Abstract
The type of reproductive isolation prevalent in the initial stages of species divergence can affect the nature and rate of emergence of additional reproductive barriers that subsequently strengthen isolation between species. Different groups of Mediterranean deceptive orchids are characterized by different levels of pollinator specificity. Whereas food-deceptive orchid species show weak pollinator specificity, the sexually deceptive Ophrys species display a more specialized pollination strategy. Comparative analyses reveal that orchids with high pollinator specificity mostly rely on premating reproductive barriers and have very little postmating isolation. In this group, a shift to a novel pollinator achieved by modifying the odour bouquet may represent the main isolation mechanism involved in speciation. By contrast, orchids with weak premating isolation, such as generalized food-deceptive orchids, show strong evidence for intrinsic postmating reproductive barriers, particularly for late-acting postzygotic barriers such as hybrid sterility. In such species, chromosomal differences may have played a key role in species isolation, although strong postmating–prezygotic isolation has also evolved in these orchids. Molecular analyses of hybrid zones indicate that the types and strength of reproductive barriers in deceptive orchids with contrasting premating isolation mechanisms directly affect the rate and evolutionary consequences of hybridization and the nature of species differentiation.
Keywords: embryo mortality, food deception, hybrid inviability, hybrid sterility, premating isolation, postmating isolation
1. Introduction
Although some evolutionary biologists have questioned whether speciation may be directly equated to the evolution of reproductive isolation (see Ehrlich & Raven 1969; Johnson 2006), there is general agreement that tempo and mode of speciation will be strongly influenced by the nature of isolating barriers (Coyne & Orr 2004). In plants, these barriers are most conveniently separated into premating (only prezygotic) and postmating (both pre- and postzygotic) isolation (Grant 1981; Snow 1994; Tiffin et al. 2001). Usually, reproductive isolation involves multiple plant life-history stages and, in most cases, a variety of reproductive barriers contribute to overall isolation (Ramsey et al. 2003; Lowry et al. 2008). However, pre- and postmating barriers strongly differ in the magnitude of their effects. Because, in nature, isolating mechanisms act sequentially, each isolating barrier can prevent only the potential gene flow that was not already eliminated by earlier acting barriers. Thus, early acting barriers (i.e. prezygotic barriers) are expected to contribute more to isolation than will late-acting barriers, all else being equal (Coyne & Orr 1989; Ramsey et al. 2003; Rieseberg & Willis 2007). In spite of several studies on animals since the pioneering work of Coyne & Orr (1989) in plants, the influence of isolation barriers that initially separate incipient species on the presence and magnitude of barriers that evolve subsequently remains unknown (but see Moyle et al. 2004).
Often, the number and nature of genetic changes involved in the origin and maintenance of reproductive barriers differ significantly between pre- and postmating isolation (Rieseberg & Willis 2007; Lowry et al. 2008) and this may influence the speed of species sorting and the strength of species boundaries. For instance, floral differences that affect pollinator attraction and cause premating isolation between species pairs within Mimulus (Scrophulariaceae) and Aquilegia (Ranunculaceae; Bradshaw et al. 1995, 1998; Whittall & Hodges 2007) are controlled by one or a few major quantitative trait loci (QTLs), indicating relatively simple genetic control (Bradshaw et al. 1995, 1998; Hodges et al. 2002), whereas reproductive isolation among Helianthus species primarily involves hybrid sterility, which seems to be related to extensive chromosomal rearrangements (Kim & Rieseberg 1999, 2001) and has a complex (polygenic) genetic basis (Lai et al. 2005). Clearly, isolation barriers controlled by few genes of major effect may evolve rapidly, whereas those having a complex genetic architecture may evolve more slowly (Coyne & Orr 2004). Furthermore, when species occur sympatrically, the genetic architecture of traits involved in their reproductive isolation can determine the outcome of introgressive hybridization (Martinsen et al. 2001; Lexer et al. 2005).
If many genes contribute to reproductive isolation, much of the genome will be sheltered from introgression as a consequence of linkage between loci directly involved in reproductive isolation and adjacent genomic regions (Coyne & Orr 2004). In sunflowers, for example, several QTLs associated with hybrid sterility or morphological differences between Helianthus annuus and Helianthus petiolaris co-localize with chromosome segments that are sheltered from introgression, suggesting that the genetic architecture of reproductive isolation operates together with different ecological adaptations to maintain species differences (Buerkle & Rieseberg 2001). By contrast, if only a few genes (and thus a few genomic regions) contribute to reproductive isolation, much of the genome will be susceptible to interspecific gene flow (Wu 2001). For instance, the two European oak species, Quercus robur and Quercus petraea, differ reliably (albeit only subtly) in morphology, but genetically they are only slightly differentiated and often hybridize when sympatric. Here, only a small percentage of their genomes shows evidence of allele frequency variation, suggesting that few genomic regions are sheltered from introgression (Scotti-Saintagne et al. 2004). This and other similar case studies (Lexer & Widmer 2008) challenge the view that species differentiation is a genome-wide phenomenon, and instead support the idea that genomes can be porous (Wu 2001).
In the present review, we summarize the recent literature on a guild of Mediterranean orchids, which have been extensively investigated in regard to their pre- and postmating isolation, to search for answers to the following questions. What is the relative importance of different isolating barriers, especially prezygotic versus postzygotic barriers, in causing reproductive isolation? Do species isolated by strong premating isolation also evolve a degree of postmating isolation comparable with those species showing weak premating isolation? Similarly, do species with weak premating and strong postzygotic isolation also evolve some form of prezygotic isolation? What are the genetic bases of reproductive isolation in orchid species with weak premating barriers? How do different patterns of reproductive isolation (premating versus postmating) impact on introgressive hybridization for species in sympatry? Is species differentiation controlled by a modest or a large number of genes?
2. Pollinator specificity and premating isolation
Orchids are a good experimental system for testing the interaction between pre- and postmating barriers owing to their species richness (suggesting that speciation occurs readily), owing to the floral diversity that characterizes this richness (indicating that premating isolation may be common), and because natural hybridization may be frequent (van der Pijl & Dodson 1966; Gill 1989; Dressler 1993).
In contrast to other plant groups, approximately one-third of the estimated 25 000 orchid species offer no reward to pollinators (Tremblay et al. 2005), suggesting an important role for floral deception in species diversification (Cozzolino & Widmer 2005a). Food deception represents the most common type of floral deception. Mediterranean members of subtribe Orchidinae are bee-pollinated species showing generalized food deception. They rely on pollinators associating bright floral signals with reward and are expected to attract a relatively wide pollinator fauna (Dafni & Bernhardt 1990; Jersáková et al. 2006). They differ from several South African deceptive orchids that have highly specialized pollination systems involving mimicry of rewarding species (food-deceptive Batesian mimicry; Johnson 2000; Jersáková et al. 2006).
The phylogenetic distribution of pollination syndromes for European members of subtribe Orchidinae suggests that generalized food deception was ancestral, so that reward pollination evolved secondarily (Cozzolino et al. 2001; Bateman et al. 2003). Sexual deception, i.e. a special type of floral deception, where the orchid flower mimics, by scent and visual clues, the female partner of particular pollinators (Kullenberg 1961; Schiestl 2005), also seems to have evolved from a food-deceptive ancestor (see Mant et al. 2002 for a similar trend found in Australian orchids). Specifically, the widespread production of a significant amount of hydrocarbons in Mediterranean Orchidinae may have represented a preadaptation for the evolution of sexual deception (Schiestl & Cozzolino 2008). Because deception is the widespread pollination mechanism in Orchidinae, the level of specificity in this type of pollination (and thus its potential as an isolation mechanism) represents a crucial factor for inferring whether reproductive isolation among these species has mainly arisen by the evolution of premating barriers.
Whether pollinator shift is the main cause of speciation in an animal-pollinated plant group can be tested by estimating how frequently sister species are served by different or shared pollinators (Armbruster 1993; Johnson et al. 1998; Whittall & Hodges 2007). Several studies of sexually deceptive orchids of the Mediterranean genus Ophrys have shown that pollination is highly specific and that closely related species always use different pollinators (reviewed in Schiestl 2005). Even for sympatric Ophrys species pollinated by the same insect species, reproductive isolation is usually maintained mechanically through the deposition of the orchid's pollen masses on different parts of the insect's body (Kullenberg 1961; Borg-Karlson 1990). This evidence strongly suggests a prominent role for pollinator shifts in reproductive isolation, and thus speciation, in sexually deceptive orchids (Paulus & Gack 1990) and in orchids in general (van der Pijl & Dodson 1966; Gill 1989). By contrast, Devey et al. (2008) recently proposed an alternative view for species delimitation in the genus Ophrys, by recognizing at the most only 10 isolated groups that can be distinguished genetically.
When compared with sexually deceptive species, the levels of pollinator specificity in Mediterranean food-deceptive orchids have been less investigated (van der Cingel 1995 and references therein), largely owing to experimental difficulties caused by infrequent pollinator visits (Neiland & Wilcock 1995) in flower-manipulation experiments. Phylogenetic analysis reveals a few switches to different pollinator classes among food-deceptive species (Cozzolino & Widmer 2005b) and recent evidence showed that several sister species of Mediterranean food-deceptive orchids co-flower and often use a wide and overlapping set of pollinator species (van der Cingel 1995; Scopece et al. 2007). Of course, different species may share pollinators and still be completely isolated owing to different pollinium placement on pollinators (Grant 1994) but molecular identification of pollinia on pollinators (Widmer et al. 2000) has demonstrated individual insects carrying pollen from several related food-deceptive orchids with the same placement on insect body (Cozzolino et al. 2005) indicating that substantial pollinator sharing occurs among sympatric, co-flowering Mediterranean food-deceptive orchids. Whereas sexual deception appears to be a highly specialized mechanism, generalized food deception is clearly a less-specific pollination mechanism.
3. Components of postmating isolation
Whether reproductive isolation evolves similarly in plant groups that differ markedly in incidence of premating isolation, such as sexually deceptive versus food-deceptive orchids, constitutes a central issue for understanding the tempo and mode of evolution of such isolating barriers, and for tracing plausible speciation patterns in these two plant groups (but see Johnson 2006, for an alternative view). Scopece et al. (2007) compared postmating isolation during a prezygotic stage (through an examination of whether pollen germination on stigmas elicits ovule development; Zhang & O'Neill 1993) with that occurring at an early postzygotic stage (in terms of formation of seeds with non-viable embryos, i.e. embryo mortality) between Mediterranean sexually and food-deceptive orchid species. This was performed between co-flowering species pairs of each plant group and involved a comparison of four phylogenetically independent genera of food-deceptive orchids (namely Anacamptis, Dactylorhiza, Orchis and Neotinea) with the unique sexually deceptive genus Ophrys (Cozzolino et al. 2001; Bateman et al. 2003).
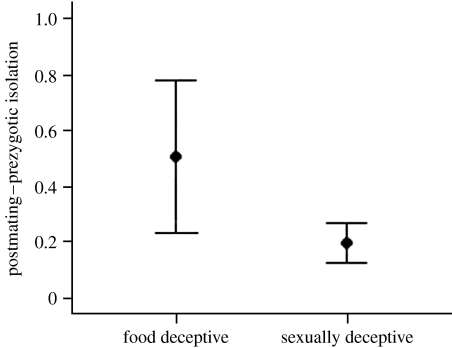
The study revealed weak premating and strong postmating isolation for food-deceptive orchids, but the converse pattern for sexually deceptive orchids. Furthermore, it was evident that the different components of postmating (both pre- and postzygotic) isolation acted with different strengths and modes between the two orchid groups. The postzygotic isolation due to embryo mortality seemed to have evolved in a clock-like manner, so that species separated by equivalent genetic distance (corresponding to low genetic diversity among species) experienced equal incidence of hybrid embryo mortality across the two orchid groups. By contrast, postmating–prezygotic isolation associated with pollen–ovule interaction did not evolve in a clock-like manner, and food-deceptive orchids exhibited greater isolation than sexually deceptive species (figure 1; Scopece et al. 2007). The presence in food-deceptive orchids of strong postmating–prezygotic isolation might be associated with their low premating isolation relative to that exhibited by sexually deceptive orchids. Clearly, if species share pollinators, other barriers are needed to limit interspecific mating and the maintenance of species in sympatry.
Figure 1.
Comparison of the strength of postmating–prezygotic isolation (pollen–ovule interaction) for food-deceptive and sexually deceptive orchids measured by crossing species pairs separated by a genetic distance of 0.008–0.032 (data from Scopece et al. 2007). Points and error bars represent means and standard errors.
Because postmating–prezygotic isolation did not vary significantly with genetic distance, we could not assess for food-deceptive orchids if pollen–ovule interaction evolved faster than embryo mortality, as has often been observed in animals for corresponding reproductive stages (egg–sperm protein interactions; Swanson & Vacquier 2002). However, the occurrence of strong postmating–prezygotic isolation in some combinations (e.g. Orchis italica–Orchis anthropophora and Orchis purpurea–Orchis simia) that produced a high percentage of viable embryos in experimental interspecific crosses suggests that, at least in some food-deceptive orchids, prezygotic isolation evolved faster than postzygotic isolation as represented by embryo mortality. By contrast, embryo mortality probably depends on genetic difference among species and, as confirmed by its clock-like evolution, depends more on the time elapsed since species diverged (Coyne & Orr 1997, 2004).
Among all stages of postzygotic isolation, including hybrid sterility and hybrid inviability, embryo mortality evolved most slowly. In fact, comparative analysis of the evolution of different stages of postzygotic isolation in the same group of Mediterranean food-deceptive orchids revealed that all postzygotic isolation stages generally evolved gradually over time and that late-acting barriers such as hybrid sterility and hybrid inviability evolved before embryo mortality (Scopece et al. 2008). Thus, although closely related species of food-deceptive orchids showed little isolation due to embryo mortality, they commonly exhibited strong hybrid sterility and hybrid inviability (figure 2), indicating that these isolation mechanisms primarily contribute to incipient stages of species isolation in such orchids. Consequently, the sequence of evolutionary events leading to complete postzygotic isolation begins with the early evolution of hybrid sterility, followed by hybrid inviability and later by embryo mortality (Scopece et al. 2008).
Figure 2.
Relationship between percentage of food-deceptive species pairs displaying total reproductive isolation for three components of postzygotic isolation and three intervals of genetic distance (from Scopece et al. 2008). White bars, embryo mortality; grey bars, hybrid inviability; black bars, hybrid sterility.
Comparisons of relative rates of evolution between the prezygotic stage of pollen–ovule interaction and the postzygotic stage of hybrid sterility are not possible because hybrid sterility evolves in a clock-like manner but pollen–ovule interaction does not. In addition, hybrid sterility can be assessed only among species pairs that lack complete isolation during earlier stages, so species pairs that display strong postmating–prezygotic isolation (pollen–ovule interaction) versus late postzygotic isolation, such as hybrid sterility, cannot be compared directly. However, some specific case studies reveal whether or not the existence of strong postmating–prezygotic isolation in food-deceptive orchids has pre-dated the evolution of hybrid sterility. For instance, interspecific pollination between Orchis mascula and Orchis provincialis does not trigger ovule formation in the experimental crosses between mainland populations (Scopece et al. 2007), but the two species occasionally hybridize in isolated areas such as the island of Sardinia (Pellegrino et al. 2005). This apparent local loss of postmating–prezygotic isolation could reflect stochastic processes (founder effect and/or genetic drift) or the absence of selection on reproductive isolation if, for example, O. provincialis occupied Sardinia in the absence of O. mascula for a protracted period. However, all examined natural hybrids were F1 or first generation backcrosses towards parental species, indicating partial hybrid sterility (Pellegrino et al. 2005). This particular case therefore suggests that, in food-deceptive orchids, even species that show strong postmating–prezygotic isolation have already evolved late postzygotic isolation.
Reproductive character displacement (sensu Butlin 1987) occurs as a consequence of interactions in sympatry among reproductively isolated species and serves to reduce gamete loss in unsuccessful heterospecific mating. The reproductive character displacement may be a plausible explanation for the observation that several food-deceptive orchids characterized by strong and often-complete postzygotic isolation have also evolved a considerable amount of prezygotic isolation (at the pollen–ovule interaction stage). This suggests that the evolution of a postmating–prezygotic barrier can be accelerated by natural selection (Coyne & Orr 1989, 1997; Sasa et al. 1998; Presgraves 2002; Moyle et al. 2004) when recently diverged species come into secondary contact. However, the predicted link between frequency of sympatry and strength of postmating–prezygotic isolation remains to be demonstrated.
Interestingly, those few food-deceptive orchids that have evolved strong premating floral isolation also show postzygotic isolation. For instance, Anacamptis pyramidalis and Orchis quadripunctata–Orchis brancifortii, which display marked differences in floral morphology and preferred pollinators compared with all other closely related species (Cozzolino et al. 2001), maintain the same extent of postzygotic isolation as other food-deceptive species that instead share pollinators (Scopece et al. 2007), suggesting that the evolution of their postzygotic isolation more likely pre-dated their pollinator shift (i.e. the evolution of premating isolation). In this particular case, a floral shift may not have originated as an isolating mechanism during speciation but evolved as a consequence of selection for reproductive performance (efficient receipt and export of pollen) in the environments where pollinator availability was limited (see Johnson 2006).
4. Genetic basis of postzygotic isolation
Intrinsic postzygotic isolation, rather than premating barriers, contributes strongly to maintain species boundaries among sympatric food-deceptive orchid species. The slow evolution of early postzygotic isolation due to embryo mortality, also noted in animals (Bolnick & Near 2005), is consistent with the gradual accumulation of many genetic incompatibilities of small effect (Carr & Dudash 2003; Coyne & Orr 2004). By contrast, hybrid sterility may be caused by changes in specific genes (as in Mimulus) and/or chromosomal rearrangements (as in Helianthus; reviewed in Rieseberg & Willis 2007). The comparison of the extent of karyotype divergence between pairs of sympatric, co-flowering orchid species that either share pollinators, or are pollinated by contrasting specific pollinators, has revealed strong chromosomal differences between species that share pollinators (Cozzolino et al. 2004). This result suggests that postzygotic reproductive barriers mediated by karyotype divergence may play a key role in the maintenance of species boundaries in food-deceptive orchids.
Chromosomal differences can evolve among geographically isolated populations, leading to allopatric speciation (Levin 2002). If such allopatric species subsequently come into contact, they either hybridize and eventually merge into a single species or maintain species integrity if reproductive isolation is sufficiently strong. Under-dominant chromosomal rearrangements that directly reduce heterozygous fitness can be established in small, inbred populations (Levin 2002; Rieseberg & Willis 2007). The fragmented habitats of the Mediterranean Basin may have accelerated chromosomal evolution in orchids, because mutations are more likely to become fixed in small populations as a consequence of genetic drift. This, however, is not a strict prerequisite: it has recently been argued that chromosomal inversions that capture locally adapted alleles may also spread in a population in the absence of drift (Kirkpatrick & Barton 2006).
The observed over-representation of chromosomal differences in sympatric orchid species pairs with the same pollinators (Cozzolino et al. 2004) represents an example of reproductive isolate selection (Rieseberg 2001), during which only species that have experienced chromosomal divergence have survived the challenge of sympatry. This specific case study thus provides the first evidence of a key role for postzygotic reproductive isolation in orchids, for which reproductive isolation has been traditionally attributed to premating barriers (van der Pijl & Dodson 1966; Gill 1989; Dressler 1993).
Chromosomal rearrangements often contribute to the sterility of hybrid animals (Noor et al. 2001) and plants (Rieseberg 2001), and hybrid sterility often maps to chromosomal rearrangements (Lai et al. 2005). Similarly, hybrid inviability can be a consequence of genetic incompatibility, which may have a simple genetic basis (Orr 2001; Bomblies & Weigel 2007). Comparative analysis (Scopece et al. 2008) revealed that hybrid lethality affected 55.6% of species pairs that produce viable embryos when experimentally crossed. For these species pairs, viable hybrids have never been recorded in the wild. Whether this hybrid lethality occurs during an early developmental stage, such as seed germination or seedling establishment, or later in the adult phase is not known.
Establishment of an association with appropriate mycorrhizal partners that provide essential nutrients is crucial for early development in orchids (Arditti 1992; Rasmussen 1995; Batty et al. 2001; Dearnaley 2007) and may contribute to hybrid inviability. Developmental and physiological processes such as fungal–plant interactions require the complex orchestration of gene expression in space and time (Bouwmeester et al. 2007). Consequently, a species’ genetic composition should include a set of alleles coadapted across gene loci that reliably produce a viable reproducing organism (Coyne & Orr 2004). Hybridization, by introducing foreign alleles, can disrupt the genetic architecture of traits (Burke & Arnold 2001) that underpin interactions with other organisms, such as fungal partners. Whether such effects underlie the genetic basis of hybrid inviability in orchids remains to be tested.
5. Reproductive isolation at work: hybrid zones
The diverse types and strength of reproductive barriers in food-deceptive and sexually deceptive orchids may shape the genetic architecture of their hybrid zones. Cozzolino et al. (2006) and Moccia et al. (2007) recently estimated hybrid indices based on AFLP (amplified fragment length polymorphism) markers in hybrid zones between two congeneric pairs of food-deceptive orchids: O. mascula and Orchis pauciflora, and Anacamptis morio and Anacamptis papilionacea. Whereas Cozzolino et al. (2006) found that most hybrids were F1, with only few backcrosses towards O. pauciflora, Moccia et al. (2007) reported no evidence for introgression, all hybrids being F1 (figure 3). In both cases, analyses of seed production by natural hybrids revealed strong (O. mascula×O. pauciflora) or complete hybrid sterility (A. morio×A. papilionacea). These results suggest that intrinsic postzygotic isolation mechanisms contribute substantially to reproductive isolation between sympatric food-deceptive orchid species in nature (see also Scopece et al. 2008).
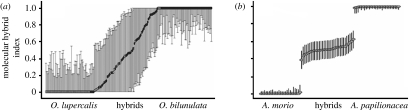
Figure 3.
Maximum-likelihood estimates of hybrid indices (calculated with the software Hindex; Buerkle 2005) for individuals in hybrid zones of (a) sexually deceptive Ophrys bilunulata×O. lupercalis (from Stökl et al. submitted) and (b) food-deceptive A. morio×A. papilionacea (from Moccia et al. 2007). Low- and high-molecular hybrid scores are indicative of parental population. Individuals within populations are ordered by increasing hybrid indices (±s.d.).
Chromosomal analyses confirmed the occurrence of a strong karyotype difference between the parental species involved in hybrid formation. For example, A. morio and A. papilionacea differ in chromosome number (2n=36 and 2n=32, respectively) and, more importantly, in the degree of karyotype asymmetry (Cozzolino et al. 2004). Consequently, hybrids of these species typically exhibit highly heteromorphic chromosomes and show irregular meiotic behaviour (D'Emerico et al. 1996), suggesting reduced homology between the parental genomes. By contrast, O. mascula and O. pauciflora have the same chromosome number (2n=42), but show strong karyotype divergence due to the presence of several large heterochromatic blocks on the chromosomes of O. mascula (D'Emerico et al. 2002). On the one hand, these studies suggest a minor role for hybridization in diversification and speciation of most Mediterranean food-deceptive orchids and, on the other hand, an important role for late postzygotic barriers in reducing or preventing gene flow among sympatric food-deceptive species.
Molecular investigation of hybrid zones between pairs of sexually deceptive orchid species found different patterns and predicted different potential evolutionary outcomes for hybridization. Hybrid zones between Ophrys lupercalis and Ophrys iricolor, Ophrys bilunulata and O. lupercalis, and O. bilunulata and Ophrys fabrella involve extensive introgression (Stökl et al. 2008, submitted; figure 3), owing to low postzygotic isolation (Scopece et al. 2007). In contrast to what was found in hybrid zones of food-deceptive orchids, F1 hybrids are rare in sexually deceptive hybrid zones (figure 3). However, even the occurrence of a single sporadic interspecific pollination in sexually deceptive orchids may lead to gene flow between parental species given the presence of weak postmating isolation. This could explain the low interspecific genetic divergence detected for such species with ribosomal markers compared with the corresponding sister clade of food-deceptive orchids (Cozzolino & Widmer 2005a). A comparison of sequence divergence for neutral loci relative to putatively selected loci, such as the odour genes involved in the production of pheromone bouquets for attracting specific pollinators, would be a promising avenue of future studies in sexually deceptive orchids.
6. Evolutionary novelty generated through hybridization
Hybridization may have a contrasting evolutionary role in the two orchid groups discussed in this paper. Hybrids between food-deceptive species have both intrinsic fitness problems (partial or total sterility) and low pollination success compared with their parents, possibly due to the intermediate floral traits they display (Cozzolino et al. 2006; Moccia et al. 2007). Ongoing studies have revealed that these hybrids use the same (or a subset) of pollinators used by their parents and occupy the same habitats, suggesting that hybridization is of limited importance as a creative force in food-deceptive orchids (also see Cozzolino & Widmer 2005b). By contrast, a more creative role for hybridization is evident from ongoing studies of hybrids of sexually deceptive orchids. For example, O. lupercalis attracts Andrena nigroaenea (Hymenoptera, Andrenidae), whereas Ophrys arachnitiformis is pollinated by Colletes cunicularius (Hymenoptera, Colletidae). Experiments with the scent bouquet of their hybrids in Southern France showed low attraction for either pollinator of the parent species, but relatively greater attraction of Andrena vaga, which does not pollinate the parents. The scent bouquet of the hybrids includes odour compounds that are either absent from those of the parent species or expressed only in very low concentrations. This evolutionary novelty in hybrids of sexually deceptive species suggests that hybridization may contribute to the high pollinator diversification in Ophrys, a hypothesis recently supported by a fine-scale phylogenetic analysis of the group (Devey et al. 2008).
7. Contrasting or supporting evidence?
The contrasting associations of strong postmating barriers (both pre- and postzygotic) with generalized food deception and premating isolation with sexual deception suggest the existence of two markedly different evolutionary patterns. However, the expected differences are not always apparent between the two orchid groups.
Despite possessing strong premating isolating mechanisms, sexually deceptive orchids also exhibit some postmating isolation (Scopece et al. 2007), which is presumably acquired secondarily as a by-product of genetic divergence (Dobzhansky 1937; Mayr 1970). In contrast to this typical pattern, O. iricolor and Ophrys incubacea, a pair of sexually deceptive species, exhibit late postzygotic isolation due to partial hybrid sterility (Cortis et al. submitted), similar to that of food-deceptive orchids. This species pair is unusual for sexually deceptive orchids, because both species attract the same bee, Andrena morio, but differ in the placement of their pollinia. These Ophrys flowers emit specific fragrances and provide tactile signals through the direction of lip hairs that align pollinators during pseudo-copulation either parallel or perpendicular to the labellum (Ågren et al. 1984; Pirstinger & Paulus 1996), thus determining whether a pollinator removes pollinia primarily with its head or abdomen. Correspondingly, hybrid zones between these species exhibit similar genetic structure to that in hybrid zones of food-deceptive species, with F1 hybrids but few backcrosses. Estimates of seed set indicate that hybrids produce few viable seeds and karyological analyses also revealed significant chromosomal divergence between the parental species. These results therefore demonstrate the presence, at least in some conditions, of significant postmating isolation in a plant group, the sexually deceptive orchids, which is generally characterized by strong premating barriers and little postmating isolation. That premating isolation of these two Ophrys species involves a mechanical barrier (i.e. different placement of pollinia) rather than an ethological barrier (different specific pollinators) suggests that mechanical mechanisms provide weaker isolation, so that integrity of the species boundary persists only in the additional presence of postmating barriers. In this specific case, postmating isolation, in the form of partial hybrid sterility, will help maintain species boundaries in secondary contact zones.
Similarly, not all food-deceptive orchid species adhere to the typical pattern for their group. Anacamptis morio and Anacamptis longicornu are two vicariant sister species (alternatively viewed as sister subspecies) with very similar floral morphology that meet on the island of Sicily. Here, hybrid zones include all possible hybrid classes—a scenario more typical of hybridizing sexually deceptive orchids. The two species have similar karyotypes, and hybrid plants and parent species exhibit equivalent pollination success and seed production. Phylogeographic and geological evidence indicates that these species have been separated on large continental platforms since the refilling of the Mediterranean basin following the reopening of the Gibraltar Straits (some 4.5 Myr ago) after the Mediterranean salinity crisis (Hsü et al. 1977), but that subsequent sporadic contacts may also have occurred during more recent glacial periods (Bell & Walker 1992). In this specific case, periods of isolation have probably been too brief to allow the evolution of strict postmating isolation (Zitari et al. in preparation). The extent to which cladogenesis in food-deceptive orchids is typically punctuated by several reticulation events involving geographical isolation and subsequent secondary population merging remains unknown.
8. Genetic bases of reproductive isolation and species differentiation
Different kinds of reproductive barriers can have contrasting effects on species richness and diversification whether or not the nature of reproductive barriers per se increases speciation rate (Rieseberg & Willis 2007). Even if orchids are unrepresentative of plants in general, the patterns described here may provide new insights into the role and interaction of different isolating barriers in plant speciation and the maintenance of species boundaries following secondary contact.
Comparative analysis of reproductive isolation within groups of sexually deceptive and food-deceptive orchids indicates that the contrasting evolution of initial isolating barriers differentially affects the subsequent evolution of other isolating barriers. Sexually deceptive orchids commonly exhibit strong premating isolation because they do not share pollinators, but usually have limited postzygotic isolation, which, when present, probably arises as a secondary effect of independent evolution after divergence. By contrast, food-deceptive orchids may initially be isolated by chromosomal rearrangements and consequent hybrid sterility, but significant postmating–prezygotic isolation associated with mechanisms of pollen–ovule interaction probably evolves once they come into contact.
The difference in the tempo of evolution of pre- and postzygotic isolation can be attributed to contrasting selection during the two critical stages. Loci or genomic regions contributing to postzygotic isolation, such as chromosomal rearrangement in food-deceptive orchids, probably evolve by drift or indirect selection alone, whereas prezygotic isolation associated with pollen–ovule interaction may also be potentially subject to direct selection particularly in contact zones. This observation explains the overall speed at which postmating–prezygotic isolation consistently accumulates in groups in which postzygotic isolation evolved first, compared with groups, such as sexually deceptive orchids, in which strong premating isolation evolved first (figure 1).
Interestingly, in the food-deceptive orchids, postmating–prezygotic isolation is more common and widespread than premating isolation. The simplest explanation for this pattern is that, although the former isolation stage involving pollen–ovule interaction may be achieved by changes in few interacting loci (as pollen coat proteins; Fiebig et al. 2004) a shift to a novel pollinator implies major changes in floral architecture, and so may require substantial mutational leaps to cross a valley of maladaptive phenotypes and low fitness (Wright 1932; Lande 1979; Bateman & Rudall 2006). By contrast, a shift to a different pollinator in sexually deceptive orchids need not require any significant change in flower shape, but only in the specific scent bouquet for male attraction. Small changes in gene expression are sufficient to change the relative ratio of alkanes and alkenes of different chain lengths that is the key to specific pollinator attraction in these species (Schiestl et al. 1999; Schiestl 2005; Stökl et al. 2008). This suggests that while food-deceptive orchids more likely evolved in allopatry, sexually deceptive orchids could also evolve in sympatry during a pollinator shift.
Finally, different reproductive isolation barriers may provide a contrasting view of species integrity in the two groups. The postzygotic barriers prevalent among Mediterranean generalized food-deceptive orchids may have a complex genetic basis, with a large part of the genome contributing to species isolation (Rieseberg & Carney 1998; Coyne & Orr 2004) as suggested by the studies on their hybrid zones. By contrast, in sexually deceptive orchids for which few loci contribute to isolation, more extensive gene exchange occurs in hybrid zones. Several sexually deceptive Ophrys species can be distinguished by scent emission, but they are genetically differentiated only weakly and their genomes largely introgress when hybridizing (Stökl et al. 2008). Thus, in sexually deceptive species, only small portions of the genome may be sheltered from introgression. Eventual co-localization of genes involved in scent emission and these areas of strong genetic differentiation among species might point towards an important role for pollinator adaptation in the maintenance of species boundaries in this orchid group, and suggests adoption of a ‘genic’ view of the speciation process for these orchids (Lexer & Widmer 2008). If so, the speciation genes are not regulatory (reviewed in Coyne & Orr 2004), with species isolation occurring as a by-product of genetic divergence and incompatibility, but rather they are the same genes that dictate the emergence of reproductive isolation.
Acknowledgments
The authors thank R. Bateman for a constructive and critical review of an earlier version of the manuscript. They also thank A. Dafni, N. Juillet, A. Widmer and L. Rieseberg for their discussions about orchids and speciation, J. Stökl for providing an unpublished figure of hybrid index of Ophrys hybrid zone and M. Longrigg for language revision. The authors are particularly grateful to Steve Johnson, Scott Armbruster and another anonymous referee who significantly contributed to improve the quality of the manuscript. Finally, the authors are also very grateful to P. Hollingsworth and R. Abbott, the organizers of the speciation symposium held in Edinburgh, for providing an opportunity to meet stimulating people and exchange ideas. Funding for this study was provided by the PRIN programme.
Footnotes
One contribution of 12 to a Theme Issue ‘Speciation in plants and animals: pattern and process’.
References
- Ågren L, Kullenberg B, Sensenbaugh T. Congruences in pilosity between three species of Ophrys (Orchidaceae) and their hymenopterean pollinators. Nova Acta Reg. Soc. Sci. Ups. Ser. 1984;V:15–25. [Google Scholar]
- Arditti J. Wiley; New York, NY: 1992. Fundamentals of orchid biology. [Google Scholar]
- Armbruster W.S. Evolution of plant pollination systems: hypotheses and tests with the neotropical vine Dalechampia. Evolution. 1993;47:1480–1505. doi: 10.1111/j.1558-5646.1993.tb02170.x. doi:10.2307/2410162 [DOI] [PubMed] [Google Scholar]
- Bateman R.M, Rudall P.J. The good, the bad, and the ugly: using naturally occurring terata to distinguish the possible from the impossible in orchid floral evolution. Aliso. 2006;22:481–496. [Google Scholar]
- Bateman R.M, Hollingsworth P.M, Preston J, Yi-Bo L, Pridgeon A.M, Chase M.W. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae) Bot. J. Linn. Soc. 2003;142:1–40. doi:10.1046/j.1095-8339.2003.00157.x [Google Scholar]
- Batty A.L, Dixon K.W, Brundrett M, Sivasithamparam K. Constraints to symbiotic germination of terrestrial orchid seeds in Mediterranean woodland. New Phytol. 2001;152:511–520. doi: 10.1046/j.0028-646X.2001.00277.x. doi:10.1046/j.0028-646X.2001.00277.x [DOI] [PubMed] [Google Scholar]
- Bell M, Walker M.J.C. Longman Scientific & Technical; Harlow, UK: 1992. Late Quaternary environmental change. [Google Scholar]
- Bolnick D.I, Near T.J. Tempo of hybrid inviability in centrarchid fishes (Teleostei: Centrarchidae) Evolution. 2005;59:1754–1767. doi:10.1554/04-563.1 [PubMed] [Google Scholar]
- Bomblies K, Weigel D. Hybrid necrosis: autoimmunity as a potential gene-flow barrier in plant species. Nat. Rev. Genet. 2007;8:382–393. doi: 10.1038/nrg2082. doi:10.1038/nrg2082 [DOI] [PubMed] [Google Scholar]
- Borg-Karlson A.-K. Chemical and ethological studies of pollination in the genus Ophrys (Orchidaceae) Phytochemistry. 1990;29:1359–1387. doi:10.1016/0031-9422(90)80086-V [Google Scholar]
- Bouwmeester H.J, Roux C, Lopez-Raez J.A, Bécard G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 2007;12:224–230. doi: 10.1016/j.tplants.2007.03.009. doi:10.1016/j.tplants.2007.03.009 [DOI] [PubMed] [Google Scholar]
- Bradshaw H.D.J, Wilbert S.M, Otto K.G, Schemske D.W. Genetic mapping of floral traits associated with reproductive isolation in monkeyflowers (Mimulus) Nature. 1995;376:762–765. doi:10.1038/376762a0 [Google Scholar]
- Bradshaw H.D.J, Otto K.G, Frewen B.E, McKay J.K, Schemske D.W. Quantitative Trait Loci affecting differences in floral morphology between two species of monkeyflower (Mimulus) Genetics. 1998;149:367–382. doi: 10.1093/genetics/149.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkle C.A. Maximum-likelihood estimation of a hybrid index based on molecular markers. Mol. Ecol. Notes. 2005;5:684–687. doi:10.1111/j.1471-8286.2005.01011.x [Google Scholar]
- Buerkle C.A, Rieseberg L.H. Low intraspecific variation for genomic isolation between hybridizing sunflower species. Evolution. 2001;55:684–691. doi: 10.1554/0014-3820(2001)055[0684:livfgi]2.0.co;2. doi:10.1554/0014-3820(2001)055[0684:LIVFGI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Burke J.M, Arnold M.L. Genetics and the fitness of hybrid. Annu. Rev. Genet. 2001;35:31–52. doi: 10.1146/annurev.genet.35.102401.085719. doi:10.1146/annurev.genet.35.102401.085719 [DOI] [PubMed] [Google Scholar]
- Butlin R. Speciation by reinforcement. Trends Ecol. Evol. 1987;2:8–13. doi: 10.1016/0169-5347(87)90193-5. doi:10.1016/0169-5347(87)90193-5 [DOI] [PubMed] [Google Scholar]
- Carr D.E, Dudash M.R. Recent approaches into the genetic basis of inbreeding depression in plants. Phil. Trans. R. Soc. B. 2003;358:1071–1084. doi: 10.1098/rstb.2003.1295. doi:10.1098/rstb.2003.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortis, P., Vereecken, N. J., Schiestl, F. P., Barone Lumaga, M. R., Scrugli, A. & Cozzolino, S. Submitted. Floral odour convergence and the nature of species boundaries in sympatric Sardinian Ophrys (Orchidaceae). [DOI] [PMC free article] [PubMed]
- Coyne J.A, Orr H.A. Patterns of speciation in Drosophila. Evolution. 1989;43:362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. doi:10.2307/2409213 [DOI] [PubMed] [Google Scholar]
- Coyne J.A, Orr H.A. Patterns of speciation in Drosophila revisited. Evolution. 1997;51:295–303. doi: 10.1111/j.1558-5646.1997.tb02412.x. doi:10.2307/2410984 [DOI] [PubMed] [Google Scholar]
- Coyne J.A, Orr H.A. Sinauer Associates; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Cozzolino S, Widmer A. Orchid diversity: an evolutionary consequence of deception? Trends Ecol. Evol. 2005a;20:487–494. doi: 10.1016/j.tree.2005.06.004. doi:10.1016/j.tree.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Cozzolino S, Widmer A. The evolutionary basis of the reproductive isolation in Mediterranean orchids. Taxon. 2005b;54:977–985. [Google Scholar]
- Cozzolino S, Aceto S, Caputo P, Widmer A, Dafni A. Speciation processes in Eastern Mediterranean Orchis s.l. species: molecular evidence and the role of pollination biology. Isr. J. Plant Sci. 2001;49:91–103. doi:10.1560/QV6M-E7A0-QFC7-G6BL [Google Scholar]
- Cozzolino S, D'Emerico S, Widmer A. Evidence for reproductive isolate selection in Mediterranean orchids: karyotype differences compensate for the lack of pollinator specificity. Proc. R. Soc. B. 2004;271:259–262. doi: 10.1098/rsbl.2004.0166. doi:10.1098/rsbl.2004.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino S, Schiestl F.P, Müller A, De Castro O, Nardella A.M, Widmer A. Evidence for pollinator sharing in Mediterranean nectar-mimic orchids: absence of premating barriers? Proc. R. Soc. B. 2005;272:1271–1278. doi: 10.1098/rspb.2005.3069. doi:10.1098/rspb.2005.3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino S, Nardella A.M, Impagliazzo S, Widmer A, Lexer C. Hybridization and orchid conservation: should we protect the orchid hybrids or the orchid hybrid zones? Biol. Conserv. 2006;129:14–23. doi:10.1016/j.biocon.2005.09.043 [Google Scholar]
- Dafni A, Bernhardt P. Pollination of terrestrial orchids of Southern Australia and the Mediterranean region: systematic, ecological, and evolutionary implications. Evol. Biol. 1990;24:193–252. [Google Scholar]
- Dearnaley J.D.W. Further advances in orchid mycorrhizal research. Mycorrhiza. 2007;17:475–486. doi: 10.1007/s00572-007-0138-1. doi:10.1007/s00572-007-0138-1 [DOI] [PubMed] [Google Scholar]
- D'Emerico S, Bianco P, Pignone D. Cytomorphological characterization of diploid and triploid individuals of Orchis xgennarii Reichenb. fil. (Orchidaceae) Caryologia. 1996;49:153–161. [Google Scholar]
- D'Emerico S, Cozzolino S, Pellegrino G, Pignone D, Scrugli A. Heterochromatin distribution in selected taxa of the 42-chromosomes Orchis s.l. (Orchidaceae) Caryologia. 2002;55:55–62. [Google Scholar]
- Devey S.D, Bateman R.M, Fay M.F, Hawkins J.A. Friends or relatives? Phylogenetics and species delimitation in the controversial European orchid genus Ophrys. Ann. Bot. 2008;101:385–402. doi: 10.1093/aob/mcm299. doi:10.1093/aob/mcm299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Columbia University Press; New York, NY: 1937. Genetics and the origin of species. [Google Scholar]
- Dressler R.L. Cambridge University Press; Cambridge, UK: 1993. Phylogeny and classification of the orchid family. [Google Scholar]
- Ehrlich P.R, Raven P.H. Differentiation of populations. Science. 1969;165:1228–1232. doi: 10.1126/science.165.3899.1228. doi:10.1126/science.165.3899.1228 [DOI] [PubMed] [Google Scholar]
- Fiebig A, Kimport R, Preuss D. Comparisons of pollen coat genes across Barssicaceae species reveal rapid evolution by repeat expansion and diversification. Proc. Natl Acad. Sci. USA. 2004;101:3286–3291. doi: 10.1073/pnas.0305448101. doi:10.1073/pnas.0305448101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D.E. Fruiting failure, pollination inefficiency, and speciation in orchids. In: Otte D, Endler J.A, editors. Speciation and its consequences. Academy of Natural Sciences Publications; Philadelphia, PA: 1989. pp. 458–481. [Google Scholar]
- Grant V. Columbia University Press; New York, NY: 1981. Plant speciation. [Google Scholar]
- Grant V. Modes and origins of mechanical and ethological isolation in angiosperms. Proc. Natl Acad. Sci. USA. 1994;91:3–10. doi: 10.1073/pnas.91.1.3. doi:10.1073/pnas.91.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges S.A, Whittall J.B, Fulton M, Yang J.Y. Genetics of floral traits influencing reproductive isolation between Aquilegia formosa and A. pubescens. Am. Nat. 2002;159:51–60. doi: 10.1086/338372. doi:10.1086/338372 [DOI] [PubMed] [Google Scholar]
- Hsü K.J, et al. History of the Mediterranean salinity crisis. Nature. 1977;267:399–403. doi:10.1038/267399a0 [Google Scholar]
- Jersáková J, Johnson S.D, Kindlmann P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 2006;81:219–235. doi: 10.1017/S1464793105006986. doi:10.1017/S1464793105006986 [DOI] [PubMed] [Google Scholar]
- Johnson S.D. Batesian mimicry in the non-rewarding orchid Disa pulchra, and its consequences for pollinator behaviour. Biol. J. Linn. Soc. 2000;71:119–132. [Google Scholar]
- Johnson S.D. Pollinator-driven speciation in plants. In: Harder L.D, Barrett S.C.H, editors. The ecology and evolution of flowers. Oxford University Press; New York, NY: 2006. pp. 296–306. [Google Scholar]
- Johnson S.D, Linder H.P, Steiner K.E. Phylogeny and radiation of pollination systems in Disa (Orchidaceae) Am. J. Bot. 1998;85:402–411. doi:10.2307/2446333 [PubMed] [Google Scholar]
- Kim S.-C, Rieseberg L.H. Genetic architecture of species differences in annual sunflowers: implications for adaptive trait introgression. Genetics. 1999;153:965–977. doi: 10.1093/genetics/153.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-C, Rieseberg L.H. The contribution of epistasis to species differences in annual sunflowers. Mol. Ecol. 2001;10:683–690. doi: 10.1046/j.1365-294x.2001.01203.x. doi:10.1046/j.1365-294x.2001.01203.x [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Barton N. Chromosome inversions, local adaptation and speciation. Genetics. 2006;173:419–434. doi: 10.1534/genetics.105.047985. doi:10.1534/genetics.105.047985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullenberg B. Almquist & Wiksells Boktryckeri AB; Uppsala, Sweden: 1961. Studies in Ophrys pollination. [Google Scholar]
- Lai Z, Nakazato T, Salmaso M, Burke J.M, Tang S, Knapp S.J, Rieseberg L.H. Extensive chromosomal repatterning and the evolution of sterility barriers in hybrid sunflower species. Genetics. 2005;171:291–303. doi: 10.1534/genetics.105.042242. doi:10.1534/genetics.105.042242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R. Quantitative genetic analysis of multivariate evolution applied to brain:body size allometry. Evolution. 1979;33:402–416. doi: 10.1111/j.1558-5646.1979.tb04694.x. doi:10.2307/2407630 [DOI] [PubMed] [Google Scholar]
- Levin D.A. Oxford University Press; New York, NY: 2002. The role of chromosomal change in plant evolution. [Google Scholar]
- Lexer C, Widmer A. The genic view of plant speciation: recent progress and emerging questions. Phil. Trans. R. Soc. B. 2008;363:3023–3036. doi: 10.1098/rstb.2008.0078. doi:10.1098/rstb.2008.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexer C, Fay M.F, Joseph J.A, Nica M.S, Heinze B. Barrier to gene flow between two ecologically divergent Populus species, P. alba (white poplar) and P. tremula (European aspen): the role of ecology and life history in gene introgression. Mol. Ecol. 2005;14:1045–1057. doi: 10.1111/j.1365-294X.2005.02469.x. doi:10.1111/j.1365-294X.2005.02469.x [DOI] [PubMed] [Google Scholar]
- Lowry D.B, Modliszewski J.L, Wright K.M, Wu C.A, Willis J.H. The strength and genetic basis of reproductive isolating barriers in flowering plants. Phil. Trans. R. Soc. B. 2008;363:3009–3021. doi: 10.1098/rstb.2008.0064. doi:10.1098/rstb.2008.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant J.G, Schiestl F.P, Peakall R, Weston P.H. A phylogenetic study of pollinator conservatism among sexually deceptive orchids. Evolution. 2002;56:888–898. doi: 10.1111/j.0014-3820.2002.tb01402.x. doi:10.1111/j.0014-3820.2002.tb01402.x [DOI] [PubMed] [Google Scholar]
- Martinsen G.D, Whitham T.G, Turek R.J, Keim P. Hybrid populations selectively filter gene introgression between species. Evolution. 2001;55:1325–1335. doi: 10.1111/j.0014-3820.2001.tb00655.x. doi:10.1111/j.0014-3820.2001.tb00655.x [DOI] [PubMed] [Google Scholar]
- Mayr E. Belknap Press; Cambridge, MA: 1970. Populations, species, and evolution: an abridgment of animal species and evolution. [Google Scholar]
- Moccia M.D, Widmer A, Cozzolino S. The strength of reproductive isolation in hybridizing food deceptive orchids. Mol. Ecol. 2007;16:2855–2866. doi: 10.1111/j.1365-294X.2007.03240.x. doi:10.1111/j.1365-294X.2007.03240.x [DOI] [PubMed] [Google Scholar]
- Moyle L.C, Olson M.S, Tiffin P. Patterns of reproductive isolation in three angiosperm genera. Evolution. 2004;58:1195–1208. doi: 10.1111/j.0014-3820.2004.tb01700.x. doi:10.1111/j.0014-3820.2004.tb01700.x [DOI] [PubMed] [Google Scholar]
- Neiland M.R.M, Wilcock C.C. Maximization of reproductive success by European Orchidaceae under conditions of infrequent pollination. Protoplasma. 1995;187:39–48. doi:10.1007/BF01280231 [Google Scholar]
- Noor M.A, Grams K.L, Bertucci L.A, Reiland J. Chromosomal inversions and the reproductive isolation of species. Proc. Natl Acad. Sci. USA. 2001;98:12 084–12 088. doi: 10.1073/pnas.221274498. doi:10.1073/pnas.221274498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H.A. The genetics of species differences. Trends Ecol. Evol. 2001;16:343–350. doi: 10.1016/s0169-5347(01)02167-x. doi:10.1016/S0169-5347(01)02167-X [DOI] [PubMed] [Google Scholar]
- Paulus H.F, Gack C. Pollinators as prepollinating isolation factors: evolution and speciation in Ophrys (Orchidaceae) Isr. J. Bot. 1990;39:43–79. [Google Scholar]
- Pellegrino G, D'Emerico S, Musacchio A, Scrugli A, Cozzolino S. Confirmation of hybridization among sympatric insular populations of Orchis mascula and O. provincialis. Plant Syst. Evol. 2005;251:131–142. doi:10.1007/s00606-004-0235-y [Google Scholar]
- Pirstinger, P. & Paulus, H. F. 1996 Congruences in pilosity between species of Ophrys (Orchidaceae) and the females of their hymenopteran pollinators (Apoidea). In Proc. XXth Int. Cong. of Entomology, Florence, Italy, 25–31 August 1996, p. 255. Basel, Switzerland: Bukhäuser.
- Presgraves D.C. Patterns of postzygotic isolation in Lepidoptera. Evolution. 2002;56:1168–1183. doi: 10.1111/j.0014-3820.2002.tb01430.x. doi:10.1111/j.0014-3820.2002.tb01430.x [DOI] [PubMed] [Google Scholar]
- Ramsey J, Bradshaw H.D, Jr, Schemske D.W. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae) Evolution. 2003;57:1520–1534. doi: 10.1111/j.0014-3820.2003.tb00360.x. doi:10.1554/01-352 [DOI] [PubMed] [Google Scholar]
- Rasmussen H.N. Cambridge University Press; Cambridge, UK: 1995. Terrestrial orchids—from seed to mycotrophic plant. [Google Scholar]
- Rieseberg L.H. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 2001;16:351–358. doi: 10.1016/s0169-5347(01)02187-5. doi:10.1016/S0169-5347(01)02187-5 [DOI] [PubMed] [Google Scholar]
- Rieseberg L.H, Carney S.E. Plant hybridization. New Phytol. 1998;140:599–624. doi: 10.1046/j.1469-8137.1998.00315.x. doi:10.1046/j.1469-8137.1998.00315.x [DOI] [PubMed] [Google Scholar]
- Rieseberg L.H, Willis J.H. Plant speciation. Science. 2007;317:910–914. doi: 10.1126/science.1137729. doi:10.1126/science.1137729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasa M.M, Chippindale P.T, Johnson N.A. Patterns of postzygotic isolation in frogs. Evolution. 1998;52:1811–1820. doi: 10.1111/j.1558-5646.1998.tb02258.x. doi:10.2307/2411351 [DOI] [PubMed] [Google Scholar]
- Schiestl F.P. On the success of a swindle: pollination by deception in orchids. Naturwissenschaften. 2005;92:255–264. doi: 10.1007/s00114-005-0636-y. doi:10.1007/s00114-005-0636-y [DOI] [PubMed] [Google Scholar]
- Schiestl F.P, Cozzolino S. Evolution of sexual mimicry in the orchid subtribe Orchidinae: the role of preadaptations in the attraction of male bees as pollinators. BMC Evol. Biol. 2008;8:27. doi: 10.1186/1471-2148-8-27. doi:10.1186/1471-2148-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl F.P, Ayasse M, Paulus H.F, Löfstedt C, Hannson B.S, Ibarra F, Francke W. Orchid pollination by sexual swindle. Nature. 1999;399:421–422. doi:10.1038/20829 [Google Scholar]
- Scopece G, Musacchio A, Widmer A, Cozzolino S. Patterns of reproductive isolation in Mediterranean deceptive orchids. Evolution. 2007;61:2623–2642. doi: 10.1111/j.1558-5646.2007.00231.x. doi:10.1111/j.1558-5646.2007.00231.x [DOI] [PubMed] [Google Scholar]
- Scopece G, Widmer A, Cozzolino S. Evolution of postzygotic reproductive isolation in a deceptive orchid lineage. Am. Nat. 2008;171:315–326. doi: 10.1086/527501. doi:10.1086/527501 [DOI] [PubMed] [Google Scholar]
- Scotti-Saintagne C, Mariette S, Porth I, Goiecoechea P.G, Barreneche T, Bodénès C, Burg K, Kremer A. Genome scanning for interspecific differentiation between two closely related oak species [Quercus robur L. and Q. petraea (Matt.) Liebl.] Genetics. 2004;168:1615–1626. doi: 10.1534/genetics.104.026849. doi:10.1534/genetics.104.026849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow A.A. Postpollination selection and male fitness in plants. Am. Nat. 1994;144:69–83. doi:10.1086/285653 [Google Scholar]
- Stökl J, Schlüter P.M, Stuessy T.F, Paulus H.F, Assum G, Ayasse M. Scent variation and hybridization cause the displacement of a sexually deceptive orchid species. Am. J. Bot. 2008;95:472–481. doi: 10.3732/ajb.95.4.472. doi:10.3732/ajb.95.4.472 [DOI] [PubMed] [Google Scholar]
- Stökl, J., Schlüter, P. M., Stuessy, T. F., Paulus, H. F., Assum, G. & Ayasse, M. Submitted. Genetic variation, hybridization, and introgression in co-flowering sexually deceptive orchids on Majorca.
- Swanson W.J, Vacquier V.D. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 2002;3:137–144. doi: 10.1038/nrg733. doi:10.1038/nrg733 [DOI] [PubMed] [Google Scholar]
- Tiffin P, Olson M.S, Moyle L.C. Asymmetrical crossing barriers in angiosperms. Proc. R. Soc. B. 2001;268:861–867. doi: 10.1098/rspb.2000.1578. doi:10.1098/rspb.2000.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay R.L, Ackerman J.D, Zimmerman J.K, Calvo R.N. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biol. J. Linn. Soc. 2005;84:1–54. doi:10.1111/j.1095-8312.2004.00400.x [Google Scholar]
- van der Cingel N.A. Balkema; Rotterdam, The Netherlands: 1995. An atlas of orchids pollination—European orchids. [Google Scholar]
- van der Pijl L, Dodson C.H. University of Miami Press; Coral Gables, FL: 1966. Orchid flowers. Their pollination and evolution. [Google Scholar]
- Whittall J.B, Hodges S.A. Pollinator shift drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–709. doi: 10.1038/nature05857. doi:10.1038/nature05857 [DOI] [PubMed] [Google Scholar]
- Widmer A, Cozzolino S, Pellegrino G, Soliva M, Dafni A. Molecular analysis of orchid pollinaria and pollinaria-remains found on insects. Mol. Ecol. 2000;9:1911–1914. doi: 10.1046/j.1365-294x.2000.01103.x. doi:10.1046/j.1365-294x.2000.01103.x [DOI] [PubMed] [Google Scholar]
- Wright, S. 1932 The roles of mutation, inbreeding, crossbreeding and selection in evolution. In Proc. Sixth Int. Congress of Genetics, vol. 1 (ed. D. F. Jones), pp. 356–366. Menasha, WI: Brooklyn Botanic Garden.
- Wu C.I. The genic view of the process of speciation. J. Evol. Biol. 2001;14:851–865. doi:10.1046/j.1420-9101.2001.00335.x [Google Scholar]
- Zhang X.S, O'Neill S.D. Ovary and gametophyte development are coordinately regulated by auxin and ethylene following pollination. Plant Cell. 1993;5:403–418. doi: 10.1105/tpc.5.4.403. doi:10.1105/tpc.5.4.403 [DOI] [PMC free article] [PubMed] [Google Scholar]