Abstract
Background and purpose:
The P2Y11 receptor, a member of the group of metabotropic nucleotide receptors, shows a stereospecific ligand recognition of Pα-substituted ATP derivatives (ATP-α-S isomers). These compounds are suitable candidates for the development of selective P2Y11 receptor agonists that might be used as immune modulators. We have analysed the binding mode of ATP at the P2Y11 receptor by molecular modeling and site-directed mutagenesis. Based on our recent findings, we decided to decipher the molecular determinants of stereoselective recognition at the P2Y11 receptor.
Experimental approach:
Two amino acid residues [Glu186 in the extracellular loop 2 and Arg268 in the transmembrane domain 6 (TM6)], which are part of the nucleotide-binding pocket, were selected and studied by mutational analyses. We expected these residues to be involved in determining the stereospecificity of the P2Y11 receptor.
Key results:
After mutation of Arg268 to alanine or glutamine, the stereospecific recognition of the ATP-α-S isomers at the P2Y11 receptor was lost. In contrast, at the Glu186Ala receptor mutant, the stereoselective differentiation between these isomers was increased. On the Arg268Gln/Glu186Ala double mutant we observed no further effect, except for additivity in the decrease in potency of both isomers, as compared with the single-point mutants.
Conclusions and implications:
Our results show that the stereospecificity of the P2Y11 receptor for Pα-substituted ATP derivatives is largely determined by the basic residue Arg268 in TM6. This will allow the design of receptor-subtype selective ligands.
Keywords: diastereoselectivity, Pα-substituted ATP, P2Y11 receptor mutants
Introduction
Stereoselective discrimination of agonists acting on G-protein-coupled receptors (GPCRs) gives insight into the preferential binding mode of the receptors. For the metabotropic nucleotide receptors (P2Y receptors; nomenclature conforms to the BJP's Guide to Receptors and Channels, Alexander et al., 2008) it was shown that specific conformations of ATP are preferred by these receptors (Kim et al., 2002). Presently, eight human receptor subtypes, P2Y1,2,4,6,11,12,13,14, have been cloned (von Kügelgen, 2006). Among these receptors, the P2Y11 receptor subtype is most interesting as it transduces signals through several different messenger systems. This receptor can couple to Gq and Gs proteins, increasing the intracellular concentrations of calcium and cAMP, respectively (Communi et al., 1997). Subsequently, the stimulation of cAMP elevation leads to activation of another signalling system, the cyclic ADP ribose-dependent calcium signalling pathway (Moreschi et al., 2006). Thus, activation of the P2Y11 receptor induces a complex signalling cascade that, among other functions, controls cell differentiation and cell movement in immune cells (Abbracchio et al., 2006). The P2Y11 receptor is present on dendritic cells (Schnurr et al., 2003), granulocytes (Moreschi et al., 2006), mast cells (Feng et al., 2004) and lymphocytes (Conigrave et al., 2001) and seems to have a modulatory function in the cellular immune response. Moreover, the receptor is also associated with pathological situations in the heart where it seems to be involved in inflammatory processes that increase the risk to myocardial infarction (Amisten et al., 2007).
The P2Y11 receptor is activated by ATP as the natural ligand (Communi et al., 1999). We have recently reported that stereoselective activation of the receptor occurs with Pα-substituted ATP derivatives (Ecke et al., 2006). In these ATP analogues, one non-bridging oxygen atom of Pα has been substituted by sulphur (ATP-α-S). This substitution introduces a new chiral centre in the ATP molecule and the resulting diastereoisomers can be separated into Sp and Rp isomers. The P2Y11 receptor shows a clear preference for the Rp-ATP-α-S isomer. In addition, the same stereoselective preference can be observed with Pα-borano-substituted ATP analogues showing that it is not only a specific interaction with sulphur, but a true stereospecific preference. Interestingly, this stereoselectivity is opposite to that of the P2Y1 receptor (Nahum et al., 2002; Major et al., 2004), the closest homologue of the P2Y11 receptor (Costanzi et al., 2004). This finding added to the understanding of the structural and conformational determinants of nucleotides, which could selectively activate the P2Y11 receptor.
To understand the molecular basis of the stereoselectivity of the P2Y11 receptor we investigated the involvement of specific amino acid residues in the putative nucleotide-binding pocket of the receptor by mutational analyses. A study focusing on the pharmacological differences of the canine and human P2Y11 receptor had already shown that the difference of a single amino acid in the transmembrane domain 6 (TM6) of the receptor (Gln268 vs Arg268) can change selectivity towards the length of the phosphate chain of adenine nucleotides (Qi et al., 2001). Here, we concentrated on a limited number of specific residues that were chosen according to their position in our recently published model of the P2Y11 receptor nucleotide-binding pocket (Zylberg et al., 2007). We found that the arginine residue in TM6 (Arg268) is involved in the stereoselective preference of the Rp-ATP-α-S isomer at the P2Y11 receptor, indicating the necessity of a basic residue at this position for proper chiral discrimination.
Materials and methods
Site-directed mutagenesis
The DNA sequence of the P2Y11 receptor (GenBankTM/EBI accession number AF030335), kindly provided by Dr D Communi, was placed between the EcoRI/BamHI restriction sites of the eGFPN1 vector (Clontech, Heidelberg, Germany). For performance of site-directed mutagenesis the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) was used. Introduction of the mutations was done using customized oligonucleotides (Quiagen, Hilden, Germany) and DNA sequencing confirmed the mutagenesis. 1321N1 human astrocytoma cells were transfected with DNA of the P2Y11–GFP (green fluorescent protein) receptor and DNA carrying the respective mutation.
Cell culture and transfection
1321N1 human astrocytoma cells were grown at 37 °C in 10% CO2 in high-glucose Dulbecco's modified Eagle's medium supplemented with 5% Foetal Calf Serum, 100 U mL−1 penicillin and 100 IU mL−1 streptomycin. Transfection of the cells with the recombinant plasmids was done using FuGENE 6 transfection reagent according to the manufacturer's protocol (Roche, Basel; Switzerland). Transfected cells were selected with 0.5 mg mL−1 G418.
Analysis of receptor expression level
Flow cytometry using a FACS LSR (BD Biosciences, Heidelberg, Germany) was applied to analyse the expression levels of wild-type and mutant receptors. Cells grown in 5 cm culture dishes (Nunc, Wiesbaden, Germany) to 80% confluency, were harvested, and resuspended in culture medium. The Flow Jo software was used to analyse the expression levels of 10 000 cells by determining the mean intensity (geometric median) of the GFP fluorescence per cell.
Calcium measurements
The cells were plated on coverslips (22 mm diameter), and single-cell measurement was made after 3 days, when the cells were 30–50% confluent. The changes of free intracellular Ca2+ concentration ([Ca2+]i) were measured, as described before (Ubl et al., 1998; Vöhringer et al., 2000). Briefly, cells were pre-incubated with 2 μM fura-2AM at 37 °C for 30 min in NaHBS (HEPES buffered saline solution: 145 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 25 mM glucose and 20 mM HEPES/Tris pH 7.4). Stimulation of the cells was done under continuous superfusion of pre-warmed NaHBS at 37 °C with different concentrations of various agonists. Fluorescence intensity was recorded alternately at 340 and 380 nm excitation and 520 nm emission with a Zeiss Axiovert microscope ( × 40/1.30 oil immersion objective) and a fluorescence imaging system from TILL Photonics (Gräfelfing; Germany).
Data analysis
The fluorescence ratio R (R=ΔF340/F380) at the two wavelengths, 340 and 380 nm, was measured. Further analysis with the resulting data was performed with the Excel program applying basal deduction to the calcium traces and calculating the peak height for each cell. The SigmaPlot program (Systat Software, Inc., Chicago, Illinois; USA) was used to derive EC50 values (half-maximal response) from the concentration–response data obtained from average values from 30 to 60 single cells. The EC50 values were calculated using the equation
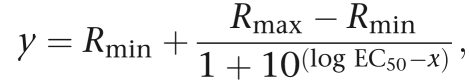 |
with a standard slope. Curve fitting was performed using the same equation. Statistical analysis used one-way ANOVA and the Tukey's test as post-test, or the unpaired t-test.
Materials
Geneticine (G418 sulphate) from Calbiochem (Nottingham; Great Britain); Rp/Sp-ATP-α-S (Biolog, Bremen, Germany); Dulbecco's modified Eagle's medium, penicillin/streptomycin (10 000/10 000 U mL−1), Trypsin/EDTA (0.05%/0.02%), foetal calf serum, (Seromed, Biochrom, Berlin); cell culture dishes (Nunc); coverslips (22 mm), (OmniLab Bremen; Germany); Fura 2-AM (Biomol, Hamburg, Germany/Molecular Probes, Carlsbad, California; USA).
Results
To decipher the molecular basis of the diastereoselectivity of the P2Y11 receptor, two mutant receptors were generated to test whether the preference of the Rp-ATP-α-S isomer is lost after mutation of the selected residues. The glutamate residue (Glu186) in the extracellular loop 2 (EL2) of the receptor was considered to be a putative candidate to take part in the determination of the P2Y11 receptor's diastereoselectivity. The corresponding residue in the P2Y1 receptor (Asp204) was suggested to coordinate an Mg2+ ion in the vicinity of the phosphate-binding pocket, which, in turn, is responsible for the diastereoselective discrimination at the P2Y1 receptor (Major et al., 2004). In addition, the arginine at position 6.55 (Arg268) in the P2Y11 receptor was selected as it was facing the glutamate residue (Glu186) in our P2Y11 receptor model (Zylberg et al., 2007). Therefore, both residues could be involved in the preferential interaction with one of the Pα conformations, as they are on opposite sides of the phosphate chain of ATP docked as a ligand in our receptor model.
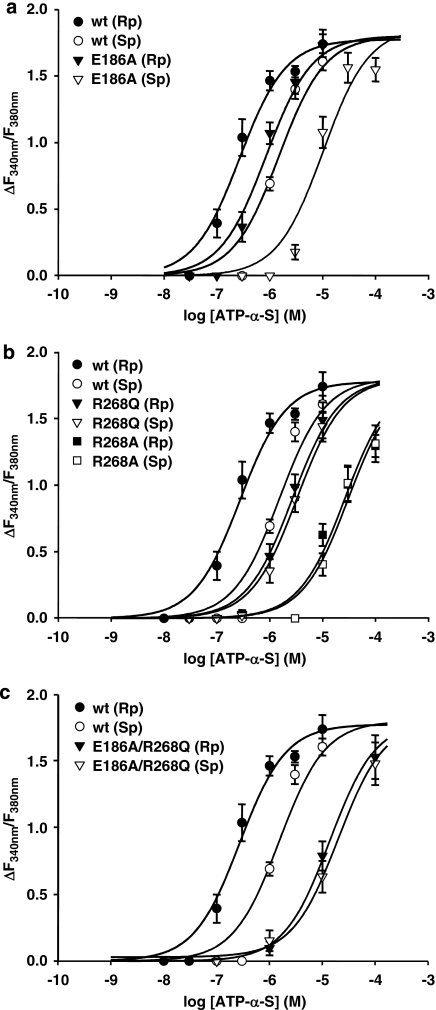
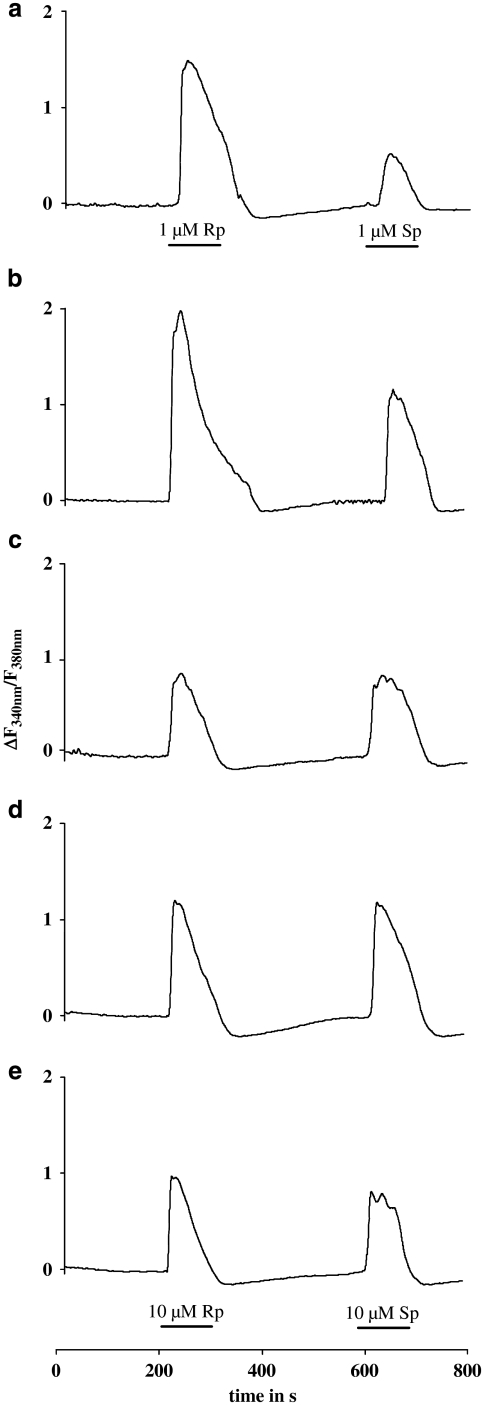
The selected amino acids were mutated to uncharged residues and the mutant receptors were stably expressed in 1321N1 cells as GFP fusion proteins. We used the GFP-tagged receptors for functional characterization by single-cell calcium measurements with fura-2AM, as described earlier (Nahum et al., 2006). Concentration–response curves for the Rp- and Sp-ATP-α-S isomers were obtained (Figure 1) and used to derive the half-maximal effective concentration (EC50) as potency measurements. The EC50 values for both isomers at different receptor mutants are summarized in Table 1. Figure 2 displays representative calcium traces for Rp and Sp isomers obtained at the wild-type and mutant P2Y11 receptors.
Figure 1.
Concentration–response curves for ATP-α-S (Rp/Sp) isomers at the wild-type and mutant P2Y11GFP receptors stably expressed in 1321N1 cells. Cells pre-incubated with 2 μM fura-2-AM were stimulated with varying concentrations of ATP-α-S (Rp/Sp) isomers and the change in fluorescence (ΔF340 nm/F380 nm) was detected. Data represent the mean values and standard error from 30 to 60 single cells. Results were obtained in at least three separate experiments. (a) Curves obtained with the Glu186Ala (E186A) receptor mutant. (b) Curves obtained with the Arg268Ala (R268A) and Arg268Gln (R268Q) receptor mutants. (c) Curves obtained with the E186A/R268Q double-mutant receptor. The maxima of the curves for both ATP-α-S isomers at the R268 mutants and at the E186A, R268Q double mutant, as well as the curve for the Rp isomer at the E186A mutant and the curve for the Sp isomer at the wild-type receptor were fixed to the plateau value of the ATP curve at the wild-type receptor.
Table 1.
Potency of ATP-α-S, Sp and Rp isomers, at mutant P2Y11GFP receptors to increase intracellular calcium was determined as described in methods
| Receptor construct | Receptor expression level (%) |
EC50 values of ATP-α-S (μM) |
|
|---|---|---|---|
| Sp isomer | Rp isomer | ||
| Wild-type | 100 | 1.71±0.55 (4) | 0.27±0.06 (5) |
| Glu186Ala | 111±10 (9)a | 10.2±1.75 (6) | 0.85±0.11 (7) |
| Arg268Ala | 143±23 (3)a | 30.8±6.43 (5) | 26.4±6.28 (4) |
| Arg268Gln | 96±8 (7)a | 3.28±0.71 (4) | 2.69±0.63 (4) |
| Glu186Ala/ Arg268Gln | 94±6 (4) | 19.8±3.92 (4) | 13.7±3.32 (5) |
Data were taken from (Zylberg et al., 2007).
Data represent mean EC50 values (μM)±s.e.m. obtained from the concentration–response curves shown in Figure 1. We analysed 1321N1 cells stably expressing the wild-type or mutated receptor in (n) numbers of experiments. Receptor expression level was determined as described before (Zylberg et al., 2007).
Figure 2.
Calcium traces for ATP-α-S (Rp/Sp) isomers at the wild-type and mutant P2Y11GFP receptors stably expressed in 1321N1 cells. Cells pre-incubated with 2 μM fura-2-AM were stimulated with ATP-α-S (Rp/Sp) isomers, and the change in fluorescence (ΔF340 nm/F380 nm) was detected. The curves show data from representative experiments using 10 μM ATP-α-S (Rp/Sp) at the receptor mutants and a 10-fold lower concentration at the wild-type receptor. (a) Wild-type receptor, (b) E186A mutant, (c) R268A mutant, (d) R268Q mutant and (e) E186A, R268Q double mutant.
The Glu186Ala P2Y11 mutant receptor did not show any loss in preference of the Rp-ATP-α-S isomer. In contrast, the potency ratio between the Sp and Rp isomers (11±0.77; P<0.0001) was even increased, as compared with the potency ratio at the wild-type receptor (6±0.69) (Table 1, Figure 1a). Interestingly, the EC50 value of the Rp isomer at the Glu186Ala mutant receptor was decreased only threefold, compared with the wild-type receptor. However, the shift in potency for the Sp isomer was more pronounced. At the Glu186Ala receptor mutant the EC50 value for the Sp-ATP-α-S isomer was six-fold higher than that at the wild-type receptor. This suggests a nearly conserved recognition of the Rp isomer of ATP-α-S after substitution of the glutamate in EL2 by alanine, whereas the Sp isomer is recognized to a lesser extent.
After mutation of Arg268 (TM6.55) to alanine or glutamine a loss of preference for the Rp-ATP-α-S isomer was observed (Table 1, Figure 1b). For the Arg268Ala P2Y11 receptor mutant the decrease in potency for the Rp isomer was about two orders of magnitude, whereas the shift in potency for the Sp isomer was only 20-fold compared with the wild-type receptor. In consequence, at the Arg268Ala receptor mutant, the isomers were found to be equipotent. Representative calcium traces show that curves with comparable time course and amplitude were obtained with Rp and Sp isomers, using the same concentration of the agonists (Figure 2c).
Furthermore, at the Arg268Gln P2Y11 receptor mutant, the activity of the Sp-ATP-α-S isomer was nearly retained, as the change in potency compared with the wild-type receptor was only twofold. The Rp isomer was 10-fold less potent at the Arg268Gln receptor mutant than at the wild-type P2Y11 receptor. Thus, the activity of both isomers was not affected to the same degree after substitution of the arginine by glutamine, and again both isomers were equipotent at this receptor mutant (Figures 1b and 2d).
To test whether a further change in the diastereoselectivity of the P2Y11 receptor could be seen after simultaneous mutation of the glutamate in EL2 and the arginine in TM6, a double mutant was constructed. The mutant receptor contained both the Glu186Ala and Arg268Gln mutations. In addition to the potency of the ATP-α-S diastereoisomers, the activity of ATP was also tested at this mutant receptor. The loss in potency for ATP at the double mutant receptor (EC50=886±231 μM, n=4) was more than two orders of magnitude compared with the wild-type receptor (EC50=2.37±0.88 μM). The diastereoselectivity of the Glu186Ala/Arg268Gln double mutant receptor showed characteristics similar to those found for the single Arg268Gln and single Arg268Ala mutant (Figures 1c and 2e). Both ATP-α-S isomers (Sp and Rp) were found to be equipotent at the double mutant receptor. The change in potency for the Sp isomer was about one order of magnitude and for the Rp isomer the change was 50-fold compared with the wild-type P2Y11 receptor (Table 1, Figure 1c). After mutating two positions in the P2Y11 receptor, the loss in potency for the Rp isomer was clearly additive, whereas the decrease in potency for the Sp isomer was not additive, when compared to the Glu186Ala single mutant receptor (Table 1).
Discussion
The purpose of this study was to decipher molecular determinants of the diastereoselectivity of the P2Y11 receptor. The two residues (Glu186, Arg268) discussed here were already shown to be important for the recognition of ATP at the P2Y11 receptor (Zylberg et al., 2007). Our new data provide more details of the function of these residues in ligand recognition and thereby validate the correctness of the recently described P2Y11 receptor model (Zylberg et al., 2007). The diastereoselectivity of the P2Y11 receptor is characterized by a discrimination between the Rp and Sp isomers of ATP-α-S analogues at which the Rp isomer is found to be more potent in activating the receptor (Ecke et al., 2006). After mutation of Glu186 and Arg268 to uncharged amino acids, the stereoselective discrimination of the P2Y11 receptor was affected.
The glutamate Glu186 in EL2 seems to be important for the activity of the Sp-ATP-α-S isomers. Substitution of Glu186 by an uncharged, nonpolar amino acid (alanine) clearly reduced the potency of the Sp isomer (P<0.001), whereas the potency of the Rp isomers was nearly conserved (P>0.05). This resulted in an increased difference in potency between both ATP-α-S diastereoisomers at the Glu186Ala receptor mutant compared with the wild-type P2Y11 receptor. Nevertheless, the recognition of both isomers (3 to 6-fold less potent than at the wild-type receptor) was not as much affected at the receptor mutant as that of ATP (14-fold less potent than at the wild-type receptor), whereas it was comparably affected to that of ATPγS (5-fold less potent than at the wild-type receptor) (Zylberg et al., 2007). This suggests a specific binding mode for adenosine-phosphorothioates at the P2Y11 receptor.
The amino acid in the EL2 of the P2Y1 receptor (Asp204) corresponding to Glu186 in the P2Y11 receptor is believed to be responsible for the preference of the Sp isomers of the Pα sulphur-substituted ATP derivatives at this receptor. Computational docking studies suggested that the Asp204 in the P2Y1 receptor coordinates an Mg2+ ion, which, in turn, interacts with the phosphate chain of a docked ATP molecule through hydrogen bonding. In this process, the Sp isomer of the ATP-α-S analogues exhibits the maximum interaction energy with the Mg2+ ion, clearly demonstrating the chiral discrimination of the P2Y1 receptor as found by computer modelling (Major et al., 2004).
If we consider these findings made at the P2Y1 receptor, the corresponding residue in the P2Y11 receptor might have a similar function. The fact that the Sp isomer shows a reduced potency at the Glu186Ala receptor mutant seems to confirm this assumption. The Pα oxygen in the Sp isomer might be able to form H-bonds with the proposed coordinated metal ion in the presence of Glu186. The sulphur atom is not able to do so, as sulphur is known to be a very weak partner in H-bonding (Platts et al., 1996). The substitution of the glutamate by alanine is therefore more significant for the potency of the Sp isomer. However, the EL2 of the P2Y11 receptor is much longer than that of the P2Y1 receptor (35 aa vs 25 aa, respectively). Therefore, different mechanisms may account for the observed differences in potency.
Another residue found to influence the diastereoselectivity of the P2Y11 receptor is an arginine in TM6 (Arg268, 6.55). Mutation of this basic residue to alanine or glutamine resulted in a loss of the chiral discrimination at the receptor. The ATP-α-S Rp/Sp isomers were found to be equipotent at these mutant receptors. This was mainly caused by a severe loss in potency of the Rp isomer at the receptor, whereas the activity of the Sp isomer was less affected. The shift in potency for the Sp-ATP-α-S isomer at the Arg268Ala mutant receptor is much less pronounced (18-fold compared with the wild-type receptor) than with ATP and ATPγS (762-fold and 238-fold, respectively compared with the wild-type receptor), which were also tested at this P2Y11 receptor mutant (Zylberg et al., 2007). Thus, the recognition of the Sp isomer is less disturbed by the mutation of this important cationic residue. Its binding mode is apparently different from that for ATP, ATPγS and Rp-ATP-α-S. Interestingly, ionic interactions do not seem to account for this specific recognition, as substitution of Arg268 by glutamine affected the potency of the Sp isomer even less.
However, the arginine at position 6.55 seems to be essential for a preferred recognition of the Rp isomer at the P2Y11 receptor, indicating the necessity of a basic residue at this position for proper chiral discrimination. Further studies are necessary to determine whether ionic interactions with the basic residue are involved in the preferred recognition of the Rp isomer. Interestingly, this arginine is also necessary for the preference of ATP over ADP at the receptor. The mutation of Arg268 to glutamine in the P2Y11 receptor induced a shift in this preference, as the mutant receptor was activated more potently by ADP than ATP (Qi et al., 2001). This was also confirmed in our study, when we compared the potency of ATP and ADP at the Arg268Gln or Arg268Ala mutant receptors (data not shown).
The Glu186Ala/Arg268Gln double mutant receptor did not show an additional change in the diastereoselectivity of the P2Y11 receptor. The influence of the Arg268Gln mutation was dominating in the receptor double mutant, so that the chiral discrimination of the receptor was lost. Interestingly, the shift to the right of the concentration–response curves for the Rp-ATP-α-S isomer was additive for the double mutant, in comparison with the two corresponding single-point mutants. This underlines that co-substitution of Glu186 and Arg268 acts synergistically in decreasing the potency of agonists at the P2Y11 receptor. In contrast, for the Sp isomer the mutation of Glu186 seems to be dominant in determining the potency of this agonist at the double mutant. No additional effect was seen, when compared when the single Glu186Ala receptor mutant.
In conclusion, the diastereoselectivity of the P2Y11 receptor is markedly determined by the presence of a basic residue in TM6 at position 6.55. The substitution of the Arg 268 (6.55) by uncharged amino acids abolished the increased potency of the Rp-ATP-α-S isomer compared with the Sp isomer at the P2Y11 receptor. In the absence of specific P2Y11 receptor radioligands, it is not possible to determine conclusively whether the decreased potency is because of a loss in affinity or efficacy. Therefore, this data cannot be unequivocally interpreted as being the result of an altered recognition at the receptor mutants. However, the P2Y11 receptor model showed that the Arg268 residue is most crucial for the recognition of agonists at the P2Y11 receptor. Novel ligands, which are designed to give increased interaction energy with this arginine residue, might be candidates for a potent and selective activation of the receptor.
Acknowledgments
We thank Ms Terhardt for excellent technical help in the complete experimental study. We thank Dr R Hartig (Institute of Immunology, Medical Faculty Magdeburg) for providing the facilities to carry out flow cytometry analysis. The study was supported by Bundesministerium für Bildung und Forschung (BMBF; Grant NBL3; FKZ 01ZZ0407) and Deutsche Forschungsgemeinschaft (Re 563/15-1).
Abbreviations
- [Ca2+]i
intracellular free calcium concentration
- 1321N1
human astrocytoma cell line
- ATP-α-S
adenosine 5′-[α-thio]triphosphate
- ATPγS
adenosine 5′-[γ-thio]triphosphate
- G-protein
GTP-binding protein
- G418
geneticine
- GFP
green fluorescent protein
- GPCR
G-protein-coupled receptor
Conflict of interest
The authors state no conflict of interest.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) 3rd edition (2008 revision) Br J Pharmacol. 2008;153 Suppl. 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amisten S, Melander O, Wihlborg AK, Berglund G, Erlinge D. Increased risk of acute myocardial infarction and elevated levels of C-reactive protein in carriers of the Thr-87 variant of the ATP receptor P2Y11. Eur Heart J. 2007;28:13–18. doi: 10.1093/eurheartj/ehl410. [DOI] [PubMed] [Google Scholar]
- Communi D, Govaerts C, Parmentier M, Boeynaems JM. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- Communi D, Robaye B, Boeynaems J-M. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave AD, Fernando KC, Gu B, Tasevski V, Zhang W, Luttrell BM, et al. P2Y11 receptor expression by human lymphocytes: evidence for two cAMP-linked purinoceptors. Eur J Pharmacol. 2001;426:157–163. doi: 10.1016/s0014-2999(01)01222-5. [DOI] [PubMed] [Google Scholar]
- Costanzi S, Mamedova L, Gao Z-G, Jacobson KA. Architecture of P2Y nucleotide receptors: structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem. 2004;47:5393–5404. doi: 10.1021/jm049914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecke D, Tulapurkar ME, Nahum V, Fischer B, Reiser G. Opposite diastereoselective activation of P2Y1 and P2Y11 nucleotide receptors by adenosine 5′-O-(α-boranotriphosphate) analogues. Br J Pharmacol. 2006;149:416–423. doi: 10.1038/sj.bjp.0706887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Mery AG, Beller EM, Favot C, Boyce JA. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol. 2004;173:7539–7547. doi: 10.4049/jimmunol.173.12.7539. [DOI] [PubMed] [Google Scholar]
- Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg AK, Erlinge D, et al. Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors. J Med Chem. 2002;45:208–218. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major DT, Nahum V, Wang Y, Reiser G, Fischer B. Molecular recognition in purinergic receptors. 2. Diastereoselectivity of the h-P2Y1-receptor. J Med Chem. 2004;47:4405–4416. doi: 10.1021/jm049771u. [DOI] [PubMed] [Google Scholar]
- Moreschi I, Bruzzone S, Nicholas RA, Fruscione F, Sturla L, Benvenuto F, et al. Extracellular NAD+ is an agonist of the human P2Y11 purinergic receptor in human granulocytes. J Biol Chem. 2006;281:31419–31429. doi: 10.1074/jbc.M606625200. [DOI] [PubMed] [Google Scholar]
- Nahum V, Tulapurkar M, Levesque SA, Sevigny J, Reiser G, Fischer B. Diadenosine and diuridine poly(borano)phosphate analogues: synthesis, chemical and enzymatic stability, and activity at P2Y1 and P2Y2 receptors. J Med Chem. 2006;49:1980–1990. doi: 10.1021/jm050955y. [DOI] [PubMed] [Google Scholar]
- Nahum V, Zündorf G, Levesque SA, Beaudoin AR, Reiser G, Fischer B. Adenosine 5′-O-(1-boranotriphosphate) derivatives as novel P2Y1 receptor agonists. J Med Chem. 2002;45:5384–5396. doi: 10.1021/jm020251d. [DOI] [PubMed] [Google Scholar]
- Platts JA, Howard ST, Bracke BRF. Directionality of Hydrogen Bonds to Sulfur and Oxygen. J Am Chem Soc. 1996;118:2726–2733. [Google Scholar]
- Qi AD, Zambon AC, Insel PA, Nicholas RA. An arginine/glutamine difference at the juxtaposition of transmembrane domain 6 and the third extracellular loop contributes to the markedly different nucleotide selectivities of human and canine P2Y11 receptors. Mol Pharmacol. 2001;60:1375–1382. doi: 10.1124/mol.60.6.1375. [DOI] [PubMed] [Google Scholar]
- Schnurr M, Toy T, Stoitzner P, Cameron P, Shin A, Beecroft T, et al. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood. 2003;102:613–620. doi: 10.1182/blood-2002-12-3745. [DOI] [PubMed] [Google Scholar]
- Ubl JJ, Vöhringer C, Reiser G. Co-existence of two types of [Ca2+]i-inducing protease-activated receptors (PAR-1 and PAR-2) in rat astrocytes and C6 glioma cells. Neuroscience. 1998;86:597–609. doi: 10.1016/s0306-4522(97)00686-6. [DOI] [PubMed] [Google Scholar]
- Vöhringer C, Schäfer R, Reiser G. A chimeric rat brain P2Y1 receptor tagged with green-fluorescent protein: high-affinity ligand recognition of adenosine diphosphates and triphosphates and selectivity identical to that of the wild-type receptor. Biochem Pharmacol. 2000;59:791–800. doi: 10.1016/s0006-2952(99)00390-1. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zylberg J, Ecke D, Fischer B, Reiser G. Structure and ligand-binding site characteristics of the human P2Y11 nucleotide receptor deduced from computational modelling and mutational analysis. Biochem J. 2007;405:277–286. doi: 10.1042/BJ20061728. [DOI] [PMC free article] [PubMed] [Google Scholar]