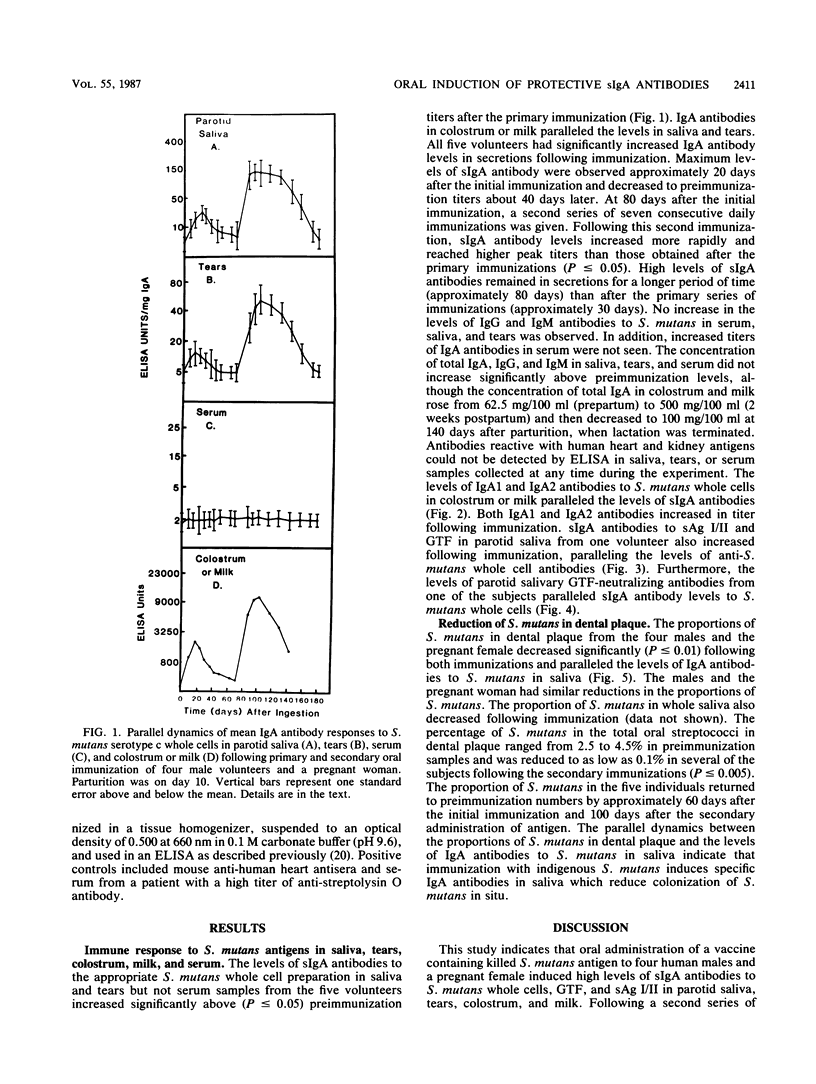
Abstract
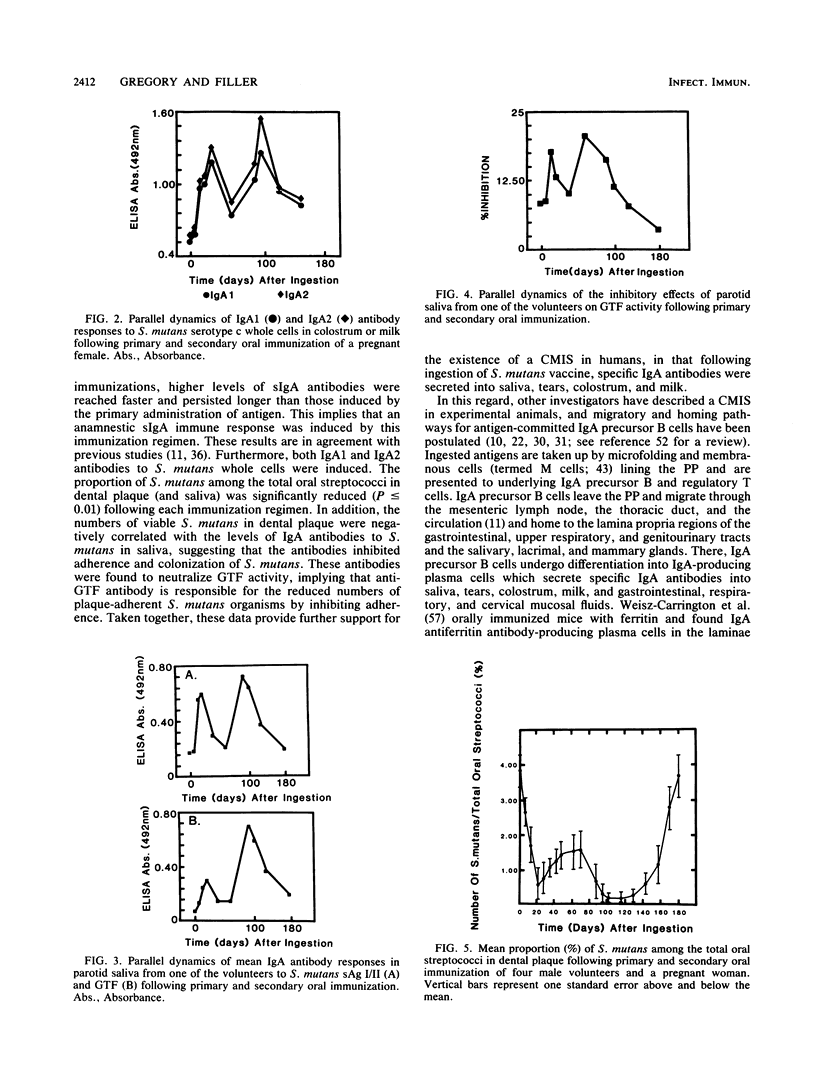
Ingestion of a vaccine containing killed Streptococcus mutans, originally isolated from each volunteer, daily for 10 consecutive days induced increased levels of specific secretory immunoglobulin A (sIgA) antibodies to S. mutans cells and two cell surface proteins, glucosyltransferase and surface antigen I/II, in parotid saliva and tears of four healthy males and in parotid saliva, tears, colostrum, and milk of a pregnant woman. In addition, these antibodies inhibited glucosyltransferase activity. Both IgA1 and IgA2 antibodies were induced. The levels of IgA antibodies in all secretions remained significantly above preimmunization levels for more than 50 days after oral administration of antigen. A second series of immunizations for 7 consecutive days resulted in even higher levels of sIgA antibodies, which peaked earlier and persisted longer than those observed after the primary immunizations. No increase in levels of antibodies in serum were detected in any subject. Antibodies reactive with human heart and kidney antigens could not be detected in saliva, tears, colostrum, milk, or serum samples collected at any time during the immunization regimen. The numbers of viable S. mutans organisms in dental plaque and whole saliva decreased after each series of immunizations, which correlated with increased levels of IgA antibodies in saliva, suggesting that IgA antibodies in saliva were responsible for the reduced adherence of this bacterium. These results indicate that ingested S. mutans antigen induces secretion of specific IgA1 and IgA2 antibodies in saliva, tears, colostrum, and milk, providing further evidence for the existence of a common mucosal immune system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allansmith M. R., Burns C. A., Arnold R. R. Comparison fo agglutinin titers for Streptococcus mutans in tears, saliva, and serum. Infect Immun. 1982 Jan;35(1):202–204. doi: 10.1128/iai.35.1.202-204.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allardyce R. A. Effect of ingested sperm on fecundity in the rat. J Exp Med. 1984 May 1;159(5):1548–1553. doi: 10.1084/jem.159.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Mestecky J., McGhee J. R. Naturally occurring secretory immunoglobulin A antibodies to Streptococcus mutans in human colostrum and saliva. Infect Immun. 1976 Aug;14(2):355–362. doi: 10.1128/iai.14.2.355-362.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLANTI J. A., ARTENSTEIN M. S., BUESCHER E. L. CHARACTERIZATION OF VIRUS NEUTRALIZING ANTIBODIES IN HUMAN SERUM AND NASAL SECRETIONS. J Immunol. 1965 Mar;94:344–351. [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- CRABBE P. A., CARBONARA A. O., HEREMANS J. F. THE NORMAL HUMAN INTESTINAL MUCOSA AS A MAJOR SOURCE OF PLASMA CELLS CONTAINING GAMMA-A-IMMUNOGLOBULIN. Lab Invest. 1965 Mar;14:235–248. [PubMed] [Google Scholar]
- Carlsson J., Newbrun E., Krasse B. Purification and properties of dextransucrase from Streptococcus sanguis. Arch Oral Biol. 1969 May;14(5):469–478. doi: 10.1016/0003-9969(69)90140-x. [DOI] [PubMed] [Google Scholar]
- Cole M. F., Emilson C. G., Hsu S. D., Li S. H., Bowen W. H. Effect of peroral immunization of humans with Streptococcus mutans on induction of salivary and serum antibodies and inhibition of experimental infection. Infect Immun. 1984 Dec;46(3):703–709. doi: 10.1128/iai.46.3.703-709.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Prince S. J., Michalek S. M., Jackson S., Russell M. W., Moldoveanu Z., McGhee J. R., Mestecky J. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D. S., Schulkind M. L., Robbins J. B. The isolation and biologic activities of purified secretory IgA and IgG anti-Salmonella typhimurium "O" antibodies from rabbit intestinal fluid and colostrum. J Immunol. 1971 Jan;106(1):181–190. [PubMed] [Google Scholar]
- Gahnberg L., Krasse B. Salivary immunoglobulin A antibodies and recovery from challenge of Streptococcus mutans after oral administration of Streptococcus mutans vaccine in humans. Infect Immun. 1983 Feb;39(2):514–519. doi: 10.1128/iai.39.2.514-519.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly R., Ogra P. L., Regas S., Waldman R. H. Rubella immunization of volunteers via the respiratory tract. Infect Immun. 1973 Oct;8(4):497–502. doi: 10.1128/iai.8.4.497-502.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., Van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973 Nov;18(11):1357–1364. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- Goldblum R. M., Ahlstedt S., Carlsson B., Hanson L. A., Jodal U., Lidin-Janson G., Sohl-Akerlund A. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975 Oct 30;257(5529):797–798. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- Gregory R. L., Michalek S. M., Filler S. J., Mestecky J., McGhee J. R. Prevention of Streptococcus mutans colonization by salivary IgA antibodies. J Clin Immunol. 1985 Jan;5(1):55–62. doi: 10.1007/BF00915169. [DOI] [PubMed] [Google Scholar]
- Gregory R. L., Rundegren J., Arnold R. R. Separation of human IgA1 and IgA2 using jacalin-agarose chromatography. J Immunol Methods. 1987 May 4;99(1):101–106. doi: 10.1016/0022-1759(87)90037-8. [DOI] [PubMed] [Google Scholar]
- Gregory R. L., Shechmeister I. L., Brubaker J. O., Smedberg C. T., Michalek S. M., McGhee J. R. Lack of cross-reactivity of antibodies to ribosomal preparations from Streptococcus mutans with human heart and kidney antigens. Infect Immun. 1984 Oct;46(1):42–47. doi: 10.1128/iai.46.1.42-47.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M., Machardy S. M., Sheppard A. J., Woods N. C. Evidence for an immunological relationship between Streptococcus mutans and human cardiac tissue. Infect Immun. 1980 Feb;27(2):576–588. doi: 10.1128/iai.27.2.576-588.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. E., Lally E. T., Nakamura M. C., Montgomery P. C. Migration of IgA-bearing lymphocytes into salivary glands. Cell Immunol. 1981 Sep 1;63(1):203–209. doi: 10.1016/0008-8749(81)90042-3. [DOI] [PubMed] [Google Scholar]
- Keren D. F., Kern S. E., Bauer D. H., Scott P. J., Porter P. Direct demonstration in intestinal secretions of an IgA memory response to orally administered Shigella flexneri antigens. J Immunol. 1982 Jan;128(1):475–479. [PubMed] [Google Scholar]
- Krasse B., Gahnberg L. Available immunoglobulin A antibodies in mouth rinses and implantation of Streptococcus mutans. Infect Immun. 1983 Sep;41(3):1360–1362. doi: 10.1128/iai.41.3.1360-1362.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutteh W. H., Koopman W. J., Conley M. E., Egan M. L., Mestecky J. Production of predominantly polymeric IgA by human peripheral blood lymphocytes stimulated in vitro with mitogens. J Exp Med. 1980 Nov 1;152(5):1424–1429. doi: 10.1084/jem.152.5.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Mehlert A., Caldwell J. Local active gingival immunization by a 3,800-molecular-weight streptococcal antigen in protection against dental caries. Infect Immun. 1986 Jun;52(3):682–687. doi: 10.1128/iai.52.3.682-687.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer R., Evans R. T., Emmings F. G., Genco R. J. Use of combined immunization routes in induction of a salivary immunoglobulin A response to Streptococcus mutans in Macaca fascicularis monkeys. Infect Immun. 1981 Jan;31(1):345–351. doi: 10.1128/iai.31.1.345-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Straffon L. H. Longitudinal investigation of the role of Streptococcus mutans in human fissure decay. Infect Immun. 1979 Nov;26(2):498–507. doi: 10.1128/iai.26.2.498-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- McDermott M. R., Clark D. A., Bienenstock J. Evidence for a common mucosal immunologic system. II. Influence of the estrous cycle on B immunoblast migration into genital and intestinal tissues. J Immunol. 1980 Jun;124(6):2536–2539. [PubMed] [Google Scholar]
- McGhee J. R., Michalek S. M. Immunobiology of dental caries: microbial aspects and local immunity. Annu Rev Microbiol. 1981;35:595–638. doi: 10.1146/annurev.mi.35.100181.003115. [DOI] [PubMed] [Google Scholar]
- McGhee, Michalek S. M., Webb J., Navia J. M., Rahman A. F., Legler D. W. Effective immunity to dental caries: protection of gnotobiotic rats by local immunization with Streptococcus mutans. J Immunol. 1975 Jan;114(1 Pt 2):300–305. [PubMed] [Google Scholar]
- McWilliams M., Phillips-Quagliata J. M., Lamm M. E. Mesenteric lymph node B lymphoblasts which home to the small intestine are precommitted to IgA synthesis. J Exp Med. 1977 Apr 1;145(4):866–875. doi: 10.1084/jem.145.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R., Arnold R. R., Michalek S. M., Prince S. J., Babb J. L. Selective induction of an immune response in human external secretions by ingestion of bacterial antigen. J Clin Invest. 1978 Mar;61(3):731–737. doi: 10.1172/JCI108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Mestecky J., Arnold R. R., Bozzo L. Ingestion of Streptococcus mutans induces secretory immunoglobulin A and caries immunity. Science. 1976 Jun 18;192(4245):1238–1240. doi: 10.1126/science.1273589. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., Morisaki I., Gregory R. L., Kiyono H., Hamada S., McGhee J. R. Oral adjuvants enhance IgA responses to Streptococcus mutans. Mol Immunol. 1983 Sep;20(9):1009–1018. doi: 10.1016/0161-5890(83)90042-1. [DOI] [PubMed] [Google Scholar]
- Montville T. J., Cooney C. L., Sinskey A. J. Measurement and synthesis of insoluble and soluble dextran by Streptococcus mutans. J Dent Res. 1977 Aug;56(8):983–989. doi: 10.1177/00220345770560082701. [DOI] [PubMed] [Google Scholar]
- Ogra P. L. Mucosal immune response to poliovirus vaccines in childhood. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S361–S368. doi: 10.1093/clinids/6.supplement_2.s361. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr Oral immunization of dogs with purified cholera toxin, crude cholera toxin, or B subunit: evidence for synergistic protection by antitoxic and antibacterial mechanisms. Infect Immun. 1982 Aug;37(2):687–694. doi: 10.1128/iai.37.2.687-694.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M. E., McWilliams M., Phillips-Quagliata J. M., Weisz-Carrington P., Lamm M. E. Origin of IgA-secreting plasma cells in the mammary gland. J Exp Med. 1977 Nov 1;146(5):1311–1322. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzik R., Clancy R. L., Perey D. Y., Day R. P., Bienenstock J. Repopulation with IgA-containing cells of bronchial and intestinal lamina propria after transfer of homologous Peyer's patch and bronchial lymphocytes. J Immunol. 1975 May;114(5):1599–1604. [PubMed] [Google Scholar]
- Schaeffer M. E., Rhodes M., Prince S., Michalek S. M., McGhee J. R. A plastic intraoral device for the collection of human parotid saliva. J Dent Res. 1977 Jul;56(7):728–733. doi: 10.1177/00220345770560070401. [DOI] [PubMed] [Google Scholar]
- Seligmann M. Membrane cell markers in human leukemias and lymphomas. Ric Clin Lab. 1976 Apr-Jun;6(2):93–98. doi: 10.1007/BF02949079. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Taubman M. A., Ebersole J. L. Preparation of glucosyltransferase from Streptococcus mutans by elution from water-insoluble polysaccharide with a dissociating solvent. Infect Immun. 1979 Feb;23(2):446–452. doi: 10.1128/iai.23.2.446-452.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Nisengard R. J., Neiders M. E., Albini B. Serology and tissue lesions in rabbits immunized with Streptococcus mutans. J Immunol. 1983 Dec;131(6):3021–3027. [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman M. A., Smith D. J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J Immunol. 1977 Feb;118(2):710–720. [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Larson L., Challacombe S., McNabb P. Mucosal immunity: The origin and migration patterns of cells in the secretory system. J Allergy Clin Immunol. 1980 Jan;65(1):12–19. doi: 10.1016/0091-6749(80)90171-2. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Ganguly R. The role of the secretory immune system in protection against agents which infect the respiratory tract. Adv Exp Med Biol. 1974;45(0):283–294. doi: 10.1007/978-1-4613-4550-3_34. [DOI] [PubMed] [Google Scholar]
- Walker J. Antibody responses of monkeys to oral and local immunization with Streptococcus mutans. Infect Immun. 1981 Jan;31(1):61–70. doi: 10.1128/iai.31.1.61-70.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. A., Isselbacher K. J., Bloch K. J. Intestinal uptake of macromolecules: effect of oral immunization. Science. 1972 Aug 18;177(4049):608–610. doi: 10.1126/science.177.4049.608. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]



