Abstract
The transient receptor potential vanilloid-1 (TRPV1) cation channel is a receptor that is activated by heat (>42 °C), acidosis (pH<6) and a variety of chemicals among which capsaicin is the best known. With these properties, TRPV1 has emerged as a polymodal nocisensor of nociceptive afferent neurones, although some non-neuronal cells and neurones in the brain also express TRPV1. The activity of TRPV1 is controlled by a multitude of regulatory mechanisms that either cause sensitization or desensitization of the channel. As many proalgesic pathways converge on TRPV1 and this nocisensor is upregulated and sensitized by inflammation and injury, TRPV1 is thought to be a central transducer of hyperalgesia and a prime target for the pharmacological control of pain. As a consequence, TRPV1 agonists causing defunctionalization of sensory neurones and a large number of TRPV1 blockers have been developed, some of which are in clinical trials. A major drawback of many TRPV1 antagonists is their potential to cause hyperthermia, and their long-term use may carry further risks because TRPV1 has important physiological functions in the peripheral and central nervous system. The challenge, therefore, is to pharmacologically differentiate between the physiological and pathological implications of TRPV1. There are several possibilities to focus therapy specifically on those TRPV1 channels that contribute to disease processes. These approaches include (i) site-specific TRPV1 antagonists, (ii) modality-specific TRPV1 antagonists, (iii) uncompetitive TRPV1 (open channel) blockers, (iv) drugs interfering with TRPV1 sensitization, (v) drugs interfering with intracellular trafficking of TRPV1 and (vi) TRPV1 agonists for local administration.
Keywords: transient receptor potential vanilloid-1 (TRPV1) cation channels, molecular nocisensors, primary afferent neurones, central nervous system, nociception, hyperalgesia, hyperthermia, thermoregulation, TRPV1 blockers, interference with TRPV1 trafficking
Pain, heat and spice
In the field of nociception there has been a long-standing debate as to whether pain arises from excitation of specific nociceptive afferent nerve fibres, as proposed by the specificity theory, or is simply the result of intense stimulation of afferent neurones as held by the intensity theory (Perl, 2007). The heat with which this debate was conducted could have been considerably lessened if it had been known that subpopulations of primary afferent neurones express specific nocisensors that transduce distinct noxious stimuli into propagated nerve activity as well as particular ion channels that control excitability and action potential propagation specifically in sensory neurones. Although pain arising from hollow viscera is in part encoded by the intensity of distension (Jänig, 2006), the specificity theory is now impressively backed by a large list of ion channels and G-protein-coupled receptors that enable afferent neurones to sense distinct modalities of pain. The pioneer that triggered this avalanche of discoveries was a family of closely related cation channels, denoted transient receptor potential (TRP), that act as molecular sensors for distinct pain, temperature, chemaesthesis and taste modalities (Table 1).
Table 1.
Overview of TRP channels, expressed by sensory neurones or cells associated with sensory neurones that are involved in nociception, thermosensation and chemaesthesis
| TRP channel | Pain modality | Thermosensation | Chemaesthesis |
|---|---|---|---|
| TRPV1 | Heat Acidosis Chemaesthetic (irritant) pain |
>42 °C | Capsaicin (red pepper), resiniferatoxin (Euphorbia), gingerol and zingerone (ginger), piperine (black pepper), eugenol (clove), camphor, vanillatoxins 1-3 (Tarantula), ethanol, acid (pH<6) |
| TRPV2 | Heat | >52 °C | Δ9-tetrahydrocannabinol (Δ9-THC) |
| TRPV3 | Chemaesthetic (irritant) pain | >33 °C (warmth) | Carvacrol (oregano), eugenol, thymol (thyme), vanillin (vanilla), camphor, menthol (mint) |
| TRPV4 | Acidosis | >25–34 °C (warmth) | Acid (pH<6) |
| TRPM8 | Cold Chemaesthetic (irritant) pain |
<25 °C | Menthol, icilin, geraniol, L-carvone, isopulegol, linalool |
| TRPA1 | Chemaesthetic (irritant) pain | <17 °C | Allicin and diallyl disulphide (garlic), allyl isothiocyanate (mustard, horseradish, wasabi), cinnamaldehyde (cinnamon), carvacrol, gingerol, eugenol, icilin, acrolein, formaldehyde, methyl salicylate, Δ9-THC |
The thermo- and chemosensory properties referred to have been delineated by studying heterologously expressed TRP channels. The list of chemicals activating TRP channels is not complete. For references see Dhaka et al. (2006) and Bandell et al. (2007).
The implication of TRP channels in pain and sensation was first heralded when in 1997 the vanilloid receptor-1 was identified at the genetic and functional level (Caterina et al., 1997). It was soon realized that this new ion channel was homologous to the TRP channel family and subsequently re-named transient receptor potential vanilloid-1 (TRPV1), which stands for transient receptor potential vanilloid-1 (Caterina and Julius, 2001; Clapham et al., 2005). The story of TRPV1, however, had begun half a century ago, when the peculiar pharmacology of capsaicin was first revealed. Responsible for the pungency of red pepper (Capsicum spp.), the vanilloid capsaicin in its pure form is one of the most painful chemicals we know, conveying the sensation of ‘hot' and ‘burning'. It was the Hungarian pharmacologist Nicolas Jancsó who first recognized that capsaicin acts specifically on nociceptive afferent neurones (Jancsó, 1960). Further work by two eminent Hungarian research groups in Szeged and Pécs provided compelling evidence that the selectivity of capsaicin's action on afferent neurones can only be accounted for by an action on specific capsaicin receptors (Szolcsányi and Jancsó-Gábor, 1975; Jancsó et al., 1977, 1987; Szolcsányi, 1982, 2004; Nagy et al., 2004). The discovery of specific vanilloid-binding sites (Szallasi and Blumberg, 1999) and a selective capsaicin antagonist (Bevan et al., 1992) were further important steps towards the identification of TRPV1 as the capsaicin receptor. Therapeutic opportunities were envisaged from the structure–activity relationship for the pain-producing effect of capsaicin (Szolcsányi and Jancsó-Gábor, 1975) and its axonal site of action following local administration to afferent neurones (Jancsó et al., 1980).
TRPV1 was soon to be joined by other TRP channels with unique sensory modalities. The group of thermo-TRP channels is able to sense the whole spectrum of temperatures from painful cold to painful heat (Dhaka et al., 2006). Moreover, they are able to detect specific chemical entities including unpleasant and/or painful toxins, whereby TRP channels subserve chemaesthesis, the chemical sense distinct from taste and smell (Bandell et al., 2007). In this context it is perplexing to note that TRP channels are sensors for many spices (Table 1). Although most people perceive spices, at least some of them, as pleasant and heightening the taste of food, it seems that—in a biological sense—the chemicals responsible for the gustatory and olfactory pleasures of spices are elaborated by plants primarily for their defence (Max, 1992; Cromer and McIntyre, 2008). By some strange perversion, however, humans have learned to enjoy low doses of these deterrent chemicals, which is opposite to the strategy of plants to discourage predators by the unusual sensory quality of spices (Max, 1992). This argument is supported by the fact that capsaicin is a pungent chemical for mammals, but not birds which are supposed to help distributing the seeds of red pepper, given that the avian orthologue of TRPV1 lacks the binding site for capsaicin (Jordt and Julius, 2002).
Analysis of the molecular and functional properties of TRPV1 has shown that this ion channel is a polymodal nocisensor, which is subject to allosteric modulation by many proalgesic pathways. This aspect and the property to become sensitized by proinflammatory mediators have raised enormous interest in TRPV1 being a prime transducer of pathological pain. Indeed, the expression and/or activity of TRPV1 has been found upregulated under conditions of chronic pain and hyperalgesia. At the same time, however, it has emerged that TRPV1 has important functions in body homeostasis, for example, in thermoregulation. As a consequence, targeting TRPV1 in chronic pain carries great potential at a heavy risk. Besides briefly outlining the functional implications of TRPV1 in health and disease, the major objective of this article is to point out that TRPV1 may be a highly useful drug target if its pathological functions can pharmacologically be differentiated from its physiological implications.
Transient receptor potential cation channels
TRPV1 belongs to the large family of TRP ion channels, so named after the function these channels have in Drosophila phototransduction. By now at least 28 different TRP subunit genes have been identified in mammals, comprising six subfamilies: the classical or canonical TRPs, the vanilloid TRPs, the melastatin TRPs (TRPMs), the mucolipin TRPs, the polycystin TRPs and the ankyrin TRPs (TRPAs). Except for some polycystin TRPs, the primary structure of the TRP channels consist of six transmembrane segments (Figure 1) with a pore domain between transmembrane segments 5 and 6 and with both the C- and N termini located intracellularly (Clapham et al., 2005; Alexander et al., 2008). This architecture is common to hundreds of ion channels but, despite the topographic similarities between the TRPs and the voltage-gated K+ channels, the TRPs are only distantly related to these channels (Clapham et al., 2005; Cromer and McIntyre, 2008). However, there is some functional relationship between these two classes of ion channels, given that peptide components of the tarantula venom, called ‘vanillatoxins', activate TRPV1 by interacting with a region that is homologous to the ‘voltage sensor' in the Kv1.2 channel (Siemens et al., 2006; Cromer and McIntyre, 2008).
Figure 1.
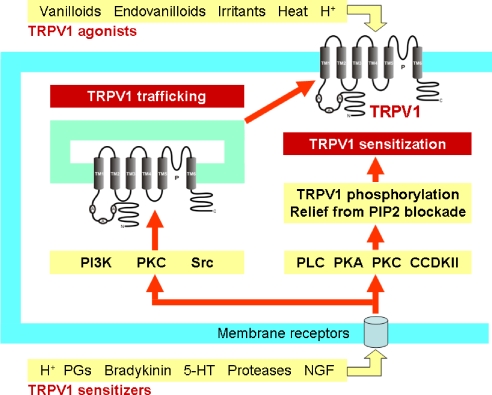
Two major pathways causing TRPV1 overactivity in sensory nerve endings: TRPV1 trafficking to the cell membrane and TRPV1 sensitization. A, ankyrin; C, C terminus; CCDKII, Ca2+-calmodulin-dependent kinase II; 5-HT, 5-hydroxytryptamine; N, N terminus; NGF, nerve growth factor; P, pore; PGs, prostaglandins; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; Src, Src kinase, TM, transmembrane domain.
Made up as tetramers of four subunits, TRP channels are opened or closed by conformational changes in the channel protein (Dhaka et al., 2006; Bandell et al., 2007). Unlike voltage-gated ion channels, TRP channels are in general only weakly sensitive to depolarization but open in response to changes in temperature, binding of ligands or other alterations of the channel protein (Clapham et al., 2005; Matta and Ahern, 2007; Nilius et al., 2007). As their activation is modulated by voltage changes, TRP channels are included in the large superfamily of voltage-gated-like ion channels (Bandell et al., 2007; Nilius et al., 2007). The ion selectivity differs markedly among the family of TRP channels, most of them being non-selective cation channels, which is also true for TRPV1 with its high permeability for Ca2+ (Caterina and Julius, 2001; Gunthorpe et al., 2002; Patapoutian et al., 2003; García-Sanz et al., 2004). Interestingly, sustained exposure to agonists increases the Ca2+ permeability of TRPV1 and causes pore dilation (Chung et al., 2008). TRPV1-bearing neurones are eventually overloaded by Ca2+, which in conjunction with other factors can result in mitochondrial swelling, long-lasting defunctionalization or even degeneration of the neurones (Szolcsányi et al., 1975; Jancsó et al., 1977, 1984, 1985; Wood et al., 1988; Szöke et al., 2002). In addition, TRPV1 allows protons to enter the cell in an acidic environment, which results in intracellular acidification (Hellwig et al., 2004; Vulcu et al., 2004).
Distinct members of the TRPV, TRPM and TRPA subunit families have turned out to be particularly relevant to nociception, thermosensation and chemaesthesis (Table 1). There is emerging evidence that members of other TRP channel subfamilies also contribute to thermo- and chemosensation, much as TRP channels are involved in sweet, bitter, sour and umami taste sensation (Zhang et al., 2003; Huang et al., 2006; Bandell et al., 2007; Montell and Caterina, 2007). Although less well studied, TRP channels may also contribute to transduction of mechanical stimuli as suggested by alterations of mechanosensation following knockout of specific TRP channels.
TRPV1, a molecular noci- and thermosensor
The existence of TRPV1 has long been envisaged from the specific action of capsaicin on nociceptive afferent neurones which, owing to this property, have become known as capsaicin-sensitive afferent neurones (Szolcsányi, 1982; Maggi and Meli, 1988; Holzer, 1991; Szallasi and Blumberg, 1999). TRPV1 is a polymodal nocisensor par excellence, being receptive to noxious heat (>42 °C), acidosis, endovanilloids and a variety of pungent compounds such as capsaicin, resiniferatoxin, piperine, gingerol, zingerone, camphor, eugenol, ethanol and the vanillatoxins 1–3 (Caterina and Julius, 2001; Trevisani et al., 2002; Patapoutian et al., 2003; Siemens et al., 2006; Szallasi et al., 2007; Cromer and McIntyre, 2008). Acid was initially thought to be the major endogenous agonist (Bevan and Geppetti, 1994), but later the cannabinoid receptor agonist anandamide and several other arachidonic acid-derived metabolites such as N-arachidonoyl-dopamine, N-oleoyldopamine, oleoylethanolamide, 12-(S)-hydroperoxyeicosatetraenoic acid, 15-(S)-hydroperoxyeicosatetraenoic acid and leukotriene B4 were found to activate TRPV1 (Zygmunt et al., 1999; Hwang et al., 2000; Huang et al., 2002; Szallasi et al., 2007). As the potency of these endovanilloids at TRPV1 is in the μmol L−1 range, the physiological and pathophysiological relevance of arachidonic acid-derived TRPV1 agonists in vivo is still debated.
The effect of acidosis on TRPV1 is two-fold. TRPV1 is gated open only if the extracellular pH is reduced below 6, in which case a sustained channel current is generated (Tominaga et al., 1998; Jordt et al., 2000). In contrast, mild acidosis in the range of pH 7–6 sensitizes TRPV1 to other stimuli such as capsaicin and heat (Tominaga et al., 1998; McLatchie and Bevan, 2001; Ryu et al., 2003; Neelands et al., 2005). As a result, the temperature threshold for TRPV1 activation is lowered under acidotic circumstances so that this cation channel becomes active at normal body temperature (Tominaga et al., 1998). Proton-induced sensitization involves both an increase in current activation and a decrease in current deactivation (Ryu et al., 2003; Neelands et al., 2005). Besides mild acidosis, many other signalling pathways (stimulated, for example, by inflammatory mediators such as prostaglandins, bradykinin, adenosine triphosphate, 5-hydroxytryptamine and nerve growth factor) cause allosteric modification of TRPV1 such that the probability of channel opening by heat, protons and capsaicin is enhanced (Caterina and Julius, 2001; Vellani et al., 2001; Gunthorpe et al., 2002; Trevisani et al., 2002; Szallasi et al., 2007). TRPV1 thus replicates the property of pain which, unlike any other sense, increases in intensity in the continued presence of a noxious stimulus (Zhang and McNaughton, 2006).
The property of TRPV1 to sensitize when exposed to painful stimuli has led to the hypothesis that TRPV1 is a prime factor in hyperalgesia (Reeh and Pethö, 2000). Sensitization of the ion channel depends on several mechanisms among which phosphorylation of TRPV1 by protein kinase A, protein kinase C and other kinases (Figure 1) is of particular importance (Premkumar and Ahern, 2000; Tominaga et al., 2001; Vellani et al., 2001; Kagaya et al., 2002; Olah et al., 2002; Bhave et al., 2003; Varga et al., 2006; Vetter et al., 2006; Zhang and McNaughton, 2006; Nilius et al., 2007). Whether pore dilation following sustained exposure to agonists (Chung et al., 2008) contributes to sensitization remains to be investigated. Another mechanism whereby bradykinin, nerve growth factor and anandamide increase TRPV1 activity involves phospholipase C-mediated hydrolysis of phosphatidylinositol-4,5-bisphosphate which normally inhibits TRPV1 gating by agonists (Prescott and Julius, 2003). In addition, TRPV1 sensitization involves the rapid recruitment of an intracellular pool of TRPV1 to the cell membrane (Figure 1), a process in which phophoinositide 3-kinase and Src kinase has an important function (Zhang et al., 2005; Stein et al., 2006).
Although phosphorylation causes sensitization, dephosphorylation of TRPV1 by protein phosphatases promotes desensitization which is a major mechanism of inhibitory regulation (Mohapatra and Nau, 2005). Desensitization of TRPV1 to capsaicin depends on extracellular Ca2+, whereas that to heat is independent of Ca2+, which attests to stimulus-dependent differences in the mechanism of desensitization (Bandell et al., 2007). Desensitization of TRPV1 to capsaicin involves a number of intracellular components including protein kinase A, adenosine triphosphate and calmodulin (Bhave et al., 2002; Mohapatra and Nau, 2003; Numazaki et al., 2003; Rosenbaum et al., 2004; Lishko et al., 2007). It appears as if a dynamic balance between phosphorylation and dephosphorylation of TRPV1 by Ca2+-calmodulin-dependent kinase II and calcineurin, respectively, controls the activation/desensitization state of the channel (Jung et al., 2004; Mohapatra and Nau, 2005). In addition, desensitization appears to be related to a depletion of phosphatidylinositol-4,5-bisphosphate (Liu et al., 2005; Stein et al., 2006), which attests to a dual function of this phosphoinositide in sensitization and desensitization of TRPV1 (Lukacs et al., 2007).
The ability of protons to sensitize TRPV1 to heat and other stimuli, on the one hand, and to activate TRPV1 per se, on the other hand, is mediated by different amino acid residues of the channel protein. Glu-600 on the extracellular side of transmembrane segment 5 is crucial for proton-induced sensitization of TRPV1, whereas Val-538 in the extracellular linker between transmembrane segments 3 and 4, Thr-633 in the pore helix and Glu-648 in the linker between the selectivity filter of the pore and transmembrane segment 6 are essential for proton-induced gating of TRPV1 (Jordt et al., 2000; Ryu et al., 2007). Mutation of the latter amino acid residues selectively abrogates proton-evoked currents but preserves the current responses to capsaicin and heat and their potentiation by mildly acidic pH (Jordt et al., 2000; Ryu et al., 2007). Thus, the sites in the TRPV1 protein targeted by protons differ from those targeted by heat and chemical ligands (Jordt et al., 2000; Welch et al., 2000; McLatchie and Bevan, 2001; Gavva et al., 2004; Ryu et al., 2007).
The vanilloid and fatty acid TRPV1 ligands bind to intracellular sites of the channel, and the amino acids critical for capsaicin/resiniferatoxin binding include Arg-491, Tyr-511, Ser-512, Ile-514, Val-518 and residue 547 (Met in rat, Leu in man), which are part of transmembrane segments 3 and 4 (Jordt and Julius, 2002; Jung et al., 2002; Sutton et al., 2005; Johnson et al., 2006; Cromer and McIntyre, 2008). Apart from transient ligand binding, there is another mechanism of TRP channel activation which involves covalent binding of ligands and stable modification of the channel protein (Bandell et al., 2007). This is also true for TRPV1 in which a single cystein residue in the N-terminal region of the protein enables pungent compounds from onion and garlic to activate the channel (Salazar et al., 2008).
Activation of TRPV1 by heat is not fully understood, although it is known that the C terminus of thermo-TRP channels has an important function in this mode of channel opening (Brauchi et al., 2006).
TRPV1 expression by the sensory system
The selectivity with which capsaicin has long been known to act on primary sensory neurones (Holzer, 1991; Szallasi and Blumberg, 1999) is due to the selective expression of TRPV1 by these nerve cells. The implications of capsaicin-sensitive afferent neurones have been extensively studied with two experimental paradigms: stimulation of afferent neurones by acute application of capsaicin and pretreatment of animals or tissues with high doses of capsaicin to ‘desensitize' afferent neurones to the excitatory action of capsaicin and other stimulants. Importantly, ‘capsaicin desensitization' is by no means equivalent to desensitization of the TRPV1 channel, but a method to defunctionalize afferent neurones by stimulation of TRPV1, which through excess influx of Ca2+ causes long-lasting functional and morphological alterations in afferent neurones. These nerve cells may even degenerate if, for example, a high dose of capsaicin is administered to newborn rats (Jancsó et al., 1977) or micromolar concentrations of capsaicin are introduced in dorsal root ganglion (DRG) cultures (Wood et al., 1988). Uptake of nerve growth factor is one of the elements that determines whether sensory neurones degenerate or undergo long-lasting defunctionalization following exposure to a high dose of capsaicin in vivo (Szöke et al., 2002). As the whole sensory neurone is knocked down by ‘capsaicin desensitization', referral to a ‘capsaicin-sensitive' structure or function in its historical connotation does not necessarily point to an implication of TRPV1. In addition, capsaicin at higher concentrations can act on ion channels unrelated to TRPV1 and cellular structures lacking TRPV1 (Holzer, 1991).
Numerous studies have confirmed that primary sensory neurones originating from the dorsal root, trigeminal and nodose ganglia are the major cellular systems expressing TRPV1 (Caterina et al., 1997; Guo et al., 1999; Michael and Priestley, 1999; Patterson et al., 2003; Robinson et al., 2004; Schicho et al., 2004). In the dorsal root and trigeminal ganglia, TRPV1 is restricted to small and medium-sized somata that are known to give rise to unmyelinated (C-) and thinly myelinated (Aδ-) fibres (Caterina et al., 1997; Guo et al., 1999; Michael and Priestley, 1999). Following synthesis in the nerve cells, TRPV1-like immunoreactivity is transported into both the central and peripheral processes of primary afferent neurones (Guo et al., 1999). The fibres of these neurones innervate virtually all tissues of the body including skin, muscle, bone, internal organs and vascular system. There are, however, regional differences in the relative proportion of sensory neurones that stain positive for TRPV1. Thus, TRPV1-immunoreactive fibres are considerably more prevalent in visceral than in somatic afferent nerves (Robinson et al., 2004; Brierley et al., 2005; Hwang et al., 2005; Christianson et al., 2006).
There are also regional and species differences in the chemical coding of primary afferent neurones expressing TRPV1. A large body of evidence indicates that calcitonin gene-related peptide (CGRP), substance P, somatostatin and other neuropeptides are messenger molecules characteristic of capsaicin-sensitive afferents (Green and Dockray, 1988; Holzer, 1991; Sternini, 1992; Szallasi and Blumberg, 1999). Immunocytochemistry has revealed that co-localization of TRPV1 with these neuropeptides varies with subpopulation of afferent neurones, region and species (Hwang et al., 2005; Price and Flores, 2007). DRG neurones can be largely differentiated by their binding of isolectin B4 and their responsiveness to different neurotrophins Guo et al., 1999; Michael and Priestley, 1999; Liu et al., 2004; Hwang et al., 2005; Price and Flores, 2007). In adult rodents, the isolectin B4-negative cell population responds to nerve growth factor, whereas isolectin B4-positive cells respond to the glial cell line-derived family of neurotrophins. However, there is no clear distinction between these populations of DRG neurones in terms of their expression of TRPV1 and the neuropeptides substance P, CGRP and somatostatin (Price and Flores, 2007). In the rat, TRPV1 is found in both populations of DRG neurones but is more prevalent in isolectin B4-positive cells (Guo et al., 1999; Michael and Priestley, 1999; Liu et al., 2004; Hwang et al., 2005; Price and Flores, 2007), whereas in the mouse TRPV1 is largely absent from isolectin B4-positive DRG cells (Zwick et al., 2002; Woodbury et al., 2004; Price and Flores, 2007). In both rat and mouse, however, TRPV1 abounds in visceral sensory neurones that bind little isolectin B4 but are rich in CGRP and substance P (Ward et al., 2003; Robinson et al., 2004; Schicho et al., 2004; Brierley et al., 2005; Hwang et al., 2005; Christianson et al., 2006).
In addition to its prominent location in sensory neurones, TRPV1 has been encountered in afferent neurone-associated cells such as epithelial cells in the urinary bladder (Birder et al., 2001, 2002), cells of the gastric mucosa (Nozawa et al., 2001; Kato et al., 2003; Kechagias et al., 2005) and keratinocytes as well as mast cells in the skin (Ständer et al., 2004; Bodó et al., 2005; Facer et al., 2007). The function of TRPV1 in these cellular systems has been less extensively studied than that in sensory neurones. It need be considered that some of the TRPV1-like immunoreactivity found in cells other than primary afferent neurones represents splice variants of TRPV1 whose function may differ from that of neuronal TRPV1 (Wang et al., 2004; Szallasi et al., 2007). Some authors have described expression of TRPV1 in neurones of the enteric nervous system whereas other authors failed to confirm this location (for a review see Holzer, 2004a), given that TRPV1 messenger ribonucleic acid (RNA) disappears from the rat stomach following extrinsic denervation (Schicho et al., 2004).
Implications of TRPV1 in inflammation, pain and hyperalgesia
In most tissues, stimulation of sensory neurones by noxious stimuli has two different effects: local release of neuropeptides from the peripheral nerve fibres in the tissue and induction of autonomic reflexes, sensation and pain (Holzer, 1988; Maggi and Meli, 1988). By releasing peptide transmitters in the periphery, sensory nerve fibres can modify vascular, immune and visceral smooth muscle functions. Following tissue irritation or injury, some of these reactions (for example, vasodilatation and plasma protein extravasation) contribute to the process of neurogenic inflammation. This efferent-like mode of operation may take place independently of nociception, and it has been hypothesized that some DRG neurones are specialized in controlling peripheral effector mechanisms only, whereas other DRG neurones may be specialized in the afferent mode of action or both (Holzer and Maggi, 1998). The neuropeptides involved in the efferent-like mode of operation include CGRP, somatostatin and the tachykinins substance P and neurokinin A (Maggi, 1995; Pintér et al., 2006). Calcitonin gene-related peptide and the tachykinins facilitate inflammation, whereas the effects of somatostatin are of an anti-inflammatory nature (Pintér et al., 2006; Helyes et al., 2007). There is an increasing body of experimental and clinical findings that TRPV1 has a function in inflammatory processes and in the pain and hyperalgesia associated with inflammation, injury, acidosis and malignancies. The evidence for this concept is severalfold as summarized in Table 2.
Table 2.
Summary of experimental and clinical findings attesting to a role of TRPV1 in inflammation and in hyperalgesia associated with inflammation, injury, acidosis and malignancies
| Activation, inhibition or deletion of TRPV1 modifies inflammatory processes in a tissue- and condition-specific manner |
| Activation of TRPV1 stimulates afferent neurones and elicits pain in humans and pain-related behaviour in animals |
| The expression of TRPV1 by sensory neurones and associated cells is upregulated under conditions of inflammation and hyperalgesia in both rodents and humans |
| Many noxious stimuli converge on TRPV1 to reduce its threshold for activation by heat, capsaicin, protons and other agonists |
| Thermal hyperalgesia in response to experimental inflammation is attenuated by TRPV1 knockout |
| Hypersensitivity to mechanical noxious stimuli following nerve injury or visceral inflammation is reduced by TRPV1 knockout |
| TRPV1 antagonists block behavioural pain responses to thermal, chemical and mechanical stimuli in experimental models of inflammatory, neuropathic, ischaemic, acidotic and cancer pain |
Table 3 presents a select overview of results that attest to an implication of TRPV1 in inflammation and in the hyperalgesia associated with inflammation, nerve injury, cancer and other disorders in a variety of tissues including skin, skeletal muscle, bone, joints and visceral organs such as the heart, respiratory system, digestive tract and urogenital system. As these implications of TRPV1 have been repeatedly reviewed elsewhere (Holzer, 2004a; Immke and Gavva, 2006; Szallasi et al., 2007; Gunthorpe and Szallasi, 2008), only some functions are exemplified here. Experimental inflammation in the skin leads to upregulation of TRPV1 expression and function in DRG neurones, particularly in the population of isolectin B4-positive somata (Carlton and Coggeshall, 2001; Ji et al., 2002; Breese et al., 2005). This sequel of inflammation depends on nerve growth factor which, by a post-transcriptional mechanism involving mitogen-activated protein kinase p38, increases the protein but not messenger RNA levels of TRPV1 in DRG neurones (Ji et al., 2002). The TRPV1 blocker SB-705498 has been found to elevate the heat pain threshold in the normal human skin and to increase the heat pain tolerance in human skin exposed to ultraviolet B irradiation (Chizh et al., 2007).
Table 3.
Select implications of TRPV1 in inflammation, pain and hyperalgesia
| Tissue | Pathophysiological process | Type of evidence | References |
|---|---|---|---|
| Skin | Experimental inflammation in rodents | Upregulation of expression and function of TRPV1 in DRG neurones | Carlton and Coggeshall (2001); Ji et al. (2002); Breese et al. (2005) |
| Skin | Inflammation-induced thermal hyperalgesia in rodents | Attenuation by TRPV1 knockout and antagonism | Caterina et al. (2000); Davis et al. (2000); Lehto et al. (2008) |
| Skin | Thermal and mechanical hyperalgesia because of thermal injury in mice | Attenuation by TRPV1 knockout | Bölcskei et al. (2005) |
| Skin | Thermal hyperalgesia of human skin because of ultraviolet B irradiation | Increase in heat pain tolerance by TRPV1 antagonism | Chizh et al. (2007) |
| Skin | Nocifensive response to intraplantar phorbol 12-myristate 13-acetate in mice | Abolition by TRPV1 knockout | Bölcskei et al. (2005) |
| Skin | Acid-induced pain in rodents | Attenuation by TRPV1 knockout and antagonism | Caterina et al. (2000); Davis et al. (2000); Pomonis et al. (2003) |
| Skin/leg | Experimental nerve injury in rodents (neuropathic pain) | Downregulation of TRPV1 in the damaged nerve fibres, but upregulation and ectopic expression of TRPV1 in non-damaged fibres, particularly those of the Aδ type | Hudson et al. (2001); Rashid et al. (2003) |
| Skin/leg | Mechanical hyperalgesia because of inflammation or nerve injury in rodents (inflammatory or neuropathic pain) | Attenuation by TRPV1 antagonism | Pomonis et al. (2003); Honore et al. (2005); Culshaw et al. (2006) |
| Skeletal muscle | Hypertensive response to muscle exercise in rodents (acid-induced pain) | Attenuation by TRPV1 antagonism | Gao et al. (2007) |
| Bone | Hyperalgesia associated with an in vivo bone cancer model in mice | Attenuation by TRPV1 knockout and antagonism | Ghilardi et al. (2005) |
| Joints | Experimental joint inflammation and mechanical hyperalgesia in rodents | Attenuation of inflammation and hyperalgesia by TRPV1 knockout | Keeble et al. (2005); Szabó et al. (2005); Barton et al. (2006) |
| Respiratory system | Bronchoconstriction, microvascular leakage, hypersecretion and cough | Induction by TRPV1 agonism | Geppetti et al. (2006); Kollarik et al. (2007); Szallasi et al. (2007); McLeod et al. (2008) |
| Respiratory system | Patients with chronic cough | Upregulation of TRPV1 in bronchi | Geppetti et al. (2006); Kollarik et al. (2007); Szallasi et al. (2007) |
| Respiratory system | Cough induced by citric acid in normal guinea-pigs or by antigen in allergic guinea-pigs | Attenuation by TRPV1 antagonism | Geppetti et al. (2006); Kollarik et al. (2007); Szallasi et al. (2007); McLeod et al. (2008) |
| Respiratory system | Patients with asthma and chronic obstructive pulmonary disease | Increased sensitivity to the protussive effect of TRPV1 | Geppetti et al. (2006); Kollarik et al. (2007); Szallasi et al. (2007) |
| Heart | Acidosis-evoked release of CGRP from sensory nerve fibres in the mouse heart | Abolition by TRPV1 knockout | Strecker et al. (2005) |
| Heart | Recovery of cardiac function after exposure to ischaemia/reperfusion (protection from and adaptation to ischaemic stress) | Attenuation by TRPV1 knockout and antagonism | Wang and Wang (2005); Zvara et al. (2006); Zhong and Wang (2007) |
| Oesophageal and gastrointestinal mucosa | Hyperaemia, secretion of bicarbonate secretion and protection from chemical injury in rats and humans | Induction by TRPV1 agonism | Holzer (1998, 2004b); Massa et al. (2006) |
| Ileal mucosa | Ileitis induced by Clostridium difficile toxin A in rodents | Attenuation by TRPV1 antagonism | McVey and Vigna (2001) |
| Colonic mucosa | Colitis induced by dextrane sulfate or trinitrobenzene sulphonic acid | Attenuation by TRPV1 antagonism | Kihara et al. (2003); Kimball et al. (2004); Miranda et al. (2007) |
| Digestive tract | Abdominal pain in rodents and humans | Induction by intraluminal capsaicin | Drewes et al. (2003); Schmidt et al. (2004); Holzer (2004a); Hammer et al. (2008) |
| Digestive tract | Acid-induced stimulation of vagal and spinal afferent nerve fibres in mice | Prevention by TRPV1 knockout and antagonism | Rong et al. (2004); Sugiura et al. (2007); Bielefeldt and Davis (2008) |
| Digestive tract | Acid-induced oesophagitis, gastric acid-evoked injury of the stomach, trinitrobenzene sulphonic acid-induced pancreatitis and colitis in rodents | Upregulation of TRPV1 in vagal and spinal afferent neurones | Schicho et al. (2004); Banerjee et al. (2007); Miranda et al. (2007); Xu et al. (2007) |
| Digestive tract | Patients with erosive gastro-oesophageal reflux disease, non-erosive reflux disease, irritable bowel syndrome, inflammatory bowel disease, idiopathic rectal hypersensitivity and faecal urgency | Upregulation of TRPV1 in the mucosa | Yiangou et al. (2001); Chan et al. (2003); Matthews et al. (2004); Bhat and Bielefeldt (2006); Akbar et al. (2008) |
| Upper gastrointestinal tract | Functional dyspepsia | Hypersensitivity to the algesic effect of capsaicin | Hammer et al. (2008) |
| Upper gastrointestinal tract | Functional dyspepsia | Beneficial effect of TRPV1 ablation | Bortolotti et al. (2002) |
| Colon | Hyperalgesia to intraluminal acid and colorectal distension in mice with acute experimental colitis | Attenuation by TRPV1 knockout and antagonism | Jones et al. (2005, 2007); Miranda et al. (2007) |
| Colon | Post-inflammatory chemical and mechanical hyperalgesia in rodents | Attenuation by TRPV1 knockout and antagonism | Eijkelkamp et al. (2007); Jones et al. (2007); Winston et al. (2007) |
| Peritoneal cavity | Behavioural pain response to intraperitoneal injection of acetic acid in rodents | Attenuation by TRPV1 antagonism | Ikeda et al. (2001); Rigoni et al. (2003); Tang et al. (2007) |
| Pancreas | Pancreatitis induced by caerulein | Attenuation by TRPV1 antagonism | Nathan et al. (2001) |
| Pancreas | Islet inflammation in non-obese diabetic mice (genetic model of type I diabetes) | Prevention by TRPV1 ablation | Razavi et al. (2006); Suri and Szallasi (2008) |
| Pancreas | Pain behaviour associated with L-arginine-induced necrotizing pancreatitis in rodents | Attenuation by TRPV1 antagonism | Wick et al. (2006) |
| Urinary bladder | Responsiveness of afferent neurones to bladder distension and intravesical acid in rodents | Attenuation by TRPV1 knockout and antagonism | Birder et al. (2002); Daly et al. (2007) |
| Urinary bladder | Increase in distension-induced afferent signalling and hyper-reflexia associated with experimental inflammation of the rodent urinary bladder | Attenuation by TRPV1 ablation and knockout | Jaggar et al. (2001); Charrua et al. (2007) |
| Urinary bladder | Patients with neurogenic bladder overactivity and sensory urgency | Upregulation of TRPV1 in the urinary bladder | Brady et al. (2004); Liu et al. (2007) |
| Urinary bladder | Patients with urinary bladder pain and hyperreflexia | Beneficial effect of TRPV1 ablation | Bley (2004); Brady et al. (2004); Avelino and Cruz (2006); Cruz and Dinis (2007) |
TRPV1 ablation refers to pretreatment with capsaicin or resiniferatoxin to defunctionalize afferent neurones.
TRPV1 in the digestive tract has been attributed diverse functions in tissue homeostasis and abdominal pain (Holzer, 2004a). Administration of capsaicin to the gastric and duodenal mucosa increases mucosal blood flow, a response which is mimicked by exposure to excess acid (Holzer, 1998). The acid-evoked hyperaemia in the duodenal mucosa is inhibited by the TRPV1 antagonist capsazepine, which indicates that acid activates TRPV1 on sensory nerve fibres that releases the vasodilator peptide CGRP (Akiba et al., 2006b). Through activation of a similar mechanism capsaicin is able to protect the oesophageal, gastric and intestinal mucosa from a variety of injurious chemical insults (Holzer, 1998). Paradoxically, knockout of TRPV1 has been reported to ameliorate acid-induced injury in the oesophagus and stomach (Fujino et al., 2006; Akiba et al., 2006a). Analysis of this observation in the stomach revealed that disruption of the TRPV1 gene causes a compensatory upregulation of other protective mechanisms in the gastric mucosa (Akiba et al., 2006a). Apart from protecting the gastrointestinal mucosa (Holzer, 1998; Massa et al., 2006), TRPV1 has also been found to exacerbate inflammation in certain models of pancreatitis, ileitis and colitis (Table 3). Emerging evidence indicates that TRPV1 contributes to pancreatic islet inflammation associated with type I diabetes and has a function in insulin-dependent glucose regulation, type II diabetes, adipogenesis and obesity (Razavi et al., 2006; Gram et al., 2007; Zhang et al., 2007; Suri and Szallasi, 2008). It awaits to be explored how these implications are reflected in the pharmacological profile of TRPV1 blockers.
Activation of TRPV1 on afferent neurones innervating the gut elicits pain in humans and pain-related behaviour in rodents, and there is emerging evidence that TRPV1 contributes to the chemical and mechanical hyperalgesia associated with gastrointestinal inflammation (Table 3). TRPV1 in afferent neurones has been found upregulated not only in inflammation but also in the absence of overt inflammation as is typical of functional gastrointestinal disorders (Holzer, 2008). This is true for patients with irritable bowel syndrome in which the increased density of TRPV1 in the rectosigmoid colon correlates with pain severity (Akbar et al., 2008). Non-erosive reflux disease (Bhat and Bielefeldt, 2006), idiopathic rectal hypersensitivity and faecal urgency (Chan et al., 2003) are other instances of TRPV1 upregulation in the absence of inflammation. Moreover, hypersensitivity to capsaicin characterizes a proportion of patients with functional dyspepsia (Hammer et al., 2008). A function of TRPV1 in this disorder is also suggested by the beneficial effect of repeated capsaicin intake (Bortolotti et al., 2002). Experimental findings have likewise shown that TRPV1 has a bearing on post-inflammatory colonic hyperalgesia in rodents, given that upregulation of TRPV1 expression and function persists long after the initial inflammatory insult has subsided (Eijkelkamp et al., 2007; Jones et al., 2007; Winston et al., 2007; Holzer, 2008). A possible function of TRPV1 in emesis (Andrews et al., 2000) requires further study.
Several experimental studies indicate that TRPV1 not only contributes to thermal and chemical nociception but also to inflammatory or neuropathic mechanical hyperalgesia in skin, bone, joint, gastrointestinal tract and urinary bladder (Table 3). This implication of TRPV1 is surprising in as much as TRPV1 channels have not yet been characterized as mechanosensitive. It may be, however, that TRPV1 has an impact on the excitability and sensory gain of TRPV1-bearing mechanosensitive nerve fibres, especially when its expression or function is upregulated.
Implications of TRPV1 in thermosensation and thermoregulation
It does not come as a surprise that the sensation caused by capsaicin is described as ‘hot' and ‘burning', given that TRPV1 is a heat sensor (Caterina et al., 1997; Tominaga et al., 1998). Surprising, however, is that TRPV1 knockout mice have a normal body temperature and do not seem to have a deficit in heat sensing (Szelényi et al., 2004; Woodbury et al., 2004; Iida et al., 2005), except that heat hyperalgesia in response to inflammation (Caterina et al., 2000; Davis et al., 2000) or heat injury (Bölcskei et al., 2005) and fever in response to the bacterial pyrogen lipopolysaccharide (Iida et al., 2005) are attenuated. The maintenance of basal thermoregulation in TRPV1 knockout mice was explained by a redundancy of heat sensors, whereas developmental compensations in heat sensing were little considered. The latter possibility, though, is a very likely explanation, given that many TRPV1 blockers cause substantial hyperthermia (Steiner et al., 2007; Gavva et al., 2007a).
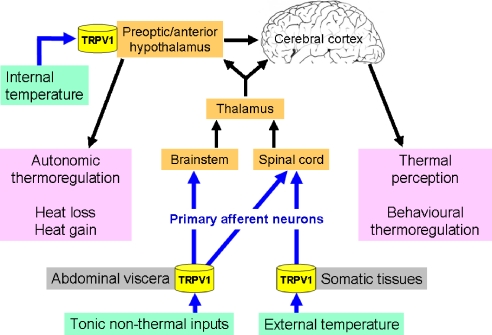
An implication of TRPV1 in heat sensing has long been envisaged from the effects of capsaicin and resiniferatoxin to cause hypothermia (Szolcsányi, 1982; Szallasi and Blumberg, 1999). In addition, pretreatment of rodents with a high dose of capsaicin to defunctionalize sensory and hypothalamic neurones leads to prolonged hyperthermia and impairs the animals' ability to recognize high ambient temperatures and to mount appropriate heat dissipation responses (Jancsó-Gábor et al., 1970a; Szolcsányi, 1982). As the thermoregulatory behaviour of rats treated with capsaicin as neonates or adults is differentially altered, it has been suggested that peripheral capsaicin-sensitive mechanisms are more relevant to body temperature regulation than central capsaicin-sensitive mechanisms (Dib, 1983; Hajós et al., 1983). It needs to be taken into account, however, that capsaicin-induced ablation of sensory neurones reduces the DRG expression of several thermo-TRP channels including TRPV1, TRPM8 and TRPA1 (Yamashita et al., 2008). TRPV1 is expressed by somatic and visceral sensory neurones that monitor the external environmental and internal body temperature, respectively, and by neurones in the preoptic/anterior hypothalamus which functions both as a sensor of local temperature and an integrator of sensory input from the periphery (Figure 2). It is now emerging that an important function of TRPV1 is to monitor and control core body temperature (Montell and Caterina, 2007). This implication is based on the ability of several TRPV1 blockers to prevent the hypothermic effect of capsaicin and to raise body temperature in rats, mice, dogs, monkeys and humans by a peripheral site of action (Steiner et al., 2007; Gavva et al., 2007a, 2007b, 2008).
Figure 2.
Involvement of peripheral and central TRPV1 in thermoregulation.
The hyperthermic action of TRPV1 blockers involves cutaneous vasoconstriction and shivering-related thermogenesis, but not warmth-seeking behaviour and is inhibited by the antipyretic drug acetaminophen (Steiner et al., 2007; Gavva et al., 2007b, 2008). As the hyperthermic effect of the TRPV1 antagonist AMG0347 is absent in TRPV1 knockout mice, it is entirely the result of TRPV1 blockade. Because the magnitude of the hyperthermic effect is independent of the baseline temperature, it has been concluded that the rise of body temperature results from blockade of tonic TRPV1 activation by non-thermal factors (Steiner et al., 2007). These non-thermal factors do not seem to include protons (Gavva et al., 2007a) but otherwise have not yet been identified. It is very likely that the site of the hyperthermic action of TRPV1 blockers is outside the blood–brain barrier (Figure 2), because i.c.v. or i.t. AMG0347 is no more effective in causing hyperthermia than intravenous administration (Steiner et al., 2007). Both brain-penetrant and peripherally restricted TRPV1 antagonists are in fact fully active in raising body temperature (Gavva et al., 2007a). The site where TRPV1 blockers act to raise body temperature is by all means on sensory nerve fibres within the abdominal cavity (Figure 2), because localized intra-abdominal ‘desensitization of TRPV1' by intraperitoneal resiniferatoxin blocks the hyperthermic action of AMG0347 (Steiner et al., 2007). Identification of the non-thermal factors that act on visceral afferent neurones to moderate body temperature may open up opportunities to differentiate between the unwanted hyperthermic and wanted analgesic effects of TRPV1 antagonists.
Phase I trials have established that the TRPV1 antagonist AMG517 (1–25 mg taken orally as a single dose) raises body temperature in humans for an approximate duration of 1 day, the magnitude of hyperthermia being related to the plasma concentration of the drug (Gavva et al., 2008). As in rats, dogs and monkeys, the hyperthermic effect of AMG517 in human volunteers was transient and attenuated following repeated dosing (2–10 mg taken orally as a single daily dose for 7 days) although, relative to placebo-treated control subjects, the mean maximal body temperatures of all subjects stayed significantly higher over the 7-day treatment period (Gavva et al., 2007b, 2008). In a phase Ib trial involving patients undergoing molar extraction in whom AMG517 (2–15 mg taken orally as a single dose) was evaluated for the control of postoperative pain, body temperatures of 39–40.2 °C were found to persist for 1–4 days in 33% of the nine subjects studied (Gavva et al., 2008). Thus, marked interindividual differences in the susceptibility to the hyperthermic effect of the TRPV1 blocker were observed. The long duration of action is likely to be related to the long plasma half life (13–23 days) of AMG517 in humans (Gavva et al., 2008). In the only other phase I trial of a TRPV1 blocker reported to date, no information on the effect of SB-705498 on body temperature was provided (Chizh et al., 2007).
TRPV1 expression and function in the central nervous system
Although sensitivity to capsaicin is a prominent feature of many primary afferent neurones, it has long been known that capsaicin can act on central neurones, notably on warm-sensitive neurones in the preoptic/anterior hypothalamus (Jancsó-Gábor et al., 1970b; Szolcsányi et al., 1971). Later it was found that a number of neurones in discrete fore- and hindbrain areas including the preoptic area of the hypothalamus are susceptible to the neurotoxic action of capsaicin (Szolcsányi, 1982; Ritter and Dinh, 1988, 1992; Kim et al., 2005). It thus did not come as a total surprise that TRPV1 messenger RNA and protein as well as TRPV1-like binding sites are widely distributed in the rodent brain (Mezey et al., 2000; Szabo et al., 2002; Roberts et al., 2004; Tóth et al., 2005; Cristino et al., 2006). A quantitative comparison, however, has shown that the levels of TRPV1 messenger RNA in the brain are substantially lower than those in the DRG ganglia (Sanchez et al., 2001). Notable levels of TRPV1 are found in the cortex, several areas of the limbic system (hippocampus, amygdala, habenula), striatum, substantia nigra, thalamus, preoptic area, hypothalamus, periaqueductal grey, reticular formation, locus coeruleus and cerebellum (Mezey et al., 2000; Sanchez et al., 2001; Szabo et al., 2002; Roberts et al., 2004; Tóth et al., 2005; Cristino et al., 2006).
The wide distribution of TRPV1 in the central nervous system raises the possibility that this ion channel could be involved in many brain functions (Steenland et al., 2006). In the preoptic/anterior hypothalamus, capsaicin stimulates and subsequently desensitizes thermosensitive neurones, which results in hypothermia and impaired thermoregulation against overheating, respectively (Jancsó-Gábor et al., 1970b; Szolcsányi et al., 1971; Szolcsányi, 1982; Hori, 1984). These actions of capsaicin are mediated by TRPV1, activation of which in the medial preoptic nucleus causes hypothermia by modification of neurotransmission through glutamate and γ-aminobutyric acid (Karlsson et al., 2005). Stimulation of glutamate release is a mechanism whereby TRPV1 activation in the paraventricular nucleus of the hypothalamus excites pre-autonomic neurones (Li et al., 2004) and in the ventral tegmental area stimulates mesolimbic dopaminergic neurones (Marinelli et al., 2005). Long-term depression in hippocampal interneurones depends on TRPV1, which points to a possible function of this ion channel in the control of learning, epileptic activity and synaptic plasticity (Gibson et al., 2008). Besides cognition, emotional processes may also involve TRPV1, given that anxiety, conditioned fear and hippocampal long-term potentiation are reduced in TRPV1 knockout mice (Marsch et al., 2007).
Increasing evidence suggests that TRPV1 participates in the processing of pain signals within the brain (Marinelli et al., 2005; Cui et al., 2006; Steenland et al., 2006; Palazzo et al., 2008). Thus, TRPV1 stimulation in the periaqueductal grey by capsaicin or anandamide causes analgesia, an effect that depends on the release of glutamate and stimulation of descending antinociceptive pathways (Palazzo et al., 2008). Other sites in the brain where TRPV1 might modify nociception include the locus coeruleus (Hajós et al., 1986), the ventral tegmental area (Marinelli et al., 2005) and the anterior cingulate cortex (Steenland et al., 2006).
The potentials and risks of TRPV1 as a drug target
Recognition of TRPV1 as a multimodal nocisensor, its sensitization by a number of proalgesic pathways and its upregulation under conditions of hyperalgesia have made this ion channel an attractive target for novel antinociceptive drugs. Pain and hyperalgesia may be attenuated at the very site of their generation, given that TRPV1 is preferentially expressed by afferent neurones and sensory neuron-associated cells. This concept is particularly attractive because it offers the opportunity to develop antinociceptive drugs with a peripherally restricted site of action, avoiding unwanted effects on the central nervous system, although there is information that brain-penetrant TRPV1 blockers are more powerful than peripherally restricted compounds (Cui et al., 2006). As any nocisensor, however, TRPV1 has an important function in the maintenance of homeostasis in the face of pending tissue injury. Interference with molecular probes that are physiologically so important is liable to have adverse effects, unless selective inhibition of ‘excess' nocisensors can be achieved whereas their physiological function is preserved. In view of these issues and the bewildering array of its functional implications, TRPV1 need be considered a pharmacological drug target of high potential and high risk.
TRPV1 function can be pharmacologically manipulated by two principal approaches: stimulant/defunctionalizing TRPV1 agonists and TRPV1 antagonists (Roberts and Connor, 2006; Gharat and Szallasi, 2008; Gunthorpe and Szallasi, 2008). The current patent literature discloses more than 1000 natural and synthetic compounds as TRPV1 activators or blockers (Gharat and Szallasi, 2008). It is important to realize that the pharmacological mechanism and biological result of the two approaches are profoundly different. Although TRPV1 antagonists specifically modify the function of the ion channel, stimulant/defunctionalizing TRPV1 agonists target the cellular function of capsaicin-sensitive afferent neurones (Holzer, 1991; Szallasi et al., 2007). The ‘desensitization' that is brought about by capsaicin, resiniferatoxin or synthetic analogues such as N-[4-(2-aminoethoxy)-3-methoxy-phenyl]-methyl-N′-[4-(1-1-dimethylethyl)phenyl]methyl-urea (SDZ 249–665) (Urban et al., 2000) reflects ‘defunctionalization' of the whole afferent neurone expressing TRPV1 for a prolonged period of time. In the case of capsaicin, the defunctionalizing action is preceded by the compound's powerful effect to cause pain and irritation, whereas resiniferatoxin and SDZ 249–665 are examples of TRPV1 agonists whose action manifests itself primarily in a defunctionalization of nociceptive neurones.
Given that sensory neurones express many nocisensors, it has been argued that local ‘desensitization' of afferent neurones by topical TRPV1 agonists is more efficacious in silencing pain and safer than just targeting one nocicensor with a systemic TRPV1 antagonist. In practice, though, the initial painful effect of TRPV1 agonists requires analgesic/anaesthetic co-medication, and a therapeutic effect is achieved only after repeated administration of the compounds for several weeks because of the low doses used and the limited absorption of the drug. Intravesical resiniferatoxin has been shown to be beneficial in patients with neurogenic bladder disorders in which activation of afferent neurones by mechanical and chemical stimuli is likely to have a function, but the results of various clinical studies have been inconsistent (Avelino and Cruz, 2006; Cruz and Dinis, 2007). Several phase II and III trials have been launched to evaluate the efficacy and safety of defunctionalizing TRPV1 agonists such as transacin and civamide for indications as diverse as post-herpetic neuropathy, human immunodeficiency virus-associated neuropathy, cluster headache, migraine and osteoarthritic, musculoskeletal as well as postoperative pain (Szallasi et al., 2007; Knotkova et al., 2008). It remains to be seen how these site-specific therapeutic regimens involving high-dose patches, intranasal formulations and injectable preparations fare in terms of onset, duration, magnitude and selectivity of action.
Most efforts have been directed at developing compounds that block TRPV1 activation in a competitive or noncompetitive manner. The first of this kind, capsazepine, has been extensively used in the exploration of the pathophysiological implications of TRPV1. However, the results obtained with this compound need to be judged with caution because the selectivity of capsazepine as a TRPV1 blocker is limited by its inhibitory action on nicotinic acetylcholine receptors, voltage-activated Ca2+ channels and other TRP channels such as TRPM8 (Docherty et al., 1997; Liu and Simon, 1997; Behrendt et al., 2004). The TRPV1 blockers that have been designed following the molecular identification of TRPV1 can be categorized into vanilloid-derived and non-vanilloid compounds (Gharat and Szallasi, 2008). The latter class of TRPV1 blockers comprises several different chemical entities (Tables 4 and 5) reviewed in detail elsewhere (Gharat and Szallasi, 2008).
Table 4.
Select classes of non-vanilloid TRPV1 blockers found in the patent literature
| Pyridyl piperazine carboxamides such BCTC |
| Tetrahydropyridine amides |
| 4-Fluopyridine amides |
| Spiroisoxazolopyridine amides |
| Piperazinyl benzimidazoles |
| Benzimidazole derivatives |
| Benzamides |
| Pyridoindazolones |
| Piperazinyl pyrimidines |
| Bicyclic pyrimidin-4-(3H)-ones |
| Phenylchromones |
| Biaryl carboxamides |
| Aminoquinazolines |
| 1,3-Disubstituted urea derivatives such as A-425619 and SB-705498 |
| Aryl cinnamides such as AMG9810 |
A-425619: 1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea; AMG9810: (E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)acrylamide; BCTC: N-(4-tertiarybutylphenyl)-4(3-cholorphyridin-2-yl)-tetrahydropyrazine-1(2H)-carboxamide; SB-705498: N-(2-bromophenyl)-N'-[((R)-1-(5-trifluoromethyl-2-pyridyl)pyrrolidin-3-yl)]urea. Detailed information on the compounds listed in the table is given by Gharat and Szallasi (2008).
Table 5.
Four classes of rat TRPV1 antagonists with distinct pharmacological action profiles
| Profile | Examples | Response to capsaicin (0.5 μmol L−1) | Response to pH 5 | Response to heat (45 °C) | Effect on body temperature in rodents |
|---|---|---|---|---|---|
| A | A-425619, AMG0347, AMG517, AMG6880, AMG7472, AMG8163, AMG9810, BCTC, iodo-resiniferatoxin, SB-705498 | Block | Block | Block | Hyperthermia |
| B | AMG0610, AMG8563, capsazepine; SB-366791 | Block | Partial block | Block | Hyperthermia |
| C | AMG8562 | Block | Potentiation | Block | No change |
| D | AMG7905 | Block | Potentiation | Potentiation | Hypothermia |
A-425619: 1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea; AMG0347: (E)-N-(7-hydroxy-5,6,7,8-tetrahydronaphthalen-1-yl)-3-(2-(piperidin-1-yl)-6-(trifluoromethyl)pyridin-3-yl)acrylamide; AMG517: N-(4-[6-(4-trifluoromethyl-phenyl)-pyrimidin-4-yloxy]-benzothiazol-2-yl)-acetamide I; AMG0610: (2E)-3-(6-tert-butyl-2-methylpyridin-3-yl)-N-(1H-indol-6-yl)acrylamide; AMG6880: (2E)-3-[2-piperidin-1-yl-6-(trifluoromethyl)pyridin-3-yl]-N-quinolin-7-ylacrylamide; AMG7472: 5-chloro-6-{(3R)-3-methyl-4-[6-(trifluoromethyl)-4-(3,4,5-trifluorophenyl)-1H-benzimidazol-2-yl]piperazin-1-yl}pyridin-3-yl)methanol; AMG7905: N-(6-(2-(cyclohexylmethylamino)-4-(trifluoromethyl)phenyl)pyrimidin-4-yl)benzo[d]thiazol-6-amine; AMG8163: tert-butyl-2-(6-([2-(acetylamino)-1,3-benzothiazol-4-yl]oxy)pyrimidin-4-yl)-5-(trifluoromethyl)phenylcarbamate; AMG8562: (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)acrylamide; AMG8563: (S,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)acrylamide; AMG9810: (E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)acrylamide; BCTC: N-(4-tertiarybutylphenyl)-4(3-cholorphyridin-2-yl)-tetrahydropyrazine-1(2H)-carboxamide; SB-366791: (2E)-3-(4-chlorophenyl)-N-(3-methoxyphenyl)acrylamide; SB-705498: N-(2-bromophenyl)-N'-[((R)-1-(5-trifluoromethyl-2-pyridyl)pyrrolidin-3-yl)]urea. Adapted from Lehto et al. (2008).
As TRPV1 is activated by multiple stimuli that interact with different domains of the channel protein, some TRPV1 blockers are stimulus-specific (Gavva et al., 2005a; Lehto et al., 2008). For instance, AMG0610 and SB-366791 inhibit the activation of rat TRPV1 by capsaicin but not acid, whereas iodo-resiniferatoxin, BCTC, AMG6880, AMG7472, AMG9810 and A-425619 are TRPV1 antagonists that do not differentiate between capsaicin and protons (Seabrook et al., 2002; Gavva et al., 2004, 2005a, 2005b; Neelands et al., 2005). On the other hand, AMG8562 does not block heat-evoked activation of rat TRPV1 (Lehto et al., 2008). Importantly, there are also species differences in the stimulus selectivity of TRPV1 blockers. For instance, capsazepine and SB-366791 are more effective in blocking proton-induced gating of human TRPV1 than of rat TRPV1 (Gunthorpe et al., 2004; Gavva et al., 2005a), and AMG8562 antagonizes heat activation of human but not rat TRPV1 (Lehto et al., 2008).
Although the vast list of emerging TRPV1 blockers (Gharat and Szallasi, 2008) attests to the antinociceptive potential that is attributed to this class of pharmacological agent, it is important to be aware of the likely drawbacks these compounds may have. It has repeatedly been argued that TRPV1 subserves important homeostatic functions, and that the challenge for an effective and safe therapy with TRPV1 blockers will be to suppress the pathological contribution of ‘excess' TRPV1 while preserving its physiological function (Holzer, 2004b; Hicks, 2006; Storr, 2007; Szallasi et al., 2007). This concept is impressively portrayed by the emerging function of TRPV1 in thermoregulation as revealed by the hyperthermic action of TRPV1 blockers (Gavva et al., 2007a, 2007b, 2008). Hyperthermia is an adverse effect of TRPV1 blockade that went unnoticed after disruption of the TRPV1 gene (Szelényi et al., 2004; Woodbury et al., 2004), most probably because of developmental compensations in heat sensing.
Apart from the thermoregulatory perils of TRPV1 antagonism (Caterina, 2008), blockade of TRPV1 will also interfere with the physiological function of this nocicensor to survey the physical and chemical environment and, if necessary, to initiate protective responses. Such a function is obvious in the gastrointestinal tract in which capsaicin-sensitive afferent neurones constitute a neural alarm system which helps maintaining mucosal homeostasis in the face of pending injury. As TRPV1 is involved in this task (Holzer, 2004a; Akiba et al., 2006b), TRPV1 antagonism may result in enhanced vulnerability of the gastrointestinal mucosa. Similarly, endotoxin-induced pulmonary inflammation, lung injury and bronchial hyper-reactivity are exacerbated in TRPV1 knockout mice, most probably because the anti-inflammatory and antinociceptive action of somatostatin released from TRPV1-bearing sensory neurones is lacking (Helyes et al., 2007). A deficiency in this protective somatostatin mechanism may also explain why the mechanical hyperalgesia associated with experimental polyneuropathy models is enhanced after TRPV1 gene deletion (Bölcskei et al., 2005). Another caveat derives from the widespread distribution of TRPV1 in the peripheral and central nervous system. Although adverse effects on the brain may be avoided by the development of peripherally restricted TRPV1 antagonists, it has been reported that a significant penetration into the brain is necessary for a TRPV1 antagonist to produce broad-spectrum analgesia (Cui et al., 2006). Recent work suggests, however, that deletion or blockade of TRPV1 in the brain affects cognitive as well as emotional-affective processes (Marsch et al., 2007; Gibson et al., 2008).
Novel approaches to TRPV1 pharmacology
The pharmacological profile of many TRPV1 antagonists to cause hyperthermia represents a hurdle to their use as first-line therapeutics (Caterina, 2008; Gavva et al., 2008). This does not discount the further development of drugs targeting TRPV1, because there are several approaches on the horizon to focus therapy specifically on those TRPV1 channels that are involved in the disease process (Table 6).
Table 6.
Approaches to focus therapy specifically on TRPV1 channels upregulated in disease while sparing their physiological function
| Site-specific TRPV1 antagonists |
| Modality-specific TRPV1 antagonists |
| Uncompetitive TRPV1 (open channel) blockers |
| Drugs interfering with the sensitization of TRPV1 |
| Drugs interfering with the intracellular trafficking of TRPV1 |
| Defunctionalizing TRPV1 agonists for local administration |
Much as the use of defunctionalizing TRPV1 agonists needs to be restricted to the region affected by inflammation and hyperalgesia, TRPV1 antagonists could be formulated such that they can be administered in an anatomically confined manner that prevents access of the drug to visceral TRPV1 channels that are most relevant to thermoregulation (Caterina, 2008). Another approach of site-specific TRPV1 blockade that has been tested experimentally is to interfere with the synthesis of new TRPV1 channels by small RNA interference (TRPV1 knockdown) or antisense oligonucleotides. Thus, i.t. administration of small interfering RNA or a TRPV1 antisense oligonucleotide attenuates visceral and neuropathic pain in rats (Christoph et al., 2006, 2007). The expression of TRPV1 by sensory neurones outside the brain offers a further pharmacological opportunity for a site-specific pharmacological intervention with sensory neuron functions. Thus, the TRPV1 channel can be used as a vehicle for the cellular influx of membrane-impermeant local anaesthetics such as the lidocaine derivative QX-314 (Binshtok et al., 2007). When TRPV1 is activated by capsaicin, QX-314 gains access to the intracellular space and, subsequently, blocks voltage-gated sodium channels and action potential conduction only in sensory neurones expressing TRPV1. In this way, local anaesthetics can be made selective for nociceptive afferent neurones, avoiding their unwanted action on non-nociceptive sensory, autonomic and motor neurones (Binshtok et al., 2007).
The property of TRPV1 to function as a multimodal nocisensor offers the opportunity to design modality-specific TRPV1 blockers, compounds that prevent activation of TRPV1 by distinct stimuli while sparing the channel's sensitivity to other stimuli. The feasibility of this approach has already been proved (Table 5), given that there are antagonists that inhibit TRPV1 activation by capsaicin and heat but not acid (Gavva et al., 2005a), whereas other compounds antagonize capsaicin but not heat (Lehto et al., 2008). On the basis of these properties, the available TRPV1 blockers have been divided into four categories with distinct pharmacological action profiles Lehto et al. (2008) as summarized in Table 5. Thus, TRPV1 antagonists that do not cause hyperthermia are in sight (Lehto et al., 2008). The existence of stimulus-dependent differences in the mechanism of channel desensitization (Bandell et al., 2007) is a further aspect relevant to the modality-specific manipulation of TRPV1.
Whereas competitive and non-competitive TRPV1 antagonists will block TRPV1 channels that are both physiologically expressed and pathologically overexpressed, uncompetitive TRPV1 antagonists may be used to differentiate between normal and exaggerated activity of TRPV1. Unlike competitive and non-competitive antagonists that prevent activation of a receptor by an agonist, uncompetitive agonists require receptor activation by an agonist before they can bind to a separate allosteric binding site. By preferentially binding to the active, open state of the channel, uncompetitive TRPV1 (open channel) blockers may preferentially silence overactive TRPV1. This type of antagonism entails that the same antagonist concentration can antagonize higher agonist concentrations better than lower agonist concentrations (Lipton, 2007). The principle of uncompetitive channel blockade is part of the general concept that drugs should be activated by the pathological state that they are intended to inhibit (Lipton, 2007). It is easily conceivable that the complex post-translational regulation of TRPV1 function may be amenable to such a disease-specific type of blockade. For instance, in an experimental model of feline interstitial cystitis, TRPV1 currents in DRG neurones are enhanced in amplitude and desensitize very slowly, because TRPV1 appears to be maximally phosphorylated by protein kinase C (Sculptoreanu et al., 2005). As the structure–activity relationship of TRPV1 agonists and antagonists is differentially modulated by phosphatase inhibition, Pearce et al. (2008) have envisaged the possibility to tailor agonists and antagonists such that they act best on TRPV1 in a specific regulatory environment.
A rational therapeutic approach would be to prevent or reverse the increase in sensitivity and activity of TRPV1 associated with the disease. Overactivity of the ion channel seems to be brought about by two principal mechanisms, TRPV1 sensitization and TRPV1 trafficking to the cell membrane (Figure 1). It is through these mechanisms that a number of pro-inflammatory mediators reduce the activation threshold of TRPV1 by heat, protons and vanilloids. Although phosphorylation and relief from phosphatidylinositol-4,5-bisphosphate blockade sensitizes TRPV1 (Premkumar and Ahern, 2000; Vellani et al., 2001; Olah et al., 2002; Prescott and Julius, 2003), dephosphorylation by protein phosphatases leads to desensitization of TRPV1. As a balance between phosphorylation and dephosphorylation appears to determine the activity of the channel (Jung et al., 2004; Mohapatra and Nau, 2005; Zhang and McNaughton, 2006; Lukacs et al., 2007), both interference with sensitization mechanisms and promotion of TRPV1 desensitization would be pharmacological opportunities to reduce the sensory gain of TRPV1.
An intriguing approach that appears increasingly feasible is interference with the rapid trafficking of TRPV1 between cytosolic membrane compartments (endosomes, vesicles) and the cell membrane (Figure 1), which will result in a reduction of the availability of TRPV1 channels on the cell surface (Morenilla-Palao et al., 2004; Planells-Cases et al., 2005; Zhang et al., 2005). Most membrane receptors reside in macromolecular complexes that include regulatory, signalling and scaffolding proteins. For instance, A-kinase-anchoring protein-150 mediates phosphorylation of TRPV1 by protein kinase A and in this way contributes to thermal hyperalgesia (Jeske et al., 2008). Phosphoinositide 3-kinase is relevant to sensitization of TRPV1 by nerve growth factor and insulin-like growth factor because—together with TRPV1 and growth factor receptors—it is part of a signal transduction complex that facilitates the translocation of TRPV1 to the plasma membrane (Van Buren et al., 2005; Zhang et al., 2005; Stein et al., 2006). Protein kinase C, Src kinase, snapin, synaptotagmin IX and soluble N-ethylmaleimide-sensitive factor attachment protein receptor also form part of the signal transduction complexes relevant to TRPV1 exocytosis (Morenilla-Palao et al., 2004; Planells-Cases et al., 2005; Van Buren et al., 2005; Zhang et al., 2005).
Thus, sensitization of TRPV1 is due not only to an enhancement of channel currents but also to a rapid translocation of TRPV1 from a cytosolic pool to the plasma membrane (Morenilla-Palao et al., 2004; Planells-Cases et al., 2005; Van Buren et al., 2005; Zhang et al., 2005; Stein et al., 2006). The trafficking of TRPV1 (and other channels) to the cell surface is blocked by botulinum neurotoxin A (Morenilla-Palao et al., 2004), which may explain why intra-detrusor injection of botulinum neurotoxin A in patients with urinary bladder overactivity reduces TRPV1- and purinoceptor P2X3-like immunoreactivity in the detrusor muscle and causes improvement of clinical and urodynamic parameters (Apostolidis et al., 2005). Intravesical administration of botulinum toxin likewise counteracts acetic acid-evoked bladder overactivity in rats (Chuang et al., 2004).
Acknowledgments
Work performed in the laboratory was supported by the Zukunftsfonds Steiermark (Grant 262), the Austrian Scientific Research Funds (FWF Grant L25-B05), the Jubilee Foundation of the Austrian National Bank (Grant 9858) and the Austrian Federal Ministry of Science and Research. I thank Ulrike Holzer-Petsche for critically reading the paper and Evelin Painsipp for graphical assistance.
Abbreviations
- CGRP
calcitonin gene-related peptide
- DRG
dorsal root ganglion
- PIP2
phosphatidylinositol-4,5-bisphosphate
- RNA
ribonucleic acid
- TRP
transient receptor potential
- TRPV1
transient receptor potential vanilloid-1
Conflict of interest
The author states no conflict of interest.
References
- Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1 expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba Y, Ghayouri S, Takeuchi T, Mizumori M, Guth PH, Engel E, et al. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology. 2006b;131:142–152. doi: 10.1053/j.gastro.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Takeuchi T, Mizumori M, Guth PH, Engel E, Kaunitz JD. TRPV-1 knockout paradoxically protects mouse gastric mucosa from acid/ethanol-induced injury by upregulating compensatory protective mechanisms. Gastroenterology. 2006a;130 Suppl 2:A-106. [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153 Suppl. 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Okada F, Woods AJ, Hagiwara H, Kakaimoto S, Toyoda M, et al. The emetic and anti-emetic effects of the capsaicin analogue resiniferatoxin in Suncus murinus, the house musk shrew. Br J Pharmacol. 2000;130:1247–1254. doi: 10.1038/sj.bjp.0703428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, et al. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- Avelino A, Cruz F. TRPV1 (vanilloid receptor) in the urinary tract: expression, function and clinical applications. Naunyn-Schmiedeberg's Arch Pharmacol. 2006;373:287–299. doi: 10.1007/s00210-006-0073-2. [DOI] [PubMed] [Google Scholar]
- Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee B, Medda BK, Lazarova Z, Bansal N, Shaker R, Sengupta JN. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol Motil. 2007;19:681–691. doi: 10.1111/j.1365-2982.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- Barton NJ, McQueen DS, Thomson D, Gauldie SD, Wilson AW, Salter DM, et al. Attenuation of experimental arthritis in TRPV1R knockout mice. Exp Mol Pathol. 2006;81:166–170. doi: 10.1016/j.yexmp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Bevan S, Hothi S, Hughes G, James I, Rang HP, Shah K, et al. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat YM, Bielefeldt K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur J Gastroenterol Hepatol. 2006;18:263–270. doi: 10.1097/00042737-200603000-00006. [DOI] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, et al. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol. 2008;294:G130–G138. doi: 10.1152/ajpgi.00388.2007. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, et al. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Bley KR. Recent developments in transient receptor potential vanilloid receptor 1 agonist-based therapies. Expert Opin Investig Drugs. 2004;13:1445–1456. doi: 10.1517/13543784.13.11.1445. [DOI] [PubMed] [Google Scholar]
- Bodó E, Bíró T, Telek A, Czifra G, Griger Z, Tóth BI, et al. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am J Pathol. 2005;166:985–998. doi: 10.1016/S0002-9440(10)62320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölcskei K, Helyes Z, Szabó A, Sándor K, Elekes K, Németh J, et al. Investigation of the role of TRPV1 receptors in acute and chronic nociceptive processes using gene-deficient mice. Pain. 2005;117:368–376. doi: 10.1016/j.pain.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Bortolotti M, Coccia G, Grossi G, Miglioli M. The treatment of functional dyspepsia with red pepper. Aliment Pharmacol Ther. 2002;16:1075–1082. doi: 10.1046/j.1365-2036.2002.01280.x. [DOI] [PubMed] [Google Scholar]
- Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, et al. Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int. 2004;93:770–776. doi: 10.1111/j.1464-410X.2003.04722.x. [DOI] [PubMed] [Google Scholar]
- Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. 2005;115:37–49. doi: 10.1016/j.pain.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, Xu L, Robinson DR, Hicks GA, et al. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol (London) 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Peripheral capsaicin receptors increase in the inflamed rat hindpaw: a possible mechanism for peripheral sensitization. Neurosci Lett. 2001;310:53–56. doi: 10.1016/s0304-3940(01)02093-6. [DOI] [PubMed] [Google Scholar]
- Caterina MJ. On the thermoregulatory perils of TRPV1 antagonism. Pain. 2008;136:3–4. doi: 10.1016/j.pain.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chan CL, Facer P, Davis JB, Smith GD, Egerton J, Bountra C, et al. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet. 2003;361:385–391. doi: 10.1016/s0140-6736(03)12392-6. [DOI] [PubMed] [Google Scholar]
- Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007;177:1537–1541. doi: 10.1016/j.juro.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Chizh BA, O'Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Christoph T, Gillen C, Mika J, Grünweller A, Schäfer MK, Schiene K, et al. Antinociceptive effect of antisense oligonucleotides against the vanilloid receptor VR1/TRPV1. Neurochem Int. 2007;50:281–290. doi: 10.1016/j.neuint.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Christoph T, Grünweller A, Mika J, Schäfer MK, Wade EJ, Weihe E, et al. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350:238–243. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Chuang YC, Yoshimura N, Huang CC, Chiang PH, Chancellor MB. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J Urol. 2004;172:1529–1532. doi: 10.1097/01.ju.0000137844.77524.97. [DOI] [PubMed] [Google Scholar]
- Chung MK, Güler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Cromer BA, McIntyre P. Painful toxins acting at TRPV1. Toxicon. 2008;51:163–173. doi: 10.1016/j.toxicon.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cruz F, Dinis P. Resiniferatoxin and botulinum toxin type A for treatment of lower urinary tract symptoms. Neurourol Urodyn. 2007;26 Suppl:920–927. doi: 10.1002/nau.20479. [DOI] [PubMed] [Google Scholar]
- Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culshaw AJ, Bevan S, Christiansen M, Copp P, Davis A, Davis C, et al. Identification and biological characterization of 6-aryl-7-isopropylquinazolinones as novel TRPV1 antagonists that are effective in models of chronic pain. J Med Chem. 2006;49:471–474. doi: 10.1021/jm051058x. [DOI] [PubMed] [Google Scholar]
- Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol (London) 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. TRP ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Dib B. Dissociation between peripheral and central heat loss mechanisms induced by neonatal capsaicin. Behav Neurosci. 1983;97:822–825. doi: 10.1037//0735-7044.97.5.822. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Piper AS. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br J Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes AM, Schipper KP, Dimcevski G, Petersen P, Gregersen H, Funch-Jensen P, et al. Gut pain and hyperalgesia induced by capsaicin: a human experimental model. Pain. 2003;104:333–341. doi: 10.1016/s0304-3959(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N, Kavelaars A, Elsenbruch S, Schedlowski M, Holtmann G, Heijnen CJ. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: spinal cord c-Fos expression and behavior. Am J Physiol. 2007;293:G749–G757. doi: 10.1152/ajpgi.00114.2007. [DOI] [PubMed] [Google Scholar]
- Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino K, de la Fuente SG, Takami Y, Takahashi T, Mantyh CR. Attenuation of acid induced oesophagitis in VR-1 deficient mice. Gut. 2006;55:34–40. doi: 10.1136/gut.2005.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol. 2007;102:2288–2293. doi: 10.1152/japplphysiol.00161.2007. [DOI] [PubMed] [Google Scholar]
- García-Sanz N, Fernández-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sánchez E, Fernández-Ballester G, et al. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci. 2004;24:5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Hovland DN, Lehto SG, Klionsky L, Surapaneni S, et al. Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J Pharmacol Exp Ther. 2007b;323:128–137. doi: 10.1124/jpet.107.125674. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Lehto SG, Gore A, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007a;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, et al. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Klionsky L, Norman MH, Louis JC, Wild KD, et al. Proton activation does not alter antagonist interaction with the capsaicin-binding pocket of TRPV1. Mol Pharmacol. 2005a;68:1524–1533. doi: 10.1124/mol.105.015727. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther. 2005b;313:474–484. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol. 2006;533:207–214. doi: 10.1016/j.ejphar.2005.12.063. [DOI] [PubMed] [Google Scholar]
- Gharat LA, Szallasi A. Advances in the design and therapeutic use of capsaicin receptor TRPV1 agonists and antagonists. Expert Opin Ther Patents. 2008;18:159–209. [Google Scholar]
- Ghilardi JR, Röhrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–759. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram DX, Ahrén B, Nagy I, Olsen UB, Brand CL, Sundler F, et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213–223. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- Green T, Dockray GJ. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse, and guinea-pig. Neuroscience. 1988;25:181–193. doi: 10.1016/0306-4522(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, et al. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Szallasi A. Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharm Des. 2008;14:32–41. doi: 10.2174/138161208783330754. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hajós M, Engberg G, Elam M. Reduced responsiveness of locus coeruleus neurons to cutaneous thermal stimuli in capsaicin-treated rats. Neurosci Lett. 1986;70:382–387. doi: 10.1016/0304-3940(86)90584-7. [DOI] [PubMed] [Google Scholar]
- Hajós M, Obál F, Jancsó G, Obál F. The capsaicin sensitivity of the preoptic region is preserved in adult rats pretreated as neonates, but lost in rats pretreated as adults. Naunyn-Schmiedeberg's Arch Pharmacol. 1983;324:219–222. doi: 10.1007/BF00503898. [DOI] [PubMed] [Google Scholar]
- Hammer J, Führer M, Pipal L, Matiasek J. Hypersensitivity for capsaicin in patients with functional dyspepsia. Neurogastroenterol Motil. 2008;20:125–133. doi: 10.1111/j.1365-2982.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Hellwig N, Plant TD, Janson W, Schäfer M, Schultz G, Schaefer M. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J Biol Chem. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Elekes K, Németh J, Pozsgai G, Sándor K, Kereskai L, et al. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol. 2007;292:L1173–L1181. doi: 10.1152/ajplung.00406.2006. [DOI] [PubMed] [Google Scholar]
- Hicks GA. TRP channels as therapeutic targets: hot property, or time to cool down. Neurogastroenterol Motil. 2006;18:590–594. doi: 10.1111/j.1365-2982.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- Holzer P. TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol. 2004a;500:231–241. doi: 10.1016/j.ejphar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Holzer P. Vanilloid receptor TRPV1: hot on the tongue and inflaming the colon. Neurogastroenterol Motil. 2004b;16:697–699. doi: 10.1111/j.1365-2982.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. TRPV1: a new target for treatment of visceral pain in IBS. Gut. 2008;57:882–884. doi: 10.1136/gut.2008.149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Maggi CA. Dissociation of dorsal root ganglion neurons into afferent and efferent-like neurons. Neuroscience. 1998;86:389–398. doi: 10.1016/s0306-4522(98)00047-5. [DOI] [PubMed] [Google Scholar]
- Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314:410–421. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- Hori T. Capsaicin and central control of thermoregulation. Pharmacol Ther. 1984;26:389–416. doi: 10.1016/0163-7258(84)90041-x. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Oh JM, Valtschanoff JG. Expression of the vanilloid receptor TRPV1 in rat dorsal root ganglion neurons supports different roles of the receptor in visceral and cutaneous afferents. Brain Res. 2005;1047:261–266. doi: 10.1016/j.brainres.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. Attenuated fever response in mice lacking TRPV1. Neurosci Lett. 2005;378:28–33. doi: 10.1016/j.neulet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Ueno A, Naraba H, Oh-ishi S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001;69:2911–2919. doi: 10.1016/s0024-3205(01)01374-1. [DOI] [PubMed] [Google Scholar]
- Immke DC, Gavva NR. The TRPV1 receptor and nociception. Semin Cell Dev Biol. 2006;17:582–591. doi: 10.1016/j.semcdb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Jaggar SI, Scott HCF, James IF, Rice ASC. The capsaicin analogue SDZ 249-665 attenuates the hyperreflexia and referred hyperalgesia associated with inflammation of the rat urinary bladder. Pain. 2001;89:229–235. doi: 10.1016/s0304-3959(00)00366-3. [DOI] [PubMed] [Google Scholar]
- Jänig W.Visceral afferent neurons and autonomic regulations The Integrative Action of the Autonomic Nervous System. Neurobiology of Homeostasis 2006Cambridge University Press: Cambridge, UK; 35–83.In: Jänig W (ed). [Google Scholar]
- Jancsó G, Karcsú S, Király E, Szebeni A, Tóth L, Bácsy E, et al. Neurotoxin induced nerve cell degeneration: possible involvement of calcium. Brain Res. 1984;295:211–216. doi: 10.1016/0006-8993(84)90969-7. [DOI] [PubMed] [Google Scholar]
- Jancsó G, Kiraly E, Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jancsó G, Király E, Jancsó-Gábor A. Direct evidence for an axonal site of action of capsaicin. Naunyn-Schmiedeberg's Arch Pharmacol. 1980;313:91–94. doi: 10.1007/BF00505809. [DOI] [PubMed] [Google Scholar]
- Jancsó G, Király E, Joó F, Such G, Nagy A. Selective degeneration by capsaicin of a subpopulation of primary sensory neurons in the adult rat. Neurosci Lett. 1985;59:209–214. doi: 10.1016/0304-3940(85)90201-0. [DOI] [PubMed] [Google Scholar]
- Jancsó G, Király E, Such G, Joó F, Nagy A. Neurotoxic effect of capsaicin in mammals. Acta Physiol Hung. 1987;69:295–313. [PubMed] [Google Scholar]
- Jancsó N. Role of the nerve terminals in the mechanism of inflammatory reactions. Bull Millard Fillmore Hosp. 1960;7:53–77. [Google Scholar]
- Jancsó-Gábor A, Szolcsányi J, Jancsó N. Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J Physiol (London) 1970a;206:495–507. doi: 10.1113/jphysiol.1970.sp009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó-Gábor A, Szolcsányi J, Jancsó N. Stimulation and desensitization of the hypothalamic heat-sensitive structures by capsaicin in rats. J Physiol (London) 1970b;208:449–459. doi: 10.1113/jphysiol.1970.sp009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, et al. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Garrett EM, Rutter R, Bonnert TP, Gao YD, Middleton RE, et al. Functional mapping of the transient receptor potential vanilloid 1 intracellular binding site. Mol Pharmacol. 2006;70:1005–1012. doi: 10.1124/mol.106.023945. [DOI] [PubMed] [Google Scholar]
- Jones RC, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Jones RC, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to ‘hot' chilli peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, et al. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;277:44448–44454. doi: 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, et al. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- Kagaya M, Lamb J, Robbins J, Page CP, Spina D. Characterization of the anandamide induced depolarization of guinea-pig isolated vagus nerve. Br J Pharmacol. 2002;137:39–48. doi: 10.1038/sj.bjp.0704840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson U, Sundgren-Andersson AK, Johansson S, Krupp JJ. Capsaicin augments synaptic transmission in the rat medial preoptic nucleus. Brain Res. 2005;1043:1–11. doi: 10.1016/j.brainres.2004.10.064. [DOI] [PubMed] [Google Scholar]
- Kato S, Aihara E, Nakamura A, Xin H, Matsui H, Kohama K, et al. Expression of vanilloid receptors in rat gastric epithelial cells: role in cellular protection. Biochem Pharmacol. 2003;66:1115–1121. doi: 10.1016/s0006-2952(03)00461-1. [DOI] [PubMed] [Google Scholar]
- Kechagias S, Botella S, Petersson F, Borch K, Ericson AC. Expression of vanilloid receptor-1 in epithelial cells of human antral gastric mucosa. Scand J Gastroenterol. 2005;40:775–782. doi: 10.1080/00365520510015782. [DOI] [PubMed] [Google Scholar]
- Keeble J, Russell F, Curtis B, Starr A, Pinter E, Brain SD. Involvement of transient receptor potential vanilloid 1 in the vascular and hyperalgesic components of joint inflammation. Arthritis Rheum. 2005;52:3248–3256. doi: 10.1002/art.21297. [DOI] [PubMed] [Google Scholar]
- Kihara N, De La Fuente SG, Fujino K, Takahashi T, Pappas TN, Mantyh CR. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut. 2003;52:713–719. doi: 10.1136/gut.52.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Lee DY, Chung ES, Oh UT, Kim SU, Jin BK. Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. J Neurosci. 2005;25:662–671. doi: 10.1523/JNEUROSCI.4166-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball ES, Wallace NH, Schneider CR, D'Andrea MR, Hornby PJ. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol Motil. 2004;16:811–818. doi: 10.1111/j.1365-2982.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- Knotkova H, Pappagallo M, Szallasi A. Capsaicin (TRPV1 agonist) therapy for pain relief: farewell or revival. Clin J Pain. 2008;24:142–154. doi: 10.1097/AJP.0b013e318158ed9e. [DOI] [PubMed] [Google Scholar]
- Kollarik M, Fei Ru F, Undem BJ. Acid-sensitive vagal sensory pathways and cough. Pulm Pharmacol Ther. 2007;20:402–411. doi: 10.1016/j.pupt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SG, Tamir R, Deng H, Klionsky L, Kuang R, Le A, et al. Antihyperalgesic effects of AMG8562, a novel vanilloid receptor TRPV1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther. 2008;326:218–229. doi: 10.1124/jpet.107.132233. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. VR1 receptor activation induces glutamate release and postsynaptic firing in the paraventricular nucleus. J Neurophysiol. 2004;92:1807–1816. doi: 10.1152/jn.00171.2004. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr Drug Targets. 2007;8:621–632. doi: 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Mansfield KJ, Kristiana I, Vaux KJ, Millard RJ, Burcher E. The molecular basis of urgency: regional difference of vanilloid receptor expression in the human urinary bladder. Neurourol Urodyn. 2007;26:433–438. doi: 10.1002/nau.20326. [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neurosci Lett. 1997;228:29–32. doi: 10.1016/s0304-3940(97)00358-3. [DOI] [PubMed] [Google Scholar]
- Liu M, Willmott NJ, Michael GJ, Priestley JV. Differential pH and capsaicin responses of Griffonia simplicifolia IB4 (IB4)-positive and IB4-negative small sensory neurons. Neuroscience. 2004;127:659–672. doi: 10.1016/j.neuroscience.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Pascucci T, Bernardi G, Puglisi-Allegra S, Mercuri NB. Activation of TRPV1 in the VTA excites dopaminergic neurons and increases chemical- and noxious-induced dopamine release in the nucleus accumbens. Neuropsychopharmacology. 2005;30:864–870. doi: 10.1038/sj.npp.1300615. [DOI] [PubMed] [Google Scholar]
- Marsch R, Foeller E, Rammes G, Bunck M, Kössl M, Holsboer F, et al. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci. 2007;27:832–839. doi: 10.1523/JNEUROSCI.3303-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa F, Sibaev A, Marsicano G, Blaudzun H, Storr M, Lutz B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J Mol Med. 2006;84:142–146. doi: 10.1007/s00109-005-0016-2. [DOI] [PubMed] [Google Scholar]
- Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol (London) 2007;585:469–482. doi: 10.1113/jphysiol.2007.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- Max B. This and that: the essential pharmacology of herbs and spices. Trends Pharmacol Sci. 1992;13:15–20. doi: 10.1016/0165-6147(92)90010-4. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Bevan S. The effects of pH on the interaction between capsaicin and the vanilloid receptor in rat dorsal root ganglia neurons. Br J Pharmacol. 2001;132:899–908. doi: 10.1038/sj.bjp.0703900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod RL, Correll CC, Jia Y, Anthes JC. TRPV1 antagonists as potential antitussive agents. Lung. 2008;186 Suppl 1:S59–S65. doi: 10.1007/s00408-007-9032-z. [DOI] [PubMed] [Google Scholar]
- McVey DC, Vigna SR. The capsaicin VR1 receptor mediates substance P release in toxin A-induced enteritis in rats. Peptides. 2001;22:1439–1446. doi: 10.1016/s0196-9781(01)00463-6. [DOI] [PubMed] [Google Scholar]
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- Montell C, Caterina MJ. Thermoregulation: channels that are cool to the core. Curr Biol. 2007;17:R885–R887. doi: 10.1016/j.cub.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Morenilla-Palao C, Planells-Cases R, García-Sanz N, Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- Nagy I, Sántha P, Jancsó G, Urbán L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004;500:351–369. doi: 10.1016/j.ejphar.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Nathan JD, Patel AA, McVey DC, Thomas JE, Prpic V, Vigna SR, et al. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol. 2001;281:G1322–G1328. doi: 10.1152/ajpgi.2001.281.5.G1322. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Jarvis MF, Han P, Faltynek CR, Surowy CS. Acidification of rat TRPV1 alters the kinetics of capsaicin responses. Mol Pain. 2005;1:28. doi: 10.1186/1744-8069-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nozawa Y, Nishihara K, Yamamoto A, Nakano M, Ajioka H, Matsuura N. Distribution and characterization of vanilloid receptors in the rat stomach. Neurosci Lett. 2001;309:33–36. doi: 10.1016/s0304-3940(01)02021-3. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah Z, Karai L, Iadarola MJ. Protein kinase C-a is required for vanilloid receptor 1 activation. Evidence for multiple signaling pathways. J Biol Chem. 2002;277:35752–35759. doi: 10.1074/jbc.M201551200. [DOI] [PubMed] [Google Scholar]
- Palazzo E, Rossi F, Maione S. Role of TRPV1 receptors in descending modulation of pain. Mol Cell Endocrinol. 2008;286 1–2 Suppl 1:S79–S83. doi: 10.1016/j.mce.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- Patterson LM, Zheng H, Ward SM, Berthoud HR. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–287. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- Pearce LV, Toth A, Ryu H, Kang DW, Choi HK, Jin MK, et al. Differential modulation of agonist and antagonist structure activity relations for rat TRPV1 by cyclosporin A and other protein phosphatase inhibitors. Naunyn-Schmiedeberg's Arch Pharmacol. 2008;377:149–157. doi: 10.1007/s00210-007-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci. 2007;8:71–80. doi: 10.1038/nrn2042. [DOI] [PubMed] [Google Scholar]
- Pintér E, Helyes Z, Szolcsányi J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther. 2006;112:440–456. doi: 10.1016/j.pharmthera.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Planells-Cases R, Garcìa-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflügers Arch. 2005;451:151–159. doi: 10.1007/s00424-005-1423-5. [DOI] [PubMed] [Google Scholar]
- Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. In vivo characterization in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:387–393. doi: 10.1124/jpet.102.046268. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science. 2003;300:1284–1288. doi: 10.1126/science.1083646. [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Kondo S, Kawashima T, Bakoshi S, Ueda H. Novel expression of vanilloid receptor 1 on capsaicin-insensitive fibers accounts for the analgesic effect of capsaicin cream in neuropathic pain. J Pharmacol Exp Ther. 2003;304:940–948. doi: 10.1124/jpet.102.046250. [DOI] [PubMed] [Google Scholar]
- Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Reeh PW, Pethö G. Nociceptor excitation by thermal sensitisation—a hypothesis. Prog Brain Res. 2000;129:39–50. doi: 10.1016/S0079-6123(00)29004-3. [DOI] [PubMed] [Google Scholar]
- Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, et al. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol. 2003;138:977–985. doi: 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter S, Dinh TT. Capsaicin-induced neuronal degeneration: silver impregnation of cell bodies, axons, and terminals in the central nervous system of the adult rat. J Comp Neurol. 1988;271:79–90. doi: 10.1002/cne.902710109. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT. Age-related changes in capsaicin-induced degeneration in rat brain. J Comp Neurol. 1992;318:103–1162. doi: 10.1002/cne.903180108. [DOI] [PubMed] [Google Scholar]
- Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Connor M. TRPV1 antagonists as a potential treatment for hyperalgesia. Recent Patents CNS Drug Discov. 2006;1:65–76. doi: 10.2174/157488906775245309. [DOI] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol (London) 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123:53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Liu B, Qin F. Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J Gen Physiol. 2003;122:45–61. doi: 10.1085/jgp.200308847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Liu B, Yao J, Fu Q, Qin F. Uncoupling proton activation of vanilloid receptor TRPV1. J Neurosci. 2007;27:12797–12807. doi: 10.1523/JNEUROSCI.2324-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar H, Llorente I, Jara-Oseguera A, García-Villegas R, Munari M, Gordon SE, et al. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11:255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JF, Krause JE, Cortright DN. The distribution and regulation of vanilloid receptor VR1 and VR1 5′ splice variant RNA expression in rat. Neuroscience. 2001;107:373–381. doi: 10.1016/s0306-4522(01)00373-6. [DOI] [PubMed] [Google Scholar]
- Schicho R, Florian W, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Hammer J, Holzer P, Hammer HF. Chemical nociception in the jejunum induced by capsaicin. Gut. 2004;53:1109–1116. doi: 10.1136/gut.2003.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Protein kinase C contributes to abnormal capsaicin responses in DRG neurons from cats with feline interstitial cystitis. Neurosci Lett. 2005;381:42–46. doi: 10.1016/j.neulet.2005.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Sutton KG, Jarolimek W, Hollingworth GJ, Teague S, Webb J, et al. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. J Pharmacol Exp Ther. 2002;303:1052–1060. doi: 10.1124/jpet.102.040394. [DOI] [PubMed] [Google Scholar]
- Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- Ständer S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain. 2006;2:34. doi: 10.1186/1744-8069-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C. Enteric and visceral afferent CGRP neurons. Targets of innervation and differential expression patterns. Ann New York Acad Sci. 1992;657:70–186. doi: 10.1111/j.1749-6632.1992.tb22766.x. [DOI] [PubMed] [Google Scholar]
- Storr M. TRPV1 in colitis: is it a good or a bad receptor? A viewpoint. Neurogastroenterol Motil. 2007;19:625–629. doi: 10.1111/j.1365-2982.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- Strecker T, Messlinger K, Weyand M, Reeh PW. Role of different proton-sensitive channels in releasing calcitonin gene-related peptide from isolated hearts of mutant mice. Cardiovasc Res. 2005;65:405–410. doi: 10.1016/j.cardiores.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Bielefeldt K, Gebhart GF. Mouse colon sensory neurons detect extracellular acidosis via TRPV1. Am J Physiol. 2007;292:C1768–C1774. doi: 10.1152/ajpcell.00440.2006. [DOI] [PubMed] [Google Scholar]
- Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. 2008;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Sutton KG, Garrett EM, Rutter AR, Bonnert TP, Jarolimek W, Seabrook GR. Functional characterisation of the S512Y mutant vanilloid human TRPV1 receptor. Br J Pharmacol. 2005;146:702–711. doi: 10.1038/sj.bjp.0706356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó A, Helyes Z, Sándor K, Bite A, Pintér E, Németh J, et al. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: in vivo study using gene-deficient mice. J Pharmacol Exp Ther. 2005;314:111–119. doi: 10.1124/jpet.104.082487. [DOI] [PubMed] [Google Scholar]
- Szabo T, Biro T, Gonzalez AF, Palkovits M, Blumberg PM. Pharmacological characterization of vanilloid receptor located in the brain. Mol Brain Res. 2002;98:51–57. doi: 10.1016/s0169-328x(01)00313-8. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Szelényi Z, Hummel Z, Szolcsányi J, Davis JB. Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur J Neurosci. 2004;19:1421–1424. doi: 10.1111/j.1460-9568.2004.03221.x. [DOI] [PubMed] [Google Scholar]
- Szöke E, Seress L, Szolcsányi J. Neonatal capsaicin treatment results in prolonged mitochondrial damage and delayed cell death of B cells in the rat trigeminal ganglia. Neuroscience. 2002;113:925–937. doi: 10.1016/s0306-4522(02)00208-7. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J.Capsaicin type pungent agents producing pyrexia Pyretics and Antipyretics. Handbook of Experimental Pharmacology, Volume 60 1982Springer: Berlin, Germany; 437–478.In: Milton AS (ed). [Google Scholar]
- Szolcsányi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38:377–384. doi: 10.1016/j.npep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J, Jancsó-Gábor A. Sensory effects of capsaicin congeners. I. Relationship between chemical structure and pain-producing potency of pungent agents. Drug Res. 1975;25:1877–1881. [PubMed] [Google Scholar]
- Szolcsányi J, Jancsó-Gábor A, Joó F. Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn-Schmiedeberg's Arch Pharmacol. 1975;287:157–169. doi: 10.1007/BF00510447. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J, Joó F, Jancsó-Gábor A. Mitochondrial changes in preoptic neurons after capsaicin desensitization of the hypothalamic thermodetectors in rats. Nature. 1971;229:116–117. doi: 10.1038/229116a0. [DOI] [PubMed] [Google Scholar]
- Tang L, Chen Y, Chen Z, Blumberg PM, Kozikowski AP, Wang ZJ. Antinociceptive pharmacology of N-(4-chlorobenzyl)-N'-(4-hydroxy-3-iodo-5-methoxybenzyl) thiourea, a high-affinity competitive antagonist of the transient receptor potential vanilloid 1 receptor. J Pharmacol Exp Ther. 2007;321:791–798. doi: 10.1124/jpet.106.117572. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina M, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth A, Boczán J, Kedei N, Lizanecz E, Bagi Z, Papp Z, et al. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Mol Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Urban L, Campbell EA, Panesar M, Patel S, Chaudhry N, Kane S, et al. In vivo pharmacology of SDZ 249-665, a novel, non-pungent capsaicin analogue. Pain. 2000;89:65–74. doi: 10.1016/S0304-3959(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Van Buren JJ, Bhat S, Rotello R, Pauza ME, Premkumar LS. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol Pain. 2005;1:17. doi: 10.1186/1744-8069-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A, Bölcskei K, Szöke E, Almási R, Czéh G, Szolcsányi J, et al. Relative roles of protein kinase A and protein kinase C in modulation of transient receptor potential vanilloid type 1 receptor responsiveness in rat sensory neurons in vitro and peripheral nociceptors in vivo. Neuroscience. 2006;140:645–657. doi: 10.1016/j.neuroscience.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol (London) 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter I, Wyse BD, Monteith GR, Roberts-Thomson SJ, Cabot PJ. The μ opioid agonist morphine modulates potentiation of capsaicin-evoked TRPV1 responses through a cyclic AMP-dependent protein kinase A pathway. Mol Pain. 2006;2:22. doi: 10.1186/1744-8069-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulcu SD, Liewald JF, Gillen C, Rupp J, Nawrath H. Proton conductance of human transient receptor potential-vanilloid type-1 expressed in oocytes of Xenopus laevis and in Chinese hamster ovary cells. Neuroscience. 2004;125:861–866. doi: 10.1016/j.neuroscience.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Wang C, Hu HZ, Colton CK, Wood JD, Zhu MX. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J Biol Chem. 2004;279:37423–37430. doi: 10.1074/jbc.M407205200. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112:3617–3623. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]
- Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol. 2003;465:121–135. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- Welch JM, Simon SA, Reinhart PH. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc Natl Acad Sci USA. 2000;97:13889–13894. doi: 10.1073/pnas.230146497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick EC, Hoge SG, Grahn SW, Kim E, Divino LA, Grady EF, et al. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am J Physiol. 2006;290:G959–G969. doi: 10.1152/ajpgi.00154.2005. [DOI] [PubMed] [Google Scholar]
- Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Wood JN, Winter J, James IF, Rang HP, Yeats J, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–1292. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Wang Z, Wang Y, Furuyama T, Kontani Y, Sato Y, et al. Impaired basal thermal homeostasis in rats lacking capsaicin-sensitive peripheral small sensory neurons. J Biochem. 2008;143:385–393. doi: 10.1093/jb/mvm233. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Dyer NH, Chan CL, Knowles C, Williams NS, et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, McNaughton PA. Why pain gets worse: the mechanism of heat hyperalgesia. J Gen Physiol. 2006;128:491–493. doi: 10.1085/jgp.200609676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhong B, Wang DH. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol. 2007;293:H1791–H1798. doi: 10.1152/ajpheart.00169.2007. [DOI] [PubMed] [Google Scholar]
- Zvara A, Bencsik P, Fodor G, Csont T, Hackler L, Jr, Dux M, et al. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: a DNA microarray study. FASEB J. 2006;20:160–162. doi: 10.1096/fj.05-4060fje. [DOI] [PubMed] [Google Scholar]
- Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, et al. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang HH, Sørgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]