependent chemiluminescence were measured. Neutrophils developed much greater luminol-dependent chemiluminescence than monocytes or alveolar macrophages. Compared with neutrophils and monocytes, alveolar macrophages developed significantly greater concentrations of superoxide, as measured by lucigenin-dependent chemiluminescence and ferricytochrome c reduction. These findings suggested that products of the myeloperoxidase-hydrogen peroxide-halide pathway were generated when peripheral blood neutrophils were stimulated and that alveolar macrophages primarily produced superoxide. When these reactive oxygen species were generated in phagocyte-free systems containing S. aureus, products of the myeloperoxidase-hydrogen peroxide-halide pathway produced denaturation of S. aureus DNA, whereas superoxide did not. Thus, differences in reactive oxygen species produced during phagocytosis may be related to the different capacities of neutrophils and alveolar macrophages to denature unopsonized adherent S. aureus DNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Allen R. C. Chemiluminescence and the study of phagocyte redox metabolism. Adv Exp Med Biol. 1982;141:411–421. doi: 10.1007/978-1-4684-8088-7_39. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Allen R. C. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 1986;133:449–493. doi: 10.1016/0076-6879(86)33085-4. [DOI] [PubMed] [Google Scholar]
- Biggar W. D., Sturgess J. M. Peroxidase activity of alveolar macrophages. Lab Invest. 1976 Jan;34(1):31–42. [PubMed] [Google Scholar]
- Brestel E. P. Co-oxidation of luminol by hypochlorite and hydrogen peroxide implications for neutrophil chemiluminescence. Biochem Biophys Res Commun. 1985 Jan 16;126(1):482–488. doi: 10.1016/0006-291x(85)90631-x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cohen A. B., Cline M. J. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C., Stendahl O. Role of myeloperoxidase in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1983 Feb;39(2):736–741. doi: 10.1128/iai.39.2.736-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Long G. D., Shirley P. S., Bass D. A., Thomas M. J., Henderson F. W., Cohen M. S. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982 Oct;129(4):1589–1593. [PubMed] [Google Scholar]
- Hoidal J. R., Schmeling D., Peterson P. K. Phagocytosis, bacterial killing, and metabolism by purified human lung phagocytes. J Infect Dis. 1981 Jul;144(1):61–71. doi: 10.1093/infdis/144.1.61. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., White J. G., Repine J. E. Influence of cationic local anesthetics on the metabolism and ultrastructure of human alveolar macrophages. J Lab Clin Med. 1979 May;93(5):857–866. [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- Lee D. A., Hoidal J. R., Clawson C. C., Quie P. G., Peterson P. K. Phagocytosis by polymorphonuclear leukocytes of Staphylococcus aureus and Pseudomonas aeruginosa adherent to plastic, agar, or glass. J Immunol Methods. 1983 Sep 30;63(1):103–114. doi: 10.1016/0022-1759(83)90213-2. [DOI] [PubMed] [Google Scholar]
- Lee D. A., Hoidal J. R., Garlich D. J., Clawson C. C., Quie P. G., Peterson P. K. Opsonin-independent phagocytosis of surface-adherent bacteria by human alveolar macrophages. J Leukoc Biol. 1984 Dec;36(6):689–701. doi: 10.1002/jlb.36.6.689. [DOI] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Mills E. L., Rholl K. S., Quie P. G. X-linked inheritance in females with chronic granulomatous disease. J Clin Invest. 1980 Aug;66(2):332–340. doi: 10.1172/JCI109861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkenberg I., Ferber E. Lucigenin-dependent chemiluminescence as a new assay for NAD(P)H-oxidase activity in particulate fractions of human polymorphonuclear leukocytes. J Immunol Methods. 1984 Jun 8;71(1):61–67. doi: 10.1016/0022-1759(84)90206-0. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Seim S. Role of myeloperoxidase in the luminol-dependent chemiluminescence response of phagocytosing human monocytes. Acta Pathol Microbiol Immunol Scand C. 1983 Apr;91(2):123–128. [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Human bronchoalveolar lavage cells and luminol-dependent chemiluminescence. J Clin Pathol. 1981 Feb;34(2):167–171. doi: 10.1136/jcp.34.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai M., Quie P. G. Chemiluminescence by polymorphonuclear leukocytes adhering to surfaces. Infect Immun. 1981 Jun;32(3):1181–1186. doi: 10.1128/iai.32.3.1181-1186.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



