Abstract
Regulation of gene expression is considered a plausible mechanism of drug addiction, given the stability of behavioural abnormalities that define an addicted state. Among many transcription factors known to influence the addiction process, one of the best characterized is ΔFosB, which is induced in the brain's reward regions by chronic exposure to virtually all drugs of abuse and mediates sensitized responses to drug exposure. Since ΔFosB is a highly stable protein, it represents a mechanism by which drugs produce lasting changes in gene expression long after the cessation of drug use. Studies are underway to explore the detailed molecular mechanisms by which ΔFosB regulates target genes and produces its behavioural effects. We are approaching this question using DNA expression arrays coupled with the analysis of chromatin remodelling—changes in the posttranslational modifications of histones at drug-regulated gene promoters—to identify genes that are regulated by drugs of abuse via the induction of ΔFosB and to gain insight into the detailed molecular mechanisms involved. Our findings establish chromatin remodelling as an important regulatory mechanism underlying drug-induced behavioural plasticity, and promise to reveal fundamentally new insight into how ΔFosB contributes to addiction by regulating the expression of specific target genes in brain reward pathways.
Keywords: chromatin remodelling, epigenetics, nucleus accumbens, orbitofrontal cortex, ventral tegmental area
Abbreviations: BNST, bed nucleus of the stria terminalis; IPAC, interstitial nucleus of the posterior limb of the anterior commissure; PAG, periaqueductal grey; VTA, ventral tegmental area; SN, substantia nigra
1. Introduction
The study of transcriptional mechanisms of addiction is based on the hypothesis that regulation of gene expression is one important mechanism by which chronic exposure to a drug of abuse causes long-lasting changes in the brain, which underlie the behavioural abnormalities that define a state of addiction (Nestler 2001). A corollary of this hypothesis is that drug-induced changes in dopaminergic and glutamatergic transmission and in the morphology of certain neuronal cell types in the brain, which have been correlated with an addicted state, are mediated in part via changes in gene expression.
Work over the past 15 years has provided increasing evidence for a role of gene expression in drug addiction, as several transcription factors—proteins that bind to specific response elements in the promoter regions of target genes and regulate those genes' expression—have been implicated in drug action. Prominent examples include ΔFosB (a Fos family protein), cAMP-response element-binding protein (CREB), inducible cAMP early repressor (ICER), activating transcription factors (ATFs), early growth response proteins (EGRs), nucleus accumbens 1 (NAC1), nuclear factor κB (NFκB) and glucocorticoid receptor (O'Donovan et al. 1999; Mackler et al. 2000; Ang et al. 2001; Deroche-Gamonet et al. 2003; Carlezon et al. 2005; Green et al. 2006, 2008). This review focuses on ΔFosB, which appears to play a unique role in the addiction process, as a way to illustrate the types of experimental approaches that have been used to investigate transcriptional mechanisms of addiction.
2. Induction of ΔFosB in nucleus accumbens by drugs of abuse
ΔFosB is encoded by the fosB gene (figure 1) and shares homology with other Fos family transcription factors, which include c-Fos, FosB, Fra1 and Fra2 (Morgan & Curran 1995). These Fos family proteins heterodimerize with Jun family proteins (c-Jun, JunB or JunD) to form active activator protein-1 (AP-1) transcription factors that bind to AP-1 sites (consensus sequence: TGAC/GTCA) present in the promoters of certain genes to regulate their transcription. These Fos family proteins are induced rapidly and transiently in specific brain regions after acute administration of many drugs of abuse (figure 2; Graybiel et al. 1990; Young et al. 1991; Hope et al. 1992). These responses are seen most prominently in nucleus accumbens and dorsal striatum, which are important mediators of the rewarding and locomotor actions of the drugs. All of these Fos family proteins, however, are highly unstable and return to basal levels within hours of drug administration.
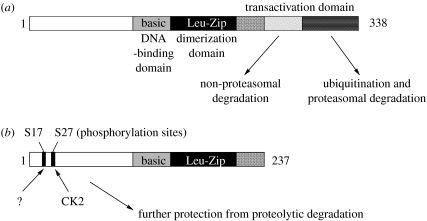
Figure 1.
Biochemical basis of ΔFosB's unique stability: (a) FosB (338 aa, Mr approx. 38 kD) and (b) ΔFosB (237 aa, Mr approx. 26 kD) are encoded by the fosB gene. ΔFosB is generated by alternative splicing and lacks the C-terminal 101 amino acids present in FosB. Two mechanisms are known that account for ΔFosB's stability. First, ΔFosB lacks two degron domains present in the C-terminus of full-length FosB (and found in all other Fos family proteins as well). One of these degron domains targets FosB for ubiquitination and degradation in the proteasome. The other degron domain targets FosB degradation by a ubiquitin- and proteasome-independent mechanism. Second, ΔFosB is phosphorylated by casein kinase 2 (CK2) and probably by other protein kinases (?) at its N-terminus, which further stabilizes the protein.
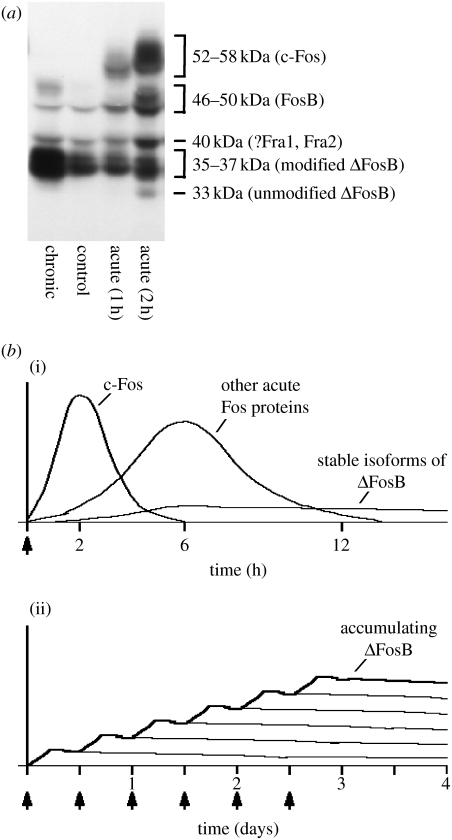
Figure 2.
Scheme showing the gradual accumulation of ΔFosB versus the rapid and transient induction of other Fos family proteins in response to drugs of abuse. (a) The autoradiogram illustrates the differential induction of Fos family proteins in the nucleus accumbens by acute stimulation (1–2 hours after a single cocaine exposure) versus chronic stimulation (1 day after repeated cocaine exposure). (b) (i) Several waves of Fos family proteins (comprising c-Fos, FosB, ΔFosB (33 kD isoform), and possibly (?) Fra1, Fra2) are induced in nucleus accumbens and dorsal striatal neurons by acute administration of a drug of abuse. Also induced are biochemically modified isoforms of ΔFosB (35–37 kD); they are induced at low levels by acute drug administration, but persist in brain for long periods due to their stability. (ii) With repeated (e.g. twice daily) drug administration, each acute stimulus induces a low level of the stable ΔFosB isoforms. This is indicated by the lower set of overlapping lines that indicate ΔFosB induced by each acute stimulus. The result is a gradual increase in the total levels of ΔFosB with repeated stimuli during a course of chronic treatment. This is indicated by the increasing stepped line in the graph.
Very different responses are seen after chronic administration of drugs of abuse (figure 2). Biochemically modified isoforms of ΔFosB (Mr 35–37 kD) accumulate within the same brain regions after repeated drug exposure, whereas all other Fos family members show tolerance (i.e. reduced induction compared with initial drug exposures; Chen et al. 1995, 1997; Hiroi et al. 1997). Such accumulation of ΔFosB has been observed for virtually all drugs of abuse (table 1; Hope et al. 1994; Nye et al. 1995; Moratalla et al. 1996; Nye & Nestler 1996; Pich et al. 1997; Muller & Unterwald 2005; McDaid et al. 2006b), although different drugs differ somewhat in the relative degree of induction seen in nucleus accumbens core versus shell and dorsal striatum (Perrotti et al. 2008). At least for some drugs of abuse, the induction of ΔFosB appears selective for the dynorphin-containing subset of medium spiny neurons located in these brain regions (Nye et al. 1995; Moratalla et al. 1996; Muller & Unterwald 2005; Lee et al. 2006), although more work is needed to establish this with certainty. The 35–37 kD isoforms of ΔFosB dimerize predominantly with JunD to form an active and long-lasting AP-1 complex within these brain regions (Chen et al. 1997; Hiroi et al. 1998; Pérez-Otan¨o et al. 1998). The drug induction of ΔFosB in the nucleus accumbens seems to be a response to the pharmacological properties of the drug per se and not related to volitional drug intake, since animals that self-administer cocaine or receive yoked drug injections show equivalent induction of this transcription factor in this brain region (Perrotti et al. 2008).
Table 1.
Drugs of abuse known to induce ΔFosB in nucleus accumbens after chronic administration.
Induction reported for self-administered drug in addition to investigator-administered drug. Drug induction of ΔFosB has been demonstrated in both rats and mice, except the following: mouse only, cannabinoids; rat only, methamphetamine, phencyclidine.
The 35–37 kD ΔFosB isoforms accumulate with chronic drug exposure due to their extraordinarily long half-lives (Chen et al. 1997; Alibhai et al. 2007). By contrast, there is no evidence that the splicing of ΔFosB or the stability of its mRNA is regulated by drug administration. As a result of its stability, therefore, the ΔFosB protein persists in neurons for at least several weeks after the cessation of drug exposure. We now know that this stability is due to the following two factors (figure 1): (i) the absence of two degron domains in ΔFosB, which are present at the C-terminus of full-length FosB and all other Fos family proteins and target those proteins to rapid degradation and (ii) the phosphorylation of ΔFosB at its N-terminus by casein kinase 2 and perhaps other protein kinases (Ulery et al. 2006; Carle et al. 2007). The stability of the ΔFosB isoforms provides a novel molecular mechanism by which drug-induced changes in gene expression can persist despite relatively long periods of drug withdrawal. We have, therefore, proposed that ΔFosB functions as a sustained ‘molecular switch’ that helps initiate and then maintain an addicted state (Nestler et al. 2001; McClung et al. 2004).
3. Role of ΔFosB in nucleus accumbens in regulating behavioural responses to drugs of abuse
Insight into the role of ΔFosB in drug addiction has come largely from the study of bitransgenic mice in which ΔFosB can be induced selectively within the nucleus accumbens and dorsal striatum of adult animals (Kelz et al. 1999). Importantly, these mice overexpress ΔFosB selectively in the dynorphin-containing medium spiny neurons, where the drugs are believed to induce the protein. The behavioural phenotype of the ΔFosB-overexpressing mice, which in certain ways resembles animals after chronic drug exposure, is summarized in table 2. The mice show augmented locomotor responses to cocaine after acute and chronic administration (Kelz et al. 1999). They also show enhanced sensitivity to the rewarding effects of cocaine and morphine in place-conditioning assays (Kelz et al. 1999; Zachariou et al. 2006), and self-administer lower doses of cocaine than littermates that do not overexpress ΔFosB (Colby et al. 2003). As well, ΔFosB overexpression in nucleus accumbens exaggerates the development of opiate physical dependence and promotes opiate analgesic tolerance (Zachariou et al. 2006). By contrast, ΔFosB-expressing mice are normal in several other behavioural domains, including spatial learning as assessed in the Morris water maze (Kelz et al. 1999).
Table 2.
Behavioural phenotype upon ΔFosB induction in dynorphin+neurons of nucleus accumbens and dorsal striatuma.
| stimulus | phenotype |
|---|---|
| cocaine | increased locomotor responses to acute administration |
| increased locomotor sensitization to repeated administration | |
| increased conditioned place preference at lower doses | |
| increased acquisition of cocaine self-administration at lower doses | |
| increased incentive motivation in progressive ratio procedure | |
| morphine | increased conditioned place preference at lower drug doses |
| increased development of physical dependence and withdrawal | |
| decreased initial analgesic responses, enhanced tolerance | |
| alcohol | increased anxiolytic responses |
| wheel running | increased wheel running |
| sucrose | increased incentive for sucrose in progressive ratio procedure |
| high fat | increased anxiety-like responses upon withdrawal of high-fat diet |
| sex | increased sexual behaviour |
The phenotypes described in this table are established upon inducible overexpression of ΔFosB in bitransgenic mice where ΔFosB expression is targeted to dynorphin+neurons of the nucleus accumbens and dorsal striatum; several-fold lower levels of ΔFosB are seen in hippocampus and frontal cortex. In many cases, the phenotype has been directly linked to ΔFosB expression in nucleus accumbens per se by use of viral-mediated gene transfer.
Specific targeting of ΔFosB overexpression to the nucleus accumbens, by use of viral-mediated gene transfer, has yielded equivalent data (Zachariou et al. 2006), which indicates that this particular brain region can account for the phenotype observed in the bitransgenic mice, where ΔFosB is also expressed in dorsal striatum and to a lesser extent in certain other brain regions. Moreover, targeting the enkephalin-containing medium spiny neurons in nucleus accumbens and dorsal striatum in different lines of bitransgenic mice which fail to show most of these behavioural phenotypes, specifically implicates dynorphin+ nucleus accumbens neurons in these phenomena. In contrast to the overexpression of ΔFosB, overexpression of a mutant Jun protein (ΔcJun or ΔJunD)—which functions as a dominant negative antagonist of AP-1-mediated transcription—by the use of bitransgenic mice or viral-mediated gene transfer produces the opposite behavioural effects (Peakman et al. 2003; Zachariou et al. 2006). These data indicate that the induction of ΔFosB in dynorphin-containing medium spiny neurons of the nucleus accumbens increases an animal's sensitivity to cocaine and other drugs of abuse, and may represent a mechanism for relatively prolonged sensitization to the drugs.
The effects of ΔFosB may extend well beyond the regulation of drug sensitivity per se to more complex behaviours related to the addiction process. Mice overexpressing ΔFosB work harder to self-administer cocaine in progressive ratio self-administration assays, suggesting that ΔFosB may sensitize animals to the incentive motivational properties of cocaine and thereby lead to a propensity for relapse after drug withdrawal (Colby et al. 2003). ΔFosB-overexpressing mice also show enhanced anxiolytic effects of alcohol (Picetti et al. 2001), a phenotype that has been associated with increased alcohol intake in humans. Together, these early findings suggest that ΔFosB, in addition to increasing sensitivity to drugs of abuse, produces qualitative changes in behaviour that promote drug-seeking behaviour, and support the view, stated above, that ΔFosB functions as a sustained molecular switch for the addicted state. An important question under current investigation is whether ΔFosB accumulation during drug exposure promotes drug-seeking behaviour after extended withdrawal periods, even after ΔFosB levels have normalized (see below).
4. Induction of ΔFosB in nucleus accumbens by natural rewards
The nucleus accumbens is believed to function normally by regulating responses to natural rewards, such as food, drink, sex and social interactions. As a result, there is considerable interest in a possible role of this brain region in so-called natural addictions (e.g. pathological overeating, gambling, exercise, etc.). Animal models of such conditions are limited; nevertheless, we and others have found that high levels of consumption of several types of natural rewards leads to the accumulation of the stable 35–37 kD isoforms of ΔFosB in nucleus accumbens. This has been seen after high levels of wheel running (Werme et al. 2002) as well as after chronic consumption of sucrose, high-fat food or sex (Teegarden & Bale 2007; Wallace et al. 2007; Teegarden et al. in press). In some cases, this induction is selective for the dynorphin+ subset of medium spiny neurons (Werme et al. 2002). Studies of inducible, bitransgenic mice and of viral-mediated gene transfer have demonstrated that overexpression of ΔFosB in nucleus accumbens increases the drive and consumption for these natural rewards, while the overexpression of a dominant negative Jun protein exerts the opposite effect (table 2; Werme et al. 2002; Olausson et al. 2006; Wallace et al. 2007). These findings suggest that ΔFosB in this brain region sensitizes animals not only for drug rewards but for natural rewards as well, and may contribute to states of natural addiction.
5. Induction of ΔFosB in nucleus accumbens by chronic stress
Given the substantial evidence that ΔFosB is induced in nucleus accumbens by chronic exposure to drug and natural rewards, it was interesting to observe that ΔFosB is also highly induced in this brain region after several forms of chronic stress, including restraint stress, chronic unpredictable stress and social defeat (Perrotti et al. 2004; Vialou et al. 2007). Unlike drugs and natural rewards, however, this induction is seen more broadly in this brain region in that it is observed prominently in both dynorphin+ and enkephalin+ subsets of medium spiny neurons. Early evidence suggests that this induction of ΔFosB may represent a positive, coping response that helps an individual adapt to the stress. This hypothesis is supported by preliminary findings that overexpression of ΔFosB in nucleus accumbens, by the use of inducible, bitransgenic mice or viral-mediated gene transfer, exerts antidepressant-like responses in several behavioural assays (e.g. social defeat, forced swim test), while ΔcJun expression causes pro-depression-like effects (Vialou et al. 2007). Moreover, the chronic administration of standard antidepressant medications exerts an effect similar to stress and induces ΔFosB in this brain region. While further work is needed to validate these findings, such a role would be consistent with the observations that ΔFosB increases the sensitivity of the brain's reward circuitry and may thereby help animals cope under periods of stress. Interestingly, this hypothesized role for ΔFosB in nucleus accumbens is similar to that which has been shown recently for periaqueductal grey where the transcription factor is also induced by chronic stress (Berton et al. 2007).
6. Target genes for ΔFosB in nucleus accumbens
Since ΔFosB is a transcription factor, it presumably produces this interesting behavioural phenotype in nucleus accumbens by enhancing or repressing expression of other genes. As shown in figure 1, ΔFosB is a truncated product of the fosB gene that lacks most of the C-terminal transactivation domain present in full-length FosB but retains the dimerization and DNA-binding domains. ΔFosB binds to Jun family members and the resulting dimer binds AP-1 sites in DNA. Some in vitro studies suggest that because ΔFosB lacks much of its transactivation domain, it functions as a negative regulator of AP-1 activity, while several others show that ΔFosB can activate transcription at AP-1 sites (Dobrazanski et al. 1991; Nakabeppu & Nathans 1991; Yen et al. 1991; Chen et al. 1997).
Using our inducible, bitransgenic mice that overexpress ΔFosB or its dominant negative ΔcJun, and analysing gene expression on Affymetrix chips, we demonstrated that, in the nucleus accumbens in vivo, ΔFosB functions primarily as a transcriptional activator, while it does serve as a repressor for a smaller subset of genes (McClung & Nestler 2003). Interestingly, this differential activity of ΔFosB is a function of the duration and degree of ΔFosB expression, with short-term, lower levels leading to more gene repression and long-term, higher levels leading to more gene activation. This is consistent with the finding that short-term and long-term ΔFosB expressions lead to opposite effects on behaviour: short-term ΔFosB expression, like the expression of ΔcJun, reduces cocaine preference, while longer term ΔFosB expression increases cocaine preference (McClung & Nestler 2003). The mechanism responsible for this shift is currently under investigation; one novel possibility, which remains speculative, is that ΔFosB, at higher levels, may form homodimers that activate AP-1 transcription (Jorissen et al. 2007).
Several target genes of ΔFosB have been established using a candidate gene approach (table 3). One candidate gene is GluR2, an alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor subunit (Kelz et al. 1999). ΔFosB overexpression in inducible bitransgenic mice selectively increases GluR2 expression in nucleus accumbens, with no effect seen on several other AMPA glutamate receptor subunits analysed, while ΔcJun expression blocks the ability of cocaine to upregulate GluR2 (Peakman et al. 2003). AP-1 complexes comprising ΔFosB (and most likely JunD) bind a consensus AP-1 site present in the GluR2 promoter. Furthermore, GluR2 overexpression via viral-mediated gene transfer increases the rewarding effects of cocaine, much like prolonged ΔFosB overexpression (Kelz et al. 1999). Since GluR2-containing AMPA channels have a lower overall conductance compared with AMPA channels that do not contain this subunit, the cocaine- and ΔFosB-mediated upregulation of GluR2 in nucleus accumbens could account, at least in part, for the reduced glutamatergic responses seen in these neurons after chronic drug exposure (Kauer & Malenka 2007; table 3).
Table 3.
Examples of validated targets for ΔFosB in nucleus accumbensa.
| target | brain region |
|---|---|
| ↑GluR2 | decreased sensitivity to glutamate |
| ↓dynorphinb | downregulation of κ-opioid feedback loop |
| ↑Cdk5 | expansion of dendritic processes |
| ↑NFκB | expansion of dendritic processes; regulation of cell survival pathways |
| ↓c-Fos | molecular switch from short-lived Fos family proteins induced acutely to ΔFosB induced chronically |
Although ΔFosB regulates the expression of numerous genes in brain (e.g. McClung & Nestler 2003), the table lists only those genes that meet at least three of the following criteria: (i) increased (↑) or decreased (↓) expression upon ΔFosB overexpression, (ii) reciprocal or equivalent regulation by ΔcJun, a dominant negative inhibitor of AP-1-mediated transcription, (iii) ΔFosB-containing AP-1 complexes bind to AP-1 sites in the promoter region of the gene, and (iv) ΔFosB causes a similar effect on gene promoter activity in vitro as seen in vivo.
Despite evidence that ΔFosB represses the dynorphin gene in drug abuse models (Zachariou et al. 2006), there is other evidence that it may act to activate the gene under different circumstances (see Cenci 2002).
Another candidate target gene of ΔFosB in nucleus accumbens is the opioid peptide, dynorphin. Recall that ΔFosB appears to be induced by drugs of abuse specifically in dynorphin-producing cells in this brain region. Drugs of abuse have complex effects on dynorphin expression, with increases or decreases seen depending on the treatment conditions used. The dynorphin gene contains AP-1-like sites, which can bind ΔFosB-containing AP-1 complexes. Moreover, we have shown that the induction of ΔFosB represses dynorphin gene expression in nucleus accumbens (Zachariou et al. 2006). Dynorphin is thought to activate κ-opioid receptors on VTA dopamine neurons and inhibit dopaminergic transmission and thereby downregulate reward mechanisms (Shippenberg & Rea 1997). Hence, the ΔFosB repression of dynorphin expression could contribute to the enhancement of reward mechanisms mediated by this transcription factor. There is now direct evidence supporting the involvement of dynorphin gene repression in ΔFosB's behavioural phenotype (Zachariou et al. 2006).
Recent evidence has shown that ΔFosB also represses the c-fos gene that helps create the molecular switch—from the induction of several short-lived Fos family proteins after acute drug exposure to the predominant accumulation of ΔFosB after chronic drug exposure—cited earlier (Renthal et al. in press). The mechanism responsible for ΔFosB repression of c-fos expression is complex and is covered below.
Another approach used to identify target genes of ΔFosB has measured the gene expression changes that occur upon the inducible overexpression of ΔFosB (or ΔcJun) in nucleus accumbens using DNA expression arrays, as described earlier. This approach has led to the identification of many genes that are up- or downregulated by ΔFosB expression in this brain region (Chen et al. 2000, 2003; Ang et al. 2001; McClung & Nestler 2003). Two genes that appear to be induced through ΔFosB's actions as a transcriptional activator are cyclin-dependent kinase-5 (Cdk5) and its cofactor P35 (Bibb et al. 2001; McClung & Nestler 2003). Cdk5 is also induced by chronic cocaine in the nucleus accumbens, an effect blocked upon ΔcJun expression, and ΔFosB binds to and activates the Cdk5 gene through an AP-1 site in its promoter (Chen et al. 2000; Peakman et al. 2003). Cdk5 is an important target of ΔFosB since its expression has been directly linked to changes in the phosphorylation state of numerous synaptic proteins including glutamate receptor subunits (Bibb et al. 2001), as well as increases in dendritic spine density (Norrholm et al. 2003; Lee et al. 2006), in the nucleus accumbens, which are associated with chronic cocaine administration (Robinson & Kolb 2004). Recently, the regulation of Cdk5 activity in nucleus accumbens has been directly linked to alterations in the behavioural effects of cocaine (Taylor et al. 2007).
Another ΔFosB target identified by use of microarrays is NFκB. This transcription factor is induced in nucleus accumbens by ΔFosB overexpression and chronic cocaine, an effect blocked by ΔcJun expression (Ang et al. 2001; Peakman et al. 2003). Recent evidence has suggested that the induction of NFκB may also contribute to cocaine's ability to induce dendritic spines in nucleus accumbens neurons (Russo et al. 2007). In addition, NFκB has been implicated in some of the neurotoxic effects of methamphetamine in striatal regions (Asanuma & Cadet 1998). The observation that NFκB is a target gene for ΔFosB emphasizes the complexity of the mechanisms by which ΔFosB mediates the effects of cocaine on gene expression. Thus, in addition to the genes regulated by ΔFosB directly via AP-1 sites on the gene promoters, ΔFosB would be expected to regulate many additional genes via altered expression of NFκB and presumably other transcriptional regulatory proteins.
The DNA expression arrays provide a rich list of many additional genes that may be targeted, directly or indirectly, by ΔFosB. Among these genes are additional neurotransmitter receptors, proteins involved in pre- and postsynaptic functions, many types of ion channels and intracellular signalling proteins, as well as proteins that regulate the neuronal cytoskeleton and cell growth (McClung & Nestler 2003). Further work is needed to confirm each of these numerous proteins as bona fide targets of cocaine acting through ΔFosB and to establish the precise role that each protein plays in mediating the complex neural and behavioural aspects of cocaine action. Ultimately, of course, it will be crucial to move beyond the analysis of individual target genes to the regulation of groups of genes whose coordinated regulation is probably required to mediate the addicted state.
7. Induction of ΔFosB in other brain regions
The discussion up to now has focused solely on nucleus accumbens. While this is a key brain reward region and important for the addicting actions of cocaine and other drugs of abuse, many other brain regions are also crucial in the development and maintenance of a state of addiction. An important question, then, is whether ΔFosB acting in other brain regions beyond the nucleus accumbens may also influence drug addiction. Indeed, there is now increasing evidence that stimulant and opiate drugs of abuse induce ΔFosB in several brain regions implicated in diverse aspects of addiction (Nye et al. 1995; Perrotti et al. 2005, 2008; McDaid et al. 2006a,b; Liu et al. 2007).
A recent study has systematically compared ΔFosB induction in these various brain regions across four different drugs of abuse: cocaine; morphine; cannabinoids; and ethanol (table 4; Perrotti et al. 2008). All four drugs induce the transcription factor to varying degrees in nucleus accumbens and dorsal striatum as well as in prefrontal cortex, amygdala, hippocampus, bed nucleus of the stria terminalis and interstitial nucleus of the posterior limb of the anterior commissure. Cocaine and ethanol alone induce ΔFosB in lateral septum, all of the drugs except for cannabinoids induce ΔFosB in the periaqueductal grey, and cocaine is unique in inducing ΔFosB in gamma-aminobutyric acid (GABA) ergic cells in the posterior ventral tegmental area (Perrotti et al. 2005, 2008). In addition, morphine has been shown to induce ΔFosB in ventral pallidum (McDaid et al. 2006a). In each of these regions, it is the 35–37 kD isoforms of ΔFosB that accumulate with chronic drug exposure and persist for relatively long periods during withdrawal.
Table 4.
Comparison of brain regions that show ΔFosB induction after chronic exposure to representative drugs of abusea.
| cocaine | morphine | ethanol | cannabinoids | |
|---|---|---|---|---|
| nucleus accumbens | ||||
| core | + | + | + | + |
| shell | + | + | + | + |
| dorsal striatum | + | + | + | + |
| ventral pallidumb | n.d. | + | n.d. | n.d. |
| prefrontal cortexc | + | + | + | + |
| lateral septum | + | − | + | − |
| medial septum | − | − | − | − |
| BNST | + | + | + | + |
| IPAC | + | + | + | + |
| hippocampus | ||||
| dentate gyrus | + | + | − | + |
| CA1 | + | + | + | + |
| CA3 | + | + | + | + |
| amygdala | ||||
| basolateral | + | + | + | + |
| central | + | + | + | + |
| medial | + | + | + | + |
| periaqueductal grey | + | + | + | − |
| ventral tegmental area | + | − | − | − |
| substantia nigra | − | − | − | − |
The table does not show the relative levels of ΔFosB induction by the various drugs. See Perrotti et al. (2008) for this information.
The effect of cocaine, ethanol and cannabinoids on ΔFosB induction in ventral pallidum has not yet been studied, but such induction has been observed in response to methamphetamine (McDaid et al. 2006b).
ΔFosB induction is seen in several subregions of prefrontal cortex, including infralimbic (medial prefrontal) and orbitofrontal cortex.
A major goal for future research is to carry out studies, analogous to those described above for nucleus accumbens, to delineate the neural and behavioural phenotypes mediated by ΔFosB for each of these brain regions. This represents an enormous undertaking, yet it is crucial for understanding the global influence of ΔFosB on the addiction process.
We have recently taken a significant step in this regard by using viral-mediated gene transfer to characterize the actions of ΔFosB in a subregion of prefrontal cortex, namely, orbitofrontal cortex. This region has been strongly implicated in addiction, in particular, in contributing to the impulsivity and compulsivity that characterize an addicted state (Kalivas & Volkow 2005). Interestingly, unlike the nucleus accumbens where self-administered and yoked cocaine induce comparable levels of ΔFosB as noted earlier, we observed that cocaine self-administration causes a several-fold greater induction of ΔFosB in orbitofrontal cortex, suggesting that this response may be related to volitional aspects of drug administration (Winstanley et al. 2007). We then used rodent tests of attention and decision-making (e.g. five-choice serial reaction time and delay-discounting tests) to determine whether ΔFosB within the orbitofrontal cortex contributes to drug-induced alterations in cognition. We found that chronic cocaine treatment produces tolerance to the cognitive impairments caused by acute cocaine. Viral-mediated overexpression of ΔFosB within this region mimicked the effects of chronic cocaine, while overexpression of the dominant negative antagonist, ΔJunD, prevents this behavioural adaptation. DNA expression microarray analyses identified several potential molecular mechanisms underlying this behavioural change, including a cocaine- and ΔFosB-mediated increase in transcription of the metabotrophic glutamate receptor mGluR5 and GABAA receptor as well as substance P (Winstanley et al. 2007). The influence of these and many other putative ΔFosB targets requires further investigation.
These findings indicate that ΔFosB helps mediate tolerance to the cognitive-disrupting effects of cocaine. Users who experience tolerance to the deleterious effects of cocaine are more likely to become cocaine dependent, whereas those who find the drug more disruptive at work or school are less likely to become addicted (Shaffer & Eber 2002). Tolerance to the cognitive disruption caused by acute cocaine in cocaine-experienced individuals may therefore facilitate the maintenance of addiction. In this way, ΔFosB induction in the orbitofrontal cortex may promote an addicted state, similar to its actions in the nucleus accumbens where ΔFosB promotes addiction by enhancing the rewarding and incentive motivational effects of the drug.
8. Epigenetic mechanisms of ΔFosB action
Until recently, all studies of transcriptional regulation in brain have relied on measurements of steady-state mRNA levels. For example, the search for ΔFosB target genes has involved identifying mRNA's up- or downregulated upon ΔFosB or ΔcJun overexpression, as stated earlier. This level of analysis has been very useful in identifying putative targets for ΔFosB; however, it is inherently limited in providing insight into the underlying mechanisms involved. Rather, all studies of mechanisms have relied on in vitro measures such as ΔFosB binding to a gene's promoter sequences in gel shift assays or ΔFosB regulation of a gene's promoter activity in cell culture. This is unsatisfying because mechanisms of transcription regulation show dramatic variations from cell type to cell type, leaving it virtually completely unknown how a drug of abuse, or ΔFosB, regulates its specific genes in the brain in vivo.
Studies of epigenetic mechanisms make it possible, for the first time, to push the envelope one step further and directly examine transcriptional regulation in the brains of behaving animals (Tsankova et al. 2007). Historically, the term epigenetics describes mechanisms by which cellular traits can be inherited without a change in DNA sequence. We use the term more broadly to encompass ‘the structural adaptation of chromosomal regions so as to register, signal or perpetuate altered activity states’ (Bird 2007). Thus, we now know that the activity of genes is controlled by the covalent modification (e.g. acetylation, methylation) of histones in the genes' vicinity and the recruitment of diverse types of coactivators or corepressors of transcription. Chromatin immunoprecipitation (ChIP) assays make it possible to take advantage of this growing knowledge of chromatin biology to determine the activation state of a gene in a particular brain region of an animal treated with a drug of abuse.
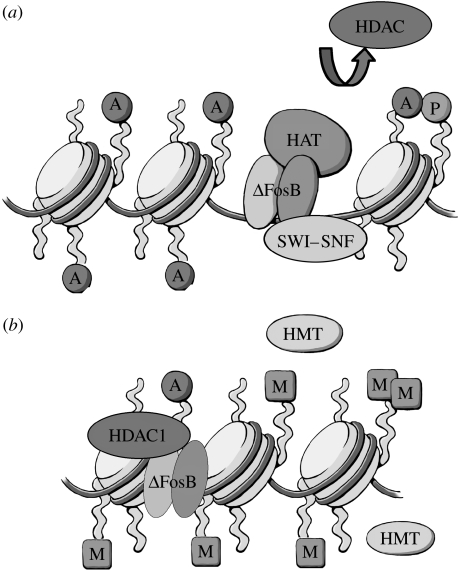
Examples of how studies of chromatin regulation can help us understand the detailed molecular mechanisms of the action of cocaine and ΔFosB are given in figure 3. As stated above, ΔFosB can function as either a transcriptional activator or repressor depending on the target gene involved. To gain insight into these actions, we analysed the chromatin state of two representative gene targets for ΔFosB, cdk5 that is induced by ΔFosB and c-fos that is repressed in nucleus accumbens. Chromatin immunoprecipitation studies demonstrated that cocaine activates the cdk5 gene in this brain region through the following cascade: ΔFosB binds to the cdk5 gene and then recruits histone acetyltransferases (HAT; which acetylate nearby histones) and SWI–SNF factors; both actions promote gene transcription (Kumar et al. 2005; Levine et al. 2005). Chronic cocaine further augments histone acetylation through the phosphorylation and inhibition of histone deacetylases (HDAC; which normally deacetylate and repress genes; Renthal et al. 2007). By contrast, cocaine represses the c-fos gene: when ΔFosB binds to this gene it recruits an HDAC and possibly histone methyltransferases (HMT; which methylate nearby histones) and thereby inhibits c-fos transcription (figure 3; Renthal et al. in press). A central question is: what determines whether ΔFosB activates or represses a gene when it binds to that gene's promoter?
Figure 3.
Epigenetic mechanisms of ΔFosB action. The figure illustrates the very different consequences when ΔFosB binds to a gene that it activates (e.g. cdk5) versus represses (e.g. c-fos). (a) At the cdk5 promoter, ΔFosB recruits HAT and SWI–SNF factors, which promote gene activation. There is also evidence for exclusion of HDACs (see text). (b) By contrast, at the c-fos promoter, ΔFosB recruits HDAC1 as well as perhaps HMTs which repress gene expression. A, P and M depict histone acetylation, phosphorylation and methylation, respectively.
These early studies of epigenetic mechanisms of drug addiction are exciting because they promise to reveal fundamentally new information concerning the molecular mechanisms by which drugs of abuse regulate gene expression in nucleus accumbens and other brain regions. Combining DNA expression arrays with so-called ChIP on chip assays (where alterations in chromatin structure or transcription factor binding can be analysed genome wide) will lead to the identification of drug and ΔFosB target genes with much greater levels of confidence and completeness. In addition, epigenetic mechanisms are particularly attractive candidates to mediate the very long-lived phenomena central to a state of addiction. In this way, drug- and ΔFosB-induced changes in histone modifications and related epigenetic alterations provide potential mechanisms by which transcriptional changes can persist long after drug exposure ceases and perhaps even after ΔFosB degrades to normal levels.
9. Conclusions
The pattern of induction of ΔFosB in nucleus accumbens by chronic exposure to natural rewards, stress or drugs of abuse raises an interesting hypothesis concerning the protein's normal functioning in this brain region. As depicted in figure 2, there is an appreciable level of ΔFosB in nucleus accumbens under normal conditions. This is unique to striatal regions, as ΔFosB is virtually undetectable elsewhere throughout brain at baseline. We hypothesize that levels of ΔFosB in nucleus accumbens represent a read-out of an individual's exposure to emotional stimuli, both positive and negative, integrated over relatively long periods of time given the temporal properties of the protein. The partial differences in the cellular specificity of ΔFosB induction by rewarding versus aversive stimuli are poorly understood, and further work is needed to elucidate the functional consequences of these distinctions. We hypothesize further that as higher levels of emotional stimulation induce more ΔFosB in nucleus accumbens neurons, the neurons' functioning is altered so that they become more sensitive to rewarding stimuli. In this way, induction of ΔFosB would promote reward-related (i.e. emotional) memory through afferent projects of the nucleus accumbens. Under normal circumstances, the induction of moderate levels of ΔFosB by rewarding or aversive stimuli would be adaptive by enhancing an animal's adjustments to environmental challenges. However, the excessive induction of ΔFosB seen under pathological conditions (e.g. chronic exposure to a drug of abuse) would lead to excessive sensitization of the nucleus accumbens circuitry and ultimately contribute to pathological behaviours (e.g. compulsive drug seeking and taking) associated with drug addiction. ΔFosB induction in other brain regions would presumably contribute to distinct aspects of an addicted state, as have been suggested by recent findings of ΔFosB action in orbitofrontal cortex.
If this hypothesis is correct, it raises the interesting possibility that levels of ΔFosB in nucleus accumbens or perhaps other brain regions could be used as a biomarker to assess the state of activation of an individual's reward circuitry, as well as the degree to which an individual is ‘addicted’, both during the development of an addiction and its gradual waning during extended withdrawal or treatment. The use of ΔFosB as a marker of a state of addiction has been demonstrated in animal models. Adolescent animals show much greater induction of ΔFosB compared with older animals, consistent with their greater vulnerability for addiction (Ehrlich et al. 2002). In addition, attenuation of the rewarding effects of nicotine with a GABAB receptor positive allosteric modulator is associated with the blockade of nicotine induction of ΔFosB in nucleus accumbens (Mombereau et al. 2007). Although highly speculative, it is conceivable that a small molecule PET ligand, with high affinity for ΔFosB, could be used to help diagnose addictive disorders as well as monitor progress during treatment.
Finally, ΔFosB itself or any of the numerous genes it regulates—identified through DNA expression arrays or ChIP on chip assays—represent potential targets for the development of fundamentally novel treatments for drug addiction. We believe that it is imperative to look beyond traditional drug targets (e.g. neurotransmitter receptors and transporters) for potential treatment agents for addiction. The genome-wide transcriptional maps capable of today's advanced technologies provide a promising source of such novel targets in our efforts to better treat and ultimately cure addictive disorders.
Acknowledgments
Disclosure. The author reports no conflicts of interest in preparing this review.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘The neurobiology of addiction: new vistas’.
References
- Alibhai I.N, Green T.A, Potashkin J.A, Nestler E.J. Regulation of fosB and ΔfosB mRNA expression: in vivo and in vitro studies. Brain Res. 2007;1143:22–33. doi: 10.1016/j.brainres.2007.01.069. doi:10.1016/j.brainres.2007.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang E, Chen J, Zagouras P, Magna H, Holland J, Schaeffer E, Nestler E.J. Induction of NFκB in nucleus accumbens by chronic cocaine administration. J. Neurochem. 2001;79:221–224. doi: 10.1046/j.1471-4159.2001.00563.x. doi:10.1046/j.1471-4159.2001.00563.x [DOI] [PubMed] [Google Scholar]
- Asanuma M, Cadet J.L. Methamphetamine-induced increase in striatal NFκB DNA-binding activity is attenuated in superoxide dismutase transgenic mice. Mol. Brain Res. 1998;60:305–309. doi: 10.1016/s0169-328x(98)00188-0. doi:10.1016/S0169-328X(98)00188-0 [DOI] [PubMed] [Google Scholar]
- Berton O, et al. Induction of ΔFosB in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. doi:10.1016/j.neuron.2007.06.033 [DOI] [PubMed] [Google Scholar]
- Bibb J.A, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. doi:10.1038/35066591 [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. doi:10.1038/nature05913 [DOI] [PubMed] [Google Scholar]
- Carle T.L, Ohnishi Y.N, Ohnishi Y.H, Alibhai I.N, Wilkinson M.B, Kumar A, Nestler E.J. Absence of conserved C-terminal degron domain contributes to ΔFosB's unique stability. Eur. J. Neurosci. 2007;25:3009–3019. doi: 10.1111/j.1460-9568.2007.05575.x. doi:10.1111/j.1460-9568.2007.05575.x [DOI] [PubMed] [Google Scholar]
- Carlezon W.A, Jr, Duman R.S, Nestler E.J. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. doi:10.1016/j.tins.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Cenci M.A. Transcription factors involved in the pathogenesis of l-DOPA-induced dyskinesia in a rat model of Parkinson's disease. Amino Acids. 2002;23:105–109. doi: 10.1007/s00726-001-0116-4. [DOI] [PubMed] [Google Scholar]
- Chen J.S, Nye H.E, Kelz M.B, Hiroi N, Nakabeppu Y, Hope B.T, Nestler E.J. Regulation of ΔFosB and FosB-like proteins by electroconvulsive seizure (ECS) and cocaine treatments. Mol. Pharmacol. 1995;48:880–889. [PubMed] [Google Scholar]
- Chen J, Kelz M.B, Hope B.T, Nakabeppu Y, Nestler E.J. Chronic FRAs: stable variants of ΔFosB induced in brain by chronic treatments. J. Neurosci. 1997;17:4933–4941. doi: 10.1523/JNEUROSCI.17-13-04933.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.S, Zhang Y.J, Kelz M.B, Steffen C, Ang E.S, Zeng L, Nestler E.J. Induction of cyclin-dependent kinase 5 in hippocampus by chronic electroconvulsive seizures: role of ΔFosB. J. Neurosci. 2000;20:8965–8971. doi: 10.1523/JNEUROSCI.20-24-08965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Newton S.S, Zeng L, Adams D.H, Dow A.L, Madsen T.M, Nestler E.J, Duman R.S. Downregulation of the CCAAT-enhancer binding protein beta in ΔFosB transgenic mice and by electroconvulsive seizures. Neuropsychopharmacology. 2003;29:23–31. doi: 10.1038/sj.npp.1300289. doi:10.1038/sj.npp.1300289 [DOI] [PubMed] [Google Scholar]
- Colby C.R, Whisler K, Steffen C, Nestler E.J, Self D.W. ΔFosB enhances incentive for cocaine. J. Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, et al. The glucocorticoid receptor as a potential target to reduce cocaine abuse. J. Neurosci. 2003;23:4785–4790. doi: 10.1523/JNEUROSCI.23-11-04785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrazanski P, Noguchi T, Kovary K, Rizzo C.A, Lazo P.S, Bravo R. Both products of the fosB gene, FosB and its short form, FosB/SF, are transcriptional activators in fibroblasts. Mol. Cell Biol. 1991;11:5470–5478. doi: 10.1128/mcb.11.11.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M.E, Sommer J, Canas E, Unterwald E.M. Periadolescent mice show enhanced ΔFosB upregulation in response to cocaine and amphetamine. J. Neurosci. 2002;22:9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A.M, Moratalla R, Robertson H.A. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc. Natl Acad. Sci. USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. doi:10.1073/pnas.87.17.6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T.A, Alibhai I.N, Hommel J.D, DiLeone R.J, Kumar A, Theobald D.E, Neve R.L, Nestler E.J. Induction of ICER expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J. Neurosci. 2006;26:8235–8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T.A, Alibhai I.N, Unterberg S, Neve R.L, Ghose S, Tamminga C.A, Nestler E.J. Induction of activating transcription factors (ATFs) ATF2, ATF3, and ATF4 in the nucleus accumbens and their regulation of emotional behavior. J. Neurosci. 2008;28:2025–2032. doi: 10.1523/JNEUROSCI.5273-07.2008. doi:10.1523/JNEUROSCI.5273-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Brown J, Haile C, Ye H, Greenberg M.E, Nestler E.J. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc. Natl Acad. Sci. USA. 1997;94:10 397–10 402. doi: 10.1073/pnas.94.19.10397. doi:10.1073/pnas.94.19.10397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, Brown J, Ye H, Saudou F, Vaidya V.A, Duman R.S, Greenberg M.E, Nestler E.J. Essential role of the fosB gene in molecular, cellular, and behavioral actions of electroconvulsive seizures. J. Neurosci. 1998;18:6952–6962. doi: 10.1523/JNEUROSCI.18-17-06952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman S.E, Nestler E.J. Regulation of IEG expression and AP-1 binding by chronic cocaine in the rat nucleus accumbens. Proc. Natl Acad. Sci. USA. 1992;89:5764–5768. doi: 10.1073/pnas.89.13.5764. doi:10.1073/pnas.89.13.5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B.T, Nye H.E, Kelz M.B, Self D.W, Iadarola M.J, Nakabeppu Y, Duman R.S, Nestler E.J. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. doi:10.1016/0896-6273(94)90061-2 [DOI] [PubMed] [Google Scholar]
- Jorissen H, Ulery P, Henry L, Gourneni S, Nestler E.J, Rudenko G. Dimerization and DNA-binding properties of the transcription factor ΔFosB. Biochemistry. 2007;46:8360–8372. doi: 10.1021/bi700494v. doi:10.1021/bi700494v [DOI] [PubMed] [Google Scholar]
- Kalivas P.W, Volkow N.D. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. doi:10.1176/appi.ajp.162.8.1403 [DOI] [PubMed] [Google Scholar]
- Kauer J.A, Malenka R.C. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. doi:10.1038/nrn2234 [DOI] [PubMed] [Google Scholar]
- Kelz M.B, et al. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. doi:10.1038/45790 [DOI] [PubMed] [Google Scholar]
- Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. doi:10.1016/j.neuron.2005.09.023 [DOI] [PubMed] [Google Scholar]
- Lee K.W, Kim Y, Kim A.M, Helmin K, Nairn A.C, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc. Natl Acad. Sci. USA. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. doi:10.1073/pnas.0511244103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Guan Z, Barco A, Xu S, Kandel E, Schwartz J. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc. Natl Acad. Sci. USA. 2005;102:19 186–19 191. doi: 10.1073/pnas.0509735102. doi:10.1073/pnas.0509735102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.F, Zhou W.H, Zhu H.Q, Lai M.J, Chen W.S. Microinjection of M(5) muscarinic receptor antisense oligonucleotide into VTA inhibits FosB expression in the NAc and the hippocampus of heroin sensitized rats. Neurosci. Bull. 2007;23:1–8. doi: 10.1007/s12264-007-0001-6. doi:10.1007/s12264-007-0001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler S.A, Korutla L, Cha X.Y, Koebbe M.J, Fournier K.M, Bowers M.S, Kalivas P.W. NAC-1 is a brain POZ/BTB protein that can prevent cocaine-induced sensitization in the rat. J. Neurosci. 2000;20:6210–6217. doi: 10.1523/JNEUROSCI.20-16-06210.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung C.A, Nestler E.J. Regulation of gene expression and cocaine reward by CREB and ΔFosB. Nat. Neurosci. 2003;11:1208–1215. doi: 10.1038/nn1143. doi:10.1038/nn1143 [DOI] [PubMed] [Google Scholar]
- McClung C.A, Ulery P.G, Perrotti L.I, Zachariou V, Berton O, Nestler E.J. ΔFosB: a molecular switch for long-term adaptation in the brain. Mol. Brain Res. 2004;132:146–154. doi: 10.1016/j.molbrainres.2004.05.014. doi:10.1016/j.molbrainres.2004.05.014 [DOI] [PubMed] [Google Scholar]
- McDaid J, Dallimore J.E, Mackie A.R, Napier T.C. Changes in accumbal and pallidal pCREB and ΔFosB in morphine-sensitized rats: correlations with receptor-evoked electrophysiological measures in the ventral pallidum. Neuropsychopharmacology. 2006a;31:1212–1226. doi: 10.1038/sj.npp.1300854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, Graham M.P, Napier T.C. Methamphetamine-induced sensitization differentially alters pCREB and ΔFosB throughout the limbic circuit of the mammalian brain. Mol. Pharmacol. 2006b;70:2064–2074. doi: 10.1124/mol.106.023051. doi:10.1124/mol.106.023051 [DOI] [PubMed] [Google Scholar]
- Mombereau C, Lhuillier L, Kaupmann K, Cryan J.F. GABAB receptor-positive modulation-induced blockade of the rewarding properties of nicotine is associated with a reduction in nucleus accumbens ΔFosB accumulation. J. Pharmacol. Exp. Therapy. 2007;321:172–177. doi: 10.1124/jpet.106.116228. doi:10.1124/jpet.106.116228 [DOI] [PubMed] [Google Scholar]
- Moratalla R, Elibol R, Vallejo M, Graybiel A.M. Network-level changes in expression of inducible Fos–Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron. 1996;17:147–156. doi: 10.1016/s0896-6273(00)80288-3. doi:10.1016/S0896-6273(00)80288-3 [DOI] [PubMed] [Google Scholar]
- Morgan J.I, Curran T. Immediate-early genes: ten years on. Trends Neurosci. 1995;18:66–67. doi:10.1016/0166-2236(95)93874-W [PubMed] [Google Scholar]
- Muller D.L, Unterwald E.M. D1 dopamine receptors modulate ΔFosB induction in rat striatum after intermittent morphine administration. J. Pharmacol. Exp. Therapy. 2005;314:148–155. doi: 10.1124/jpet.105.083410. doi:10.1124/jpet.105.083410 [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y, Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991;64:751–759. doi: 10.1016/0092-8674(91)90504-r. doi:10.1016/0092-8674(91)90504-R [DOI] [PubMed] [Google Scholar]
- Nestler E.J. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. doi:10.1038/35053570 [DOI] [PubMed] [Google Scholar]
- Nestler E.J, Barrot M, Self D.W. ΔFosB: a sustained molecular switch for addiction. Proc. Natl Acad. Sci. USA. 2001;98:11 042–11 046. doi: 10.1073/pnas.191352698. doi:10.1073/pnas.191352698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm S.D, Bibb J.A, Nestler E.J, Ouimet C.C, Taylor J.R, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. doi:10.1016/S0306-4522(02)00560-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye H.E, Nestler E.J. Induction of chronic Fras (Fos-related antigens) in rat brain by chronic morphine administration. Mol. Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- Nye H, Hope B.T, Kelz M, Iadarola M, Nestler E.J. Pharmacological studies of the regulation by cocaine of chronic Fra (Fos-related antigen) induction in the striatum and nucleus accumbens. J. Pharmacol. Exp. Therapy. 1995;275:1671–1680. [PubMed] [Google Scholar]
- O'Donovan K.J, Tourtellotte W.G, Millbrandt J, Baraban J.M. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22:167–173. doi: 10.1016/s0166-2236(98)01343-5. doi:10.1016/S0166-2236(98)01343-5 [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch J.D, Tronson N, Neve R, Nestler E.J, Taylor J.R. ΔFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J. Neurosci. 2006;26:9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. doi:10.1523/JNEUROSCI.1124-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakman M.-C, et al. Inducible, brain region specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res. 2003;970:73–86. doi: 10.1016/s0006-8993(03)02230-3. doi:10.1016/S0006-8993(03)02230-3 [DOI] [PubMed] [Google Scholar]
- Pérez-Otan¨o I, Mandelzys A, Morgan J.I. MPTP-Parkinsonism is accompanied by persistent expression of a Δ-FosB-like protein in dopaminergic pathways. Mol. Brain Res. 1998;53:41–52. doi: 10.1016/s0169-328x(97)00269-6. doi:10.1016/S0169-328X(97)00269-6 [DOI] [PubMed] [Google Scholar]
- Perrotti L.I, Hadeishi Y, Ulery P, Barrot M, Monteggia L, Duman R.S, Nestler E.J. Induction of ΔFosB in reward-related brain regions after chronic stress. J. Neurosci. 2004;24:10 594–10 602. doi: 10.1523/JNEUROSCI.2542-04.2004. doi:10.1523/JNEUROSCI.2542-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti L.I, et al. ΔFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur. J. Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. doi:10.1111/j.1460-9568.2005.04110.x [DOI] [PubMed] [Google Scholar]
- Perrotti L.I, et al. Distinct patterns of ΔFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. doi:10.1002/syn.20500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti, R., Toulemonde, F., Nestler, E. J., Roberts, A. J. & Koob, G. F. 2001 Ethanol effects in ΔFosB transgenic mice. Soc. Neurosci. Abs 745.16.
- Pich E.M, Pagliusi S.R, Tessari M, Talabot-Ayer D, hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. doi: 10.1126/science.275.5296.83. doi:10.1126/science.275.5296.83 [DOI] [PubMed] [Google Scholar]
- Renthal W, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. doi:10.1016/j.neuron.2007.09.032 [DOI] [PubMed] [Google Scholar]
- Renthal, W., Carle, T. L., Maze, I., Covington III, H. E., Truong, H.-T., Alibhai, I., Kumar, A., Olson, E. N. & Nestler, E. J. In press. ΔFosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine. J. Neurosci [DOI] [PMC free article] [PubMed]
- Robinson T.E, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:S33–S46. doi: 10.1016/j.neuropharm.2004.06.025. doi:10.1016/j.neuropharm.2004.06.025 [DOI] [PubMed] [Google Scholar]
- Russo, S. J. et al 2007 NFκB signaling regulates cocaine-induced behavioral and cellular plasticity. Soc. Neurosci. Abs, 611.5.
- Shaffer H.J, Eber G.B. Temporal progression of cocaine dependence symptoms in the US National Comorbidity Survey. Addiction. 2002;97:543–554. doi: 10.1046/j.1360-0443.2002.00114.x. doi:10.1046/j.1360-0443.2002.00114.x [DOI] [PubMed] [Google Scholar]
- Shippenberg T.S, Rea W. Sensitization to the behavioral effects of cocaine: modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol. Biochem. Behav. 1997;57:449–455. doi: 10.1016/s0091-3057(96)00450-9. doi:10.1016/S0091-3057(96)00450-9 [DOI] [PubMed] [Google Scholar]
- Taylor J.R, Lynch W.J, Sanchez H, Olausson P, Nestler E.J, Bibb J.A. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor activating and incentive motivational effects of cocaine. Proc. Natl Acad. Sci. USA. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. doi:10.1073/pnas.0610288104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden S.L, Bale T.L. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol. Psychiatry. 2007;61:1021–1029. doi: 10.1016/j.biopsych.2006.09.032. doi:10.1016/j.biopsych.2006.09.032 [DOI] [PubMed] [Google Scholar]
- Teegarden, S. L., Nestler, E. J. & Bale, T. L. In press. ΔFosB-mediated alterations in dopamine signaling are normalized by a palatable high fat diet. Biol. Psychiatry [DOI] [PMC free article] [PubMed]
- Tsankova N, Renthal W, Kumar A, Nestler E.J. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. doi:10.1038/nrn2132 [DOI] [PubMed] [Google Scholar]
- Ulery P.G, Rudenko G, Nestler E.J. Regulation of ΔFosB stability by phosphorylation. J. Neurosci. 2006;26:5131–5142. doi: 10.1523/JNEUROSCI.4970-05.2006. doi:10.1523/JNEUROSCI.4970-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou, V. F., Steiner, M. A., Krishnan, V., Berton, O. & Nestler, E. J. 2007 Role of ΔFosB in the nucleus accumbens in chronic social defeat. Soc. Neurosci. Abs, 98.3.
- Wallace, D., Rios, L., Carle-Florence, T. L., Chakravarty, S., Kumar, A., Graham, D. L., Perrotti, L. I., Bolaños, C. A. & Nestler, E. J. 2007 The influence of ΔFosB in the nucleus accumbens on natural reward behavior. Soc. Neurosci. Abs, 310.19. [DOI] [PMC free article] [PubMed]
- Werme M, Messer C, Olson L, Gilden L, Thorén P, Nestler E.J, Brené S. ΔFosB regulates wheel running. J. Neurosci. 2002;22:8133–8138. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C.A, et al. ΔFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J. Neurosci. 2007;27:10 497–10 507. doi: 10.1523/JNEUROSCI.2566-07.2007. doi:10.1523/JNEUROSCI.2566-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J, Wisdom R.M, Tratner I, Verma I.M. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc. Natl Acad. Sci. USA. 1991;88:5077–5081. doi: 10.1073/pnas.88.12.5077. doi:10.1073/pnas.88.12.5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S.T, Porrino L.J, Iadarola M.J. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc. Natl Acad. Sci. USA. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. doi:10.1073/pnas.88.4.1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, et al. An essential role for ΔFosB in the nucleus accumbens in morphine action. Nat. Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. doi:10.1038/nn1636 [DOI] [PubMed] [Google Scholar]