Abstract
Background and purpose:
The slow delayed rectifier K+ current (IKs) contributes to ventricular repolarization when the action potential (AP) is prolonged. IKs block during drug-induced AP prolongation may promote Torsades de Pointes (TdP), but whether this is due to additional AP prolongation is uncertain.
Experimental approach:
In bradycardic perfused rabbit ventricles, the incidence of spontaneous TdP, monophasic AP duration at 90% repolarization (MAPD90) and ECG interval between the peak and the end of T wave (Tpeak−end) (index of dispersion of repolarization) were measured after the administration of veratridine (125 nM, slows Na+ channel inactivation), dofetilide (7.5 or 10 nM, a rapid delayed rectifier blocker) and HMR 1556 (HMR, 100 nM, an IKs blocker), alone or in combinations (n=6 each).
Key results:
HMR did not prolong MAPD90, whereas veratridine or 7.5 nM dofetilide prolonged MAPD90 (P<0.01) without inducing TdP. Veratridine+7.5 nM dofetilide additively prolonged MAPD90 (P<0.05), induced 4±6 TdP per heart and prolonged Tpeak−end by 12±10 ms. Subsequent addition of HMR did not further prolonged MAPD90, but increased the number of TdP to 22±18 per heart and increased Tpeak−end by 39±21 ms (P<0.05). Increasing dofetilide concentration from 7.5 to 10 nM (added to veratridine) produced a longer MAPD90, but fewer TdP (5±5 per heart) and less Tpeak−end prolongation (17±8 ms) compared to the veratridine+7.5 nM dofetilide+HMR group (P<0.05).
Conclusions and implications:
Adding IKs block markedly increases TdP incidence in hearts predisposed to TdP development by increasing the dispersion of repolarization, but without additional AP prolongation.
Keywords: Torsades de Pointes, action potential prolongation, dispersion of repolarization, IKs, HMR 1556, dofetilide, veratridine, perfused rabbit ventricles
Introduction
The balance of inward and outward ionic currents of the heart is crucial in forming a normal cardiac action potential (AP). The delayed rectifier K+ current (IK) is the key outward current for cardiac repolarization and is composed of rapid (IKr) and slow (IKs) components (Sanguinetti and Jurkiewicz, 1990). The presence of both components is postulated to provide some redundancy or ‘repolarization reserve', thereby preventing excessive prolongation of repolarization (Roden, 2006).
Torsades de Pointes (TdP) is a serious polymorphic ventricular tachycardia caused by reductions in repolarizing currents or increases in depolarizing currents, resulting in prolonged AP duration (APD). Reductions in IKr owing to genetic mutations can lead to congenital long QT-2 syndrome and TdP, and this subtype accounts for 39% of the total long QT syndrome cases (Napolitano et al., 2005). Nevertheless, IKr blockers, which prolong cardiac refractoriness, are used clinically to treat atrial and ventricular tachyarrhythmias, but they can sometimes induce TdP. IKr blockade is also responsible for the increased incidence of TdP and unexpected death associated with non-cardiac drugs, such as certain antihistamines and antimicrobials (Woosley et al., 1993; Bischoff et al., 2000; Heist and Ruskin, 2005).
Unlike IKr, IKs contributes to ventricular repolarization as a function of heart rate and the extent of β-adrenoceptor stimulation (Marx et al., 2002; Silva and Rudy, 2005). IKs blockade produces little AP prolongation in rabbit, canine and human ventricles (Biliczki et al., 2002; Jost et al., 2005; So et al., 2006), unless it is in the presence of catecholamines (Jost et al., 2005; So et al., 2007). Moreover, IKs acts as a ‘repolarization reserve' (Roden, 2006), and its blockade further prolongs the AP when repolarizing currents are reduced or when depolarizing currents are increased (Biliczki et al., 2002; Nakashima et al., 2004; Jost et al., 2005; So et al., 2005, 2006). Long QT-1 syndrome caused by genetic mutation of IKs is the most common subtype of long QT syndrome (49% of the total long QT syndrome cases) (Napolitano et al., 2005). Although long QT-1 patients usually develop TdP owing to inability to shorten the QT interval during β-adrenoceptor stimulation (for example, during exercise), previous studies have shown that adding IKs block to IKr block increased the incidence of TdP (Lengyel et al., 2007; Michael et al., 2007), indicating that IKs may provide ‘repolarization reserve' against drug-induced TdP. It has been proposed that variability in IKs expression among individuals may lead to differences in the extent of ‘repolarization reserve', which may not be reflected by the resting QT interval (Roden, 2006). This may explain the variable susceptibility to develop drug-induced TdP in individuals with similar baseline QT interval.
Although previous findings suggest that IKs block can be proarrhythmic in the presence of drug-induced AP prolongation (Lengyel et al., 2007; Michael et al., 2007), it is unclear whether it was caused by additional QT prolongation or other arrhythmogenic mechanisms, such as increase in dispersion of ventricular repolarization. IKs is heterogeneously expressed across the ventricular layers, with the mid-myocardium expressing the least IKs compared with the epicardium and endocardium (Liu and Antzelevitch, 1995). Adding IKs block to IKr block has been shown to increase dispersion of ventricular repolarization, a condition that promotes re-entrant tachyarrhythmias (Burashnikov and Antzelevitch, 2002).
To investigate the mechanisms by which IKs blockade promote TdP, we have established a proarrhythmic perfused rabbit heart model through the combination of IKr block and slowing of sodium channel (INa) inactivation, in the presence or absence of IKs block. This study investigated whether adding IKs block to drug-induced AP prolongation promotes TdP, and whether it is owing to additional AP prolongation or increase in dispersion of ventricular repolarization.
Methods
Langendorff perfused rabbit hearts
All animal procedures were in accordance with the UK Animals Act 1986 for scientific procedures and approved by the Animal Care Committee of St Michael's Hospital. All animals were maintained with standard food, water and light/dark cycles according to the ethical guidelines. Hearts isolated from 42 New Zealand White rabbits (3.0–4.0 kg, male) were perfused at constant pressure using the Langendorff method as previously described (So et al., 2006).
Electrophysiological studies
To facilitate the development of drug-induced TdP at slow ventricular rates, the atrioventricular node was ablated by crushing with forceps. To determine the rate-corrected monophasic AP (MAP) duration, the ventricles were paced at a cycle length (CL) of 500 ms using a 7F quadripolar contact Ag-AgCl MAP catheter (EP Technologies Inc., Sunnyvale, CA, USA) and a programmable stimulator (Medtronic 5325, Minneapolis, MN, USA) with 2 ms pulse width and twice the diastolic threshold. The MAP duration at 90% repolarization (MAPD90) and QT interval were measured after a 20 s conditioning train at CL of 500 ms. As drug-induced changes in conduction might affect the inducibility of proarrhythmic events, QRS duration at pacing CL of 500 ms was measured from the left ventricular (LV) epicardial bipolar electrogram and did not change in any group.
Spontaneous TdP or R on T premature beats were measured during ventricular escape rhythm without pacing for 10 min. Spontaneous TdP was defined as at least four consecutive beats of polymorphic QRS complexes (including the first beat following a pause), with an initiating beat falling on the T wave of the preceding beat after a pause (‘pause-dependent' initiation). Spontaneous ‘R on T premature beats' were defined as any premature beats whose onset occurred before the MAP of the preceding beat had recovered back to the baseline, but did not lead to the initiation of spontaneous TdP.
An increase in dispersion of repolarization is a known risk factor for TdP development. The interval between the peak and the end of the T wave (Tpeak−end) is a proposed surrogate measure of dispersion of ventricular repolarization (Yan and Antzelevitch, 1998). Tpeak−end was measured from the unipolar LV electrogram during pacing at CL of 500 ms. Instability of ventricular repolarization is another predictor of TdP development, which can be reflected by beat-to-beat variability in AP (Hondeghem et al., 2001; Thomsen et al., 2006). The standard deviation of 10 consecutive MAPD90 during pacing at CL of 500 ms was measured as an index of beat-to-beat variability of ventricular repolarization.
Drug administration
Untreated control experiments were performed to assess the electrophysiological stability of the preparation over time (n=6). The effects of a selective IKs blocker HMR 1556 (HMR, Sanofi-Aventis, Frankfurt am Main, Germany), veratridine (Sigma-Aldrich, St Louis, MO, USA), which slows INa inactivation or an IKr blocker dofetilide (Pfizer Canada Inc, Quebec, Canada) were studied alone or in combination. The single drug groups included HMR (100 nM), veratridine (125 nM) and dofetilide (7.5 nM) (n=6 each). The combined drug groups included 125 nM veratridine+7.5 nM dofetilide, 125 nM veratridine+7.5 nM dofetilide+100 nM HMR and 125 nM veratridine+10 nM dofetilide (n=6 each). After baseline measurements were complete, the above drugs and their combinations were administered to the circulating perfusate, and a 15 min equilibration time was allowed before any measurements. HMR was dissolved in dimethylsulphoxide as a stock solution of 16 mM. Veratridine was dissolved in dimethylsulphoxide as a stock solution of 24.7 mM. The highest dimethylsulphoxide concentration in the perfusate was <0.001%. Dofetilide was dissolved in saline as a stock solution of 22.7 μM.
Coronary flow rate and LV systolic pressure measurement
As any drug-induced alterations in cardiac hemodynamics and contractility might affect the inducibility of proarrhythmic events, coronary flow rate and LV systolic pressure were continuously monitored. Coronary flow rate (mL min−1) was determined 15 min after each drug administration, by measuring the volume of perfusate flowing out of the heart per minute. The LV systolic pressure was measured as the peak pressure generated when the ventricles were paced at CL of 500 ms. There were no differences in the coronary flow rate and LV systolic pressure between the untreated control group and all the treatment groups.
Data analysis and statistics
The normality of data was tested using the Kolmogorov–Smirnov test. As the data passed the normality test, parametric tests were used and the results are expressed as mean±s.d. Student's unpaired t-test was used to compare results of two different groups. Multiple group comparisons were performed by one-way ANOVA. Bonferroni post hoc corrections were performed when P-values were <0.05, which was considered statistically significant. Correlation analysis was performed using the Pearson's correlation. All statistical analyses and curve-fitting were performed using GraphPad Prism version 4.01 for Windows (GraphPad Software, San Diego, CA, USA).
Results
To investigate the protective function of IKs in preventing TdP development when AP is substantially prolonged, we perfused rabbit hearts with veratridine (which slows INa inactivation), the IKr blocker dofetilide and the IKs blocker HMR, alone or in combination. Representative MAP tracings obtained in untreated control experiments and after the individual administration of 100 nM HMR, 125 nM veratridine or 7.5 nM dofetilide are shown in Figure 1a. Control hearts had no change in MAPD90 over time (P=0.25, n=6). The IKs blocker HMR alone also had no effect on MAPD90 (P=0.63, n=6, Figure 1b). In contrast, administration of veratridine or dofetilide alone significantly prolonged MAPD90 (P<0.01, n=6, Figure 1b). No proarrhythmic events were observed when HMR, veratridine or dofetilide were administered alone.
Figure 1.
(a) Representative monophasic action potential (MAP) recordings in perfused rabbit hearts during baseline and after the individual administration of HMR 1556 (100 nM), veratridine (125 nM) or dofetilide (7.5 nM) at a pacing cycle length (CL) of 500 ms. Endocardial MAP signals were obtained from the left ventricular apex. Amplitude of the MAP recordings was rescaled for the figure. The variability in MAP morphology and amplitude can be caused by changes in contact pressure between the MAP catheter and tissue, and do not necessarily reflect changes in specific ionic currents. Such variability is consistent with the known reported feature of MAP (Franz, 1999). (b) Individual effects of HMR 1556, veratridine or dofetilide on MAP duration at 90% repolarization (MAPD90) at pacing CL of 500 ms. Mean±s.d., n=6. **P<0.01 vs untreated control.
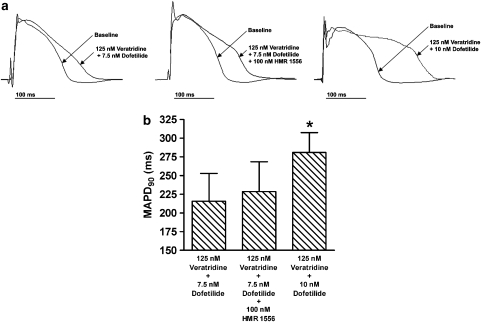
As our previous studies indicate that adding IKs block to either dofetilide or veratridine produced a modest APD prolongation without TdP induction in perfused rabbit hearts (So et al., 2005, 2006), we combined veratridine and dofetilide in an attempt to achieve greater APD prolongation. As expected, veratridine (125 nM) and dofetilide (7.5 nM) additively prolonged MAPD90 (P<0.05, n=6) compared with 125 nM veratridine or 7.5 nM dofetilide alone (Figure 2). Moreover, premature beats occurred and interrupted the pacing sequence. This occasionally caused the pacing stimulus to be applied on the T wave of the preceding premature beat (‘stimulus on T'), sometimes leading to pacing-induced TdP. As the incidence of TdP during pacing was dependent on the occurrence of premature beats and the number of ‘stimulus on T' events, we measured the incidence of TdP in spontaneously beating hearts without pacing. The intrinsic heartbeats originate from slow ventricular escape rhythm, as the atrioventricular node had been ablated (see Methods). The spontaneous heart rate was 41±13 beats per minute in the 125 nM veratridine+7.5 nM dofetilide group and was not different from the other combined treatment groups (P=0.19, n=6).
Figure 2.
(a) Representative monophasic action potential (MAP) recordings during baseline and after the combined administration of veratridine (125 nM), dofetilide (7.5 or 10 nM) and with or without HMR 1556 (100 nM) in perfused rabbit hearts. Endocardial MAP signals were obtained from the left ventricular apex. Amplitude of the MAP recordings was rescaled for the figure. The variability in MAP morphology and amplitude can be caused by changes in contact pressure between the MAP catheter and tissue, and do not necessarily reflect changes in specific ionic currents. Such variability is consistent with the known reported feature of MAP (Franz, 1999). (b) Effects of combined administration of veratridine (125 nM), dofetilide (7.5 or 10 nM) and with or without HMR 1556 (100 nM) on MAP duration at 90% repolarization (MAPD90) at pacing cycle length of 500 ms. Mean±s.d., n=6. *P<0.05 vs 125 nM veratridine+7.5 nM dofetilide or 125 nM veratridine+7.5 nM dofetlide+100 nM HMR 1556.
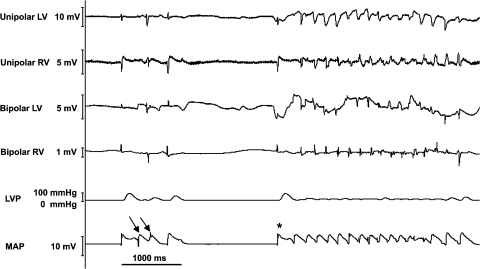
A representative episode of spontaneous TdP and R on T premature beats after the administration of 125 nM veratridine and 7.5 nM dofetilide is illustrated in Figure 3. Although further administration of HMR to this combination increased both the number of spontaneous TdP episodes (P<0.05) and spontaneous R on T premature beats (P<0.05; Table 1), the additional MAPD90 prolongation is not significant when compared with 125 nM veratridine+7.5 nM dofetilide (P=0.58, Figure 2).
Figure 3.
Representative episode of spontaneous Torsades de Pointes (TdP, indicated by *) and spontaneous R on T premature beat (indicated by arrow) in perfused rabbit hearts after the administration of 125 nM veratridine+7.5 nM dofetilide. Unipolar and bipolar left ventricular (LV) and right ventricular (RV) electrograms were obtained from the epicardial surface, and left ventricular pressure (LVP) was obtained by inserting a fluid-filled balloon into the left ventricle. Monophasic action potential (MAP) signal was obtained from the left ventricular apex.
Table 1.
Number of spontaneous Torsades de Pointes episodes and R on T premature beats in perfused rabbit ventricles
|
Torsades de Pointes episodes |
R on T premature beats |
|||||
|---|---|---|---|---|---|---|
| V+7.5D | V+7.5D+H | V+10D | V+7.5D | V+7.5D+H | V+10D | |
| Mean±s.d. | 4±6 | 22±18† | 5±5 | 11±16 | 55±45‡ | 21±16 |
| Median (range) | 2 (0–16) | 20 (0–45) | 3 (0–12) | 3 (0–37) | 56 (1–119) | 25 (0–45) |
| No. of heartsa | 4 in 6 | 5 in 6 | 5 in 6 | 4 in 6 | 6 in 6 | 5 in 6 |
Number of hearts with at least one episode of TdP or R on T premature beats.
V=125 nM veratridine; 7.5D=7.5 nM dofetilide; 10D=10 nM dofetilide; H=100 nM HMR 1556.
†P<0.05 vs V+7.5D or V+10D; ‡P<0.05 vs V+7.5D.
To investigate if a comparable amount of TdP could be induced by further reduction in IKr instead of blocking IKs (that is, to study the specificity of IKs in protecting against TdP development), we examined the incidence of spontaneous TdP in hearts treated with a higher concentration of dofetilide (10 nM) in the presence of 125 nM veratridine. As shown in Figure 2, despite substantial further prolongation of MAPD90 (P<0.05, n=6) from 216±37 to 281±27 ms when the concentration of dofetilide was increased from 7.5 to 10 nM (added to 125 nM veratridine), no further increase in the number of TdP episodes per heart (P=0.73) or spontaneous R on T premature beats per heart was observed (P=0.32, Table 1). Despite a longer MAPD90 (P<0.05) in hearts treated with veratridine+10 nM dofetilide than in those treated with veratridine+7.5 nM dofetilide+HMR (Figure 2), fewer spontaneous TdP episodes (P<0.05) were observed in the group without HMR than with HMR (Table 1). The mean number of spontaneous R on T premature beats was also less in the group without HMR than with HMR, although the difference was not statistically significant (P=0.12).
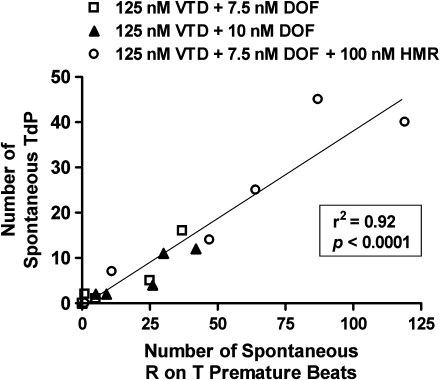
As spontaneous TdP and R on T premature beats may originate from a common cause (for example, early afterdepolarizations), we predicted that the greater the number of R on T premature beats, the higher would be the TdP incidence. There was a strong positive correlation (R2=0.92, P<0.0001, n=18) between the number of spontaneous TdP episodes and the number of R on T premature beats (Figure 4), and that both types of proarrhythmic events were proportionately increased in hearts treated with HMR (in the presence of veratridine and dofetilide). There were no differences in the mean duration of TdP (P=0.17), the pause duration preceding the onset of TdP (P=0.31) or the morphology of TdP between the three treatment groups.
Figure 4.
Correlation analysis between the number of spontaneous Torsades de Pointes (TdP) and R on T premature beats after combined administration of veratridine (VTD, 125 nM), dofetilide (DOF, 7.5 or 10 nM) and with or without HMR 1556 (HMR, 100 nM) in perfused rabbit hearts (Pearson's R2=0.92, P<0.0001). The atrioventricular nodes of the rabbit hearts were ablated to generate slow ventricular escape rhythm and the number of spontaneous TdP and R on T premature beats were measured without pacing for 10 min.
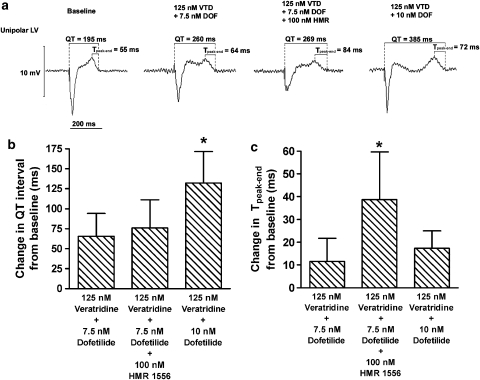
As dispersion of ventricular repolarization is a predictor of TdP, we measured the baseline and post-treatment Tpeak−end interval, which is a proposed surrogate measure of dispersion of ventricular repolarization (Yan and Antzelevitch, 1998). We also measured the QT interval at pacing CL of 500 ms. Figure 5a illustrates the representative LV unipolar electrograms showing the QT and Tpeak−end interval during baseline and after drug administration. The baseline QT interval of 199±19 ms was not different among the three combined treatment groups. As observed with MAPD90, addition of 100 nM HMR to 125 nM veratridine+7.5 nM dofetilide did not produce a significant additional QT prolongation, whereas increasing the concentration of dofetilide from 7.5 to 10 nM (in the presence of veratridine) further prolonged the QT interval (P<0.05, n=6; Figure 5b). The baseline Tpeak−end of 51±10 ms was not different among the three combined treatment groups. However, the increase in Tpeak−end interval was greater (P<0.05, n=6) in the group treated with HMR compared with the other two groups without HMR (Figure 5c), indicating that adding IKs blocks significantly increased the dispersion of ventricular repolarization. There was a weak but significant correlation between the number of spontaneous TdP and the change in Tpeak−end from baseline (R2=0.30, P<0.05, n=18).
Figure 5.
(a) Representative unipolar left ventricular (LV) electrograms during baseline and after the combined administration of veratridine (VTD, 125 nM), dofetilide (DOF, 7.5 or 10 nM) and with or without HMR 1556 (HMR, 100 nM) in perfused rabbit hearts. The QT interval and the interval between the peak and the end of T wave (Tpeak−end) were measured, the later being a proposed index of dispersion of ventricular repolarization. (b) Summary data on the change in QT interval from baseline. (c) Summary data on the change in Tpeak−end from baseline. The baseline QT interval of 199±19 ms and the baseline Tpeak−end of 51±10 ms were not different among the three groups. QT and Tpeak−end were measured from the unipolar left ventricular electrogram during pacing at cycle length of 500 ms. Mean±s.d., n=6. *P<0.05 vs veratridine+7.5 nM dofetilide or veratridine+10 nM dofetilide.
As beat-to-beat instability in ventricular repolarization is another predictor of TdP (Hondeghem et al., 2001; Thomsen et al., 2006), we also assessed the stability of repolarization by measuring the s.d. of 10 consecutive measurements of MAPD90 during pacing at a CL of 500 ms. As shown in Figure 6a, the beat-to-beat variability in MAPD90 is greater in the veratridine+7.5 nM dofetilide+HMR group compared with the veratridine+7.5 nM dofetilide group (3.3±3.6 vs 0.3±0.3 ms, respectively, P<0.05, n=6), but not statistically significantly different from the veratridine+10 nM dofetilide group (1.0±0.8, P=0.18, n=6). The pattern of beat-to-beat variability appeared to occur in a random manner, as illustrated by Figure 6b, which shows a representative plot of individual MAPD90 values vs the beat number in a rabbit heart treated with veratridine+7.5 nM dofetilide+100 nM HMR 1556.
Figure 6.
Effects of combined administration of veratridine (125 nM), dofetilide (7.5 or 10 nM) and with or without HMR 1556 (100 nM) on the beat-to-beat variability of ventricular repolarization during pacing at cycle length (CL) of 500 ms. (a) The beat-to-beat variability was measured as the s.d. of 10 consecutive monophasic action potential (MAP) duration at 90% repolarization (MAPD90) during pacing CL of 500 ms. (b) A representative plot of individual MAPD90 values vs the beat number in a rabbit heart treated with veratridine+7.5 nM dofetilide+100 nM HMR 1556. Mean±s.d., n=6. *P<0.05 vs veratridine+7.5 nM dofetilide.
Discussion
This study demonstrates that adding IKs block in the presence of drug-induced AP prolongation promotes TdP in perfused rabbit hearts, but it was unlikely to be caused by additional APD prolongation. Fewer TdP were observed when APD was prolonged to a greater extent by increasing IKr block than with IKs block, indicating that there is a dissociation between the extent of drug-induced AP prolongation and incidence of TdP. Adding IKs block promotes proarrhythmia by increasing the dispersion and beat-to-beat variability in ventricular repolarization during drug-induced AP prolongation.
Reductions in IKs induced by HMR 1556 had no effect on APD when administered alone (Figure 1) and did not induce TdP in rabbit ventricles, which agrees with previous studies showing that i.v. administration of HMR 1556 alone produced little QT prolongation and did not induce TdP in anaesthetized rabbits (Lengyel et al., 2007; Michael et al., 2007; So et al., 2007). Drug-induced AP prolongation (for example, IKr block) prolongs the duration during which the membrane stays at a voltage range which allows IKs to activate, especially at slow heart rates (So et al., 2006). IKs acts as an important ‘repolarization reserve' that activates and prevents excessive AP prolongation (Biliczki et al., 2002; Nakashima et al., 2004; Jost et al., 2005; So et al., 2006). However, it is unclear whether the proarrhythmic effect of IKs block during drug-induced AP prolongation is caused by additional AP prolongation or other arrhythmogenic mechanisms, such as increase in dispersion of repolarization. If adding the IKs blocker further increased APD prolongation in the presence of IKr block plus slowing of INa inactivation, one would clearly expect that the incidence of TdP would be increased. However, adding the IKs blocker did not cause further APD prolongation; thus, the incidence of TdP would not be expected to increase if additional APD prolongation is a sine qua non for increase in TdP occurrence. Therefore, the ability of IKs to prevent TdP development may not be solely related to its ability to limit drug-induced AP prolongation, as a longer AP produced by increasing IKr block (from 7.5 to 10 nM dofetilide in the presence of veratridine) produces less TdP compared with the hearts treated with the IKs blocker (Figure 2 and Table 1). There is, thus, a dissociation between the extent of drug-induced AP prolongation and TdP incidence, and other arrhythmogenic mechanisms may be responsible for the proarrhythmic effect of IKs block. In a recent study by Lengyel et al. (2007), adding IKs block to IKr block produced an additional corrected QT prolongation by 8% in rabbits in vivo, and the combined IKr and IKs block elevated the incidence of TdP (82%) compared with either IKr block (28%) or IKs block alone (0%). The threefold increase in TdP (from 28 to 82%) is unlikely to be caused by the relatively small increase in corrected QT interval alone, suggesting that other proarrhythmic mechanisms of IKs blockade may be responsible. By contrast, under our experimental conditions, which involve previous blockade of IKr and slowing of INa inactivation, IKs block did not produce a significant additional AP prolongation. However, adding IKs block markedly increased the number of TdP episodes, from a median of 2 to 20 episodes per heart (Table 1). Our data suggest that IKs block promotes TdP out of proportion to its AP prolonging effect, and that there is not a simple linear relationship between the incidence of TdP and the absolute amount of AP prolongation. Hondeghem (2007) has proposed that QT prolongation itself is antiarrhythmic, but it is usually associated with other proarrhythmic indices, such as triangulation of the AP, reverse use dependence, AP instability and dispersion of repolarization (known as TRIaD); QT prolongation appears to account for only some of the phenomena associated with TdP.
In the combined drug studies, we increased the concentration of dofetilide from 7.5 to 10 nM in an attempt to produce a small increment in APD. However, we observed that MAPD90 prolonged by ∼30%, indicating that the concentration range was likely at the very steep portion of the concentration–response curve. The large magnitude of response may also be owing to the synergistic effects of veratridine and dofetilide. Veratridine+10 nM dofetilide was able to produce a much longer MAPD90, but nevertheless induced less TdP compared with the HMR 1556-treated group (Figures 2 and Table 1).
Why would adding IKs block to veratridine and 7.5 nM dofetilide highly promote the TdP incidence without causing significant APD prolongation, whereas increasing IKr block from 7.5 to 10 nM dofetilide (in the presence of veratridine) substantially further prolonged APD without increasing the incidence of TdP? One mechanism may be that IKs block, but not additional IKr block, increases dispersion of ventricular repolarization. This is supported by our observation that adding an IKs blocker to veratridine and 7.5 nM dofetilide increased the Tpeak−end interval to a greater extent than increasing IKr block from 7.5 to 10 nM dofetilide (in the presence of veratridine) (Figure 5). This also agrees with an in vitro study that directly measured transmural dispersion in a canine ventricular wedge model, where combined IKr and IKs blockade increased transmural dispersion of ventricular repolarization compared with IKr block alone (Burashnikov and Antzelevitch, 2002). The mechanism for the apparent increase in transmural dispersion of repolarization by adding the IKs blocker is unclear. IKs is heterogeneously expressed with less IKs in the mid-myocardium than the epicardium and endocardium (Liu and Antzelevitch, 1995). On the basis of this observation, one would predict that IKs block may induce less APD prolongation in the mid-myocardium (and thus less dispersion of repolarization). However, Shimizu and Antzelevitch (1998) showed that administering the selective IKs blocker chromanol 293B alone prolonged APD homogeneously across the three myocardial layers and therefore did not affect the transmural dispersion. The same research group (Liu and Antzelevitch, 1995) suggested that the lowest IKs expression in the mid-myocardium may cause the APD to be the longest, but a long APD may allow more functional IKs activation during positive membrane potentials. Moreover, the effect of adding an IKs blocker to IKr block on dispersion may be different from that of IKs block alone. IKr blockers have been shown to exert the greatest APD prolongation in the mid-myocardium, particularly during slow heart rates (Shimizu and Antzelevitch, 1997). Therefore, the combined IKr block and slowing of INa inactivation is likely to induce greater APD prolongation in the mid-myocardium, and hence allow more time for IKs to activate in this myocardial layer. IKs is therefore expected to be most functionally activated in the mid-myocardium and has a critical function as the ‘safety current', thereby minimizing transmural dispersion of repolarization during bradycardia.
Another potential contributing factor to the increased TdP incidence is the increase in beat-to-beat variability of APD observed following IKs block, as variability in ventricular repolarization is another known predictor of TdP (Hondeghem et al., 2001; Thomsen et al., 2006). Our results are consistent with the recent study by Lengyel et al. (2007), where adding IKs block to IKr block greatly increased the beat-to-beat variability of the QT interval in anaesthetized rabbits. We observed that the pattern of beat-to-beat variation is random rather than occurring regularly on alternating beats, which agrees with previous studies (Thomsen et al., 2006; Lengyel et al., 2007). The magnitude of the beat-to-beat variability of APD is small, probably because it was measured at a period with electrical pacing at a constant CL, without interruptions by premature beats or spontaneous changes in heart rate as observed in vivo. The mechanism underlying the instability of ventricular repolarization with IKs block may be related to an increase in the slope of ventricular effective refractory period vs CL relationship (reverse rate-dependence) after combined IKr+IKs block compared with IKr block alone (So et al., 2006). Increase in the steepness of the reverse rate-dependence enhances the oscillations in ventricular repolarization, which may be associated with proarrhythmias (Hondeghem et al., 2001).
Our studies are based on the experimental approach of drug perfusion in isolated rabbit ventricles using the measurement of MAP without direct measurement of the transmembrane AP or ionic currents. Unlike the transmembrane AP, variation in the MAP can be caused by changes in contact pressure between the MAP catheter and the tissue, and do not necessarily reflect changes in specific ionic currents (Franz, 1999). Thus, the use of MAP measurement is limited to estimating the overall duration of ventricular repolarization, and the morphology of MAP provides little direct evidence regarding ionic mechanisms compared with transmembrane AP measurements. There are well-known species differences in the expression and kinetics of IKs; although there are several important differences between the human and rabbit hearts (for example the transient outward K+ current (Ito) in rabbits has a slower recovery from inactivation than in humans (Lu et al., 2001)), rabbit hearts are similar to human hearts in terms of the activation and deactivation kinetics of IKs (Jost et al., 2007). Moreover, episodes of TdP may inhibit the onset of subsequent TdP because the QT is transiently shortened. As there were more TdP episodes in the group treated with HMR 1556 (plus IKr block and slowing of INa inactivation), such a mechanism would lead to an underestimation of the number of TdP episodes in this experimental group.
Our data suggest that IKs preservation is crucial in protecting against TdP development. This result agrees with the suggestion by Salata et al. (1998) that IKs activation may prevent the development of proarrhythmia by limiting excessive APD prolongation caused by other K+ channel blockers. During the rapid phase 3 repolarization, IKs channels deactivate slowly (Sanguinetti and Jurkiewicz, 1990; Lengyel et al., 2001), leading to a residual IKs conductance after the onset of ventricular repolarization, which decreases membrane excitability (Davidenko et al., 1994; Beaumont et al., 1995). This may counterbalance the depolarizing calcium current, which can reactivate during the late phase of repolarization to trigger early afterdepolarizations. A similar mechanism has been proposed for preventing triggered arrhythmias by the inward rectifier K+ current (Pogwizd et al., 2001). This mechanism is also consistent with previous studies showing that adding IKs blockers to an already prolonged APD promotes early afterdepolarizations (Burashnikov and Antzelevitch, 2002), whereas adding an IKs activator (L3) decreases the incidence of early afterdepolarizations in dofetilide-treated and hypertrophied ventricular myocytes (Xu et al., 2002). IKs block may also change the AP morphology such that the membrane potential resides longer at the voltage range that may promote activation of depolarizing currents and trigger TdP. Future studies using direct transmembrane AP measurements or computer modelling are needed to assess the change in AP morphology in these settings. Early afterdepolarizations and TdP generation may thus reflect an imbalance between inward and outward currents during phase 3 repolarization, rather than being purely a consequence of APD prolongation (Antzelevitch, 2004; Hondeghem, 2006). Sympathetic activation has also been suggested to induce early afterdepolarizations by reactivation of L-type calcium current, which may act as a trigger for TdP (January and Riddle, 1989). It may also increase the dispersion of repolarization owing to heterogeneous distribution of both sympathetic innervation and IKs channels in human myocardium (Kawano et al., 2003; Szentadrassy et al., 2005). On the other hand, β-adrenoceptor stimulation increases IKs magnitude and accelerates its activation kinetics (Volders et al., 2003), which may be a protective mechanism against TdP induction during drug-induced APD prolongation. Sympathetic denervation in perfused hearts or other in vitro preparations limits the ability to assess the role of β-adrenoceptor stimulation in L-type calcium current and IKs activation, and their overall effects on TdP induction. Therefore, future in vivo studies are necessary to investigate the role of sympathetic activation in the torsadogenic mechanism of IKs block.
In conclusion, this study highlights the function of IKs in protecting against TdP development under the condition of substantial APD prolongation. IKs acts as a ‘safety current', or repolarization reserve, which may prevent the development of proarrhythmic events when APD is prolonged. Because IKs contributes little to ventricular repolarization during baseline state (‘latent nature') (Biliczki et al., 2002; Jost et al., 2005; So et al., 2006), some individuals with IKs channel mutations may have normal baseline QT interval but a higher risk of TdP development upon exposure to drugs that inhibit repolarizing currents, especially IKr (Roden, 2006). The use of IKs blockers could promote the occurrence of TdP, especially when other repolarizing currents are reduced by drugs, genetic mutations or heart failure.
Acknowledgments
This study was supported by the Heart and Stroke Foundation of Ontario. Petsy Pui-Sze So is a recipient of a scholarship from the Natural Sciences and Engineering Research Council (NSERC) of Canada. We thank Xu-Dong Hu for technical support, Susan O'Donnell for statistical advice, Latoya Austin, Marta Gadacz and Zana Mariano for administrative assistance, Pfizer Canada Inc. and Sanofi-Aventis for providing dofetilide and HMR 1556, respectively.
Abbreviations
- AP
action potential
- APD
AP duration
- CL
cycle length
- HMR
HMR 1556
- IK
delayed rectifier K+ current
- IKr
the rapid component of IK
- IKs
the slow component of IK
- INa
sodium current
- LV
left ventricular
- LVP
LV pressure
- MAP
monophasic AP
- MAPD90
MAP duration at 90% repolarization
- Torsades de Pointes
TdP
- Tpeak−end
the interval between the peak and the end of T wave
Conflict of interest
The authors state no conflict of interest.
References
- Antzelevitch C. Arrhythmogenic mechanisms of QT prolonging drugs: is QT prolongation really the problem. J Electrocardiol. 2004;37:S15–S24. doi: 10.1016/j.jelectrocard.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Beaumont J, Michaels DC, Delmar M, Davidenko J, Jalife J. A model study of changes in excitability of ventricular muscle cells: inhibition, facilitation, and hysteresis. Am J Physiol. 1995;268:H1181–H1194. doi: 10.1152/ajpheart.1995.268.3.H1181. [DOI] [PubMed] [Google Scholar]
- Biliczki P, Virag L, Iost N, Papp JG, Varro A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br J Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff U, Schmidt C, Netzer R, Pongs O. Effects of fluoroquinolones on HERG currents. Eur J Pharmacol. 2000;406:341–343. doi: 10.1016/s0014-2999(00)00693-2. [DOI] [PubMed] [Google Scholar]
- Burashnikov A, Antzelevitch C. Prominent IKs in epicardium and endocardium contributes to development of transmural dispersion of repolarization but protects against development of early afterdepolarizations. J Cardiovasc Electrophysiol. 2002;13:172–177. doi: 10.1046/j.1540-8167.2002.00172.x. [DOI] [PubMed] [Google Scholar]
- Davidenko JM, Delmar M, Beaumont J, Michaels DC, Lorente P, Jalife J. Electrotonic inhibition and active facilitation of excitability in ventricular muscle. J Cardiovasc Electrophysiol. 1994;5:945–960. doi: 10.1111/j.1540-8167.1994.tb01134.x. [DOI] [PubMed] [Google Scholar]
- Franz MR. Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res. 1999;41:25–40. doi: 10.1016/s0008-6363(98)00268-5. [DOI] [PubMed] [Google Scholar]
- Heist EK, Ruskin JN. Drug-induced proarrhythmia and use of QTc-prolonging agents: clues for clinicians. Heart Rhythm. 2005;2:S1–S8. doi: 10.1016/j.hrthm.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM. Thorough QT/QTc not so thorough: removes torsadogenic predictors from the T-wave, incriminates safe drugs, and misses profibrillatory drugs. J Cardiovasc Electrophysiol. 2006;17:337–340. doi: 10.1111/j.1540-8167.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM. Relative contributions of TRIaD and QT to proarrhythmia. J Cardiovasc Electrophysiol. 2007;18:655–657. doi: 10.1111/j.1540-8167.2007.00827.x. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
- January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- Jost N, Papp JG, Varró A. Slow delayed rectifier potassium current (IKs) and the repolarization reserve. Ann Noninvasive Electrocardiol. 2007;12:64–78. doi: 10.1111/j.1542-474X.2007.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, et al. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112:1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 2003;18:32–39. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- Lengyel C, Iost N, Virag L, Varro A, Lathrop DA, Papp JG. Pharmacological block of the slow component of the outward delayed rectifier current (IKs) fails to lengthen rabbit ventricular muscle QT(c) and action potential duration. Br J Pharmacol. 2001;132:101–110. doi: 10.1038/sj.bjp.0703777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel C, Varro A, Tabori K, Papp JG, Baczko I. Combined pharmacological block of IKr and IKs increases short-term QT interval variability and provokes torsades de pointes. Br J Pharmacol. 2007;151:941–951. doi: 10.1038/sj.bjp.0707297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial and endocardial myocytes: a weaker IKs contributes to the longer action potential of the M cell. Circ Res. 1995;76:351–365. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- Lu Z, Kamiya K, Opthof T, Yasui K, Kodama I. Density and kinetics of IKr and IKs in guinea pig and rabbit ventricular myocytes explain different efficacy of IKs blockade at high heart rate in guinea pig and rabbit: implications for arrhythmogenesis in humans. Circulation. 2001;104:951–956. doi: 10.1161/hc3401.093151. [DOI] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- Michael G, Dempster J, Kane KA, Coker SJ. Potentiation of E-4031-induced torsade de pointes by HMR1556 or ATX-II is not predicted by action potential short-term variability or triangulation. Br J Pharmacol. 2007;152:1215–1227. doi: 10.1038/sj.bjp.0707513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H, Gerlach U, Schmidt D, Nattel S. In vivo electrophysiological effects of a selective slow delayed-rectifier potassium channel blocker in anesthetized dogs: potential insights into class III actions. Cardiovasc Res. 2004;61:705–714. doi: 10.1016/j.cardiores.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Napolitano C, Priori SG, Schwartz PJ, Bloise R, Ronchetti E, Nastoli J, et al. Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice. JAMA. 2005;294:2975–2980. doi: 10.1001/jama.294.23.2975. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- Roden DM. Long QT syndrome: reduced repolarization reserve and the genetic link. J Intern Med. 2006;259:59–69. doi: 10.1111/j.1365-2796.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- Salata JJ, Jurkiewicz NK, Wang J, Evans BE, Orme HT, Sanguinetti MC. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol Pharmacol. 1998;54:220–230. doi: 10.1124/mol.54.1.220. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade des pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long-QT syndrome: effects of beta-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- Silva J, Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation. 2005;112:1384–1391. doi: 10.1161/CIRCULATIONAHA.105.543306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So PP, Backx P, Dorian P.The slowed delayed rectifier K+ repolarizing current (IKs) is accentuated following slowed Na+ channel inactivation Visions in Pharmacology 2005University of Toronto (Abstract)
- So PP, Backx PH, Hu XD, Dorian P. IKs block by HMR 1556 lowers ventricular defibrillation threshold and reverses the repolarization shortening by isoproterenol without rate-dependence in rabbits. J Cardiovasc Electrophysiol. 2007;18:750–756. doi: 10.1111/j.1540-8167.2007.00812.x. [DOI] [PubMed] [Google Scholar]
- So PP, Hu XD, Backx PH, Puglisi JL, Dorian P. Blockade of IKs by HMR 1556 increases the reverse rate-dependence of refractoriness prolongation by dofetilide in isolated rabbit ventricles. Br J Pharmacol. 2006;148:255–263. doi: 10.1038/sj.bjp.0706721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentadrassy N, Banyasz T, Biro T, Szabo G, Toth BI, Magyar J, et al. Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc Res. 2005;65:851–860. doi: 10.1016/j.cardiores.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Thomsen MB, Volders PG, Beekman JD, Matz J, Vos MA. Beat-to-Beat variability of repolarization determines proarrhythmic outcome in dogs susceptible to drug-induced torsades de pointes. J Am Coll Cardiol. 2006;48:1268–1276. doi: 10.1016/j.jacc.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Volders PG, Stengl M, van Opstal JM, Gerlach U, Spätjens RL, Beekman JD, et al. Probing the contribution of IKs to canine ventricular repolarization: key role for beta-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- Woosley RL, Chen Y, Freiman JP, Gillis RA. Mechanism of the cardiotoxic actions of terfenadine. JAMA. 1993;269:1532–1536. [PubMed] [Google Scholar]
- Xu X, Salata JJ, Wang J, Wu Y, Yan GX, Liu T, et al. Increasing IKs corrects abnormal repolarization in rabbit models of acquired LQT2 and ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2002;283:H664–H670. doi: 10.1152/ajpheart.00076.2002. [DOI] [PubMed] [Google Scholar]
- Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]