Abstract
Background and purpose:
Tachykinin NK3 receptors are widely expressed in the mouse gastrointestinal tract but their functional role in enteric neuromuscular transmission remains unstudied in this species. We investigated the involvement of NK3 receptors in cholinergic neurotransmission in the mouse stomach and small intestine.
Experimental approach:
Muscle strips of the mouse gastric fundus and ileum were mounted in organ baths for tension recordings. Effects of NK3 agonists and antagonists were studied on contractions to EFS of enteric nerves and to carbachol.
Key results:
EFS induced frequency-dependent tetrodotoxin-sensitive contractions, which were abolished by atropine. The cholinergic contractions to EFS in the stomach were enhanced by the NK3 antagonist SR142801, but not affected by the NK3 agonist senktide or neurokinin B. The cholinergic contractions to EFS in the small intestine were not affected by SR142801, but dose-dependently inhibited by senktide and neurokinin B. This inhibitory effect was prevented by SR142801 but not by hexamethonium. SR142801, senktide or neurokinin B did not induce any response per se in the stomach and small intestine and did not affect contractions to carbachol.
Conclusions and implications:
NK3 receptors modulate cholinergic neurotransmission differently in the mouse stomach and small intestine. Blockade of NK3 receptors enhanced cholinergic transmission in the stomach but not in the intestine. Activation of NK3 receptors inhibited cholinergic transmission in the small intestine but not in the stomach. This indicates a physiological role for NK3 receptors in mouse stomach contractility and a pathophysiological role in mouse intestinal contractility.
Keywords: cholinergic nerves, enteric nervous system, ileum, neurokinin B, NK3 receptors, senktide, SR142801, tachykinin
Introduction
Tachykinins are a family of neuropeptides that have an important function in the gut by acting on tachykinin NK1, NK2 and NK3 receptors. These receptors are widely expressed in the gastrointestinal tract and have distinct pharmacological features in physiological and pathophysiological conditions (Holzer and Holzer-Petsche, 1997a, 1997b, 2001). The exact location and function of tachykinin receptors varies upon the species and gastrointestinal region that is studied. Naturally occurring ligands of tachykinin receptors are substance P, neurokinin A and neurokinin B, all of which are present in the gut. Substance P preferentially activates tachykinin NK1 receptors, whereas neurokinin A and neurokinin B (NKB) preferentially activate NK2 and NK3 receptors, respectively (Maggi, 2000). Of the three known tachykinin receptors in the gastrointestinal tract, NK3 receptors have gained considerable attention because they mediate intrinsic primary afferent nerve activity and are regarded as interesting targets for the treatment of functional bowel disorders (Sanger, 2004).
Immunohistochemical studies in rats, mice and guinea pigs showed moderate tachykinin NK3 receptor immunoreactivity in the stomach (Grady et al., 1996; Mann et al., 1997; Wang et al., 2002) and extensive NK3 receptor immunoreactivity in the small intestine, mainly in myenteric and submucosal neurons and on the surface of smooth muscle cells (Vannucchi and Faussone-Pellegrini, 2000). In the guinea-pig and rat intestine, tachykinin NK3 receptors appear to be located mainly on intrinsic sensory nerves (Mann et al., 1997; Jenkinson et al., 1999) where they mediate afferent tachykinin signalling. Functional studies on guinea-pig intestinal preparations with intact intrinsic afferent nerve signalling showed that tachykinin NK3 receptors on intrinsic primary afferent neurons modulate slow synaptic transmission at nicotinic synapses (Johnson et al., 1996, 1998; Alex et al., 2001). In the guinea pig, contractility studies on isolated intestinal muscle strips, in which the sensory nerve network is generally disrupted, have shown that activation of tachykinin NK3 receptors induces a contraction that is mediated by acetylcholine and by tachykinins released from enteric nerves (Yau and Youther, 1982; Jacoby et al., 1986; Guard and Watson, 1987; Guard et al., 1988; Yau et al., 1992; Patacchini et al., 1995). This is in agreement with the finding that intrinsic primary afferent neurons with immunoreactivity for tachykinin NK3 receptors are also choline acetyl transferase immunoreactive (Mann et al., 1999).
Overall, these results suggest an important modulatory role for tachykinin NK3 receptors in intrinsic neuro-neuronal enteric signalling. However, studies on the functional role of NK3 receptors in enteric neurotransmission were almost exclusively conducted in intestinal preparations from the guinea pig. It is not clear whether similar NK3 receptor-mediated modulatory mechanisms are also operational in the intestine of other species. We previously provided evidence that tachykinin NK1, NK2 and NK3 receptors modulate the contraction to exogenous tachykinins in the mouse small intestine (De Schepper et al., 2005). Because tachykinin NK3 receptors are promising therapeutic targets for the treatment of intestinal motility disorders, we investigated their functional role in mediating cholinergic excitatory transmission in the mouse stomach fundus and small intestine.
Methods
Animals
All animal procedures and experimental protocols received approval of the Committee for Medical Ethics of the University of Antwerp. Swiss OF1 mice (25–30 g) were fasted for 24 h with free access to water before experimentation. Mice were anaesthetized with diethyl ether and exsanguinated from the carotid artery. The gastrointestinal tract was rapidly removed and put in ice-cold aerated Krebs–Ringer solution (118.3 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 25 mM NaCHO3, 0.026 mM CaEDTA and 11.1 mM glucose). The stomach and a ∼10 cm long segment of the ileum, located ∼10 cm above the ileocolonic junction, were removed and prepared further (see below).
Pharmacological studies: tissue preparation and isometric tension recording
The stomach was opened along the lesser curvature, the mucosa removed by sharp dissection and longitudinal muscle strips were cut. The ileal segment was gently flushed with Krebs–Ringer solution and opened longitudinally along the mesenteric border. The mucosa was removed and muscle strips were cut in the longitudinal direction. A silk thread was attached at the upper and lower end of the muscle strips after which they were mounted in organ baths (volume 5 mL) filled with Krebs–Ringer solution (37 °C, aerated with 5% CO2/95% O2). The muscle strips in the organ baths were positioned between two platinum ring electrodes (diameter of rings: 5 mm) for electrical stimulation of the tissues. The lower end of the muscle strip was fixed and the other end connected to a strain gauge transducer (Scaime transducers, Annemasse, France) for the recording of isometric tension. The strips were stretched to optimal length–tension relationship as described previously (De Man et al., 2001, 2003). Briefly, strips were contracted with carbachol (0.1 μM) and the amplitude of the contraction was recorded. Carbachol was washed out of the organ bath and when tension returned to baseline, the strips were stretched (increment of 2.5 mN). After stabilization of the muscle strip tension, 0.1 μM carbachol was added again and the amplitude of the contraction was recorded. This procedure was repeated until the amplitude of the contraction to carbachol reached a maximum. This point was taken as the optimal length–tension. The tissues were then allowed to equilibrate for 60 min before starting the experimentation. During the equilibration period, the preparations were washed every 15 min with fresh Krebs–Ringer solution.
Experimental protocols
Frequency–response curves to EFS (0.25–4 Hz, train duration 10 s, pulse width 1 ms) and concentration–response curves to carbachol were constructed in muscle strips from the gastric fundus and ileum. Stimulation trains for EFS were delivered at decreasing frequency (starting at 4 Hz and finishing at 0.25 Hz, see Figure 1), as we found that this resulted in more reproducible responses especially at lower frequency stimulation (De Man et al., 2001, 2003; De Schepper et al., 2005). The aim of our study was to induce excitatory cholinergic responses, and therefore all experiments were conducted in the presence of the NOS inhibitor L-NOARG to avoid interference from inhibitory nitrergic neurotransmission. In a preliminary series of experiments, the effect of 1 μM atropine and 1 μM tetrodotoxin was investigated on the frequency–response curves to EFS in gastric fundus and ileal muscle strips. All further experiments, unless indicated otherwise, were performed in the presence of the tachykinin NK1 and NK2 receptor antagonists RP67580 (2 μM) and nepadutant (1 μM) to avoid interference of NK1 and NK2 receptors. At the end of each experiment, muscle strips were challenged with 50 mM KCl. Preliminary experiments showed that the tachykinin NK3 receptor agonists and antagonists under study did not affect the contraction to KCl.
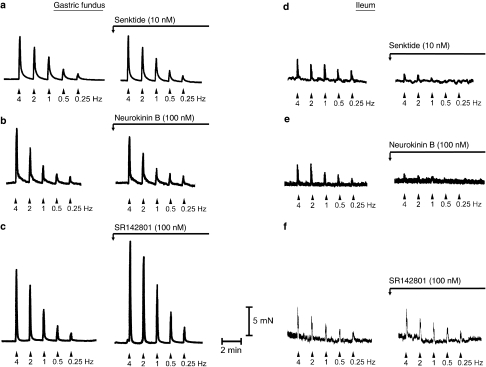
Figure 1.
Typical experimental recordings of isolated muscle strips from the mouse gastric fundus (a–c) and ileum (d–f) showing cholinergic nerve-mediated contractions to electrical field stimulation (0.25–4 Hz) in control conditions (saline) and after incubation with 10 nM senktide (a and d), 100 nM neurokinin B (NKB; b and e) and 100 nM SR142801 (c and f). Horizontal bar represents scale of time (2 min) and vertical bar represents scale of force (5 mN). Stimulation trains for EFS were delivered at decreasing frequency as this results in more reproducible responses, especially at low-frequency stimulation (see Methods).
In a first series of experiments, the effect of the tachykinin NK3 receptor agonist senktide (0.1, 1 or 10 nM; Guard and Watson, 1987; Guard et al., 1988; Patacchini et al., 1995; Alexander et al., 2008) and NKB (1, 10 or 100 nM; Jacoby et al., 1986; Emonds-Alt et al., 1995; Smits and Lefebvre, 1994; Alexander et al., 2008) was studied on the frequency–response curves to EFS and on the dose–response curves to carbachol in gastric fundus and ileal muscle strips. In these experiments, individual muscle strips were challenged only once with a respective concentration of senktide or NKB to avoid desensitization of NK3 receptors because of repetitive challenging with NK3 receptor agonists. The incubation time for senktide and NKB was 10 min.
In a second series of experiments, the effect of the selective tachykinin NK3 receptor antagonist SR142801 (0.03–0.1 μM; Emonds-Alt et al., 1995; Patacchini et al., 1995; Holzer et al., 1998) was investigated on the frequency–response curves to EFS and on the dose–response curves to carbachol in gastric fundus and ileal muscle strips. Because the antagonistic actions of SR142801 on tachykinin NK3 receptors may be slow in developing (Emonds-Alt et al., 1995), the incubation time with SR142801 was 30 min.
Presentation of results and statistical analysis
The amplitude of the contractions was expressed as percentage of contraction of the reference contraction in response to 50 mM KCl. Results are shown as mean±s.e.mean for the number (n) of mice indicated. For statistical analysis, Student's t-test for paired or unpaired values or one-way ANOVA was used, followed by Dunnett's or Bonferroni post hoc testing as appropriate. P-values of ⩽0.05 were considered to be significant.
Solutions and drugs
Receptor and drug nomenclature in this study conform to the Guide to Receptors and Channels of the British Journal of Pharmacology (Alexander et al., 2008). The following drugs were used: β-A-NKA, RP67580, senktide (Tocris Bioscience, Bristol, UK); septide (Calbiochem, San Diego, CA, USA); atropine sulphate, carbachol, hexamethonium, L-NOARG, neurokinin B, tetrodotoxin (Sigma-Aldrich, St Louis, MO, USA); SR142801 was kindly provided by Sanofi-Synthelabo Recherche, Chilly-Mazarin, France and nepadutant was kindly provided by Dr Criscuoli, Menarini, Florence, Italy. A stock solution of 1 μM SR142801 (osanetant) was dissolved in 100% DMSO. All other drugs were dissolved in water. Solutions were injected in the 5 mL organ bath in volumes of 2.5–5 μL. The final volume of DMSO in the organ bath did not exceed 0.1%, which did not affect the contractions of the muscle strips.
Results
Mouse gastric fundus
In the presence of the NOS blocker, L-NOARG (300 μM), EFS (0.25–4 Hz) of gastric fundus muscle strips induced frequency-dependent contractions. These contractions were abolished by either tetrodotoxin (1 μM) or atropine (1 μM) (Table 1) demonstrating that they result from the activation of cholinergic enteric nerves. All further experiments were performed in the presence of the tachykinin NK1 and NK2 receptor antagonists RP67580 (2 μM) and nepadutant (1 μM). The neurogenic contractions to EFS in the mouse gastric fundus were not affected by RP67580 plus nepadutant (1 Hz: 22.8±2.2% vs 24.8±1.6%; 2 Hz: 31.2±2.7% vs 34.1±2.3%; control (n=10) vs RP67580 plus nepadutant (n=18), unpaired Student's t-test).
Table 1.
Effect of tetrodotoxin (TTX; 1 μM) and atropine (1 μM) on the contractions induced by electrical field stimulation (EFS) in the mouse gastric fundus and small intestine
| EFS | Control | TTX | Control | Atropine |
|---|---|---|---|---|
| Gastric fundus | ||||
| 0.5 Hz | 12.5±2.6 | 0±0* | 12.0±2.3 | 0±0* |
| 1 Hz | 23.7±3.4 | 0±0* | 22.1±3.0 | 0±0* |
| 2 Hz | 32.3±3.9 | 0±0* | 30.3±3.8 | 0±0* |
| 4 Hz | 48.7±4.2 | 0±0* | 45.4±3.3 | 0±0* |
| Small intestine | ||||
| 0.5 Hz | 24.3±2.2 | 0±0* | 27.4±1.9 | 0±0* |
| 1 Hz | 36.8±2.8 | 0±0* | 36.3±3.3 | 0±0* |
| 2 Hz | 46.0±2.7 | 0±0* | 45.3±2.4 | 0±0* |
| 4 Hz | 57.4±3.8 | 1±1* | 57.8±4.3 | 1±1* |
Contractions are expressed as percentage of a 50 mM KCl contraction and shown as mean±s.e.mean. *P⩽0.05, Student's t-test for paired observations (n=5).
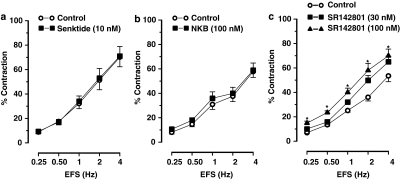
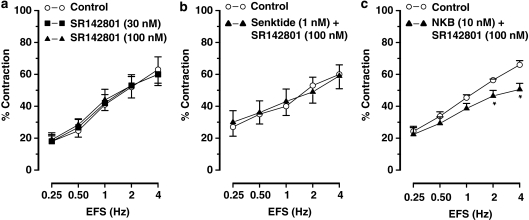
The tachykinin NK3 receptor agonist senktide (0.1–10 nM) and NKB (1–100 nM) did not induce any response per se in gastric fundus muscle strips and they did not affect the contractions to EFS (Figures 1a, b, 2a and b) or carbachol (results not shown).
Figure 2.
Frequency–response curves showing the cholinergic nerve-mediated contractions to electrical field stimulation (EFS) of muscle strips from the mouse gastric fundus. (a and b) show the effect of the NK3 receptor agonist senktide (10 nM) and neurokinin B (NKB; 100 nM), respectively. (c) Shows the effect of the NK3 receptor antagonist SR142801 (30–100 nM) on EFS-induced contractions. Results are expressed as mean±s.e.mean for six experiments. Student's t-test for paired observations did not show differences in the effect of senktide and NKB. *P⩽0.05, One-way ANOVA followed by Dunnett's post hoc test.
The tachykinin NK3 receptor antagonist SR142801 (30–100 nM) dose dependently enhanced the contractions to EFS (Figures 1c and 2c) without affecting the dose–response curves to carbachol (0.01–1 μM) (results for 0.03 μM carbachol: 33±4% in controls and 28±3% in strips treated with 100 nM SR142801, n=5).
Mouse ileum
In the presence of the NOS blocker, L-NOARG (300 μM), EFS (0.25–4 Hz) of ileal muscle strips induced frequency-dependent contractions that were blocked by either tetrodotoxin (1 μM) or atropine (1 μM) (Table 1) demonstrating the neurogenic and cholinergic nature of these contractions. All further experiments were performed in the presence of RP67580 (2 μM) and nepadutant (1 μM). Comparison of the neurogenic contractions to EFS in the mouse ileum showed that NK1 plus NK2 receptor blockade did not affect these contractions (1 Hz: 36.9±1.9% vs 36.6±1.5%; 2 Hz: 45.4±1.9% vs 45.0±1.8%, control (n=25) vs RP67580 plus nepadutant (n=47), unpaired Student's t-test).
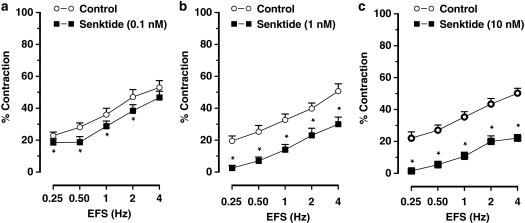
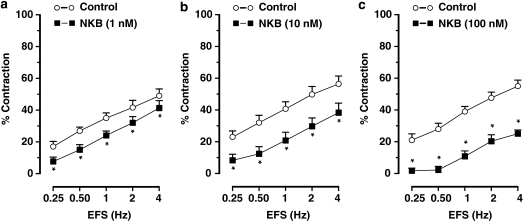
Senktide (0.1–10 nM) or NKB (1–100 nM) did not induce any response per se in ileal muscle strips but dose dependently inhibited the cholinergic nerve-mediated contractions to EFS (Figures 1d, e, 3 and 4). Senktide or NKB did not affect the dose–response curve to carbachol (0.01–1 μM; results for 0.3 μM carbachol: 38±2% in control vs 40±3% after 10 nM senktide and 42±3% in controls vs 37±2% after 100 nM neurokinin B, n=4–6).
Figure 3.
Frequency–response curves showing the cholinergic nerve-mediated contractions to electrical field stimulation (EFS) of muscle strips from the mouse ileum showing the effect of the NK3 receptor agonist senktide (a) 0.1 nM, (b) 1 nM and (c) 10 nM, obtained in different muscle strips to avoid desensitization of NK3 receptors. Results are expressed as mean±s.e.mean for six experiments. *P⩽0.05, Student's t-test for paired observations.
Figure 4.
Frequency–response curves showing the cholinergic nerve-mediated contractions to electrical stimulation (EFS) of muscle strips from the mouse ileum showing the effect of neurokinin B (NKB; a) 1 nM, (b) 10 nM and (c) 100 nM, obtained in different muscle strips to avoid desensitization of NK3 receptors. Results are expressed as mean±s.e.mean for five experiments. *P⩽0.05, Student's t-test for paired observations.
SR142801 (30–100 nM) by itself did not affect the contractions to electrical stimulation (Figures 1f and 5a), but blocked the inhibitory effect of senktide on the contractions to EFS (Figure 5b). SR142801 also abolished the inhibitory effect of NKB on contractions to lower frequency stimulation and reduced the effect of NKB on contractions to higher frequency stimulation (Figure 5c).
Figure 5.
Frequency–response curves showing the cholinergic nerve-mediated contractions to electrical field stimulation (EFS) of muscle strips from the mouse ileum showing the effect of (a) SR142801 per se, (b) the effect of senktide in the presence of SR142801 and (c) the effect of neurokinin B (NKB) in the presence of SR142801. Results are expressed as mean±s.e.mean for five experiments. *P⩽0.05, Student's t-test for paired observations.
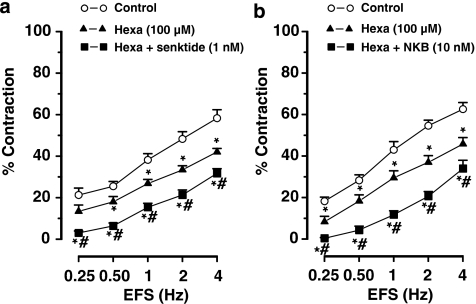
Blockade of synaptic transmission by the nicotinic receptor antagonist, hexamethonium (100 μM), inhibited the cholinergic nerve-mediated contractions to EFS (Figure 6). In the presence of hexamethonium, the inhibitory effect of the NK3 receptor agonist senktide and NKB was still evident (Figure 6) and comparable to their effect in the absence of hexamethonium (Figures 3b and 4b).
Figure 6.
Frequency–response curves showing the cholinergic nerve-mediated contractions to electrical field stimulation (EFS) of muscle strips from the mouse ileum showing the effect of hexamethonium (hexa) and (a) hexamethonium plus senktide and (b) hexamethonium plus neurokinin B (NKB). Results are expressed as mean±s.e.mean for four to six experiments. One-way ANOVA followed by Bonferroni post hoc test, *P⩽0.05, significantly different from results obtained in control conditions; #P⩽0.05, significantly different from results obtained in the presence of hexamethonium.
Because cholinergic neurotransmission in the mouse small intestine is modulated by tachykinin NK3 receptor activation, we additionally investigated the effect of agonists of tachykinin NK1 and NK2 receptors. These experiments were performed in the absence of the tachykinin NK1 and NK2 receptor antagonists RP67580 and nepadutant but in the continuous presence of the NOS blocker L-NOARG (300 μM). The NK1 agonist septide (0.1 μM) and the NK2 agonist β-A-NKA (0.1 μM) induced a transient contraction per se of 37±5% and 31±4%, respectively (n=6). These contractions returned to baseline within 8–10 min after which a frequency–response curve to EFS was constructed. Contractions to EFS of mouse ileal muscle strips were not affected by septide or β-A-NKA (Table 2).
Table 2.
Effect of the NK1 receptor agonist septide (0.1 μM) and the NK2 receptor agonist β-A-NKA (0.1 μM) on the contractions induced by electrical field stimulation (EFS) in the mouse small intestine, obtained in the absence of NK1 and NK2 receptor antagonists RP67580 and nepadutant, respectively
| EFS | Control | Septide | Control | β-A-NKA |
|---|---|---|---|---|
| 0.5 Hz | 26.0±4.8 | 24.6±4.7 | 31.0±5.4 | 27.8±4.6 |
| 1 Hz | 36.1±5.0 | 32.3±5.5 | 39.4±4.9 | 35.8±4.4 |
| 2 Hz | 42.9±5.1 | 40.7±4.9 | 47.5±4.8 | 44.8±6.1 |
| 4 Hz | 50.0±7.9 | 53.8±8.0 | 52.2±5.1 | 48.9±8.5 |
Contractions are expressed as percentage of a 50-mM KCl contraction and shown as mean±s.e.mean. Student's t-test for paired observations did not show significant differences (n=6).
Discussion and conclusions
Previous findings on the functional role of intestinal tachykinin NK3 receptors in enteric neurotransmission were obtained almost exclusively from experiments in the guinea-pig small intestine. These studies showed a role for tachykinin NK3 receptors in neuro-neuronal signalling from intrinsic primary afferent nerves to myenteric interneurons and motor nerves (Johnson et al., 1996, 1998; Alex et al., 2001). This agrees with the presence of tachykinin NK3 receptor immunoreactivity in intrinsic primary afferent neurons, interneurons and excitatory motor neurons innervating the longitudinal muscle of the guinea-pig small intestine (Jenkinson et al., 1999). In the mouse small intestine, immunohistochemistry showed a wide distribution of NK3 receptors in the myenteric and submucosal plexus and smooth muscle layers (Vannucchi and Faussone-Pellegrini, 2000; Wang et al., 2002), but a functional role for tachykinin NK3 receptors in mouse gastrointestinal contractility remained to be shown.
In this study, we demonstrated that the tachykinin NK3 receptor agonist senktide and neurokinin B, which preferentially activates NK3 receptors, had no effect per se in mucosa-free muscle strips of the mouse gastric fundus. This suggests that tachykinin NK3 receptors are not present in smooth muscle cells of the mouse gastric fundus. In the rat, mucosa-intact muscle strips of the gastric fundus contract dose dependently to neurokinin B, and these contractions are insensitive to tetrodotoxin and are mediated by muscular NK3 receptors (Smits and Lefebvre, 1994). There is evidence from electrophysiological studies that the activation of tachykinin NK3 receptors excites guinea-pig gastric neurons (Schemann and Kayser, 1991). To examine whether NK3 receptors have a function in enteric neurotransmission in the mouse gastric fundus, the effect of tachykinin NK3 receptor agonists and antagonists was investigated on cholinergic neurotransmission. Previous findings in the mouse whole stomach showed the involvement of nitric oxide (NO), acetylcholine and tachykinins in the response to EFS (Mulè and Serio, 2002). In our study on mucosa-free longitudinal muscle strips of the mouse gastric fundus, we aimed to investigate cholinergic excitatory neurotransmission and the parameters for electrical stimulation were therefore chosen to induce contractions of cholinergic origin. Consequently, the contractions to EFS were fully sensitive to atropine and tetrodotoxin. To avoid the interference of NK1 and NK2 receptors when studying the modulatory role of NK3 receptors, all further experiments were performed in the presence of NK1 and NK2 receptor antagonists. Blockade of NK1 and NK2 receptors did not affect the cholinergic neurogenic contractions to EFS.
We found that SR142801, a selective antagonist of tachykinin NK3 receptors (Emonds-Alt et al., 1995), significantly enhanced the cholinergic nerve-mediated contractions in the gastric fundus. The potentiating effect of SR142801 on cholinergic nerve-mediated contractions in the gastric fundus was most likely at a presynaptic site as SR142801 did not alter the direct smooth muscle contractions to the muscarinic receptor agonist carbachol. Our findings suggest that endogenous tachykinins released from enteric nerves inhibit cholinergic nerve activity in the mouse gastric fundus through the activation of prejunctional NK3 receptors. Blockade of this modulatory mechanism then results in an increased response to electrical stimulation.
It should be noted that the pharmacological activity of SR142801 is highly species dependent, showing high affinity binding to guinea-pig but not to rat NK3 receptors (Emonds-Alt et al., 1995). In addition, SR142801 may lose selectivity for tachykinin NK3 receptors when used at high doses (Emonds-Alt et al., 1995; Lecci et al., 1996). We, however, previously reported that SR142801 up to 0.3 μM does not affect contractions to tachykinin NK1 and NK2 receptor agonists in the mouse small intestine (De Man et al., 2008). SR142801 in the concentration range that we have used in this study (30–100 nM) also showed NK3 receptor selectivity in other studies on isolated intestinal tissues (Giuliani and Maggi, 1995; Patacchini et al., 1995; Johnson et al., 1998; Alex et al., 2001) and it effectively reversed the action of senktide in the mouse small intestine in this study (Figure 5b).
Treatment of mouse gastric fundus muscle strips with the NK3 receptor agonist senktide or NKB did not affect the electrically induced cholinergic nerve-mediated contractions. This may indicate that endogenous tachykinins, released by electrical stimulation of enteric nerves, optimally activate NK3 receptors thereby preventing any additional effect of exogenously added NK3 receptor agonists.
In muscle strips of the mouse small intestine, the NK3 receptor agonist senktide and NKB failed to induce any contraction per se. Previous findings in longitudinal muscle strips of the guinea-pig ileum and colon (Guard and Watson, 1987; Guard et al., 1988; Giuliani and Maggi, 1995) showed dose-dependent contractions in response to senktide and neurokinin B, and these contractions are abolished by either tetrodotoxin or a combination of muscarinic and tachykinin NK1 receptor blockers. This suggests that the activation of tachykinin NK3 receptors in the guinea-pig intestine induces the release of acetylcholine and tachykinins from enteric nerves thereby inducing an indirect smooth muscle contraction. The failure of senktide to induce a contraction per se in mouse ileal muscle strips suggests that such an NK3 receptor-mediated activation of excitatory enteric nerves does not exist in the mouse small intestine. Possibly, the presence of tachykinin NK1 and NK2 receptor antagonists in our study may have prevented a direct smooth muscle response to senktide. However, also in the absence of NK1 and NK2 receptor antagonists, senktide did not induce changes in tension of longitudinal and circular muscle strips of the mouse small intestine (De Schepper et al., 2005; De Man et al., 2008). In mucosa-intact circular segments of the guinea-pig ileum, blockade of NK1 and NK2 receptors partially inhibited the contractile effect of senktide (Johnson et al., 1998), but it did not affect this contraction in mucosa-free circular muscle strips of the guinea-pig colon (Giuliani and Maggi, 1995).
The effect of tachykinin NK3 receptor agonists and antagonists was investigated on nerve-mediated contractions induced by EFS of the mouse ileum. Previous findings in the mouse small intestine demonstrated a cholinergic and tachykininergic component in the contractions to EFS in mucosa-intact circular segments (Saban et al., 1999) and mucosa-free circular muscle strips (De Schepper et al., 2005). As our aim was to study cholinergic excitatory neurotransmission, the parameters for electrical stimulation were chosen to induce a neurogenic contraction of cholinergic origin. Consequently, the contractions to EFS were blocked by atropine and by tetrodotoxin. Although these contractions were essentially cholinergic in origin, further experiments on the modulatory role of NK3 receptors were conducted in the presence of NK1 and NK2 receptor antagonists. Blockade of NK1 and NK2 receptors had no effect on the cholinergic neurogenic contractions to EFS.
In contrast to our findings in the mouse stomach, NK3 receptor blockade by SR142801 did not affect the response to cholinergic nerve stimulation in the mouse small intestine. However, senktide and NKB induced a remarkably pronounced and dose-dependent inhibition of cholinergic nerve-mediated contractions and this effect was reversed by SR142801. This is in line with previous findings in mucosa-free muscle strips of the guinea-pig colon where senktide dose dependently inhibits electrically induced cholinergic twitch contractions, and this effect is partially reversed by SR142801 (Giuliani and Maggi, 1995). Our results suggested that the activation of NK3 receptors reduced excitatory cholinergic neurotransmission in the mouse small intestine, but this mechanism may not be operative in physiological conditions. This correlates well with the assumption that tachykinin NK3 receptors have a minor function in normal gut reflexes, as deduced from the lack of effect of NK3 receptor antagonists on intestinal peristalsis in the guinea pig, pig and rat (Holzer et al., 1998; Schmidt and Hoist, 2002; Shafton et al., 2004). Interestingly, Sanger et al. (2007) recently confirmed these findings but additionally showed that tachykinin NK3 receptors do modulate the peristaltic reflex but only at intestinal pressures that are associated with a defensive behaviour of the intestine. This suggests that tachykinin NK3 receptors in the small intestine come into play only in pathological conditions, such as rescue responses or inflammation. Inflammation of the gut is associated with tachykinin release from various sources (Holzer and Holzer-Petsche, 1997b, 2001), and NK3 receptor activation in these conditions may represent an important protective mechanism, decreasing the excitatory input to intestinal smooth muscles in the diseased intestine.
We found that the modulatory effect of senktide and NKB on cholinergic neurogenic contractions was not mimicked by the NK1 and NK2 receptor agonist septide and β-A-NKA, respectively. This does not exclude the possibility that tachykinin NK1 and NK2 receptors modulate excitatory transmission in the mouse small intestine, but it further demonstrated that the effect of senktide was specifically on NK3 receptors and did not involve non-specific or secondary effects on NK1 and NK2 receptors.
Studies in the guinea-pig intestine provided strong evidence that tachykinin NK3 receptors mainly mediate reflex transmission between intrinsic primary afferent nerves. In our study, we found that hexamethonium, which blocks synaptic transmission to interneurons and to enteric motor neurons (Johnson et al., 1996), did not affect the NK3 receptor-mediated inhibition of cholinergic nerve-mediated contractions. In vivo experiments in the rat demonstrated that the hexamethonium-resistant inhibitory effect of NK3 receptor activation on colonic contractions involves NO (Lecci et al., 1996). Our in vitro results do not exclude a modulatory role of NO at NK3 receptor-mediated synaptic transmission. However, the inhibition of cholinergic contractions by tachykinin NK3 receptor activation was observed in the presence of the NOS blocker L-NOARG, excluding the possibility that NO mediated this inhibitory effect. Owing to the lack of effect of hexamethonium and because signalling of intrinsic primary afferent nerves is largely disrupted in mucosa-free muscle strips, we propose that the observed action of senktide and NKB is on cholinergic motor neurons that directly supply the smooth muscle. This suggests the presence of tachykinin NK3 receptors on postganglionic cholinergic neurons in the mouse small intestine. This is in agreement with immunohistochemical evidence showing NK3 receptor immunoreactivity in myenteric neurons of this tissue (Vannucchi and Faussone-Pellegrini, 2000).
In conclusion, we demonstrated that tachykinin NK3 receptors modulate cholinergic nerve activity in the mouse gastrointestinal tract, but this modulation shows regional differences. Blockade of NK3 receptors enhanced cholinergic transmission in the stomach fundus but not in the small intestine. This indicates that endogenous tachykinins tonically inhibit cholinergic motor nerve activity in the stomach fundus by acting on presynaptic NK3 receptors. Activation of tachykinin NK3 receptors inhibited cholinergic transmission in the small intestine but not in the stomach. Thus, NK3 receptors have the potential to modulate neuromuscular transmission in the small intestine, but this effect may become prominent only in pathophysiological conditions that are associated with an upregulation of tachykinin release. Our results, therefore, suggest that tachykinin NK3 receptors in the mouse gastrointestinal tract have a physiological modulatory function in stomach fundus contractility and a pathophysiological modulatory function in the contractility of the small intestine.
Acknowledgments
This study was supported by Grant no. P5/20 of the Interuniversity Attraction Pole of the Belgian Federal Science Policy and by Grant no. G 0200.05 from the Fund for Scientific Research—Flanders (FWO-Vlaanderen).
Abbreviations
- β-A-NKA
[bAla8]-neurokinin A(4-10)
- EFS
electrical field stimulation
- SR142801 (osanetant)
N-[1-[3[1-benzoyl-3-(3, 4-dichlorophenyl)-3-piperidinyl]propyl]-4-phenyl-4-piperidinyl]-N-methyl-, monohydrochloride
Conflicts of interest
The authors state no conflict of interest.
References
- Alex G, Kunze WA, Furness JB, Clerc N. Comparison of the effects of neurokinin-3 receptor blockade on two forms of slow synaptic transmission in myenteric AH neurons. Neuroscience. 2001;104:263–269. doi: 10.1016/s0306-4522(01)00064-1. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Man JG, Boeckx S, Anguille S, De Winter BY, de Schepper HU, Herman AG, et al. Functional study on TRPV1-mediated signalling in the mouse small intestine: involvement of tachykinin receptors. Neurogastroenterol Motil. 2008;20:546–556. doi: 10.1111/j.1365-2982.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- De Man JG, Moreels TG, De Winter BY, Bogers JJ, Van Marck EA, Herman AG, et al. Disturbance of the prejunctional modulation of cholinergic neurotransmission during chronic granulomatous inflammation of the mouse ileum. Br J Pharmacol. 2001;133:695–707. doi: 10.1038/sj.bjp.0704115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Man JG, Seerden TC, De Winter BY, Van Marck EA, Herman AG, Pelckmans PA. Alteration of the purinergic modulation of enteric neurotransmission in the mouse ileum during chronic intestinal inflammation. Br J Pharmacol. 2003;139:172–184. doi: 10.1038/sj.bjp.0705218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper HU, De Winter BY, Seerden TC, Herman AG, Pelckmans PA, De Man JG. Functional characterisation of tachykinin receptors in the circular muscle layer of the mouse ileum. Regul Pept. 2005;130:105–115. doi: 10.1016/j.regpep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Emonds-Alt X, Bichon D, Ducoux JP, Heaulme M, Miloux B, Poncelet M, et al. SR 142801, the first potent non-peptide antagonist of the tachykinin NK3 receptor. Life Sci. 1995;56:PL27–PL32. doi: 10.1016/0024-3205(94)00413-m. [DOI] [PubMed] [Google Scholar]
- Giuliani S, Maggi CA. Effect of SR 142801 on nitric oxide-dependent and independent responses to tachykinin NK3 receptor agonists in isolated guinea-pig colon. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:512–519. doi: 10.1007/BF00169385. [DOI] [PubMed] [Google Scholar]
- Grady EF, Baluk P, Bohm S, Gamp PD, Wong H, Payan DG, et al. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard S, Watling KJ, Watson SP. Neurokinin3-receptors are linked to inositol phospholipid hydrolysis in the guinea-pig ileum longitudinal muscle-myenteric plexus preparation. Br J Pharmacol. 1988;94:148–154. doi: 10.1111/j.1476-5381.1988.tb11509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard S, Watson SP. Evidence for neurokinin-3 receptor-mediated tachykinin release in the guinea-pig ileum. Eur J Pharmacol. 1987;144:409–412. doi: 10.1016/0014-2999(87)90398-0. [DOI] [PubMed] [Google Scholar]
- Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther. 1997a;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part II. Roles in neural excitation, secretion and inflammation. Pharmacol Ther. 1997b;73:219–263. doi: 10.1016/s0163-7258(96)00196-9. [DOI] [PubMed] [Google Scholar]
- Holzer P, Holzer-Petsche U. Tachykinin receptors in the gut: physiological and pathological implications. Curr Opin Pharmacol. 2001;1:583–590. doi: 10.1016/s1471-4892(01)00100-x. [DOI] [PubMed] [Google Scholar]
- Holzer P, Lippe IT, Heinemann A, Bartho L. Tachykinin NK1 and NK2 receptor-mediated control of peristaltic propulsion in the guinea-pig small intestine in vitro. Neuropharmacology. 1998;37:131–138. doi: 10.1016/s0028-3908(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Jacoby HI, Lopez I, Wright D, Vaught JL. Differentiation of multiple neurokinin receptors in the guinea pig ileum. Life Sci. 1986;39:1995–2003. doi: 10.1016/0024-3205(86)90323-1. [DOI] [PubMed] [Google Scholar]
- Jenkinson KM, Morgan JM, Furness JB, Southwell BR. Neurons bearing NK(3) tachykinin receptors in the guinea-pig ileum revealed by specific binding of fluorescently labelled agonists. Histochem Cell Biol. 1999;112:233–246. doi: 10.1007/s004180050411. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Bornstein JC, Burcher E. Roles of neuronal NK1 and NK3 receptors in synaptic transmission during motility reflexes in the guinea-pig ileum. Br J Pharmacol. 1998;124:1375–1384. doi: 10.1038/sj.bjp.0701967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Bornstein JC, Yuan SY, Furness JB. Analysis of contributions of acetylcholine and tachykinins to neuro-neuronal transmission in motility reflexes in the guinea-pig ileum. Br J Pharmacol. 1996;118:973–983. doi: 10.1111/j.1476-5381.1996.tb15495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecci A, Giuliani S, Tramontana M, Meini S, De Giorgio R, Maggi CA. In vivo evidence for the involvement of tachykinin NK3 receptors in the hexamethonium-resistant inhibitory transmission in the rat colon. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:671–679. doi: 10.1007/BF00167186. [DOI] [PubMed] [Google Scholar]
- Maggi CA. The troubled story of tachykinins and neurokinins. Trends Pharmacol Sci. 2000;21:173–175. doi: 10.1016/s0165-6147(00)01463-2. [DOI] [PubMed] [Google Scholar]
- Mann PT, Furness JB, Southwell BR. Choline acetyltransferase immunoreactivity of putative intrinsic primary afferent neurons in the rat ileum. Cell Tissue Res. 1999;297:241–248. doi: 10.1007/s004410051352. [DOI] [PubMed] [Google Scholar]
- Mann PT, Southwell BR, Ding YQ, Shigemoto R, Mizuno N, Furness JB. Localisation of neurokinin 3 (NK3) receptor immunoreactivity in the rat gastrointestinal tract. Cell Tissue Res. 1997;289:1–9. doi: 10.1007/s004410050846. [DOI] [PubMed] [Google Scholar]
- Mulè F, Serio R. Spontaneous mechanical activity and evoked responses in isolated gastric preparations from normal and dystrophic (mdx) mice. Neurogastroenterol Motil. 2002;14:667–675. doi: 10.1046/j.1365-2982.2002.00368.x. [DOI] [PubMed] [Google Scholar]
- Patacchini R, Bartho L, Holzer P, Maggi CA. Activity of SR 142801 at peripheral tachykinin receptors. Eur J Pharmacol. 1995;278:17–25. doi: 10.1016/0014-2999(95)00090-8. [DOI] [PubMed] [Google Scholar]
- Saban R, Nguyen N, Saban MR, Gerard NP, Pasricha PJ. Nerve-mediated motility of ileal segments isolated from NK(1) receptor knockout mice. Am J Physiol. 1999;277:G1173–G1179. doi: 10.1152/ajpgi.1999.277.6.G1173. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. Neurokinin NK1 and NK3 receptors as targets for drugs to treat gastrointestinal motility disorders and pain. Br J Pharmacol. 2004;141:1303–1312. doi: 10.1038/sj.bjp.0705742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger GJ, Tuladhar BR, Brown J, Aziz E, Sivakumar D, Furness JB. Modulation of peristalsis by NK(3) receptor antagonism in guinea-pig isolated ileum is revealed as intraluminal pressure is raised. Auton Autacoid Pharmacol. 2007;27:105–111. doi: 10.1111/j.1474-8673.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- Schemann M, Kayser H. Effects of tachykinins on myenteric neurones of the guinea-pig gastric corpus: involvement of NK-3 receptors. Pflugers Arch. 1991;419:566–571. doi: 10.1007/BF00370296. [DOI] [PubMed] [Google Scholar]
- Schmidt PT, Hoist JJ. Tachykinin NK1 receptors mediate atropine-resistant net aboral propulsive complexes in porcine ileum. Scand J Gastroenterol. 2002;37:531–535. doi: 10.1080/00365520252903062. [DOI] [PubMed] [Google Scholar]
- Shafton AD, Bogeski G, Kitchener PD, Lewis VA, Sanger GJ, Furness JB. Effects of the peripherally acting NK receptor antagonist, SB-235375, on intestinal and somatic nociceptive responses and on intestinal motility in anaesthetized rats. Neurogastroenterol Motil. 2004;16:223–231. doi: 10.1111/j.1365-2982.2004.00501.x. [DOI] [PubMed] [Google Scholar]
- Smits GJ, Lefebvre RA. Tachykinin receptors involved in the contractile effect of the natural tachykinins in the rat gastric fundus. J Auton Pharmacol. 1994;14:383–392. doi: 10.1111/j.1474-8673.1994.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Vannucchi MG, Faussone-Pellegrini MS. NK1, NK2 and NK3 tachykinin receptor localization and tachykinin distribution in the ileum of the mouse. Anat Embryol. 2000;202:247–255. doi: 10.1007/s004290000106. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang YQ, Ding YQ, Zhang JS. Localization of neurokinin B receptor in mouse gastrointestinal tract. World J Gastroenterol. 2002;8:172–175. doi: 10.3748/wjg.v8.i1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau WM, Mandel KG, Dorsett JA, Youther ML. Neurokinin3 receptor regulation of acetylcholine release from myenteric plexus. Am J Physiol. 1992;263:G659–G664. doi: 10.1152/ajpgi.1992.263.5.G659. [DOI] [PubMed] [Google Scholar]
- Yau WM, Youther ML. Direct evidence for a release of acetylcholine from the myenteric plexus of guinea pig small intestine by substance P. Eur J Pharmacol. 1982;81:665–668. doi: 10.1016/0014-2999(82)90357-0. [DOI] [PubMed] [Google Scholar]