Abstract
Background and purpose:
Ultra-low doses of opioid receptor antagonists augment spinal morphine antinociception and block the induction of tolerance. Considering the evidence demonstrating functional and physical interactions between the opioid and α2-adrenoceptors, this study investigated whether ultra-low doses of α2-adrenoceptor antagonists also influence spinal morphine analgesia and tolerance.
Experimental approach:
Effects of low doses of the competitive α2-adrenoceptor antagonists—atipamezole (0.08, 0.8 ng), yohimbine (0.02, 2 ng), mirtazapine (0.02 ng) and idazoxan (0.08 ng) were investigated on intrathecal morphine analgesia, as well as acute and chronic morphine antinociceptive tolerance using the rat tail flick and paw pressure tests.
Key results:
At doses markedly lower than those producing α2-adrenoceptor blockade, atipamezole, yohimbine, mirtazapine and idazoxan, prolonged the antinociceptive effects of morphine. When co-administered with repeated acute spinal injections of morphine, all four agents blocked the induction of acute tolerance. Co-injection of atipamezole with morphine for 5 days inhibited the development of tolerance in a chronic treatment paradigm. Spinal administration of atipamezole also reversed established antinociceptive tolerance to morphine as indicated by the restoration of morphine antinociceptive potency. The effects of atipamezole on spinal morphine tolerance were not influenced by treatment with 6-hydroxydopamine.
Conclusions and implications:
Low doses of competitive α2-adrenoceptor antagonists can augment acute morphine analgesia and block or reverse tolerance to spinal administration of morphine. These actions are interpreted in terms of their interaction with an opioid-α2-adrenoceptor complex, whose activity may have a function in the genesis of analgesic tolerance.
Keywords: adrenoceptor antagonists, spinal analgesia, morphine tolerance
Introduction
Spinal administration of opioid drugs such as morphine to experimental animals and humans produces potent analgesia, an effect mediated by pre- and post-synaptic opioid receptors localized on terminals of the nociceptive primary afferents and the dorsal horn spinal neurons, respectively. Repeated spinal administration of opioids, however, produces tolerance, a phenomenon manifesting as a decrease in both the magnitude of the pharmacological response and the agonist potency (an increase in ED50 value). These acute and chronic effects of opioids are also observable after treatment with agonists acting on the spinal α2-adrenoceptors (Reddy et al., 1980; Milne et al., 1985; Takano and Yaksh, 1992; Quartilho et al., 2004, all receptor nomenclature follows Alexander et al., 2008) known to be physiological targets of noradrenaline, a neurotransmitter present in descending noradrenergic projections to the dorsal horn and other spinal regions.
Previous pharmacological and molecular studies have provided considerable evidence for functional interaction between the opioid receptors and α2-adrenoceptors in the spinal cord. Animals tolerant to antinociceptive actions of intrathecal morphine show reduced α2-adrenoceptor agonist-induced antinociception (Milne et al., 1985; Stevens et al., 1988). Competitive opioid receptor antagonists such as naloxone or naltrindole can antagonize the antinociceptive effect of spinal noradrenaline and other α2-adrenoceptor agonists (Loomis et al., 1985; Takano et al., 1996), attenuate their inhibitory effects on spinal nociceptive neuronal discharge (Omote et al., 1991), and disrupt the analgesic synergy between the opioid and α2-adrenoceptor agonists (Roerig et al., 1992; Sullivan et al., 1992). Taken together, these studies support a role for opioid receptor activity in the expression of antinociceptive actions of α2-adrenoceptor agonists. Conversely, adrenoceptor antagonists, including yohimbine, an α2-adrenoceptor antagonist, have been reported to antagonize opioid-induced analgesia (Browning et al., 1982; Bentley et al., 1983). Aley and Levine (1997) showed that antagonists selective for the μ opioid receptor and α2-adrenoceptors produced ‘cross' blockade of the peripheral antinociceptive actions of agonists activating the opposite receptor. The authors proposed the existence of a multireceptor complex incorporating μ opioid receptors and α-adrenoceptor units, at which the effects of an agonist acting on one receptor component could be reciprocally influenced by an antagonist acting on the other component. Indeed, recent studies have provided biochemical evidence for the physical association of α2-adrenoceptors with opioid receptors (Jordan et al., 2003; Rios et al., 2004) and have identified that functional μ-α2-adrenoceptor complexes can form in brain and spinal cord neurons. However, the significance of such a receptor complex in the production of acute or chronic responses produced by activation of its constituent receptor units is presently unknown.
Recent studies have demonstrated that ultra-low doses of the non-selective opioid antagonist naltrexone paradoxically augment morphine antinociception and inhibit the development of opioid tolerance (Powell et al., 2002), effects shared not only by low doses of the μ opioid receptor antagonists, but also the δ opioid receptor antagonists such as naltrindole (Abul-Husn et al., 2007; McNaull et al., 2007). The crossover effects of low-dose naltrindole on the actions of morphine, an agonist with preference for the μ opioid receptor, have been explained on the basis of its antagonist interaction with the δ opioid component in the μ-δ receptor complex (Gomes et al., 2004). Low doses of a δ opioid receptor antagonist have been reported to increase the potency and efficacy of μ opioid receptor signalling in cells expressing the μ-δ receptor complex (Gomes et al., 2004). Thus, given the evidence for a significant interplay between the α2-adrenoceptor and opioid receptor activity in the behavioural models of spinal antinociception and tolerance and the existence of a μ-α2-adrenoceptor complex in the spinal neurons, the present study examined whether ultra-low doses of competitive α2-adrenoceptors antagonists modulated the acute and chronic effects of morphine in the spinal analgesia model. An additional goal was to determine if such actions of the α2-adrenoceptor antagonists were dependent on the integrity of the spinal noradrenergic input.
Methods
Animals
The experiments were performed in accordance with Guidelines of the Canadian Council on Animal Care using a protocol approved by the Queen's University Animal Care Committee. All experiments in this study were conducted on male Sprague–Dawley rats (275–300 g) obtained from Charles River Canada, Montreal PQ, Canada. Animals were acclimatized 3–4 days before surgery, given free access to food and water ad libitum and maintained under a 12 h light/dark cycle at room temperature (21–23 °C). Each animal was used only in one experiment.
Intrathecal catheter implantation
The indwelling intrathecal catheter implantation was performed under anaesthesia using the procedure described by Yaksh and Rudy (1976). Briefly, each animal was anaesthetized with 2.5% halothane and placed prone in a stereotaxic frame for the insertion of a catheter in the subarachinoid space through an opening in the cisterna magna. The cisternal membrane was surgically exposed and punctured carefully using a blunt needle curved at the tip. A polyethylene catheter (7.5 cm long, PE-10) was then inserted through the cisternal opening and advanced caudally such that the tip rested at the lumbar enlargement of the spinal cord. The rostral tip of the catheter was inserted under the skin overlying the skull and exteriorized to protrude at the top of the head. The surgical opening was then closed, the implanted catheter flushed with 10 μL sterile saline and plugged. Animals were allowed to recover in the home cage for 4–5 days, closely monitored for signs of injury and given injections of lactated Ringer to prevent dehydration. Animals showing neurological deficits—visible forelimb or hindlimb deficits or limb paralysis—were excluded from the study. Drugs under investigation were administered through the exteriorized portion of the catheter in 10 μL volume and flushed in with 10 μL of sterile saline (0.9%). Control animals received equivalent injections of vehicle (saline) alone.
Behavioural assessment of nociception
Before testing, all animals used in the drug investigations were conditioned to the testing environment. The drug effects were evaluated using thermal and mechanical nociceptive tests as previous studies have shown low-dose opioid antagonists influence acute and chronic spinal morphine tolerance in both paradigms. All behavioural testing was performed without knowledge of the treatments. The tail flick test (D'Amour and Smith, 1941) evaluated the response to a brief thermal stimulus applied to the base of the tail using an antinociception meter (Owen et al., 1984). The time latency for tail removal from the stimulus source was recorded. Baseline latency was set between 2–3 s and cutoff time set at 10 s to prevent tissue damage. The paw pressure test evaluated response to a brief mechanical nociceptive stimulus applied to the dorsal hind paw using an inverted air-filled syringe linked to a pressure gauge (Loomis et al., 1987). The pressure was gradually increased until a withdrawal response was elicited. Baseline responses in this test ranged between 80–100 mm Hg and the maximal cutoff time was set at 300 mm Hg. The measurements of tail flick responses preceded the paw pressure responses in each animal and previous experience has revealed no significant interaction between responses in these tests (Loomis et al., 1985). All testing occurred between 0800–1400 h.
Any motor impairment that may have occurred as a result of pharmacological studies was assessed by visual inspection of the animals in their home cage, so as to minimize stress to the animals: effects included in the inspection were gait, grip strength and righting reflex.
Acute spinal morphine tolerance
Acute tolerance to the action of intrathecal morphine (15 μg) was induced by administration of three successive agonist injections delivered at 90 min intervals (McNaull et al., 2007). Control animals received similar injections of vehicle (0.9% saline). Prior to the drug injection, baseline response values were obtained in the tail flick and paw pressure test and animals were tested over 270 min. The response to drug administration was evaluated at 30-min intervals as peak morphine effect in both tests occurs at this time following the drug injection (Powell et al., 2002). The rats were placed in their home cages between the testing intervals. Twenty-four hours after the repeated injections of morphine or saline, cumulative dose–response curves for the acute action of morphine were derived to obtain quantitative estimates of the agonist potency (ED50 values). These curves were obtained by administering ascending cumulative doses (2.5, 5, 10 and 20 μg in the saline group and 12.5, 25, 50 and 100 μg in the morphine treatment group) of intrathecal morphine every 30 min until a maximal antinociceptive response was obtained in both nociception tests. Morphine ED50 values, indicators of the agonist potency, were derived from the dose–response curves obtained in these tests.
Tolerance to the agonist was indicated by a progressive decrease in the level of the antinociceptive effect (day 1) and a significant increase in the morphine ED50 value (day 2) over the corresponding value obtained in the saline-injected control animals.
To determine the action of low-dose α2-adrenoceptor antagonists (atipamezole, yohimbine, idazoxan and mirtazapine) on acute tolerance, each drug was co-injected with the first, second and third dose of intrathecal morphine. Its action on induction of acute morphine tolerance was evaluated by the ability to influence the magnitude of the morphine-induced response during the 270 min testing period and on the morphine potency (ED50 value) determined 24 h after administration of the drug combination.
Chronic morphine tolerance
Induction
Chronic opioid tolerance was induced by administration of intrathecal morphine (15 μg) once daily between 1000 and 1100 h for 5 days, as described by Powell et al. (2000). The response to the agonist was evaluated in the tail flick and paw pressure test 30 min after the daily injection (see Methods). To determine actions of low-dose α2-adrenoceptor antagonists on the tolerance induction, each agent under study was co-injected with the daily dose of morphine for 5 days. Nociceptive testing was performed 30 min after each injection. On day 6, dose–response curves for morphine were derived as described above and the ED50 values were determined from these curves. The ability of the α2-adrenoceptor antagonists to influence the induction of chronic tolerance to morphine was assessed by examining their effect on (a) the progressive decline of morphine antinociception, and (b) on the morphine potency value (ED50).
Reversal
The potential of an α2-adrenoceptor antagonist to reverse established spinal morphine tolerance was examined in animals rendered tolerant to chronic morphine (15 μg) for 5 days. From days 6–10, the antagonist was administered either alone (control) or in combination with morphine. The morphine ED50 values were determined on day 11 from the cumulative dose–response curves generated (as described above). The ability of drug treatments to reverse established morphine tolerance was indicated by recovery of the morphine antinociception and restoration of morphine potency (normalization of ED50).
Treatment with 6-OHDA
Animals were allowed to recover for 4–6 days after intrathecal surgery before they were treated with 6-hydroxydopamine (6-OHDA) as described earlier (Howe and Yaksh, 1982). Briefly, 6-OHDA (20 μg) or vehicle (0.01% ascorbic acid in 0.9% saline) was injected through intrathecal administration. To ensure the neurotoxic lesion was complete, the antinociceptive effects of intrathecal administration of L-noradrenaline (10 μg) were assessed 7 days following 6-OHDA treatment. Subsequently, animals were subjected to tolerance studies 19 days post-lesion.
Data analysis
Tail flick and paw pressure values were converted to percentage of maximum possible effect (M.P.E.): M.P.E.=100 × (post-drug response−baseline response)/(maximum response−baseline response). Data in the figures are expressed as mean±s.e.mean. ED50 values were determined using a non-linear regression analysis (Prism 2, GraphPad Software Inc., San Diego, CA, USA). Statistical significance (P<0.05) was determined using a one-way analysis of variance (ANOVA) followed by a Student Newman–Keuls post hoc test for multiple comparisons between groups.
Drugs
Morphine sulphate (BDH Pharmaceuticals, Toronto, Canada), clonidine, atipamezole (Farmos, Turku, Finland) yohimbine, idazoxan and mirtazapine and L-noradrenaline (Sigma Chemical Co, St Louis, MO, USA) were dissolved in 0.9% saline. In all drug interaction experiments, the α2-adrenoceptor antagonists and morphine were administered as a single solution prepared on the day of the experiment. 6-Hydroxydopamine (6-OHDA) was dissolved in 0.01% ascorbic acid solution prepared with 0.9% saline.
Results
Effects of α2-adrenoceptor antagonists on clonidine-induced antinociception
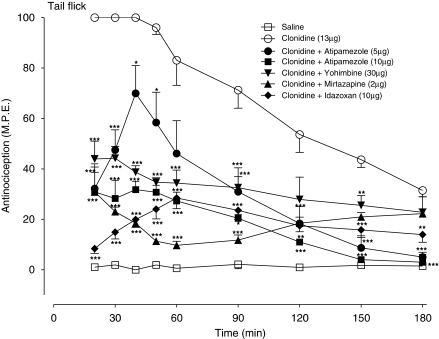
To establish that the α2-adrenoceptor antagonists selected for study (atipamezole, yohimbine, idazoxan and mirtazepine) would interact with the spinal α2-adrenoceptor sites under experimental conditions used in the present study, the effects of these agents were first assessed on the antinociceptive effects of the α2-adrenoceptor agonist, clonidine. The dose of clonidine used in these experiments was selected on the basis of pilot experiments to produce a maximal antinociceptive effect without producing motor impairment. In the tail flick test (Figure 1), intrathecal clonidine (13 μg) produced a sustained antinociceptive response that peaked at 15 min post-injection and continued to produce antinociception for up to 180 min. Addition of atipamezole (5, 10 μg) produced a dose-dependent inhibition of clonidine-induced thermal antinociception, with the highest dose (10 μg) abolishing the response. In subsequent experiments, yohimbine (30 μg), mirtazapine (2 μg) and idazoxan, (10 μg) also significantly attenuated the clonidine-induced thermal antinociceptive effect. Under similar test conditions, all four agents also produced a significant decrease in the effect of clonidine in the paw pressure test (data not shown). All subsequent experiments, exploring the interaction of these agents with spinal morphine, were performed using doses that were significantly lower than those producing blockade of clonidine-induced antinociceptive effects.
Figure 1.
Effects of intrathecal administration of α2-adrenoceptor antagonists, atipamezole (5, 10 μg) yohimbine (30 μg), mirtazapine (2 μg) and idazoxan (10 μg) on the acute antinociceptive action of a maximal dose of the α2-adrenoceptor agonist clonidine (13 μg) in the tail flick test. The antagonists were administered with clonidine as a single injection. Nociceptive testing was performed every 10 min after the injection for the first hour and every 30 min for the following 2-h test period. The data are expressed as mean±s.e.mean for four to six animals. Significant difference from the action of clonidine alone at the corresponding time point: *P<0.05, **P<0.01, ***P<0.001.
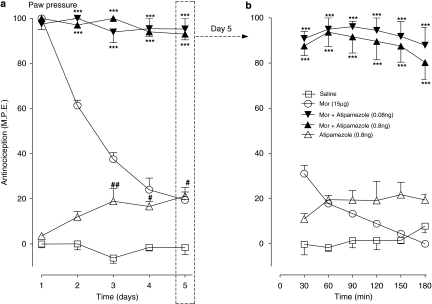
Effects of low-dose α2-adrenoceptor antagonists on acute morphine antinociception
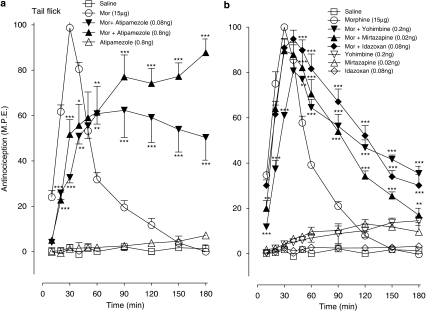
The effects of low doses of atipamezole and related α2-adrenoceptor antagonists on the morphine (15 μg, i.t.) antinociception in the tail flick and paw pressure tests are represented in Figures 2 and 3, respectively. In the tail flick test (Figure 2a), morphine elicited a peak antinociceptive response at 30 min post-drug injection and thermal nociceptive thresholds returned to baseline values by 90 min post-drug injection. The co-injection of the opioid agonist with atipamezole (0.08, 0.8 ng) initially reduced the peak antinociceptive response to approximately 60% of the maximal value; however, the response of the combination of morphine with atipamezole (0.8 ng) subsequently attained 80–90% of the maximal value and remained elevated significantly above that produced by morphine alone until the end of the test period (Figure 2a). Atipamezole (alone, 0.8 ng) did not produce a significant effect on thermal withdrawal latencies. Low doses of yohimbine (0.2 ng), mirtazapine (0.02 ng) and idazoxan (0.08 ng) when administered with morphine did not decrease in the peak response; however, all three agents significantly extended the morphine-induced thermal antinociceptive effect (Figure 2b).
Figure 2.
Time course of the effects of ultra-low doses of intrathecal (a) atipamezole (0.08, 0.8 ng), (b) yohimbine (0.2 ng), mirtazapine (0.02 ng) and idazoxan (0.08 ng) on the acute antinociceptive action of a maximal dose of morphine (Mor, 15 μg) in the tail flick test. Each agent was administered with morphine as a single injection. Nociceptive testing was every 10 min post-injected for the first 60 min and every 30 min for the following 2 h period. The data are expressed as mean±s.e.mean for four to seven animals. Significant difference is presented as difference from the action of morphine at the corresponding time point. *P<0.05, **P<0.01, ***P<0.001.
Figure 3.
Time course of the effects of ultra-low doses of intrathecal (a) atipamezole (0.08, 0.8 ng), (b) yohimbine (0.2 ng), mirtazapine (0.02 ng) and idazoxan (0.08 ng) on the acute antinociceptive action of a maximal dose of morphine (Mor, 15 μg) in the paw pressure test. Each agent was administered with morphine as a single injection. Nociceptive testing was every 10 min post-injected for the first 60 min and every 30 min for the following 2 h period. The data are expressed as mean±s.e.mean for four to seven animals. Significant difference is presented as difference from the action of morphine at the corresponding time point. *P<0.05, ***P<0.001.
In the paw pressure test (Figure 3a) atipamezole (0.08, 0.8 ng) significantly extended the morphine effect without producing initial depression of the morphine-induced mechanical antinociception. Administration of both atipamezole doses combined with morphine produced a maximal antinociceptive response 30-min post-injection and maintained it above that produced by the opioid agonist alone (Figure 3a). Indeed, the response to the combination of 0.8 ng of atipamezole with morphine was sustained at its maximal value during the 180-min testing period. As shown in Figure 3b, yohimbine, mirtazapine and idazoxan similarly extended the morphine-induced antinociceptive response, which exhibited a slower recovery to baseline thresholds than that in the preceding tail flick test (Figure 2b). The administration of the four antagonists alone elicited no significant antinociceptive response in either the thermal or mechanical nociceptive tests, or produced a weak response approximating 5–15% of the maximal value.
Thus, in these acute experiments, the combined administration of a maximal dose of intrathecal morphine with each of the four α2-adrenoceptor antagonists, at doses well below those producing spinal α2-adrenoceptor blockade, resulted in a significant augmentation of the antinociceptive response in both the tail flick and paw pressure tests. This augmentation was not associated with any visible sign of motor impairment.
Effects of ultra-low doses of α2-adrenoceptor antagonists on the induction of acute morphine tolerance
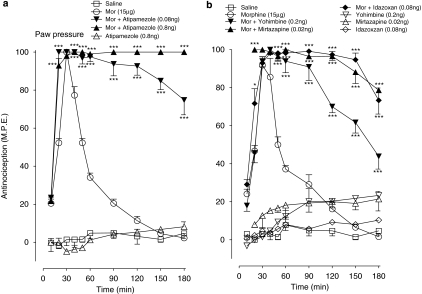
The effects of α2-adrenoceptor antagonists on the magnitude of the mechanical antinociceptive response to repeated acute injections of a maximal antinociceptive dose of morphine are shown in Figures 4a and b. The effects of these treatments on the potency of morphine (ED50 value), estimated 24 h after cessation of the drug administration are represented in Table 1. As shown in Figure 4a, three successive injections of morphine (15 μg), delivered at 90 min intervals, resulted in a progressive decline of the antinociceptive effect, with the peak response after the third injection reaching only 30% of the maximal response. Remarkably, the co-injection of atipamezole (0.8 ng) with each injection of morphine abolished the rapid decline of the pharmacological response and sustained this response near maximal value for the duration of the 240-min test period. Repeated administration of atipamezole (0.8 ng) alone initially produced little effect, but the second and third dosing elicited a weak antinociceptive response approximating 10–15% of the maximal value. Serial injections of saline did not produce a significant effect on mechanical withdrawal thresholds. In similar experiments (Figure 4b), yohimbine (0.2 ng), mirtazapine (0.02 ng) and idazoxan (0.08 ng) administered in combination with intrathecal morphine (15 μg) also attenuated the decline of the response produced by repeated morphine treatment. In these experiments, the response to first dosing with the drug combinations ranged between 80 and 95% of the maximal value and it was generally maintained at this level after subsequent dosings. All three agents produced non-significant effects on mechanical withdrawal thresholds when administered alone. The actions of α2-adrenoceptor antagonists were also evaluated in the tail flick test and all four agonists inhibited the decline of the antinociceptive response (data not shown but see Table 1).
Figure 4.
Effects of intrathecal (a) atipamezole (0.8 ng), (b) yohimbine (0.02 ng), mirtazapine (0.02 ng) and idazoxan (0.08 ng) on the induction of acute tolerance to morphine (Mor) in the paw pressure test. Tolerance was induced by intrathecal injection of morphine (15 μg) at 0, 90 and 180 min. Morphine was administered with atipamezole, yohimbine, mirtazapine and idazoxan as a singe injection. Nociceptive testing was performed every 30 min post-injection. The data are presented as mean±s.e.mean; n=five to six animals per treatment group. Significant difference from the action of morphine **P< 0.01, ***P< 0.001.
Table 1.
Effect of low dose i.t. atipamezole, idazoxan, mirtazapine and yohimbine on the induction of acute tolerance to morphine
| Acute treatment | Tail flick ED50 (μg; i.t.) (mean±s.e.mean) | Paw pressure ED50 (μg; i.t.) (mean±s.e.mean) |
|---|---|---|
| Saline | 5.5±0.2*** | 5.9±0.1*** |
| Morphine (15 μg) | 27.9±1.9 | 29.0±1.0 |
| Morphine/Atipamezole (0.8 ng) | 3.3±0.1*** | 3.7±0.1*** |
| Morphine/Idazoxan (0.08 ng) | 6.1±0.2*** | 4.5±0.5*** |
| Morphine/Mirtazapine (0.02 ng) | 15.2±0.8*** | 7.2±2.3*** |
| Morphine/Yohimbine (0.02 ng) | 2.8±0.2*** | 3.6±0.2*** |
| Atipamezole (0.8 ng) | 4.8±0.5*** | 8.6±2.1*** |
| Idazoxan (0.08 ng) | 7.2±2.9*** | 2.9±0.2*** |
| Mirtazapine (0.02 ng) | 6.8±0.3*** | 5.1±0.3*** |
| Yohimbine (0.02 ng) | 3.4±0.3*** | 4.6±0.1*** |
The morphine ED50 values were obtained from the cumulative dose–response curves to anti-nociceptive action of the opioid agonist 24 h after termination of drug treatment in the animal groups represented in Figures 4a and b.
The data presented are means ± s.e.mean; n=5–6 animals per treatment group.
Significant difference from the morphine (15 μg) group *** (P<0.001).
Table 1 shows the morphine ED50 values obtained from the cumulative morphine dose–response curves (not shown) in the drug treatment groups. In both tests, morphine ED50 value in the groups that had been treated with the opioid agonist alone showed approximately sixfold increase over the values in the control group treated with saline, reflecting a significant loss of the drug potency. It is noteworthy that the ED50 values (5.5, and 5.9 μg) obtained from cumulative dose–response curves in saline-treated rats are similar to the dose required to produce a 50% M.P.E. in opioid naive rats (Powell et al., 2002). This increase in ED50 was, however, abolished in the group receiving a combination of atipamezole with morphine. Repeated treatment with atipamezole alone did not produce a significant change in morphine ED50 relative to the treatment with saline. Similarly, yohimbine, mirtazapine or idazoxan, when combined with morphine, also abolished the increase in morphine ED50. Like atipamezole, none of these three agents administered alone had a significant influence on the ED50 value.
Thus, in these experiments, successive acute injections of morphine led to a very rapid decrease in magnitude of the antinociceptive response and a significant loss of drug potency, as reflected in elevated morphine ED50 values. The introduction of a low dose of an α2-adrenoceptor antagonist with each dose of morphine significantly reduced the loss of pharmacological effect and drug potency. None of the animals in these tests showed visible signs of motor impairment such as forelimb or hindlimb weakness or abnormality of gait.
Effects of ultra-low doses of an α2-adrenoceptor antagonist on the induction of chronic morphine tolerance
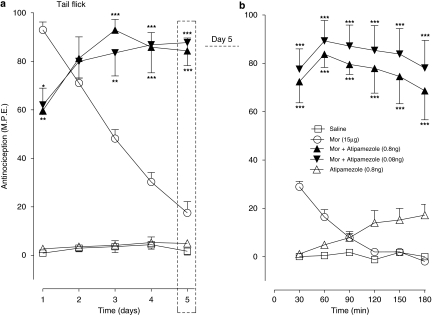
The actions of atipamezole (0.08, 0.8 ng) on the antinociceptive responses produced by the maximal dose of intrathecal morphine (15 μg) administered once daily for 5 days, in the tail flick and paw pressure test are shown in Figures 5a and 6a, respectively. As illustrated in Figure 5a, the administration of morphine on day 1 produced a maximal antinociceptive effect, but successive daily injections of morphine resulted in a progressive loss of the drug-induced antinociceptive response, which approached baseline value on day 5. Co-injection of atipamezole (0.08 or 0.8 ng) with morphine on day 1 initially reduced the peak response on day 1, but continuation of the drug combination resulted in its recovery to nearly 80–85% of the maximal value; the response remaining at this level until completion of the 5-day test period. Daily administration of atipamezole alone produced a response comparable with that produced by saline. Figure 5b illustrates the time course of the antinociceptive response in all treatment groups on day 5 of the drug administration. As shown, the peak effect of morphine, measured 30 min post-drug injection, reached only 25% of the value on day 1 and fell to the baseline level by 90 min post-injection. In sharp contrast, the response to atipamezole–morphine combinations approximated to 80% of the maximal value and it was sustained at this level during the 180 min test period. Atipamezole by itself elicited a delayed response that attained 20% of the maximal value towards the end of the test period.
Figure 5.
Effects of atipamezole on the induction of tolerance to chronic antinociceptive effects of morphine (Mor) in the tail flick test. (a) Time course of the action of chronic intrathecal morphine (5 μg) delivered in combination with saline or atipamezole (0.08, 0.8 ng) once daily for 5 days. Nociceptive testing was performed 60 min after each injection. The data are expressed as mean±s.e.mean for four to seven animals. *P<0.05, **P<0.01, ***P<0.001. (b) Time course of the effect of morphine–saline and morphine–atipamezole combination on day 5 in a subgroup of animals that had received chronic treatment with these drug treatments (panel a). Nociceptive testing was performed every 30 min after the drug injection. The data are expressed as mean±s.e.mean for four to five animals. Significant difference from morphine at the corresponding time point ***P<0.001.
Figure 6.
Effects of atipamezole on the induction of tolerance to chronic antinociceptive effects of morphine (Mor) in the paw pressure test. (a) Time course of the action of chronic intrathecal morphine (15 μg) delivered in combination with saline or atipamezole (0.08, 0.8 ng) once daily for 5 days. Nociceptive testing was performed 60 min after each injection. The data are expressed as mean±s.e.mean for four to seven animals. ***P<0.001. (b) Time course of the effect of morphine–saline and morphine–atipamezole combination on day 5 in a subgroup of animals that had received chronic treatment with these drug treatments (panel a). Nociceptive testing was performed every 30 min after the drug injection. The data are expressed as mean±s.e.mean for four to five animals. Significant difference from morphine at the corresponding time point ***P<0.001. Significant difference from saline at the corresponding time point. #P<0.05, ##P<0.01.
In the paw pressure test (Figure 6a), the 5-day morphine treatment was associated with a rapid decay of the mechanical withdrawal antinociceptive response similar to that observed in the preceding tail flick test. Administration of the opioid agonist with low doses of atipamezole produced 90% of the maximal response, which was maintained until completion of the 5-day test period. The time course of the response to drug treatments on day 5 is represented in Figure 6b. As illustrated, the response to morphine alone at 30 min approximated to 30% of the maximal value and it returned to the baseline value at 90 min. In marked contrast, the response to atipamezole–morphine combinations approached the maximal value and persisted at this level for the duration of the 180 min test period. The injection of atipamezole alone elicited only a weak antinociceptive effect.
Table 2 illustrates the morphine ED50 values obtained from the cumulative dose–response curves (not shown) for the action of morphine in groups of animals represented in Figures 5 and 6. The morphine ED50 in the 5-day morphine treatment group increased nearly sevenfold over the corresponding value in saline treatment group in the tail flick test and fivefold in the paw pressure test. This elevation in ED50 did not occur in groups receiving low doses of atipamezole with morphine in both tests. The ED50 value in the atipamezole only groups was comparable with that in saline treatment groups.
Table 2.
Effect of low dose atipamezole on the induction of tolerance to chronic antinociceptive effects of i.t. morphine
| Chronic treatment | Tail flick ED50 (μg; i.t.) (mean±s.e.mean) | Paw pressure ED50 (μg; i.t.) (mean±s.e.mean) |
|---|---|---|
| Saline | 5.4±0.1*** | 8.7±1.5*** |
| Morphine (15 μg) | 38.6±0.9 | 42.0±1.4 |
| Morphine+Atipamezole (0.08 ng) | 1.5±0.3*** | 2.5±0.4*** |
| Morphine+Atipamezole (0.8 ng) | 1.8±0.1*** | 2.7±0.2*** |
| Atipamezole (0.8 ng) | 4.2±0.2*** | 7.2±0.2*** |
Chronic i.t. morphine (15 μg) was delivered alone and in combination with atipamezole once daily for 5 days. The morphine ED50 values were obtained from the cumulative dose–response curves for the antinociceptive action of the opioid agonist 24 h after termination of drug treatment in the animal groups represented in Figures 5a and 6a.
The data are presented as mean±s.e.mean; n=4–7 animals per treatment group.
Significant difference from the morphine (15 μg) group ***(P<0.001).
Thus, in these chronic drug experiments, 5-day exposure to a maximal dose of morphine resulted in a progressive decline of the pharmacological effect and a marked loss of the opioid drug potency in the two nociception tests. Introduction of low doses of the α2-adrenoceptor antagonist, atipamezole, with chronic morphine (a) prevented loss of the peak antinociceptive effect, (b) significantly increased the duration of this effect, and (c) abolished the characteristic loss of opioid agonist potency. None of the animals treated with atipamezole alone or in combination with morphine showed visible signs of motor dysfunction.
Effects of ultra-low doses of α2-adrenoceptor antagonists on reversal of chronic morphine tolerance
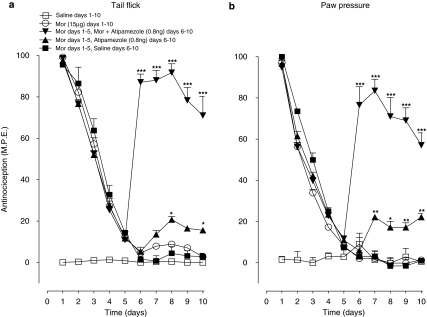
Effects of intervention with atipamezole plus morphine in animals showing loss of sensitivity to morphine in the tail flick and paw pressure tests following chronic treatment with the opioid agonist are illustrated in Figures 7a and b, respectively. In both tests, a single daily injection of morphine (15 μg) resulted in a rapid decline of the antinociceptive response, which approached baseline values on day 5. Continuation of treatment with the opioid agonist from day 6 to 10 maintained the response near baseline levels. However, in animals unresponsive to morphine following a 5-day morphine treatment, the co-injection of morphine (15 μg) with atipamezole (0.8 ng) from day 6 to 10 produced a rapid and robust recovery of antinociceptive response in both tests, the peak effect reaching nearly 90% of maximal value in the tail flick test (Figure 7a) and 80% in the paw pressure test (Figure 7b). The response to combined drug treatment from day 6 to 10 remained elevated well above the baseline value. Comparable intervention with atipamezole alone produced only a weak effect, the peak response in both tests attaining only 20% of the maximal value. Intervention with saline alone from day 6 to 10 yielded no significant recovery of the response.
Figure 7.
Effect of low-dose atipamezole (0.8 ng) on the antinociceptive effect and the morphine (Mor) potency in animals tolerant to the opioid agonist in the tail flick (a) and paw pressure test (b). Time course for the effects of atipamezole on the morphine-induced antinociception in the opioid-tolerant animals. Tolerance was induced by a single daily injection of morphine (15 μg) from day 1 to 5. On day 6, morphine was administered alone or in combination with atipamezole as a single injection until day 10. Nociceptive testing was performed after 60 min after each injection. The data are expressed as mean±s.e.mean of six to seven animals. Significant difference from the morphine alone (10 days). *P<0.05, **P<0.01, ***P<0.001.
The morphine ED50 values, obtained from the cumulative dose–response curves (not shown) derived on day 11 in the tail flick and paw pressure test, are represented in Table 3. In both tests, the morphine ED50 value in the groups that had received 10-day treatment with the opioid agonist alone was nearly 10-fold greater than the value in the control groups receiving saline alone, reflecting a highly significant loss of the agonist potency. However, administration of atipamezole with morphine from day 6 to10, in the groups pre-exposed to the opioid agonist alone for 5 days, abolished the increase in morphine ED50 value, reflecting a complete reversal of the drug potency loss. The morphine ED50 values in animals that had received the opioid agonist for 5 days followed by treatment with saline or atipamezole alone from day 6 to 10 were comparable with or slightly greater than those in animals that had received only saline for 10 days, reflecting spontaneous recovery of sensitivity to morphine.
Table 3.
Effect of low dose atipamezole on the reversal of tolerance to chronic morphine
|
Chronic treatment |
Tail flick ED50 (μg; i.t.) (mean±s.e.mean) | Paw pressure ED50 (μg; i.t.) (mean±s.e.mean) | |
|---|---|---|---|
| Days 1–5 | Days 6–10 | ||
| Saline | Saline | 5.1±0.3*** | 6.4±0.3*** |
| Morphine (15 μg) | Morphine (15 μg) | 51.2.±3.7 | 53.4±5.2 |
| Morphine (15 μg) | Morphine+Atipamezole (0.8 ng) | 4.8±0.8*** | 5.7±0.8*** |
| Morphine (15 μg) | Atipamezole (0.8 ng) | 5.3.±0.6*** | 5.3±0.8*** |
| Morphine (15 μg) | Saline | 7.2±0.2*** | 18.1±3.9*** |
The morphine ED50 values were obtained from the cumulative dose–response curves for the antinociceptive action of the opioid agonist 24 h after termination of drug treatment in the animal groups represented in Figures 7a and b.
The data presented are means±s.e.mean; n=6–7 animals per treatment group.
Significant difference from the morphine (15 μg) group ***(P<0.001). Tolerance was induced by a single daily injection of morphine (15 μg) from day 1–5.
Thus, delayed administration of a low dose of the α2-adrenoceptor antagonist, atipamezole, with a maximal antinociceptive dose of morphine to animals that had become unresponsive to the opioid agonist following a 5-day drug exposure not only produced a robust recovery of the pharmacological response in both nociception tests, but also reversed the loss of morphine potency.
Effects of an α2-adrenoceptor antagonist on acute morphine tolerance following 6-OHDA treatment
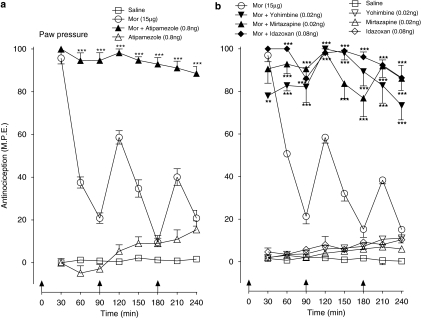
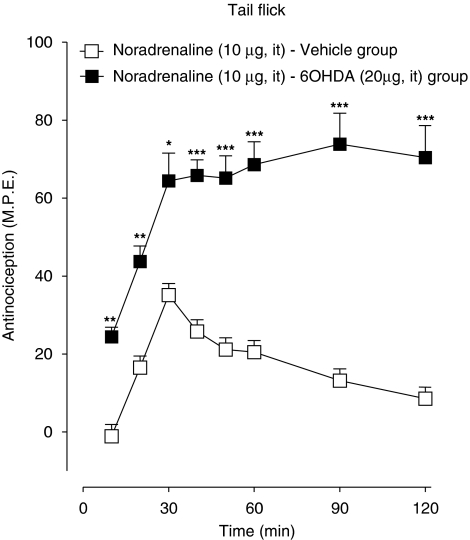
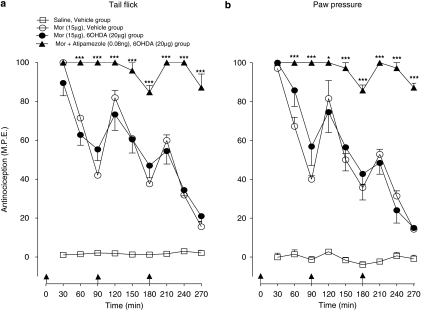
The effects of low-dose atipamezole on induction of acute spinal morphine tolerance (see Figure 4) were measured in animals pretreated with intrathecal 6-OHDA, a neurotoxin known to deplete the spinal levels of L-noradrenaline and induce supersensitivity to its antinociceptive effects (Howe and Yaksh, 1982). Figure 8 shows the sensitivity of 6-OHDA pretreated animals to a single intrathecal injection of L-noradrenaline (10 μg). As illustrated, administration of L-noradrenaline to the vehicle-pretreated control animals produced an antinociceptive response that peaked at 30% of maximal value followed by a recovery at 90 min. Administration of the same dose to 6-OHDA pretreated animals produced a response that peaked at 60% of maximal value and remained at this level for duration of the 120-min test period. Figure 9 shows effects of atipamezole on the tail flick and paw pressure response elicited by serial injections of morphine in the vehicle and 6-OHDA-pretreated animals. In both pretreatment groups, delivery of three successive injections of morphine dose (15 μg) at 90 min intervals produced a highly comparable decline of the antinociceptive response, its profile corresponding to that in preceding tests on un-pretreated animals (see Figure 4a). The 6-OHDA treatment did not prevent the augmented response of atipamezole (0.08 ng) with intrathecal morphine (15 μg). The maximal response elicited by the first injection of the drug combination was fully maintained for the duration of the 240-min test period (Figure 9) as without 6-OHDA treatment. The effect of atipamezole thus corresponded closely to its previously observed effect on morphine in un-pretreated animals (see Figure 4a).
Figure 8.
Anti-nociceptive effect of intrathecal L-noradrenaline (10 μg) in animals pretreated with intrathecal 6-hydroxydopamine (6-OHDA). Animals in the 6-OHDA group received a single injection of the neurotoxin (20 μg) 7 days before assessment of the effects of L-noradrenaline. Control animals received an injection of the vehicle. Anti-nociceptive testing was performed every 10 min for 60 min and every 30 min for 120 min after the drug injection. Significant difference from corresponding response in the vehicle-treated group. *P<0.05, **P<0.01, ***P<0.001.
Figure 9.
Influence of intrathecal 6-hydroxydopamine (6-OHDA) pretreatment on the induction of acute spinal morphine (Mor) tolerance and the effect of low-dose atipamezole on this response in (a) the tail flick test and (b) the paw pressure test. Morphine (15 μg) or saline was administered at 0, 90 and 180 min to groups of animals pretreated with a single injection of 6-OHDA (20 μg) or the vehicle 14 days before testing. Atipamezole (0.08 ng) was administered with morphine to 6-OHDA pretreated animals as a single injection. Nociceptive testing was performed every 30 min post-injection. The data are presented as mean±s.e.mean; n=six to seven animals per treatment group. Significant difference from the action of morphine alone at the corresponding time point in the 6-OHDA-pretreated animals *P<0.05, ***P<0.001.
The morphine ED50 values obtained from cumulative morphine dose–response curves derived in the above treatment groups are represented in Table 4. Repeated injections of the opioid agonist in the vehicle-pretreated group led to nearly fourfold (tail flick) and threefold (paw pressure) increase in ED50 values relative to injections of saline. Similar injections of morphine in 6-OHDA pretreated group led to a comparable elevation in the morphine ED50 values. Importantly, the combination of atipamezole with morphine in the 6-OHDA group abolished the increase in morphine ED50 value in both nociception tests. The effect of atipamezole on morphine in the 6-OHDA pretreated group was comparable to its earlier observed effect in un-pretreated animals (see Table 1).
Table 4.
Influence of spinal 6-hydroxydopamine (6-OHDA) pretreatment on the effect of low-dose atipamezole on acute tolerance
| Pretreatment | Treatment | Tail flick ED50 (μg; i.t.) | Paw pressure ED50 (μg; i.t.) |
|---|---|---|---|
| Vehicle | Vehicle | 5.2±0.1*** | 7.1±1.2*** |
| Vehicle | Morphine (15 μg) | 24.5±0.8 | 25.5±1.5 |
| 6-OHDA | Morphine (15 μg) | 27.8±1.3 | 29.8±1.3 |
| 6-OHDA | Morphine+Atipamezole (0.08 ng) | 6.4±0.6*** | 5.3±0.8*** |
The morphine ED50 values were obtained from the cumulative dose–response curves for the antinociceptive action of the opioid agonist 24 h after termination of drug treatment in the animal groups represented in Figures 9a and b.
The data presented are means±s.e.mean; n=6–7 animals per treatment group.
Significant difference from the morphine (15 μg) group ***(P<0.001).
Thus, consistent with results of a previous study (Howe and Yaksh, 1982), pretreatment with spinal 6-OHDA significantly increased the sensitivity to intrathecal L-noradrenaline, an effect reflecting the noradrenergic denervation. Repeated administration of acute morphine to the 6-OHDA pretreated animals produced the loss of antinociceptive effect and the opioid agonist potency comparable to that in the vehicle-pretreated or un-pretreated animals. Atipamezole fully prevented the loss of morphine effect and potency in the 6-OHDA pretreated animals.
Discussion
The spinal administration of ultra-low doses of the non-selective opioid antagonist, naltrexone (Powell et al., 2002), or the μ or δ opioid receptor-selective antagonists (Abul-Husn et al., 2007) has been shown to augment acute antinociceptive effects of morphine and block or reverse tolerance to these effects. Previous studies have provided extensive evidence demonstrating functional interactions between opioid and α2-adrenoceptors in different models of analgesia and analgesic tolerance (Browning et al., 1982; Bentley et al., 1983; Milne et al., 1985; Loomis et al., 1987; Takano and Yaksh, 1992; Aley and Levine, 1997; Stone et al., 1997, 2007), and recent investigations have shown that α2-adrenoceptors physically associate with μ or δ receptors to form functional binary units (Jordan et al., 2003, Rios et al., 2004). These findings prompted us to investigate whether low doses of the competitive α2-adrenoceptor antagonists share the paradoxical actions of their opioid counterparts on the acute and chronic effects of morphine in the spinal analgesia model.
The results of this study show that four structurally distinct α2-adrenoceptor antagonists, when administered at doses significantly below those producing blockade of spinal clonidine antinociception can, to a remarkable degree, replicate the previously reported actions of low-dose opioid antagonists on the effects of intrathecal morphine in tests of thermal and mechanical nociception. Atipamezole, a highly selective α2-adrenoceptor antagonist (Virtanen et al., 1989), markedly augmented acute antinociceptive effects of morphine both in the tail flick and paw pressure test, blocked the induction of acute and chronic tolerance to these effects and restored the effect of morphine in animals exhibiting chronic tolerance to the agonist. The acute or chronic tolerance to morphine in both nociception tests was signalled by a rapid and progressive decrease in magnitude of the pharmacological response and a highly significant loss of the drug potency, as indicated by a marked elevation in morphine ED50 values obtained 24 h post-drug treatment. Atipamezole administered with morphine, but not alone, influenced the appearance of both characteristics of the analgesic tolerance and substantially reversed these in the tolerant animals. The effects of atipamezole on the antinociception and tolerance produced by acute morphine were reproducible with low doses of yohimbine, mirtazapine and idazoxan; these drugs were chosen based on the diversity of their chemical structure. In view of the exceedingly low doses at which these agents influenced morphine effects, it appears unlikely that their actions originated solely from a direct interaction with the opioid receptors. That such doses of all four agents, with a common ability to produce spinal clonidine blockade in higher doses, exerted qualitatively similar effects on morphine in the two distinct nociception tests, suggests that they influenced opioid activity indirectly, most likely through an interaction with the α2-adrenoceptors associated with spinal neurons. However, as yohimbine has been shown to possess affinity for the central opioid receptor sites and to antagonize morphine antinociception in the mouse model (Browning et al., 1982), its effects could also involve a direct low-dose naltrexone-like action on the spinal opioid receptors (Powell et al., 2002; McNaull et al., 2007). The anatomical locus of the receptor sites or nature of the α2-adrenoceptor subtypes (Fairbanks and Wilcox, 1999; Fairbanks et al., 2002; Stone et al., 2007) and mechanisms underlying the unusual effects of the α2-adrenoceptor antagonists observed in this study remain unknown.
One possibility is these antagonists, by preferentially blocking the presynaptic α2-autoreceptors, mediating feedback inhibition of transmitter release, increased the spinal level of L-noradrenaline which in turn augmented the effects of morphine. This possibility, however, is minimized by several factors: the antinociceptive effects of intrathecal L-noradrenaline itself are partially expressed through spinal opioid activity, as these are sensitive to naloxone (Loomis et al., 1985) and are significantly attenuated in animals tolerant to spinal morphine (Milne et al., 1985); intrathecal 6-OHDA treatment or cervical hemisection, resulting in a significant depletion of tissue L-noradrenaline levels and noradrenergic supersensitivity, does not reduce spinal α2-adrenoceptor binding, suggesting that the autoreceptor population is not a major contributor to the α2-adrenoceptor population (Howe et al., 1987); and pretreatment of animals with intrathecal 6-OHDA in the current study, did not significantly affect the ability of atipamezole to prevent attrition of the morphine-induced antinociceptive response or loss of the agonist potency in the acute tolerance model. Thus, it would appear that the effects of low-dose atipamezole, and probably of the other α2-adrenoceptor antagonists, on the actions of intrathecal morphine are not dependent on integrity of adreno-autoreceptors on the spinal noradrenergic neurons. Their effects, however, could involve interaction with the α2-adrenoceptors that are present on nociceptive neurons in the dorsal horn (Howe et al., 1987) and that share the operational characteristics of opioid receptors also present on these neurons (North and Yoshimura, 1984; Kuraishi et al., 1985; Pang and Vasko, 1986). Recent biochemical experiments have revealed that μ and α2-adrenoceptors associated with the central neurons form a binary receptor complex whose level of signalling response to a μ opioid agonist is influenced by exposure to the α2-adrenoceptor agonist (Jordan et al., 2003). Thus, it is conceivable that the adrenoceptor antagonists used in the present study influenced the actions of morphine, an agonist preferring μ opioid receptors, by targeting the α2-adrenoceptor component of this receptor complex (see Discussion below).
Bentley et al. (1983), observing that the opioid-induced antinociceptive effects in a mouse model of visceral nociception could be antagonized by adrenergic antagonists, remarked that the ‘opioid receptor is linked to its effector mechanism through an α-adrenoceptor and that opioid agonists in some way activate the α-adrenoceptor'. In 1997, Aley and Levine (1997) showed that antinociceptive effects of locally administered μ opioid and α2-adrenoceptor agonists on the prostaglandin-induced peripheral hyperalgesia in the rat could be cross antagonized by the competitive antagonists of α2-adrenoceptors and opioid receptors, or by anti-sense oligodeoxynucleotides producing knockdown of these receptors. They additionally demonstrated the development of cross-tolerance as well as cross-dependence between the two classes of receptor agonists in this model of nociception. The authors postulated that the agonists exerted their inhibitory effects on the peripheral sensory neurons by acting on a multireceptor complex incorporating μ opioid and α2-adrenoceptor components linked through a common second messenger pathway. Jordan et al. (2003) reported the presence of an immunoprecipitable μ opioid-α2-adrenoceptor complex in the cultured spinal and hippocampal neurons and showed that in heterologous cells expressing this complex, simultaneous activation with clonidine and morphine resulted in a reduced signalling response relative to activation with the morphine alone. The authors suggested that the presence of an inactive α2-adrenoceptor component in the binary receptor may promote active state of the μ opioid component, resulting in an enhanced signalling response. Thus, in context of this study, a low-dose exposure to an α2-adrenoceptor antagonist such as atipamezole could promote a receptor state in which activation of the μ opioid receptor component leads to an enhanced and sustained signalling response. Functionally, such a state could involve a slower or reduced desensitization of the μ opioid receptor unit in the binary receptor complex.
Another possibility, prompted by the observed duality of opioid receptor activity in the dorsal root ganglion neuron model (Crain and Shen, 1995, 2000), is that activation of the μ opioid-α2-adrenoceptor complex by the opioid agonist produces a latent stimulatory response that behaviourally manifests as hyperalgesia and that physiologically antagonizes the characteristic analgesia produced by the agonist. Extensive evidence from numerous animal studies has implicated the development of a latent hyperalgesia in the induction of analgesic tolerance to the opioid drugs (see Angst and Clark, 2006). Low doses of the α2-adrenoceptor (or opioid) antagonists thus may block the latent stimulatory response to the agonist, thus preventing hyperalgesia and augmenting analgesia (see Discussion below).
In their extensive electrophysiological studies on cultured dorsal root ganglion neurons, Crain and Shen (1995, 2000) have demonstrated that opioid agonists, depending on the dose, can elicit stimulatory or inhibitory effects that are differentially sensitive to low and high doses of a competitive opioid receptor antagonist. Thus, ultra-low concentrations of an opioid agonist produced a stimulatory action through activation of a Gs-coupled mode sensitive to ultra-low doses of an opioid antagonist whereas higher concentrations acting through Go/Gi produced the classical inhibitory action, sensitive to larger antagonist doses. In intact animals, both the electrophysiological and behavioural studies have shown that low doses of morphine can exert stimulatory effects. Dickenson and Sullivan (1986) reported that in anaesthetized rats administration of a low dose of morphine increased whereas higher doses decreased firing of the dorsal horn nociceptive neurons driven by a noxious input. Similarly, Wiesenfeld-Hallin et al. (1991) showed that in the decerebrate, spinalized and unanaesthetized rat, low doses of intrathecal morphine facilitated the flexor reflex, a response blocked by naloxone as well as a substance P receptor antagonist. Behaviourally, extremely low doses of systemic morphine were reported to produce hyperalgesia that was blocked by an antagonist dose of naloxone (Kayser et al., 1987). Similarly, Van Elstraete et al. (2005) reported the induction of a delayed ketamine-sensitive hyperalgesia following a single analgesic dose of morphine. In recent years, ultra-low doses of systemic (Crain and Shen, 2001) or spinal morphine (McNaull et al., 2007) have been found to elicit a tail flick hyperalgesia sensitive to ultra-low doses of systemic or spinal naltrexone. Thus, the ability of low doses of naltrexone and other opioid antagonists to augment morphine antinociception and inhibit or reverse of tolerance may be a consequence of blockade of the latent hyperalgesia induced by the opioid agonist exposure. However, if such hyperalgesia were attributable to activation of the opioid-α2-adrenoceptor complex, predictably it would be sensitive to an α2-adrenoceptor antagonist, and, additionally, be inducible by a low dose of an α2-adrenoceptor agonist. Our preliminary experiments have demonstrated that the tail flick hyperalgesia produced by a very low dose of spinal morphine (0.05 ng) is sensitive to an atipamezole dose (0.08 ng) that affected the development of tolerance to morphine antinociception in the current study (unpublished results). Additional work is in progress to confirm this finding and determine if the atipamezole effect is shared by related antagonists. Whether low doses of α2-adrenoceptor agonists produce thermal hyperalgesia is unclear but an electrophysiological study on the rat spinal cord has shown that, a low dose of clonidine, such as a similar dose of morphine, increases activity of the dorsal horn neurons driven by nociceptive input (Sullivan et al., 1987). The sensitivity of this response to low doses of α2-adrenoceptor antagonists is unknown. In a similar vein, a sustained spinal administration of clonidine to rats (Quartilho et al., 2004), like opioids (Mao et al., 1994; Gardell et al., 2002), induces thermal hypersensitivity, reflecting hyperalgesia. The sensitivity of this response to low doses of α2-adrenoceptor antagonists also remains unknown. In view of these observations, it would be useful to determine if low doses of α2-adrenoceptor agonists produce neuronal excitatory responses and behavioural hyperalgesia and whether such responses are sensitive to low doses of the α2-adrenoceptor antagonists.
In conclusion, this study on the spinal analgesia model reveals that at doses considerably below those producing blockade of the α2-adrenoceptor-mediated analgesia, the α2-adrenoceptor antagonists increase acute antinociceptive effects of morphine, block the induction of acute as well as chronic tolerance and effectively reverse established tolerance. The effects of these agents thus are highly comparable to earlier reported effects of low-dose opioid receptor antagonists. Recent studies have examined the contributions of α-adrenoceptor subtypes, specifically α2a and α2c, in expression of the synergistic interaction between antinociceptive actions of the opioid and α2-adrenoceptor agonists (Fairbanks and Wilcox, 1999; Fairbanks et al., 2002; Stone et al., 2007). Thus, it would be of interest to examine in future studies relative contributions of different α2-adrenoceptor subtypes to the opioid facilitatory actions of non-selective α2-adrenoceptor antagonists observed in this study. Such actions, however, may have applicability in design and development of novel agents with potential to produce potent analgesic effects lacking the ability to induce tolerance. In addition, these agents may be useful in optimizing actions of morphine in conditions such as neuropathic pain in which there is a decline in sensitivity to the antinociceptive actions of morphine.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Canada Research Chairs Program, and the Ontario Innovation Trust/Canadian Foundation for Innovation.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- M.P.E.
maximum possible effect
Conflict of interest
The research reported in this paper is the basis of a patent application filed by PARTEQ, Queen's University, Kingston, Ontario, Canada.
References
- Abul-Husn NS, Sutak M, Milne B, Jhamandas K. Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists. Br J Pharmacol. 2007;151:877–887. doi: 10.1038/sj.bjp.0707277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA.Guide to Receptors and Channels (GRAC) Br J Pharmacol 2008153Suppl 2S1–S209.3rd edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Multiple receptors involved in peripheral alpha 2, mu, and A1 anti-nociception, tolerance, and withdrawal. J Neurosci. 1997;17:735–744. doi: 10.1523/JNEUROSCI.17-02-00735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Bentley GA, Newton SH, Starr J. Studies on the anti-nociceptive action of alpha-agonist drugs and their interactions with opioid mechanisms. Br J Pharmacol. 1983;79:125–134. doi: 10.1111/j.1476-5381.1983.tb10504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning S, Lawrence D, Livingston A, Morris B. Interactions of drugs active at opiate receptors and drugs active at alpha 2-receptors on various test systems. Br J Pharmacol. 1982;888:75–82. doi: 10.1111/j.1476-5381.1982.tb09322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its anti-nociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci USA. 1995;92:10540–10544. doi: 10.1073/pnas.92.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Antagonists of excitatory opioid receptor functions enhance morphine′s analgesic potency and attenuate opioid tolerance/dependence liability. Pain. 2000;84:121–131. doi: 10.1016/s0304-3959(99)00223-7. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Acute thermal hyperalgesia elicited by low-dose morphine in normal mice is blocked by ultra-low-dose naltrexone, unmasking potent opioid analgesia. Brain Res. 2001;888:75–82. doi: 10.1016/s0006-8993(00)03010-9. [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DD. A method for determining loss of pain sensation. J Pharmacol Expt Ther. 1941;72:74–79. [Google Scholar]
- Dickenson AH, Sullivan AF. Electrophysiological studies on the effects of intrathecal morphine on nociceptive neurons in the rat dorsal horn. Pain. 1986;24:211–222. doi: 10.1016/0304-3959(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Stone LS, Kitto KF, Nguyen HO, Posthumus IJ, Wilcox GL. alpha(2C)-Adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J Pharmacol Exp Ther. 2002;300:282–290. doi: 10.1124/jpet.300.1.282. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Wilcox GL. Spinal anti-nociceptive synergism between morphine and clonidine persists in mice made acutely or chronically tolerant to morphine. J Pharmacol Exp Ther. 1999;288:1107–1116. [PubMed] [Google Scholar]
- Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP, Jr, et al. Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 2002;22:6747–6755. doi: 10.1523/JNEUROSCI.22-15-06747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci USA. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JR, Yaksh TL. Changes in sensitivity to intrathecal norepinephrine and serotonin after 6-hydroxydopamine (6-OHDA), 5,6-dihydroxytryptamine (5,6-DHT) or repeated monoamine administration. J Pharmacol Exp Ther. 1982;220:311–321. [PubMed] [Google Scholar]
- Howe JR, Yaksh TL, Tyce GM. Intrathecal 6-hydroxydopamine or cervical spinal hemisection reduces norepinephrine content, but not the density of alpha 2-adrenoceptors, in the cat lumbar spinal enlargement. Neuroscience. 1987;21:377–384. doi: 10.1016/0306-4522(87)90128-x. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Mol Pharmacol. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- Kayser V, Besson JM, Guilbaud G. Paradoxical hyperalgesic effect of exceedingly low doses of systemic morphine in an animal model of persistent pain (Freund's adjuvant-induced arthritic rats) Brain Res. 1987;414:155–157. doi: 10.1016/0006-8993(87)91338-2. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–182. doi: 10.1016/0006-8993(85)91426-x. [DOI] [PubMed] [Google Scholar]
- Loomis CW, Cervenko FW, Jhamandas K, Sutak M, Milne B. Analgesia and autonomic function following intrathecal administration of morphine and norepinephrine to the rat. Can J Physiol Pharmacol. 1985;63:656–662. doi: 10.1139/y85-109. [DOI] [PubMed] [Google Scholar]
- Loomis CW, Jhamandas K, Milne B, Cervenko F. Monoamine and opioid interactions in spinal analgesia and tolerance. Pharmacol Biochem Behav. 1987;26:445–451. doi: 10.1016/0091-3057(87)90146-8. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaull B, Trang T, Sutak M, Jhamandas K. Inhibition of tolerance to spinal morphine anti-nociception by low doses of opioid receptor antagonists. Eur J Pharmacol. 2007;560:132–141. doi: 10.1016/j.ejphar.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Milne B, Cervenko F, Jhamandas K, Loomis C, Sutak M. Analgesia and tolerance to intrathecal morphine and norepinephrine infusion via implanted mini-osmotic pumps in the rat. Pain. 1985;22:165–172. doi: 10.1016/0304-3959(85)90176-9. [DOI] [PubMed] [Google Scholar]
- North RA, Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 1984;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote K, Kitahata LM, Collins JG, Nakatani K, Nakagawa I. Interaction between opiate subtype and alpha-2 adrenergic agonists in suppression of noxiously evoked activity of WDR neurons in the spinal dorsal horn. Anesthesiology. 1991;74:737–743. doi: 10.1097/00000542-199104000-00018. [DOI] [PubMed] [Google Scholar]
- Owen JA, Tasker RA, Nakatsu K. A simple, less stressful rat restrainer. Experientia. 1984;40:306–308. doi: 10.1007/BF01947596. [DOI] [PubMed] [Google Scholar]
- Pang IH, Vasko MR. Morphine and norepinephrine but not 5-hydroxytryptamine and gamma-aminobutyric acid inhibit the potassium-stimulated release of substance P from rat spinal cord slices. Brain Res. 1986;376:268–279. doi: 10.1016/0006-8993(86)90189-7. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Abul-Husn NS, Jhamandas A, Olmstead MC, Beninger RJ, Jhamandas K. Paradoxical effects of the opioid antagonist naltrexone on morphine analgesia, tolerance, and reward in rats. J Pharmacol Exp Ther. 2002;300:588–596. doi: 10.1124/jpet.300.2.588. [DOI] [PubMed] [Google Scholar]
- Powell KJ, Ma W, Sutak M, Doods H, Quirion R, Jhamandas K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonists. Br J Pharmacol. 2000;131:875–884. doi: 10.1038/sj.bjp.0703655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Ossipov MH, Lai J, et al. Production of paradoxical sensory hypersensitivity by alpha 2-adrenoreceptor agonists. Anesthesiology. 2004;100:1538–1544. doi: 10.1097/00000542-200406000-00029. [DOI] [PubMed] [Google Scholar]
- Reddy SV, Maderdrut JL, Yaksh TL. Spinal cord pharmacology of adrenergic agonist-mediated anti-nociception. J Pharmacol Exp Ther. 1980;213:525–533. [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. Interactions between delta opioid receptors and alpha-adrenoceptors. Clin Exp Pharmacol Physiol. 2004;31:833–836. doi: 10.1111/j.1440-1681.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- Roerig SC, Lei S, Kitto K, Hylden JK, Wilcox GL. Spinal interactions between opioid and noradrenergic agonists in mice: multiplicativity involves delta and alpha-2 receptors. J Pharmacol Exp Ther. 1992;262:365–374. [PubMed] [Google Scholar]
- Stevens CW, Monasky MS, Yaksh TL. Spinal infusion of opiate and alpha-2 agonists in rats: tolerance and cross-tolerance studies. J Pharmacol Exp Ther. 1988;244:63–70. [PubMed] [Google Scholar]
- Stone LS, Kelley F, Kitto F, Eisenach JC, Fairbanks CA, Wilcox GL. ST91 [2-(2,6-Diethylphenylamino)-2-imidazoline hydrochloride]-mediated spinal anti-nociception and synergy with opioids persists in the absence of functional α-2A- or α-2C-adrenergic receptors. J Pharmacol Exp Ther. 2007;323:899–906. doi: 10.1124/jpet.107.125526. [DOI] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AF, Dashwood MR, Dickenson AH. Alpha 2-adrenoceptor modulation of nociception in rat spinal cord: location, effects and interactions with morphine. Eur J Pharmacol. 1987;138:169–177. doi: 10.1016/0014-2999(87)90430-4. [DOI] [PubMed] [Google Scholar]
- Sullivan AF, Kalso EA, McQuay HJ, Dickenson AH. Evidence for the involvement of the mu but not delta opioid receptor subtype in the synergistic interaction between opioid and alpha 2 adrenergic anti-nociception in the rat spinal cord. Neurosci Lett. 1992;139:65–68. doi: 10.1016/0304-3940(92)90859-6. [DOI] [PubMed] [Google Scholar]
- Takano Y, Takano M, Sato I, Yaksh TL. Effects of spinal naloxone and naltrindole on the anti-nociceptive acton of intrathecally administered dexmedetomidine. J Anesth. 1996;10:194–198. doi: 10.1007/BF02471390. [DOI] [PubMed] [Google Scholar]
- Takano Y, Yaksh TL. Characterization of the pharmacology of intrathecally administered alpha-2 agonists and antagonists in rats. J Pharmacol Exp Ther. 1992;261:764–772. [PubMed] [Google Scholar]
- Van Elstraete AC, Sitbon P, Trabold F, Mazoit JX, Benhamou D. A single dose of intrathecal morphine in rats induces long-lasting hyperalgesia: the protective effect of prior administration of ketamine. Anesth Analg. 2005;101:1750–1756. doi: 10.1213/01.ANE.0000184136.08194.9B. [DOI] [PubMed] [Google Scholar]
- Virtanen R, Savola JM, Saano V. Highly selective and specific antagonism of central and peripheral alpha 2-adrenoceptors by atipamezole. Arch Int Pharmacodyn Ther. 1989;297:190–204. [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ, Hakanson R, Feng DM, Folkers K. Low-dose intrathecal morphine facilitates the spinal flexor reflex by releasing different neuropeptides in rats with intact and sectioned peripheral nerves. Brain Res. 1991;551:157–162. doi: 10.1016/0006-8993(91)90928-o. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]