Abstract
Background and purpose:
Reactive oxygen and nitrogen species play an important role in the development of diabetic cardiomyopathy. They can activate matrix metalloproteinases (MMPs), and MMP-2 in particular is known to mediate early consequences of oxidative stress injury in the heart. Therefore, we investigated the role of MMP-2 and the effect of the MMP inhibitor doxycycline on the changes of heart function caused by diabetes.
Experimental approach:
Using streptozotocin-induced diabetic rats, we evaluated the effect of doxycycline on both mechanical and electrical function of isolated hearts, papillary muscle and cardiomyocytes.
Key results:
Doxycycline abolished the diabetes-induced depression in left ventricular developed pressure and the rates of changes in developed pressure in isolated hearts and normalized the prolongation of the action potential in papillary muscles. In cardiomyocytes isolated from doxycycline-treated diabetic rats, the altered kinetic parameters of Ca2+ transients, depressed Ca2+ loading of sarcoplasmic reticulum and basal intracellular Ca2+ level, and the spatio-temporal properties of Ca2+ sparks were significantly restored. Gelatin zymography and western blot data indicated that the diabetes-induced alterations in MMP-2 activity and protein level, level of tissue inhibitor of matrix metalloproteinase-4 and loss of troponin I were restored to control levels with doxycycline.
Conclusions and implications:
Our data suggest that these beneficial effects of doxycycline on the mechanical, electrical and biochemical properties of the diabetic rat heart appear, at least in part, to be related to inhibition of MMP activity, implying a role for MMPs in the development of diabetic cardiomyopathy.
Keywords: metalloproteinases, diabetes mellitus, cardiomyopathy, calcium, calcium sparks, electrophysiology
Introduction
Both type 1 and type 2 diabetes are accepted as disorders affecting normal heart function and leading to congestive heart failure, even in the absence of coronary artery disease (Rubler et al., 1972). Several mechanisms for the development of diabetic cardiomyopathy have been postulated including enhanced oxidative stress (Wold et al., 2006). Hyperglycaemia increases the production of reactive oxygen and nitrogen species (ROS and RNS) and alters the cellular redox state and causes rapid changes in membrane function, followed by contractile dysfunction within weeks in hearts of diabetic animals (Nishikawa et al., 2000; Ayaz and Turan, 2006). The ROS- and RNS-induced endothelial and myocardial dysfunction associated with diabetic cardiomyopathy results in enhanced myocardial nitrotyrosine formation (Ceriello et al., 2002) and activation of downstream effector pathways including poly (ADP-ribose) polymerase (Pacher et al., 2002). The increase in ROS/RNS leads to a decrease in the antioxidant capacity of the diabetic myocardium (Ulusu and Turan, 2005), contributing significantly to oxidative stress and the resultant myocardial damage.
Enhanced oxidative stress activates matrix metalloproteinases (MMPs) (Okamoto et al., 2001; Wang et al., 2002a). MMPs, a group of zinc-dependent neutral endopeptidases, are expressed in a wide variety of cells (Nagase and Woessner, 1999) and MMP-2 is found in all cardiac cells including cardiac myocytes, fibroblasts, endothelial and smooth muscle cells. In addition, MMPs are critically involved in remodelling the extracellular matrix of different tissues in pathological conditions (Lemaitre and D'Armiento, 2006). Often this occurs in conjunction with increased oxidative stress as shown by reduced levels of glutathione (Cantin et al., 1989). Thus, it is of great importance to explore the role of MMPs in oxidative stress-induced changes in diabetes-induced cardiac dysfunction.
Although MMPs are best known for their actions in remodelling the extracellular matrix, it has been shown that the acute contractile dysfunction resulting from myocardial ischaemia–reperfusion injury is caused in part by activation of MMP-2 in cardiac myocytes that act intracellularly by proteolytic cleavage of susceptible intracellular targets including troponin I (TnI) (Cheung et al., 2000; Wang et al., 2002a, 2002b; Schulz, 2007). In addition, the role of pro-oxidant species in MMP-2 activation and the subsequent loss of contractile function, either as a result of the endogenous biosynthesis of peroxynitrite in myocardial ischaemia–reperfusion injury, or the direct infusion of peroxynitrite into isolated hearts, was shown by the ability of either the peroxynitrite scavenger glutathione or inhibitors of MMP activity to prevent contractile dysfunction (Cheung et al., 2000; Wang et al., 2002a, 2002b). However, whether activation of MMPs has a role in diabetes-induced cardiac dysfunction is unknown.
To address this question, we investigated the effect of the MMP inhibitor doxycycline (Golub et al., 1998) on mechanical and electrophysiological function, as well as Ca2+ handling properties, of heart preparations from rats with streptozotocin (STZ)-induced diabetes. Doxycycline is the most potent MMP inhibitor of the tetracycline class of antibiotics and exhibits MMP inhibition in vivo at blood levels lower than those required for its antibacterial effect (Golub et al., 1990). It effectively crosses cell membranes (Liu et al., 2001) and accumulates preferentially in cardiomyocytes (Modrak and Rovang, 1981), and was shown to be protective in several models of myocardial oxidative stress injury (Cheung et al., 2000; Wang et al., 2002b; Schulz, 2007). In this study, we found doxycycline treatment to have beneficial effects on the mechanical, electrical and biochemical properties of the diabetic rat heart, which appear to be because of its MMP inhibitory property.
Methods
Experimental model
All animal procedures and experiments were approved by the Ankara University School of Medicine Animal Ethics Committee. Male Wistar rats were used (200–250 g). Diabetes was induced in the diabetic group as described earlier (Yaras et al., 2005). One week after injection of STZ, blood glucose level was measured and rats with threefold higher levels of blood glucose than pre-injection levels were used in these experiments. Diabetic and non-diabetic animals received either doxycycline (15 mg kg−1 day−1; intragastrically) or vehicle (carboxymethylcellulose) for 4 weeks in an identical fashion. All rats had free access to standard chow and water.
Langendorff-perfused hearts
After the 5-week experimental period, the animals were killed and left ventricular developed pressure (LVDP), left ventricular end diastolic pressure (LVEDP) and the rates of changes in developed pressure (±dP/dt) of isolated hearts were measured as described earlier (Tuncay et al., 2007). Briefly, isolated hearts were electrically stimulated (DCS, Harvard Instruments, Commat Ltd., Ankara, Turkey) at 300 beats min−1 by a square wave of two times the threshold voltage of 1.5 ms duration. Hearts were perfused for a total of 50–60 min and functional parameters were determined at 40 min.
Electron microscopy
For electron microscopic evaluations, longitudinal sections of cardiac muscle tissue were removed from the left ventricles and fixed in 2.5% glutaraldehyde in phosphate buffer for 2–4 h at 4 °C and postfixed in 1% osmium tetroxide. The samples were then dehydrated in graded alcohol solutions and embedded in Araldite 6005. Ultrathin sections (60–80 μm) were cut with a glass knife on LKB III ultramicrotome (Leica, Gantenbein Ltd., Ankara, Turkey) and stained with uranyl acetate–lead citrate and examined using a Leo 906 E transmission electron microscope.
Action potentials in isolated papillary muscle
Isolation of papillary muscles and measurement of action potential were performed as described earlier (Yaras et al., 2005). Muscle strips were stimulated and intracellular action potential was measured using a conventional glass microelectrode connected to a preamplifier. Repolarization phases of the action potential duration (APD25,50,75,90) were determined and compared between groups.
Isolation of cardiomyocytes
Cell isolation was performed as described elsewhere (Yaras et al., 2005). Briefly, ventricles were removed from rapidly excised hearts and minced into small pieces and gently passed through a nylon mesh. Following digestion with collagenase, dissociated cardiomyocytes were washed with collagenase-free solution. Subsequently, Ca2+ in the medium was increased in a graded manner to a final concentration of 1.3 mM. The cells were kept in this solution at 37 °C and only Ca2+-tolerant cells were used in the experiments.
Patch-clamp experiments
Whole-cell patch clamp recordings were carried out as described earlier (Ozdemir et al., 2005). K+ currents (transient outward current, Ito, steady-state outward current, Iss and inward rectifier current, IK1) were recorded. From a holding potential of −80 mV, voltage pulses (duration of 600 ms) between −120 and +70 mV (with 10 mV steps) were applied at a frequency of 0.14 Hz. K+ currents were normalized with cell capacitance and expressed as pA pF−1. A detailed kinetic analysis of the Ito was performed. Ito activation characteristics were determined by applying 6 ms prepulses within the range −40 to +50 mV in 10 mV increments that were followed by a −40 mV, 120 ms pulse. Its inactivation was established by applying a 200 ms prepulse within the range −85 mV to +30 mV in 10 mV increments that was followed by a +50 mV pulse 300 ms long.
Simultaneous measurement of intracellular Ca2+ transients and L-type Ca2+ currents (ICa)
Cardiomyocytes were whole-cell patch-clamped in an external solution containing (in mM): NaCl 135, MgCl2 1, CsCl 20, CaCl2 1.8, glucose 10, HEPES 10; pH 7.4. Patch-clamp recording of ICa was made using a HEKA EPC8 amplifier. Patch pipettes (1.0–1.2 MΩ) were filled with a solution that contained (mM): CsCl 130, MgC12 0.4, TEA-Cl 20, MgATP 4, HEPES 10 (pH 7.2) and 50 μM Fura-2. Cells were held at −80 mV and five sequential step-depolarizations to 0 mV to keep the sarcoplasmic reticulum load constant and to allow the penetration of dye into the cells. To inhibit Na+ channels, cells were then ramped to −50 mV over 500 ms and then held at −50 mV for 50 ms before the test pulses were tried (between −50 mV and +60 mV in 10 mV increments). ICa was recorded using pClamp8 software. Current amplitude was estimated as the difference between peak inward current and the current level at the end of the 250 ms pulse. Currents were normalized to the membrane capacitance to obtain current density. Intracellular Ca2+ transients were monitored by using a Ca2+-sensitive dye, Fura-2, at room temperature. Fluorescence was recorded using a micro-spectrophotometer and FELIX software (Photon Technology International, Birmingham, NJ, USA). Cells were sequentially excited at 340/380 nm at 10 Hz and emission was measured at 510 nm. The ratio of the fluorescence at 340 nm excitation to the fluorescence at 380 nm was calculated and used as an indicator of intracellular [Ca2+].
Ca2+ sparks measurement
Ca2+ sparks were measured as described earlier (Yaras et al., 2005). Fluorescence images were acquired with a Zeiss LSM 510 NLO confocal system. Fluo-3 was excited with an argon laser at 488 nm, and the emitted fluorescence was recorded at 505 nm. Potential spark areas were empirically identified using an autodetection algorithm (Yaras et al., 2005). The mean fluorescence intensity (F) of the images was calculated by summing and averaging the temporal F at each spatial location while ignoring the potential spark area. This F value then used to create a ΔF F−1 image pixel by pixel. Following a three-point smoothing routine, potential spark locations were visualized manually and analysed for spatio-temporal properties as described (Yaras et al., 2005). Ca2+ sparks were manually detected and converted to temporal lines by averaging the fluorescence intensity of two to three pixels, aligning the peak of fluorescence intensity over time. Then the temporal profiles were fitted to the γ function to analyse time-to-peak (TP), peak amplitude of fluorescence intensity (max) and the decrease time to half-maximum (DT50).
Biochemical analyses
Preparation of heart homogenates
Frozen hearts were pulverized in liquid N2 and then homogenized (Cheung et al., 2000). Protein content was analysed using the Bradford method (Bio-Rad) and bovine serum albumin was used as the standard.
Gelatin zymography
Gelatin zymography for MMP activity was performed as described (Sawicki et al., 1998). Briefly, non-reduced proteins were loaded onto an 8% polyacrylamide gel containing gelatin. Gelatinolytic activities were detected as transparent bands against the background of Coomassie blue-stained gelatin. To quantify the activities zymograms were imaged by a Raytest camera attached to a computer with AIDA software. Gelatinolytic activity was identified using HT1080 cell culture medium as a standard. The intensities of the bands were analysed using SigmaGel (Jandel) and reported as normalized to non-treated control values.
Western blotting
MMP-2, TIMP-4 and TnI protein levels were determined by western blotting. Briefly, equal amount of proteins from samples were loaded and separated on 8–15% sodium dodecyl sulphate-polyacrylamide gel electrophoresis under reducing conditions (Laemmli, 1970). After electrophoresis (150 V, 20 °C) the samples were electroblotted onto a PVDF membrane. MMP-2, MMP-9, TIMP-4 and TnI content in the samples were identified using anti-MMP-2, anti-MMP-9, anti-TIMP-4 or anti-TnI antibodies, respectively. Immunoreactive protein bands were visualized using the ECL plus detection system.
Data analysis
Results are expressed as mean±s.e.mean, from the number of experiments shown. Statistical analysis was performed using analysis of variance followed by Newman–Keuls as the post hoc test. P<0.05 was considered statistically significant.
Chemicals
Unless otherwise specified, the reagents used were obtained from Sigma-Aldrich or Fisher Scientific. Anti-MMP-2, -MMP-9 and -TIMP-4 antibodies were obtained from Chemicon International (Temecula, CA, USA). Monoclonal anti human TnI (clone 81−7) was purchased from Spectral Diagnostics (Toronto, ON, Canada). Goat anti-rabbit and anti-mouse IgG conjugated with horseradish peroxidase was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Results
Characteristics of experimental animals
Diabetic animals had blood glucose levels fivefold higher than those of control rats (Table 1). The heart weight/body weight ratio of diabetics was not significantly different from the controls (data not shown). The weight loss of untreated diabetics within the total 5-week observation period was about 18%. Treatment with doxycycline for 4 weeks reduced the weight loss by 50%. Doxycycline, however, did not affect the high blood glucose level in diabetic animals as observed at the end of the 5-week experimental period (Table 1). In the non-diabetic group (n=8) doxycycline treatment did not affect weight gain (269.8±9.2 vs 275.8±7.1 g, initial vs final body weights, P>0.05 vs vehicle-treated control group) nor blood glucose level (1.110±0.025 vs 1.196±0.033 mg mL−1, initial vs final, P>0.05 vs vehicle-treated control group). Diabetic animals did not show abnormal behaviour or any sign of heart failure.
Table 1.
Changes in body weight and blood glucose levels during the experimental period
| Group |
Weight (g) |
Blood glucose (mg mL−1) |
||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Control (n=32) | 224.8±7.9 | 234.4±8.0* | 0.990±0.021 | 1.043±0.034 |
| DM (n=41) | 237.2±8.4 | 195.0±7.6*,** | 1.005±0.016 | 5.400±0.109*,** |
| Doxy (n=43) | 257.4±5.6 | 233.9±8.3* | 1.022±0.035 | 5.260±0.206*,** |
DM, diabetic; Doxy, doxycycline treated diabetic; n, number of rats.
'Initial' and ‘Final' represent the observation times of the end of first and fifth weeks of the experimental period, respectively.
Values are expressed as mean±s.e.mean.
*P<0.05 vs initial; **P<0.05 vs control.
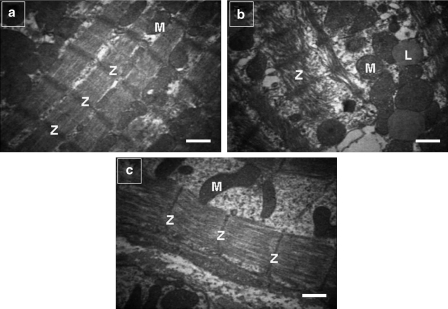
The ultrastructure of diabetic hearts (Figure 1b) showed various morphological changes including a loss in cardiomyocyte diameter, alterations in myofilaments and Z-lines of myofibres, myofibrillary degeneration and destruction and loss of myofibrils over sarcomere lengths when compared with control hearts (Figure 1a). In the diabetic group most of the mitochondria of the cardiomyocytes showed loss of cristae and granular matrix and also increased numbers of lipid droplets (Figure 1b). Doxycycline treatment normalized the alterations in the myofilaments (Figure 1c). We did not see any signs of tissue necrosis in any group.
Figure 1.
Electron micrographs of left ventricular heart muscle sections from control, diabetic and doxycycline-treated diabetic rats. (a) Regularly arranged myofilaments and Z-lines (Z) in myofibres and mitochondria (M), rich in cristae, were observed in control hearts. (b) Diabetes-induced changes in myofilaments and the Z-lines of myofibres, degeneration of myofibrils and destruction and loss of myofibrils over sarcomere units. Mitochondria showed loss of cristae and a granular matrix. There were also increased numbers of lipid droplets (L). (c) Doxycycline treatment normalized alterations in myofilaments, Z-lines and mitochondria. Scale bar: 2 μm.
Doxycycline improved STZ-induced cardiac contractile dysfunction
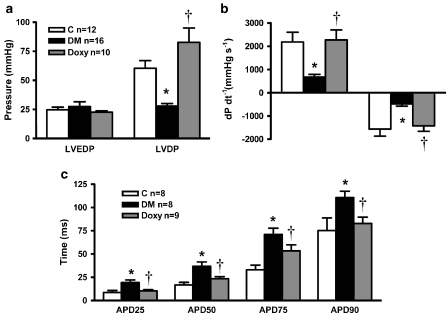
Effects of doxycycline on LVDP, LVEDP and the ±dP/dt were determined in Langendorff-perfused rat hearts isolated at the end of the 5-week experimental period. Diabetes caused a significant depression in LVDP, which was abolished by doxycycline treatment (Figure 2a). No changes in LVEDP were observed in any group. Doxycycline also abolished the diabetes-induced reductions in both +dP/dt and −dP/dt (Figure 2b). In summary, inhibition of MMP activity by doxycycline exerted beneficial effects on diabetes-induced mechanical dysfunction. In the non-diabetic group (n=8) doxycycline treatment did not show any significant effect on either LVDP (47.8±8.6 mm Hg) or LVEDP (27.3±2.4 mm Hg). As doxycycline treatment of the non-diabetic rats for 4 weeks had no significant effect on mechanical activity of the heart, we did not measure any other electrical parameters.
Figure 2.
Effect of doxycycline on mechanical function of hearts and action potential kinetics of papillary muscle strips isolated from diabetic rats. Despite no changes in LVEDP, both the reductions in LVDP (a) and ±dP/dt (b) induced by diabetes were abolished by doxycycline. The diabetes-induced increase in action potential duration (APD) was also restored by doxycycline (c). Bar graphs represent mean±s.e.mean values from control (C; nheart=12, npapillary=8); diabetic (DM; nheart=16, npapillary=8); doxycycline-treated diabetic (Doxy; nheart=10, npapillary=9) groups. *P<0.01 vs control, †P<0.01 vs diabetic, analysis of variance.
Doxycycline improved diabetes-induced alterations in action potential characteristics of left ventricular papillary muscle
Diabetes significantly prolonged the action potential duration in isolated papillary muscle strips (Figure 2c) as observed earlier (Pacher et al., 1999). Doxycycline significantly decreased the prolongation in action potential duration. Parameters of the repolarization phase of the action potentials were markedly shorter (46% in APD25, 36% in APD50, 25% in APD75 and 25% in APD90, P<0.05, ANOVA) in the doxycycline-treated diabetics than those in the untreated diabetic animals. Doxycycline did not cause any significant alterations in other parameters such as resting membrane potential and peak depolarization potential, which were also not affected by diabetes itself (data not shown).
Doxycycline restored depressed K+ currents in diabetic cardiomyocytes
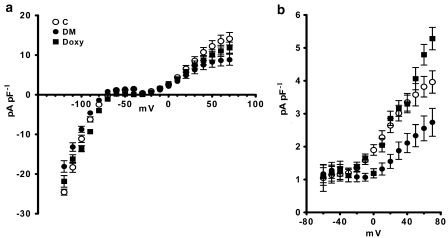
Three major K+ currents (Ito, IK1 and Iss) were evaluated in the cardiomyocytes. The current densities were estimated for IK1 at −120 mV and for Ito and Iss at +70 mV. These K+ current densities (Ito, IK1 and Iss, respectively) were significantly reduced in diabetics (8.82±1.40 pA pF−1, −18.09±1.46 pA pF−1 and 2.74±0.42 pA pF−1, respectively P<0.05, ANOVA) compared with controls (14.12±1.56, −24.57±0.75 and 3.96±0.34 pA pF−1, respectively, P<0.05, ANOVA). Doxycycline significantly restored these inhibited K+ currents (11.85±1.40, −21.84±1.46 and 5.28±0.34 pA pF−1, respectively P<0.05, ANOVA). Current–voltage relationships of these currents are shown for comparison in Figures 3a and b. Here we show that the lengthening of the action potential duration induced by diabetes results mainly from a decrease in the Ito and, to a lesser extent, IK1 and Iss. Doxycycline prevented the prolongation in action potential duration, an action likely to be because of the restoration of these inhibited K+ currents.
Figure 3.
Effect of doxycycline on K+ currents in ventricular cardiomyocytes isolated from diabetic rat hearts. (a) Current–voltage (I–V) relationships of peak current densities (Ito and IK1) in cardiomyocytes isolated from control (C; n=22 cells from five rats), diabetic (DM; n=30 cells from six rats) and doxycycline-treated diabetic groups (Doxy; n=24 cells from five rats). Voltage pulses were applied from a holding potential of 80 mV to between 120 and +70 mV, with 10 mV steps. The current densities were estimated for IK1 at −120 mV, and for Ito, which was estimated as the difference between peak and steady state currents (Nagase et al.) elicited at +70 mV. Doxycyline significantly recovered IK1 and Ito density. (b) I–V characteristics of Iss were also improved in doxycycline-treated diabetic rats. Data points on the graphs represent mean±s.e.mean values.
Doxycycline restored global Ca2+ release in cardiomyocytes isolated from diabetic rats
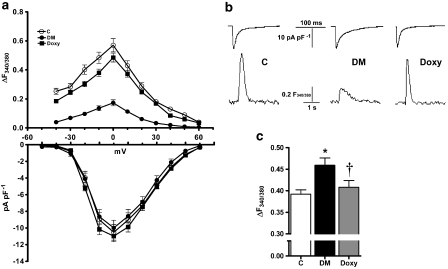
To determine whether doxycycline treatment has an effect on altered cellular Ca2+ signalling, which underlies diabetes-induced cardiac dysfunction, we simultaneously recorded intracellular Ca2+ transients and ICa in isolated cardiomyocytes at various membrane potentials (between −50 and +60 mV with 10 mV increments). Figure 4a (upper) shows the corresponding calculated Ca2+ transients of cardiomyocytes from control, diabetic and doxycycline-treated groups as a function of the depolarization potentials. The Ca2+ transients of diabetics were markedly smaller in amplitude and decayed more slowly with respect to the corresponding controls at all depolarizing potentials. Doxycycline significantly restored these altered parameters. As we obtained maximal amplitudes for both Ca2+ transients and ICa at 0 mV depolarization, we compared the data between groups as follows: averaged data indicate a significant reduction in the maximum amplitude of the Ca2+ transients (max) at 0 mV in diabetics (0.173±0.021) compared with controls (0.570±0.046) and significant restoration in this inhibition with doxycycline (0.485±0.031) (P<0.05, ANOVA) (Figure 4a-upper).
Figure 4.
Doxycycline restored the depressed intracellular Ca2+ homoeostasis transients in diabetic rat ventricular cardiomyocytes. (a) Intracellular Ca2+ transients measured through Fura-2 under voltage-clamp conditions (upper part) and I–V relationships of ICaL density (lower part) were obtained from control (C; n=35 myocytes from 11 hearts), diabetic (DM; n=33 myocytes from seven hearts) and doxycycline-treated diabetic (Doxy; n=37 myocytes from seven hearts) groups (between −50 and +60 mV with 10 mV increments). (b) Representative ICaL traces (upper part) and Ca2+ transients (lower part) recorded at 0 mV depolarization potential from the experimental groups. (c) Changes of basal fluorescence intensity indicating intracellular basal Ca2+ levels of the groups. Data points on the graphs and bar graphs represent mean± s.e.mean values. *P<0.01 vs control, †P<0.01 vs diabetic, analysis of variance.
The half-decay time (DT50) and time-to-peak amplitude of fluorescence intensity (TP) of the Ca2+ transients (at 0 mV depolarization potential) were significantly longer in cardiomyocytes from diabetics (0.610±0.033 and 0.262±0.009 ms, respectively) than those of the controls (0.492±0.028 and 0.166±0.005 ms). Thus, diabetic cardiomyocytes have depressed contractile properties and decreased [Ca2+]i transients and doxcycyline significantly restored DT50 (0.524±0.025 ms) and TP (0.185±0.007 ms) to normal values.
To characterize the effect of doxycycline on diabetes-induced impairment of the Ca2+ transients in more detail, we analysed the function of L-type Ca2+ channels (ICa) and measured basal Ca2+ levels, thus examining the critical steps in diabetes-induced altered cardiac Ca2+ cycling more comprehensively. ICa was not significantly changed in diabetic cardiomyocytes compared with controls (recorded at all voltages) (Figure 4a-lower). The averaged current densities of ICa (as pA pF−1 at 0 mV) were −10.98±0.66, −9.98±0.92 and −10.51±0.94 for doxycycline-treated, diabetic and control groups, respectively. We also analysed steady-state activation and inactivation of ICa. Neither steady-state activation nor inactivation was significantly different among the three groups (data not shown).
Figure 4b shows examples of the Ca2+ transients and ICa recorded simultaneously in the three groups. Figure 4c shows the averaged data of the basal Ca2+ levels and confirms Ca2+ overload in diabetics compared to the controls. Doxycycline normalized Ca2+ overload in diabetic cardiomyocytes.
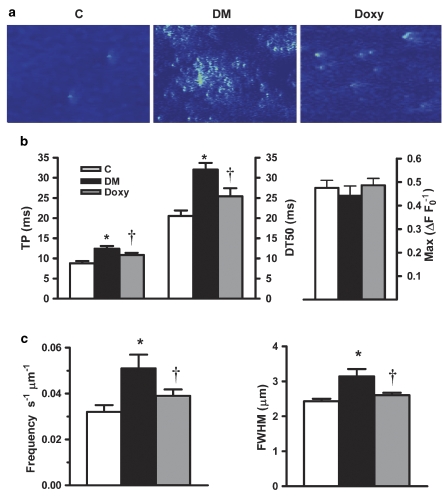
Doxycycline normalized the altered parameters of local Ca2+ release in diabetic cardiomyocytes
To get an insight into the possible effects of doxycycline on cardiac ryanodine receptors (RyR2) activity, we recorded Ca2+ sparks in cardiomyocytes. Figure 5a shows examples of line-scan images recorded in cardiomyocytes from control, diabetic and doxycycline-treated diabetic animals. Quantitative data for Ca2+ spark characteristics are summarized in Figures 5b and c. The maximum peak amplitude of the diabetic group (ΔF F0−1) was not significantly changed by diabetes (Figure 5b) but the time-to-peak amplitude was significantly slower as well as the half-decay time was prolonged (Figure 5b). The spatial spread (sparks width at half maximal amplitude) was significantly higher in diabetic cardiomyocytes with respect to control (Figure 5c). The spontaneous Ca2+ sparks frequency was also elevated in diabetic cardiomyocytes compared with control cells (Figure 5c). Doxycycline caused a near-complete restoration in the prolonged time-to-peak amplitude, the half-decay time, the spontaneous Ca2+ sparks frequency and the sparks width at half maximal amplitude (Figures 5b and c).
Figure 5.
Doxycycline restored diabetes-induced alterations of Ca2+ sparks parameters in cardiomyocytes. (a) Line-scan images with spontaneously arising Ca2+ sparks recorded using Fluo-3AM in representative cardiomyocytes from control (C; n=30 myocytes from six hearts), diabetic (DM; n=27 myocytes from five hearts) and doxycycline-treated diabetic rats (Doxy; n=28 myocytes from five hearts). (b) Averaged spatial and temporal values of Ca2+ sparks. Time-to-peak amplitude (TP; left-hand axis) and half-decay time (DT50; right-hand axis), of Ca2+ sparks were improved by doxycycline. Diabetes itself did not affect peak amplitude (Max; right-hand graph). (c) Left: Comparison of Ca2+ sparks frequency. Right: Spatial distribution of the sparks represented as Ca2+ sparks width at half maximal amplitude (FWHM). Bar graphs represent mean±s.e.mean values. *P<0.01 vs control, †P<0.01 vs diabetic, analysis of variance.
Activity and expression of MMPs in heart homogenates
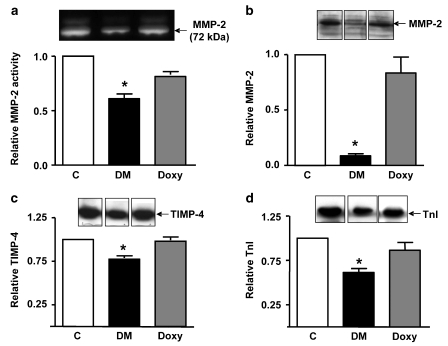
A 72-kDa MMP-2 activity was detected by gelatin zymography in all heart homogenates as verified by comparison with MMP standards from HT1080 cell culture medium, in accordance with earlier observations (Cheung et al., 2000; Wang et al., 2002b). Diabetes caused a significant decrease in myocardial MMP-2 activity (Figure 6a) that reflects its activation by oxidative stress and subsequent release from the heart (Cheung et al., 2000; Schulz, 2007). Doxycycline abolished the loss in MMP-2 activity. MMP-9 activity was not found in any of the heart extracts (data not shown).
Figure 6.
Effect of doxycycline on MMP-2 and troponin I in diabetic hearts. Heart homogenates were assayed for MMP-2 activity by gelatin zymography (a), or MMP-2 (b), TIMP-4 (c) and TnI (d) protein levels by Western blotting. Upper panels show representative gelatin zymogram (a) and Western blots (b–d) bottom panels show the summary of the densitometric analysis. C: control; DM: diabetic; Doxy: doxycycline-treated diabetic. Bar graphs represent mean±s.e.mean values. *P<0.05 vs control (n=3–6), analysis of variance.
Western blot analysis showed that the level of MMP-2 protein in diabetic heart extracts was also significantly reduced and was normalized by doxycycline treatment (Figure 6b). As the balance between MMPs and TIMPs may be impaired in the diabetic heart as we observed earlier in ischemic-reperfused hearts (Schulze et al., 2003) we examined myocardial TIMP-4 levels, which were found to be significantly decreased in diabetic hearts. Treatment with doxycycline reduced the loss of TIMP-4 (Figure 6c).
TnI degradation in diabetic hearts
We have shown earlier that MMP-2 is localized to cardiac sarcomeres and is responsible for TnI degradation, which causes contractile dysfunction in the setting of acute myocardial oxidative stress injury (Wang et al., 2002b). We determined whether doxycycline would alter the degradation of TnI in hearts. Analysis of TnI protein level showed a significant loss of 31 kDa TnI in the diabetic heart extracts, which was prevented by doxycycline (Figure 6d).
Discussion
The major novel result of our study is that doxycycline exerts a pronounced protective action against cardiac dysfunction in STZ-induced diabetic rats. Induction of diabetes caused alterations in mechanical, biochemical and electrical functions of the heart. Inhibition of MMP activity with doxycycline, a property that is distinct from its antibacterial action (Golub et al., 1990, 1998; Schulz, 2007), improved cardiac contractile function, the changes in the repolarization phase of action potentials and restored K+ currents in the diabetic rat heart. Besides improvement in cardiac function, inhibition of MMP activity also prevented the typical diabetes-induced ultrastructural alterations in the cardiac myocytes. Doxycycline treatment also normalized MMP-2, TIMP-4 and TnI levels in the diabetic rat heart.
This study addresses two main aspects of diabetic cardiomyopathy: electrical abnormalities in cardiac function and mechanical dysfunction through effects on both Ca2+ homoeostasis and contractile machinery (Belke et al., 2000; Bell, 2003). It has been shown that high glucose induces superoxide anion production through multiple pathways including xanthine and NAD(P)H oxidases, uncoupled NO synthase, glucose autoxidation and the mitochondrial respiratory chain (Rosen et al., 2001; Evans et al., 2002). Superoxide leads to increased expression of pro-inflammatory cytokines, angiotensin II, endothelin-1 and NAD(P)H oxidases, which in turn generate more superoxide through multiple mechanisms and favours increased expression of iNOS, which increases the generation of NO. The reaction between superoxide and NO forms the highly reactive oxidant, peroxynitrite (Pacher et al., 2007), which induces cell damage through lipid peroxidation, inactivation of several enzymes and damage to many proteins by oxidation/nitration, and activation of downstream pathways including poly (ADP) ribose polymerase, which contributes to diabetic cardiomyopathy (Pacher et al., 2002). Therefore, hyperglycaemia and other disturbances caused by diabetes increases oxidative stress in a variety of tissues including the heart. Endogenous antioxidant defence mechanisms are also impaired and therefore, the balance between pro- and antioxidants is disturbed (Bonnefont-Rousselot et al., 2000).
Diabetes-induced oxidative stress is one of the major modulators of cardiac K+ currents (Rozanski and Xu, 2002; Ayaz et al., 2004) and it has been associated with a reversible increase in the duration of the action potential attributable to a reduced transient outward K+ current (IK1) whereas the L-type Ca2+ current (ICaL) was unaffected (Shimoni et al., 1994; Choi et al., 2002; Yaras et al., 2005). Despite the fact that ICaL is not modified by diabetes, cardiomyocytes from diabetic rats demonstrate significant alterations in Ca2+ homoeostasis (Wold et al., 2006) such as an increase in rise time and half-decay time of Ca2+ transient together with decrease in Ca2+ transient amplitude and sarcoplasmic reticulum Ca2+ load as well as an increase in diastolic Ca2+. In addition, it is known that abnormalities in contractile function have been linked to changes in Ca2+ homoeostasis and contractility (Fein et al., 1980; Shimoni et al., 1994; Ayaz et al., 2004; Ozdemir et al., 2005). ROS/RNS are known to react with some thiol groups of the ryanodine-sensitive receptors and increase Ca2+ release from the sarcoplasmic reticulum through the ryanodine-sensitive receptors (Anzai et al., 1998; Liu et al., 1998).
We have shown earlier that Ca2+ sparks in ventricular cardiomyocytes from STZ-induced diabetic rats are slower and have higher frequency compared with controls, consistent with alterations in Ca2+ handling and cardiac dysfunction (Yaras et al., 2005). Moreover, defective intracellular Ca2+ signalling as well as increased basal Ca2+ and Zn2+ levels in cardiomyocytes from diabetic animals can be normalized with an antioxidant dose of selenium through the restoration of the cell redox state (Ayaz and Turan, 2006). Our results showed that treatment of diabetic rats with doxycycline restored the altered electrical activities, normalized the increased basal Ca2+ level, depressed Ca2+ loading of sarcoplasmic reticulum, and altered parameters of the Ca2+ transients and Ca2+ sparks.
Previous studies have shown that doxycycline also has some ability to inhibit ROS (Akamatsu et al., 1992; Ramamurthy et al., 1993). Ramamurthy et al. (1993) showed that at least 50–100 μM tetracycline was necessary to prevent hypochlorite activation of pro-collagenase, whereas less than 30 μM was sufficient to inhibit MMP activity. Akamatsu et al. (1992) showed that 2 μM doxycycline would inhibit in vitro systems generating superoxide, hydroxyl or hydrogen peroxide by 0, 16 and 22%, respectively. As our dose of 15 mg kg−1 day−1 doxycycline is reported to give approximately 2 μM peak plasma concentration (Lorne Golub, SUNY, Stonybrook NY, USA; personal communication) we suggest that MMP inhibition, as opposed to ROS scavenging, may be an important mechanism of the protective action of doxycycline in this study. Nonetheless, we cannot exclude the possibility that doxycycline may have other protective effects in diabetic cardiomyopathy because of its reported pleiotropic actions (Golub et al., 1998). Further experiments examining other MMP inhibitors, whether doxycycline has any effect on ROS/RNS generation or acts only downstream of these and the full spectrum of proteolytic targets of MMP activation (Schulz, 2007), will be required to resolve this.
MMP-2, which can be activated by oxidative stress (Cheung et al., 2000), not only degrades extracellular matrix proteins, thereby impairing the normal structural support of cardiomyocytes (Nagase et al., 2006), but also specifically targets intracellular substrates such as TnI (Wang et al., 2002b) which contributes to the acute impairment in contractile function following myocardial ischaemia–reperfusion injury. We have shown that reperfusion after ischaemia or exposure of hearts to pro-inflammatory cytokines causes a rapid and significant increase in 72 kDa activity in isolated heart perfusate in conjunction with a decrease of MMP-2 activity and protein in the myocardial tissue (Cheung et al., 2000). Similarly, we observed a clear-cut depletion of MMP-2 in the diabetic heart. In keeping with the loss of MMP-2 from the heart observed here as a result of diabetes, activation of MMP-2 in the heart as a result of oxidative stress in the form of ischaemia–reperfusion injury resulted in enhanced transcription and translation of MMP-2 (Alfonso-Jaume et al., 2006). The time course of this response (seen as early as 30 min of reperfusion following ischaemia) is in keeping with the rapid activation and release of MMP-2 from the heart seen in the first 5 min of reperfusion (Cheung et al., 2000) and is probably intended to replenish stores of MMP-2 lost from the myocyte. Myocardial-specific overexpression of constitutively active MMP-2 results in severe systolic dysfunction, which is accompanied by disruption of the sarcomere, lysis of myofilaments and loss of TnI (Bergman et al., 2007). Consistent with our previous data we also observed a loss of myocardial TIMP-4, the predominant intracellular TIMP in the heart (Greene et al., 1996; Schulze et al., 2003). Doxycycline prevented the activation of MMP-2 and normalized TnI, TIMP-4 and MMP-2 levels in the myocardium. This suggests that the activation of MMP-2 contributes to the development of mechanical dysfunction in the diabetic myocardium.
In this study we have focused on MMP-2 as it is robustly expressed in cardiomyocytes and in all heart cell types. We found no evidence for MMP-9 by zymography or immunoblot, in accordance with earlier studies in rat hearts including those exposed to pro-inflammatory cytokines or endotoxin (Cheung et al., 2000; Lalu et al., 2004). Doxycycline preferentially inhibits MMP-2, -9 and -8 activities and is a much weaker inhibitor of MMP-1; it does not inhibit MMP-3 or MMP-7 (Nip et al., 1993; Golub et al., 1998; Smith et al., 1999; Kivela-Rajamaki et al., 2003). Interestingly, doxycycline has a unique property in that it rapidly accumulates in cardiac myocytes (Modrak and Rovang, 1981). On account of its inhibitory profile on MMPs, we can only suggest that MMP-2 is likely to be an important inhibitory target of doxycycline in the heart, especially in terms of its intracellular actions to prevent cleavage of TnI. We cannot, however, exclude possible effects of doxycycline on other MMPs, or other proteolytic targets.
In conclusion, diabetes results in the activation of MMP-2 and loss of TIMP-4 in rat heart, which results in significant degradation of TnI in the heart tissue. Although doxycycline did not significantly alter hyperglycaemia, it normalized cardiac dysfunction of diabetic rat heart through restoring the altered parameters of Ca2+ homoeostasis. Therefore, doxycycline may prevent diabetic cardiomyopathy because of its salutary effects on both mechanical and electrical heart function. Although cardiomyopathy is a complicated disorder, and several factors have been associated with its development, we conclude that MMPs are at least in part involved in the development of diabetes-induced cardiac dysfunction.
Acknowledgments
We thank Dr B Can for assistance with electron microscopy. This study has been supported by grants from Ankara University (2006-080-9233; 2003-080-9120); State Planning Organization (DPT, 2003K120-9025-6); TUBITAK-SBAG-107S304, -SBAG-107S427 to BT and by the Canadian Institutes of Health Research (FRN 66953) to RS. MS was supported by a trainee travel stipend provided by the Heart and Stroke Foundation of Alberta, NWT and Nunavut. RS is an Alberta Heritage Foundation for Medical Research Scientist.
Abbreviations
- ±dP/dt
rates of changes in developed pressure
- APD
action potential duration
- DT50
half-decay time
- F
fluorescence intensity
- ICa
Ca2+ currents
- IK1
inward rectifier K+ current
- Iss
steady-state outward K+ current
- Ito
transient outward K+ current
- LVDP
left ventricular developed pressure
- LVEDP
left ventricular end diastolic pressure
- MMP
matrix metalloproteinase
- ROS/RNS
reactive oxygen/nitrogen species
- STZ
streptozotocin
- TIMP
tissue inhibitor of metalloproteinase
- TnI
troponin I
- TP
time-to-peak amplitude
Conflict of interest
The authors state no conflict of interest.
References
- Akamatsu H, Asada M, Komura J, Asada Y, Niwa Y. Effect of doxycycline on the generation of reactive oxygen species: a possible mechanism of action of acne therapy with doxycycline. Acta Derm Venereol. 1992;72:178–179. [PubMed] [Google Scholar]
- Alfonso-Jaume MA, Bergman MR, Mahimkar R, Cheng S, Jin ZQ, Karliner JS, et al. Cardiac ischemia-reperfusion injury induces matrix metalloproteinase-2 expression through the AP-1 components FosB and JunB. Am J Physiol Heart Circ Physiol. 2006;291:H1838–H1846. doi: 10.1152/ajpheart.00026.2006. [DOI] [PubMed] [Google Scholar]
- Anzai K, Ogawa K, Kuniyasu A, Ozawa T, Yamamoto H, Nakayama H. Effects of hydroxyl radical and sulfhydryl reagents on the open probability of the purified cardiac ryanodine receptor channel incorporated into planar lipid bilayers. Biochem Biophys Res Commun. 1998;249:938–942. doi: 10.1006/bbrc.1998.9244. [DOI] [PubMed] [Google Scholar]
- Ayaz M, Ozdemir S, Ugur M, Vassort G, Turan B. Effects of selenium on altered mechanical and electrical cardiac activities of diabetic rat. Arch Biochem Biophys. 2004;426:83–90. doi: 10.1016/j.abb.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Ayaz M, Turan B. Selenium prevents diabetes-induced alterations in [Zn2+]i and metallothionein level of rat heart via restoration of cell redox cycle. Am J Physiol Heart Circ Physiol. 2006;290:H1071–H1080. doi: 10.1152/ajpheart.00754.2005. [DOI] [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- Bell DS. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–2951. doi: 10.2337/diacare.26.10.2949. [DOI] [PubMed] [Google Scholar]
- Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, Nguyen A, et al. Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1847–H1860. doi: 10.1152/ajpheart.00434.2006. [DOI] [PubMed] [Google Scholar]
- Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000;26:163–176. [PubMed] [Google Scholar]
- Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Quagliaro L, D'Amico M, Di Filippo C, Marfella R, Nappo F, et al. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002;51:1076–1082. doi: 10.2337/diabetes.51.4.1076. [DOI] [PubMed] [Google Scholar]
- Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, et al. Defective intracellular Ca2+ signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1398–H1408. doi: 10.1152/ajpheart.00313.2002. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Fein FS, Kornstein LB, Strobeck JE, Capasso JM, Sonnenblick EH. Altered myocardial mechanics in diabetic rats. Circ Res. 1980;47:922–933. doi: 10.1161/01.res.47.6.922. [DOI] [PubMed] [Google Scholar]
- Golub LM, Ciancio S, Ramamamurthy NS, Leung M, McNamara TF. Low-dose doxycycline therapy: effect on gingival and crevicular fluid collagenase activity in humans. J Periodontal Res. 1990;25:321–330. doi: 10.1111/j.1600-0765.1990.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271:30375–30380. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- Kivela-Rajamaki M, Maisi P, Srinivas R, Tervahartiala T, Teronen O, Husa V, et al. Levels and molecular forms of MMP-7 (matrilysin-1) and MMP-8 (collagenase-2) in diseased human peri-implant sulcular fluid. J Periodontal Res. 2003;38:583–590. doi: 10.1034/j.1600-0765.2003.00688.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalu MM, Csont T, Schulz R. Matrix metalloproteinase activities are altered in the heart and plasma during endotoxemia. Crit Care Med. 2004;32:1332–1337. doi: 10.1097/01.ccm.0000127778.16609.ec. [DOI] [PubMed] [Google Scholar]
- Lemaitre V, D'Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo Today. 2006;78:1–10. doi: 10.1002/bdrc.20065. [DOI] [PubMed] [Google Scholar]
- Liu W, Pasek DA, Meissner G. Modulation of Ca2+-gated cardiac muscle Ca2+-release channel (ryanodine receptor) by mono- and divalent ions. Am J Physiol. 1998;274:C120–C128. doi: 10.1152/ajpcell.1998.274.1.C120. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ramamurthy N, Marecek J, Lee HM, Chen JL, Ryan ME, et al. The lipophilicity, pharmacokinetics, and cellular uptake of different chemically-modified tetracyclines (CMTs) Curr Med Chem. 2001;8:243–252. doi: 10.2174/0929867013373525. [DOI] [PubMed] [Google Scholar]
- Modrak JB, Rovang KS. Estimation of infarct size by determination of myocardial 3H-tetracycline accumulation in the coronary ligated rat. Res Commun Chem Pathol Pharmacol. 1981;34:149–152. [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nip LH, Uitto VJ, Golub LM. Inhibition of epithelial cell matrix metalloproteinases by tetracyclines. J Periodontal Res. 1993;28:379–385. doi: 10.1111/j.1600-0765.1993.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- Ozdemir S, Ugur M, Gurdal H, Turan B. Treatment with AT(1) receptor blocker restores diabetes-induced alterations in intracellular Ca2+ transients and contractile function of rat myocardium. Arch Biochem Biophys. 2005;435:166–174. doi: 10.1016/j.abb.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- Pacher P, Ungvari Z, Nanasi PP, Kecskemeti V. Electrophysiological changes in rat ventricular and atrial myocardium at different stages of experimental diabetes. Acta Physiol Scand. 1999;166:7–13. doi: 10.1046/j.1365-201x.1999.00538.x. [DOI] [PubMed] [Google Scholar]
- Ramamurthy NS, Vernillo AT, Greenwald RA, Lee HM, Sorsa T, Golub LM, et al. Reactive oxygen species activate and tetracyclines inhibit rat osteoblast collagenase. J Bone Miner Res. 1993;8:1247–1253. doi: 10.1002/jbmr.5650081013. [DOI] [PubMed] [Google Scholar]
- Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- Rozanski GJ, Xu Z. Sulfhydryl modulation of K+ channels in rat ventricular myocytes. J Mol Cell Cardiol. 2002;34:1623–1632. doi: 10.1006/jmcc.2002.2112. [DOI] [PubMed] [Google Scholar]
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- Sawicki G, Sanders EJ, Salas E, Wozniak M, Rodrigo J, Radomski MW. Localization and translocation of MMP-2 during aggregation of human platelets. Thromb Haemost. 1998;80:836–839. [PubMed] [Google Scholar]
- Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- Schulze CJ, Wang W, Suarez-Pinzon WL, Sawicka J, Sawicki G, Schulz R. Imbalance between tissue inhibitor of metalloproteinase-4 and matrix metalloproteinases during acute myocardial ischemia-reperfusion injury. Circulation. 2003;107:2487–2492. doi: 10.1161/01.CIR.0000065603.09430.58. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Firek L, Severson D, Giles W. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res. 1994;74:620–628. doi: 10.1161/01.res.74.4.620. [DOI] [PubMed] [Google Scholar]
- Smith GN, Jr, Mickler EA, Hasty KA, Brandt KD. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: relationship to structure of the enzyme. Arthritis Rheum. 1999;42:1140–1146. doi: 10.1002/1529-0131(199906)42:6<1140::AID-ANR10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tuncay E, Seymen AA, Tanriverdi E, Yaras N, Tandogan B, Ulusu NN, et al. Gender related differential effects of Omega-3E treatment on diabetes-induced left ventricular dysfunction. Mol Cell Biochem. 2007;304:255–263. doi: 10.1007/s11010-007-9508-4. [DOI] [PubMed] [Google Scholar]
- Ulusu NN, Turan B. Beneficial effects of selenium on some enzymes of diabetic rat heart. Biol Trace Elem Res. 2005;103:207–216. doi: 10.1385/BTER:103:3:207. [DOI] [PubMed] [Google Scholar]
- Wang W, Sawicki G, Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res. 2002a;53:165–174. doi: 10.1016/s0008-6363(01)00445-x. [DOI] [PubMed] [Google Scholar]
- Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002b;106:1543–1549. doi: 10.1161/01.cir.0000028818.33488.7b. [DOI] [PubMed] [Google Scholar]
- Wold LE, Ceylan-Isik AF, Fang CX, Yang X, Li SY, Sreejayan N, et al. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med. 2006;40:1419–1429. doi: 10.1016/j.freeradbiomed.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Yaras N, Ugur M, Ozdemir S, Gurdal H, Purali N, Lacampagne A, et al. Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes. 2005;54:3082–3088. doi: 10.2337/diabetes.54.11.3082. [DOI] [PubMed] [Google Scholar]