Abstract
Relapse, the resumption of drug taking after periods of abstinence, remains the major problem for the treatment of addiction. Even when drugs are unavailable for long periods or when users are successful in curbing their drug use for extended periods, individuals remain vulnerable to events that precipitate relapse. Behavioural studies in humans and laboratory animals show that drug-related stimuli, drugs themselves and stressors are powerful events for the precipitation of relapse. Molecular, neurochemical and anatomical studies have identified lasting neural changes that arise from mere exposure to drugs and other enduring changes that arise from learning about the relationship between drug-related stimuli and drug effects. Chronic drug exposure increases sensitivity of some systems of the brain to the effects of drugs and stressful events. These changes, combined with those underlying conditioning and learning, perpetuate vulnerability to drug-related stimuli. Circuits of the brain involved are those of the mesocorticolimbic dopaminergic system and its glutamatergic connections, and the corticotropin-releasing factor and noradrenergic systems of the limbic brain. This paper reviews advances in our understanding of how these systems mediate the effects of events that precipitate relapse and of how lasting changes in these systems can perpetuate vulnerability to relapse.
Keywords: relapse to drug seeking, drug-related stimuli, stress, dopamine (DA), glutamate, corticotropin-releasing factor
1. Introduction
In the context of drug addiction, relapse refers to the reinitiation of drug seeking and drug taking after abstinence. The central questions that are being addressed by researchers in the field of drug addiction are: what are the primary triggers for relapse; which systems of the brain mediate the effects of these triggers; and what maintains the vulnerability to these triggers in individuals even after drugs have been unavailable for long periods of time or when users are successful in curbing their own drug use for extended periods? Is it a set of physiological changes brought about by being exposed to the effects of drugs, per se? Is it drug-related memories that can be reactivated by drug-related cues and thoughts? Does it arise from something within individuals that makes them initially vulnerable to the effects of drugs of abuse and which simply remains or is exaggerated after the termination of drug taking? No doubt, factors such as these all contribute. In fact, exposure to a drug can initiate neurochemical changes with enduring molecular and anatomical consequences that affect subsequent responses to events that induce relapse; drugs that are abused activate appetitive motivational systems of the brain, inducing behaviours and emotions that very rapidly become associated with stimuli and events in the environment where they are experienced, and drug effects can be different in different individuals and differentially experienced by them.
2. Primary triggers for relapse
Studies carried out in humans and laboratory animals have demonstrated that craving (Wikler 1973; Jaffe et al. 1989; Childress et al. 1992; de Wit 1996; Leyton et al. 2002, 2005; Sinha et al. 2000; Duncan et al. 2007) and the reinitiation of drug seeking (See 2002; Shalev et al. 2002; Spealman et al. 2004; Stewart 2004; Weiss 2005) can be induced by re-exposure to cues previously associated with drug exposure, by acute exposure to stressors and by re-exposure to the drug itself. In experimental studies in humans, various means are used to present to drug users events that are suspected of triggering relapse and subjective ratings are used to assess drug craving or wanting. Such methods are now being complemented by brain-imaging techniques to assess regions of the brain that are differentially activated by these triggering events. In laboratory animals trained to self-administer drugs such as cocaine or heroin (when drug cues or responding are associated with obtaining the drug) and then subjected to a period of extinction training, or simply to the passage of time, the presentation of cues that have been explicitly paired with drug delivery, brief exposure to a stressor or an experimenter delivered injection of the drug all result in an increase in increased drug-seeking behaviour. Clearly, under non-laboratory conditions, the reinitiation of drug seeking after abstinence occurs before exposure to the drug itself, and is instigated by environmental cues, thoughts or stressors. It is well recognized, however, that re-exposure to the drug spurs on further drug seeking; thus, it is important to study in an experimental setting how the action of the drug itself, increases subsequent drug seeking.
In the reinstatement model of relapse (de Wit & Stewart 1981; Spealman et al. 2004; Stewart 2004; Epstein et al. 2006), animals are trained to self-administer a drug by pressing one of two levers, and are then exposed to a period when the drug is no longer available. During this abstinence period, animals may simply be left in their home cages (Grimm et al. 2001; Fuchs et al. 2006) or they may be free to try to obtain drug in the testing chambers (extinction training). In extinction training, the sight of the lever and stimuli previously associated with drug delivery are usually present; in the case of tests for cue-induced reinstatement, however, the cues previously associated with drug delivery are absent. When animals reduce responding to very low levels, tests for reinstatement can begin. During these tests, animals are given access to the levers, but drugs remain unavailable. It is on this background of renewed drug seeking, or reinstatement, that we are able to begin a search for pharmacological and neurochemical manipulations that can block or attenuate such behaviour. Using this procedure, the periods of self-administration training, abstinence, extinction and reinstatement can be separated by days and weeks allowing for the study of factors such as the extent and amount of initial exposure to drug taking and the effect of the passage of time since last exposure on the susceptibility to relapse.
3. How might drugs and stressors initiate reinstatement or relapse in experienced drug users
The observation that a brief exposure to stress or an abused drug reinstates drug-seeking behaviour implies a change in the motivational state of the animal that alters responses to stimuli in its environment. Traditionally, the term motivation is invoked by the observation that a particular goal-directed behaviour, such as food seeking, occurs at some times and not others, with more or less vigour and persistence. The ease with which a behaviour is engaged by environmental stimuli, its persistence and the energy expended to obtain the goal all appear to depend on internal changes that alter stimulus effectiveness and readiness to act.
We have argued, on the basis of our studies showing that a priming injection of previously self-administered drug in experienced drug users can reinstate drug seeking, that the priming injection acts to renew the significance or salience of the learned stimulus–drug associations. Such drug-related stimuli gain conditioned incentive value, drawing the animal to approach the lever and to engage in lever pressing (Stewart et al. 1984; Stewart 1992). Thus, after extinction, a priming injection of the previously self-administered drug (and presumably exposure to stress) acts to renew the salience of the drug-associated lever and the surrounding stimuli. We have used the conditioned place preference (CPP) procedure to explore this hypothesis directly.
In this procedure, a particular stimulus environment is paired with the effects of the drug, without the animal having to learn to make a response to obtain the drug, and a second environment is explicitly paired with the absence of the drug. In the test trial, the animal is allowed, while in a drug-free state, to move freely between the area previously paired with the drug and the unpaired environment. Using this procedure, we have tested the idea that a priming injection of the drug used to develop the CPP, given after an extinction training, acts to restore the salience or attractiveness of the environment previously paired with drug. It has been found that following the extinction of the CPP by repeatedly pairing both the compartments with saline or by giving repeated tests in the absence of drug, the former preference for the ‘drug-paired’ compartment can be completely reinstated by giving a single injection of the drug before the test (Mueller & Stewart 2000; Parker & McDonald 2000; Mueller et al. 2002).
Results from a study on stress-induced reinstatement lend further support to this idea (Liu & Weiss 2002). During training, ethanol-reinforced responses were accompanied by a light that served as a conditioned stimulus. After extinction, given in the absence of both ethanol and the light, lever pressing was reinstated by response-contingent presentations of the conditioned light stimulus or by prior brief exposure to intermittent footshock stress. Rats tested with the conditioned light stimulus present after a brief period of footshock showed greatly enhanced responding compared with those tested with either the light stimuli or footshock alone, suggesting that the stress state induced by footshock enhanced the salience of the drug-related stimuli augmenting drug-seeking behaviour.
Another more direct approach to this question was taken in an experiment in which rats were given pairings of a compound stimulus with passive intravenous infusions of cocaine (0, 0.5 or 1.0 mg kg−1 infusion−1). After training, rats were allowed to lever press for the conditioned stimulus under extinction conditions, and the amount of pressing was shown to be dependent on the training dose. After extinction of lever pressing, a single priming injection of cocaine (20 mg kg−1 intraperitoneally) or exposure to footshock stress reinstated lever pressing for the conditioned stimulus, in a training dose-dependent manner, even though the rats had never been trained to administer cocaine (Goddard & Leri 2006). Again, these data support the view that both a priming drug injection and exposure to stress induce reinstatement by restoring the incentive salience or value of the drug-related cues that previously activated appetitive behaviour. In cocaine-dependent men, it was shown using fMRI that the activation by cocaine cues of brain regions associated with reward processing and attention was enhanced in the presence of stress (Duncan et al. 2007).
4. Primary neural pathways mediating relapse
Studies carried out in a number of laboratories have provided evidence that the brain systems mediating the effects of conditioned stimuli, priming injections of drugs and stress on the reinitiation of drug seeking are to some degree dissociable (Shaham et al. 2000a; Stewart 2000; McFarland & Kalivas 2001), although common pathways are beginning to be identified (McFarland & Kalivas 2001; See 2002; Saal et al. 2003; McFarland et al. 2004; Stewart 2004; Wang et al. 2005; Weiss 2005; Rodaros et al. 2007). In the following sections, I briefly review evidence concerning the role of different brain systems, regions and transmitters involved in cue-, drug- and stress-induced reinstatement.
5. Drug-induced reinstatement
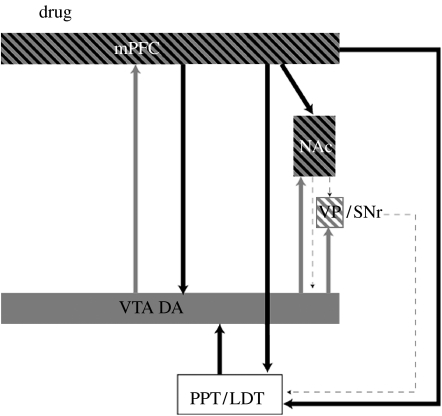
The reinstatement of drug craving or seeking by priming injections of the abused or training drug, drugs of a similar class or drugs that activate pathways in common with the training drug is a robust phenomenon in both humans and laboratory animals. As expected, specific receptor antagonists for drugs opioids and nicotine or, in the case of cocaine and amphetamines, dopamine (DA) receptor antagonists, will block reinstatement induced by priming injections. Furthermore, and inasmuch as most, if not all, addictive drugs activate the mesocorticolimbic pathways of the brain, it is not surprising that, in general, DA receptor agonists induce the reinstatement of drug seeking in experienced users, whereas antagonists attenuate or block drug-induced reinstatement. A sketch of the circuits identified and the primary neurotransmitters implicated in drug-induced reinstatement is shown in figure 1. These include DAergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), and glutamatergic inputs to VTA from mPFC, peduncular pontine and laterodorsal tegmental nuclei and from the mPFC to the NAc. There is evidence that DA receptor antagonists attenuate drug-induced reinstatement more effectively when given into the shell region of the NAc (Anderson et al. 2003, 2006; Schmidt et al. 2006), although there is some evidence that they are effective in the core as well (Bachtell et al. 2005). Evidence that DA in the shell is effective in inducing reinstatement is consistent with previous work showing the importance of the shell in stimulant drug-induced enhancement of responding in the presence of conditioned stimuli (Parkinson et al. 1999). Interestingly, however, reversible inactivation of either core or shell blocks drug-induced reinstatement (McFarland & Kalivas 2001), suggesting, as discussed in §6, that increases in DA in the shell facilitate the effectiveness of cues acting through the core.
Figure 1.
Diagram showing the primary circuits and neurotransmitters implicated in drug-induced reinstatement. VTA, ventral tegmental area, cell body regions of mesocorticolimbic DA pathway; NAc, nucleus accumbens; mPFC, medial prefrontal cortex; VP/SNr, ventral pallidum/substantia nigra reticulata; PPT/LDT, peduncular pontine and laterodorsal tegmental nuclei. Grey, dopamine; black, glutamate.
A great number of studies have shown the importance of glutamatergic projections in this circuitry for reinstatement. Though studies have found effects of glutamate agonists and antagonists in tests for drug-induced reinstatement (Cornish & Kalivas 2000), it is not clear to what extent these effects are important to the mediation of the drug effects, per se, or to the mediation of the effects of drug-related cues in drug-induced reinstatement (see §6).
6. Cue- and context-induced reinstatement
Contexts or environments where drugs are used can serve as conditioned stimuli (cues), eliciting expectations, thoughts, neural and neurochemical responses, emotional and motivational responses, and behavioural responses such as approach. Discrete stimuli such as odours, sounds, etc. can have similar effects. Although these stimuli are paired with the effects of drugs when they are self-administered, their effectiveness can best be studied using classical conditioning procedures where stimuli are explicitly paired with drug injections. Discrete stimuli paired with either passive infusions (classical conditioning) or response-contingent presentation of a drug (instrumental learning) can come to serve as conditioned reinforcers, maintaining responses such as lever pressing in the absence of drugs. Finally, cues that predict the availability/non-availability of drugs (discriminative stimuli) can differentially control the occurrence of drug-seeking/taking behaviours.
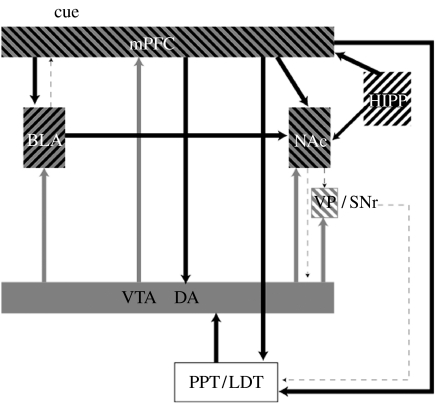
The neural systems involved in mediating cue-induced reinstatement have been studied using systemic injections of receptor antagonists, intracranial infusions of receptor agonists and antagonists and reversible or non-reversible lesions of specific regions. A sketch of the circuits identified and the primary neurotransmitters implicated in cue-induced reinstatement is shown in figure 2. The principal regions associated with cue- and context-induced reinstatement are the basolateral amygdala (BLA), the hippocampus, the mPFC, the NAc core, the DAergic inputs to BLA, mPFC and NAc from the VTA, glutamatergic inputs to the VTA and glutamatergic inputs from the BLA and PFC to the NAc (Ito et al. 2000; Fuchs & See 2002; See 2002; Bossert et al. 2004, 2006; Fuchs et al. 2007; Weiss 2005). In a recent study, an attempt was made using reversible inactivation to assess the role of the NAc core and shell and dorsal striatum in a number of behaviours controlled by a conditioned reinforcer (Di Ciano et al. 2008). Cue-induced reinstatement of responding was dependent on the NAc core, as were all other conditioned stimulus-controlled behaviours studied (see also Di Ciano & Everitt 2001, 2004), again suggesting a special role for the circuits involving the NAc core in cue-induced reinstatement and relapse.
Figure 2.
Diagram showing the primary circuits and neurotransmitters implicated in cue-induced reinstatement. VTA, ventral tegmental area, cell body regions of mesocorticolimbic DA pathway; NAc, nucleus accumbens; mPFC, medial prefrontal cortex; VP/SNr, ventral pallidum/substantia nigra reticulata; PPT/LDT, peduncular pontine and laterodorsal tegmental nuclei; BLA, basolateral amygdala; HIPP, hippocampus. Grey, dopamine; black, glutamate.
Recently, Kalivas and colleagues have argued for a key role for glutamate projections from the PFC to the core of the NAc in the precipitation of relapse to drug seeking in general (Kalivas & McFarland 2003; Kalivas 2004), serving as the final common pathway for all events that induce relapse. Glutamatergic agonists given into the NAc induce reinstatement (Cornish & Kalivas 2000), whereas antagonists (Backstrom & Hyytia 2007) and mGLU 2/3 receptor agonists, which reduce glutamate release, given systemically or into the NAc (Bossert et al. 2006) block cue-induced reinstatement. mGLU 2/3 receptor agonists given into the VTA block both cue-induced reinstatement of heroin seeking (Bossert et al. 2004) and cue- and nicotine-induced reinstatement of nicotine (Liechti et al. 2007), suggesting that the glutamatergic activation of DAergic neurons may play an important role in both cue and drug seeking especially when the drugs have their effects by activating DAergic neurons. In a recent study, it was shown that the initiation of self-administration in cocaine-trained rats was accompanied by a sharp transient release of glutamate in the VTA and that this was a conditioned response associated with drug-related cues and that it disappeared after extinction training (You et al. 2007), suggesting that drug-related cues normally activate glutamate release in the VTA where they would serve to activate the VTA DAergic system. Thus, cue-induced reinstatement probably involves the activation of glutamatergic receptors in both the NAc and the VTA.
7. Stress-induced reinstatement
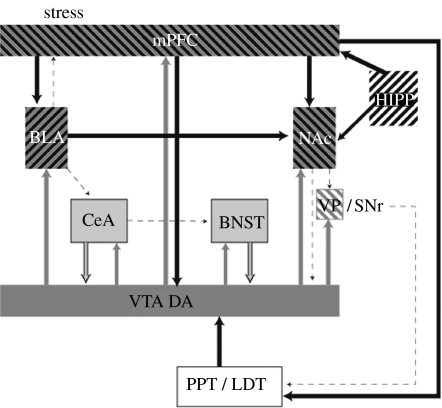
In our initial work on stress-induced reinstatement, rats trained to self-administer heroin intravenously were given 7–10 days of extinction training and were then exposed to 10 min of intermittent footshock (acute stress). Footshock reinstated heroin seeking as it did again four to six weeks later (Shaham & Stewart 1995). Similar effects of footshock were seen in cocaine-trained rats (Erb et al. 1996) and in rats trained to self-administer nicotine (Buczek et al. 1999) and ethanol (Lê et al. 1998), but interestingly not in rats trained to lever press for food (Ahmed & Koob 1997) or sucrose solutions (Buczek et al. 1999). These findings show that exposure to stress reinstates drug seeking in animals experienced in the self-administration of drugs of abuse from several different pharmacological classes. In a search for the hormonal and neurochemical systems involved in stress-induced relapse, we found that stress-induced corticosterone release was not responsible for the effect in either cocaine- or heroin-trained rats (Shaham et al. 1997; Erb et al. 1998). This finding led us to explore the role of corticotropin-releasing factor (CRF) systems of the brain. We found that infusions of CRF given intracerebroventricularly (i.c.v.) or into the bed nucleus of the stria terminalis (BNST) induce reinstatement in the absence of an external stressor, whereas infusions into the CRF-containing regions of the amygdala, central nucleus of the amygdala (CeA), have no effect. Infusions of CRF receptor antagonists block footshock-induced reinstatement when given i.c.v. (Shaham et al. 1997; Erb et al. 1998) or into the ventrolateral BNST, but have no effect in the amygdala (Erb & Stewart 1999). Similar effects for central CRF systems have been found for rats trained to self-administer alcohol (Lê et al. 2000; Liu & Weiss 2002; figure 3).
Figure 3.
Diagram showing the primary circuits and neurotransmitters implicated in stress-induced reinstatement. VTA, ventral tegmental area, cell body regions of mesocorticolimbic DA pathway; NAc, nucleus accumbens; mPFC, medial prefrontal cortex; VP/SNr, ventral pallidum/substantia nigra reticulata; PPT/LDT, peduncular pontine and laterodorsal tegmental nuclei; BLA, basolateral amygdala; HIPP, hippocampus; CeA, central nucleus of the amygdala; BNST, bed nucleus of the stria terminalis. Dark grey, dopamine; black, glutamate; light grey, CRF.
In studies of the role of central noradrenergic systems in stress-induced relapse, we and others found that systemic injections of agents that reduce cell firing and the release of noradrenaline in the brain, such as the α2-adrenoceptor agonists, clonidine and lofexidine, block stress-induced reinstatement in cocaine- (Erb et al. 2000), heroin- (Shaham et al. 2000b) and alcohol-trained rats (Lê et al. 2005). Interestingly, in both rats and monkeys, the α2-antagonist, yohimbine, induces reinstatement, acting like a stressor (Lee et al. 2004; Shepard et al. 2004; Gass & Olive 2007). In additional experiments, we determined that noradrenergic neurons arising from the lateral tegmental nuclei and projecting to the CeA and BNST were of primary importance in stress-induced reinstatement (Shaham et al. 2000b). These findings, combined with those showing the importance of extra-hypothalamic CRF activity, led us to study the role of noradrenergic activity in the BNST and CeA regions in stress-induced reinstatement. We found a dose-dependent reduction of stress-induced reinstatement after infusions of β-receptor antagonists into the BNST, and a complete blockade after infusions into the CeA at all doses tested without an effect on cocaine-induced reinstatement at either site (Leri et al. 2002).
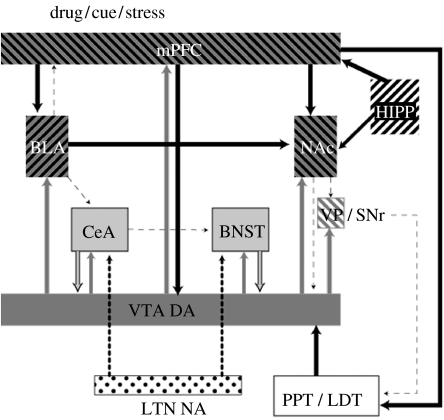
These data suggest that the mediation of the effects of footshock on reinstatement of drug seeking is via the release of noradrenaline in the amygdala and the BNST. Through the effects at β-noradrenergic receptors, noradrenaline may activate CRF-containing cells in both the regions. Some of these CRF neurons appear to project from the CeA to the BNST and others are intrinsic to the BNST itself. Interference in this circuit has no effect on cocaine-induced relapse, suggesting that the brain systems mediating stress-induced relapse could be dissociated from those mediating drug-induced relapse. Furthermore, we had found that stress-induced reinstatement of heroin seeking was relatively unaffected by systemic injections of DA D1 or D2 receptor antagonists, and that only sustained treatment with a mixed antagonist was effective (Shaham & Stewart 1996). However, the role of DA in stress-induced relapse was shown in subsequent studies to include the mPFC, where infusions of a D1 receptor antagonist, SCH23390, into the prelimbic (PL) region block footshock stress-induced, but not cocaine-induced, reinstatement (Capriles et al. 2003). mPFC infusions of the D1/D2 antagonist fluphenazine block footshock stress-induced reinstatement and, interestingly, the inactivation of PL by tetrodotoxin infusions blocked both footshock (McFarland et al. 2004) and cocaine-induced reinstatement (McFarland & Kalivas 2001). These findings, combined with those showing that inactivation of the shell or core blocks stress-induced reinstatement (McFarland et al. 2004), establish a role for the DAergic system in stress-induced reinstatement. In addition, these findings, taken together with those discussed above for cue-induced reinstatement, confirm the idea that the PL region of the mPFC serves as a common pathway for cue-, drug- and stress-induced reinstatement of drug seeking. Sketches of the circuits and neurotransmitters implicated in stress-induced reinstatement are shown in figures 3 and 4.
Figure 4.
Diagram showing the primary circuits and neurotransmitters implicated in reinstatement by drugs, cues and stressors. VTA, ventral tegmental area, cell body regions of mesocorticolimbic DA pathway; NAc, nucleus accumbens; mPFC, medial prefrontal cortex; VP/SNr, ventral pallidum/substantia nigra reticulata; PPT/LDT, peduncular pontine and laterodorsal tegmental nuclei; BLA, basolateral amygdala; HIPP, hippocampus; CeA, central nucleus of the amygdala; BNST, bed nucleus of the stria terminalis; LTN, lateral tegmental nuclei; NA, noradrenaline. Dark grey, dopamine; black, glutamate; light grey, CRF; black dots, noradrenaline.
In an earlier section, we saw how drugs and stressors might have their effects on relapse by renewing the effectiveness of drug-related cues in the instigation of appetitive, drug-seeking, behaviours. Another issue is how the activation of the CRF systems, found to be critical for stress-induced relapse, gain access to those systems that mediate appetitive behaviours such as drug seeking.
CRF systems are known to be activated in response to stressors and to mediate a wide variety of physiological and behavioural responses to stress including fear and anxiety (Schulkin et al. 2005; Davis 2006); in addition, CRF has been shown to facilitate locomotor activity (Kalivas et al. 1987; Cador et al. 1993) and responses to positive incentive stimuli (Pecina et al. 2006), responses involved in appetitive behaviour. Little is known about the pathways through which the activation of CRF systems facilitates appetitive behaviour. In a recent study, it was found, however, that CRF is released directly into the VTA during footshock stress and that, in cocaine-experienced rats, intra-VTA infusions of a CRF receptor antagonist block stress-induced reinstatement (Wang et al. 2005). These findings point to an interaction between the CRF-containing cell groups and the DAergic neurons in the VTA, providing a possible pathway for stress activation of CRF to modulate appetitive behaviour. Interestingly, prior exposure to stress facilitates glutamatergic synaptic transmission in DAergic neurons in the VTA, in a manner similar to prior exposure to drugs of abuse (Saal et al. 2003). Furthermore, CRF applied directly to a VTA slice preparation has a similar effect (Ungless et al. 2003). Little is known, however, about the sources of CRF-containing fibres in the VTA. An understanding of the sources of the CRF innervation of the VTA would help explain the role of stress and CRF in the modulation of appetitive behaviours. We recently found using a fluorescent retrograde tracer and fluorescence immunocytochemistry for CRF that the VTA region receives CRF projections from the oval nucleus of the BNST, the CeA and the paraventricular nucleus of the hypothalamus (Rodaros et al. 2007), pointing to a means whereby stressor activation of CRF systems of the brain could facilitate the DAergic activity in the VTA and, thus, appetitive behaviour. A final summary sketch showing all these circuits and primary neurotransmitters implicated in drug-, cue- and stress-induced reinstatement is shown in figure 4.
8. Sources of plasticity within pathways mediating relapse
The major sources of plasticity within pathways mediating relapse derive from exposure to the pharmacological effects of the drugs themselves, and from conditioning and learning associated with drugs.
The idea that long-term changes within specific circuitry might alter the motivational effects of drugs has received considerable attention within the field of drug abuse (e.g. Piazza et al. 1990; Robinson & Berridge 2000; Nestler et al. 2001). The circuitry found to undergo lasting changes as a result of repeated exposure to stimulant drugs is the mesocorticolimbic DAergic system and its targets in striatum, amygdala and mPFC. Stimulant and opioid drugs induce increases in extracellular DA in all of these regions, as well as in the BNST (see Di Chiara et al. 1999).
Repeated exposure to stimulant drugs, such as amphetamine and cocaine, results in the enhancement of their behavioural activating effects. This phenomenon, known as behavioural sensitization, develops over time, is observed months after the termination of drug treatment (Paulson et al. 1991; Castner & Goldman-Rakic 1999) and is accompanied by an increased responsiveness of the mesolimbic DAergic system (see Robinson & Becker 1986; Kalivas & Stewart 1991). This enhancement develops gradually, is long-lasting, and appears to result from a series of changes within the DAergic system and its targets that occur over time after the termination of drug treatment. Importantly, it has been found that these changes in behaviour and DAergic function can be mimicked by the direct application of amphetamine in the VTA (see Vezina 2004), demonstrating that processes initiated in the cell body region of DAergic neurons are responsible for sensitized functioning within the system. The relevance of such drug-induced sensitization within the mesolimbic DAergic system to the motivational effects of drugs and drug-related stimuli has recently been reviewed by Vezina (2007).
The long-lasting changes induced by stimulant drugs suggest structural modifications in neuronal circuitry and, in fact, studies have shown selective and persistent changes in transcription factors known to be involved in neuroplasticity (Chen et al. 1995; Nestler et al. 1999, 2001), drug-induced changes in synaptic facilitation and long-term potentiation of DA neurons in the VTA (Bonci & Williams 1996; Ungless et al. 2001; Borgland et al. 2004; Liu et al. 2005), long-term depression and potentiation in the NAc (Thomas et al. 2001; Kourrich et al. 2007), and structural changes in the VTA, NAc and mPFC neurons following repeated exposure to these drugs (Robinson & Kolb 1997; Hu et al. 2002; Mueller et al. 2006; Sarti et al. 2007). The fact that many of the important long-lasting changes are enhanced by the passage of time after the termination of drug exposure and involve structural changes in neurons suggests that neurotrophic factors are involved. We found, for example, that amphetamine induces increases in the neurotrophic factor, basic fibroblast growth factor (bFGF or FGF-2), in astrocytes in the VTA, which are seen early after the termination of drug treatment and last for at least one month (Flores et al. 1998). As is the case for the development of behavioural sensitization to amphetamine (see Wolf 1998), the induction of bFGF by amphetamine is prevented by the co-administration of glutamate antagonists, and the inactivation of bFGF in the VTA prevents the development of behavioural sensitization to amphetamine (Flores et al. 2000). We proposed that repeated exposure to stimulant drugs increases the demands on DAergic cell functioning, and by stimulating glutamate release recruits neurotrophic and neuroprotective substances such as bFGF (Flores & Stewart 2000). More recent studies have pointed to a major role for brain-derived neurotrophic factor (BDNF) in the long-lasting changes induced by drugs of abuse (Grimm et al. 2003; Lu et al. 2004; Liu et al. 2006; Berglind et al. 2007; Graham et al. 2007). Earlier studies suggested that BDNF can induce long-lasting changes in the sensitivity of the mesolimbic DAergic pathway to motivationally significant stimuli (Shen et al. 1994; Horger et al. 1999). Support for this view comes from a study showing that the potentiation of excitatory synapses at DAergic neurons in the VTA after withdrawal from cocaine is dependent on BDNF TrkB receptor signalling (Pu et al. 2006). A time-dependent enhancement of cue-induced reinstatement in rats has been found days and months after the termination of cocaine self-administration (Grimm et al. 2001). This phenomenon, referred to as ‘an incubation effect’, is accompanied by the increased expression of BDNF in the VTA, NAc and amygdala over many days (Grimm et al. 2003). Recently, it was found that if BDNF was infused into the NAc of rats immediately following daily cocaine self-administration sessions, reinstatement of cocaine seeking was greatly enhanced after presentations of drug-associated cues, priming injections of cocaine or footshock stress (Graham et al. 2007).
Time-dependent effects have been found for stress-induced reinstatement of both heroin (Shalev et al. 2001) and cocaine seeking (Sorge & Stewart 2005). It is likely that a number of systems are involved in these changes over time, including DAergic, CRF (Orozco-Cabal et al. 2008) and noradrenergic systems (Leri et al. 2002; Macey et al. 2003; Dumont & Williams 2004). Whether BDNF plays a role in these effects is not known.
9. Drug-induced plasticity in glutamatergic function
As discussed previously, there is evidence that BDNF plays a role in the facilitation of NMDA receptor-mediated glutamatergic transmission at DAergic neurons in the VTA after the termination of cocaine exposure (Pu et al. 2006), an effect observed at 10–15 days, but not at 1 day, after cocaine exposure. Furthermore, increases in NMDAR1 subunits have been reported in the VTA for up to 90 days after the termination of cocaine (Lu et al. 2003). The blockade of glutamate receptors in the VTA decreases cocaine-induced reinstatement of cocaine seeking (Sun et al. 2005a), and intra-VTA infusions of a group II metabotropic glutamate receptor agonist, thought to reduce glutamate release, block cue-induced reinstatement in heroin-trained rats (Bossert et al. 2004). In a study discussed previously, it was shown that after cocaine self-administration, CRF released in the VTA during exposure to stress causes glutamate release (an effect not seen in cocaine-naive rats) and that stress-induced reinstatement could be blocked by a glutamate receptor antagonist (Wang et al. 2005). This study provides another example of long-lasting facilitation of glutamatergic activity in the VTA after cocaine exposure, in this case via changes in the effectiveness of CRF. It is likely that similar changes, perhaps mediated by other peptides, will be found within those brain regions already identified as playing critical roles in the reinstatement of drug seeking induced by various triggers (e.g. Dumont et al. 2005, 2008).
Changes in glutamatergic functioning have already been found to play a critical role in the reinstatement of drug seeking. Marked increases in glutamate release in the NAc core in response to drugs or stress have been found after extinction in rats trained to self-administer cocaine or heroin. Simple exposure to drugs does not seem to be sufficient to induce this effect, again suggesting that learning about drug-associated cues may be critical (McFarland et al. 2003, 2004). These researchers have argued that this enhanced release of glutamate arises from the activation of mPFC inputs to the NAc, and there is other evidence for changes in glutamate receptors in the NAc after cocaine exposure that would make cells more sensitive to glutamatergic input (Boudreau & Wolf 2005; Sun et al. 2005b; Gao et al. 2006). Interestingly, reported increases in cell surface glutamatergic AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors are not seen immediately after the discontinuation of cocaine (they were in fact decreased), but are seen after 14 days (Boudreau et al. 2007): temporal changes that parallel changes in sensitivity to glutamatergic input to NAc neurons (Kourrich et al. 2007).
It has been proposed that drug-induced changes in cystine–glutamate exchange in the NAc, which would induce changes in glutamatergic tone in the NAc, may underlie these lasting changes in glutamatergic function (Baker et al. 2002, 2003). It is considered that the reduced tone affects presynaptic mGlu receptors causing dysregulation of glutamatergic function (Moran et al. 2005). The restoration of cystine–glutamate exchange blocks cocaine-induced reinstatement of cocaine seeking (Baker et al. 2003; Madayag et al. 2007) and cue- and heroin-induced reinstatement of heroin seeking (Zhou & Kalivas 2008). Interestingly, chronic treatment with the partial opioid agonist, buprenorphine, a drug used in the treatment of heroin and cocaine abuse, blocks drug-induced reinstatement of both heroin and cocaine seeking and reduces responding to drug-paired cues, and we have found that chronic infusions of this drug increase basal levels of both DA and glutamate in the NAc, suggesting that it may have its ‘therapeutic’ effect by stabilizing dysregulated transmitter function following the termination of drug taking (Sorge et al. 2005; Sorge & Stewart 2006; Placenza et al. in press). In addition, as mentioned above, a group II mGluR agonist given into the NAc blocks cue-induced reinstatement of heroin seeking and, given systemically, cue- and stress-induced ethanol seeking (Zhao et al. 2006). Together, these data lend strong support to the idea that long-lasting dysregulation of glutamatergic function involving the mPFC and the NAc contributes importantly to sensitivity to triggers for, and thus vulnerability to, relapse.
10. Summary
Experience with drug self-administration promotes long-lasting changes in systems of the brain mediating the effects of events that trigger relapse to drug seeking. These lasting changes are induced, in part, by mere exposure to the pharmacological effects of these drugs and, in part, through conditioning and learning. Circuits of the brain involved in relapse are those of the mesocorticolimbic DAergic system and its glutamatergic inputs, and the CRF and noradrenergic systems of the limbic brain. Exposure to drugs changes sensitivity to subsequent exposure to drugs and to the effects of stressors. Many neurochemical and molecular changes have been found to underlie drug-induced plasticity. These changes develop with repeated exposure, invade more brain regions over time (Porrino et al. 2004) and have progressive consequences on behaviour (Everitt & Robbins 2005; Kalivas & O'Brien 2008). Environmental stimuli that acquire conditioned incentive properties through pairings with the effects of drugs maintain their capacity to instigate drug seeking in spite of long-term abstinence. After extinction training, when the capacity of these conditioned stimuli to induce relapse is diminished or absent, exposure to a stressor or the drug itself is able to reinstate the effectiveness of cues and drug-seeking behaviours. Although a number of manipulations have been found to reduce reinstatement by cues, drugs and stressors, few are sufficiently broad in their effects to serve as effective treatments.
Acknowledgments
Experimental procedures comply with the guidelines of the Canadian Council on Animal Care.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and Le Fonds de la recherche en santé du Québec (FRSQ).
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘The neurobiology of addiction: new vistas’.
References
- Ahmed S.H, Koob G.F. Cocaine- but not food- seeking behavior is reinstated by stress after extinction. Psychopharmacology. 1997;132:289–295. doi: 10.1007/s002130050347. doi:10.1007/s002130050347 [DOI] [PubMed] [Google Scholar]
- Anderson S.M, Bari A.A, Pierce R.C. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology (Berl.) 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. doi:10.1007/s00213-002-1298-5 [DOI] [PubMed] [Google Scholar]
- Anderson S.M, Schmidt H.D, Pierce R.C. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. doi:10.1038/sj.npp.1300922 [DOI] [PubMed] [Google Scholar]
- Bachtell R.K, Whisler K, Karanian D, Self D.W. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl.) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. doi:10.1007/s00213-005-0133-1 [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl.) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. doi:10.1007/s00213-007-0753-8 [DOI] [PubMed] [Google Scholar]
- Baker D.A, Shen H, Kalivas P.W. Cystine/glutamate exchange serves as the source for extracellular glutamate: modifications by repeated cocaine administration. Amino Acids. 2002;23:161–162. doi: 10.1007/s00726-001-0122-6. doi:10.1007/s00726-001-0122-6 [DOI] [PubMed] [Google Scholar]
- Baker D.A, McFarland K, Lake R.W, Shen H, Tang X.C, Toda S, Kalivas P.W. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. doi:10.1038/nn1069 [DOI] [PubMed] [Google Scholar]
- Berglind W.J, See R.E, Fuchs R.A, Ghee S.M, Whitfield T.W, Jr, Miller S.W, McGinty J.F. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur. J. Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. doi:10.1111/j.1460-9568.2007.05692.x [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams J.T. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–639. doi: 10.1016/s0896-6273(00)80082-3. doi:10.1016/S0896-6273(00)80082-3 [DOI] [PubMed] [Google Scholar]
- Borgland S.L, Malenka R.C, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J. Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. doi:10.1523/JNEUROSCI.1312-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert J.M, Liu S.Y, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J. Neurosci. 2004;24:10 726–10 730. doi: 10.1523/JNEUROSCI.3207-04.2004. doi:10.1523/JNEUROSCI.3207-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert J.M, Gray S.M, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. doi:10.1038/sj.npp.1300977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau A.C, Wolf M.E. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. doi:10.1523/JNEUROSCI.2252-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau A.C, Reimers J.M, Milovanovic M, Wolf M.E. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J. Neurosci. 2007;27:10 621–10 635. doi: 10.1523/JNEUROSCI.2163-07.2007. doi:10.1523/JNEUROSCI.2163-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczek Y, Le A.D, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology. 1999;144:183–188. doi: 10.1007/s002130050992. doi:10.1007/s002130050992 [DOI] [PubMed] [Google Scholar]
- Cador M, Cole B.J, Koob G.F, Stinus L, Le Moal M. Central administration of corticotropin releasing factor induces long-term sensitization to d-amphetamine. Brain Res. 1993;606:181–186. doi: 10.1016/0006-8993(93)90982-s. doi:10.1016/0006-8993(93)90982-S [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge R.E, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl.) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. doi:10.1007/s00213-002-1283-z [DOI] [PubMed] [Google Scholar]
- Castner S.A, Goldman-Rakic P.S. Long-lasting psychotomimetic consequences of repeated low-dose amphetamine exposure in rhesus monkeys. Neuropsychopharmacology. 1999;20:10–28. doi: 10.1016/S0893-133X(98)00050-5. doi:10.1016/S0893-133X(98)00050-5 [DOI] [PubMed] [Google Scholar]
- Chen J, Nye H.E, Kelz M.B, Hiroi N, Nakabeppu Y, Hope B.T, Nestler E.J. Regulation of delta FosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments. Mol. Pharmacol. 1995;48:880–889. [PubMed] [Google Scholar]
- Childress A.R, Ehrman R, Rohsenow D.J, Robbins S.J, O'Brien C.P. Classically conditioned factors in drug dependence. In: Lowinson J.W, Luiz P, Millman R.B, Langard G, editors. Substance abuse: a comprehensive textbook. Williams and Wilkins; Baltimore, MD: 1992. pp. 56–69. [Google Scholar]
- Cornish J.L, Kalivas P.W. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J. Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am. Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. doi:10.1037/0003-066X.61.8.741 [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp. Clin. Psychopharmacol. 1996;4:5–10. doi:10.1037/1064-1297.4.1.5 [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. doi:10.1007/BF00432175 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C, Carboni E. Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Ann. NY Acad. Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. doi:10.1111/j.1749-6632.1999.tb09283.x [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt B.J. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. doi:10.1016/S0893-133X(01)00235-4 [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt B.J. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J. Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. doi:10.1523/JNEUROSCI.1581-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Robbins T.W, Everitt B.J. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. doi:10.1038/sj.npp.1301522 [DOI] [PubMed] [Google Scholar]
- Dumont E.C, Williams J.T. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J. Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. doi:10.1523/JNEUROSCI.0425-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont E.C, Mark G.P, Mader S, Williams J.T. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat. Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. doi:10.1038/nn1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont E.C, Rycroft B.K, Maiz J, Williams J.T. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. doi:10.1016/j.neuroscience.2008.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, Hu X, Drexler K, Kilts C. An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am. J. Addict. 2007;16:174–182. doi: 10.1080/10550490701375285. doi:10.1080/10550490701375285 [DOI] [PubMed] [Google Scholar]
- Epstein D.H, Preston K.L, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl.) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. doi:10.1007/s00213-006-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. See also pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. doi:10.1007/s002130050150 [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J. Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott P.K, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. doi:10.1016/S0893-133X(99)00158-X [DOI] [PubMed] [Google Scholar]
- Everitt B.J, Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. doi:10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- Flores C, Stewart J. Basic fibroblast growth factor as a mediator of the effects of glutamate in the development of long-lasting sensitization to stimulant drugs: studies in the rat. Psychopharmacology. 2000;151:152–165. doi: 10.1007/s002130000417. doi:10.1007/s002130000417 [DOI] [PubMed] [Google Scholar]
- Flores C, Rodaros D, Stewart J. Long-lasting induction of astrocytic basic fibroblast growth factor by repeated injections of amphetamine: blockade by concurrent treatment with a glutamate antagonist. J. Neurosci. 1998;18:9547–9555. doi: 10.1523/JNEUROSCI.18-22-09547.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Samaha A.N, Stewart J. Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine. J. Neurosci. 2000;20:RC55. doi: 10.1523/JNEUROSCI.20-02-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R.A, See R.E. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl.) 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. doi:10.1007/s00213-001-0997-7 [DOI] [PubMed] [Google Scholar]
- Fuchs R.A, Branham R.K, See R.E. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J. Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. doi:10.1523/JNEUROSCI.5146-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R.A, Eaddy J.L, Su Z.I, Bell G.H. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur. J. Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. doi:10.1111/j.1460-9568.2007.05674.x [DOI] [PubMed] [Google Scholar]
- Gao C, Sun X, Wolf M.E. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J. Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. doi:10.1111/j.1471-4159.2006.03999.x [DOI] [PubMed] [Google Scholar]
- Gass J.T, Olive M.F. Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcohol. Clin. Exp. Res. 2007;31:1441–1445. doi: 10.1111/j.1530-0277.2007.00480.x. doi:10.1111/j.1530-0277.2007.00480.x [DOI] [PubMed] [Google Scholar]
- Goddard B, Leri F. Reinstatement of conditioned reinforcing properties of cocaine-conditioned stimuli. Pharmacol. Biochem. Behav. 2006;83:540–546. doi: 10.1016/j.pbb.2006.03.015. doi:10.1016/j.pbb.2006.03.015 [DOI] [PubMed] [Google Scholar]
- Graham D.L, Edwards S, Bachtell R.K, DiLeone R.J, Rios M, Self D.W. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat. Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. doi:10.1038/nn1929 [DOI] [PubMed] [Google Scholar]
- Grimm J.W, Hope B, Wise R.A, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. doi:10.1038/35084134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm J.W, Lu L, Hayashi T, Hope B.T, Su T.P, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger B.A, Iyasere C.A, Berhow M.T, Messer C.J, Nestler E.J, Taylor J.R. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J. Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.T, Koeltzow T.E, Cooper D.C, Robertson G.S, White F.J, Vezina P. Repeated ventral tegmental area amphetamine administration alters dopamine D1 receptor signaling in the nucleus accumbens. Synapse. 2002;45:159–170. doi: 10.1002/syn.10095. doi:10.1002/syn.10095 [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley J.W, Howes S.R, Robbins T.W, Everitt B.J. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J. Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe J.H, Cascell N.G, Kumor K.M, Sherer M.A. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. doi:10.1007/BF00443414 [DOI] [PubMed] [Google Scholar]
- Kalivas P.W. Glutamate systems in cocaine addiction. Curr. Opin. Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. doi:10.1016/j.coph.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Kalivas P.W, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl.) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. doi:10.1007/s00213-003-1393-2 [DOI] [PubMed] [Google Scholar]
- Kalivas P.W, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. doi:10.1038/sj.npp.1301564 [DOI] [PubMed] [Google Scholar]
- Kalivas P.W, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. doi:10.1016/0165-0173(91)90007-U [DOI] [PubMed] [Google Scholar]
- Kalivas P.W, Duffy P, Latimer G. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J. Pharmacol. Exp. Ther. 1987;242:757–763. [PubMed] [Google Scholar]
- Kourrich S, Rothwell P.E, Klug J.R, Thomas M.J. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J. Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. doi:10.1523/JNEUROSCI.1859-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê A.D, Quan B, Juzytsch W, Fletcher P.J, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. doi:10.1007/s002130050498 [DOI] [PubMed] [Google Scholar]
- Lê A.D, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. doi:10.1007/s002130000411 [DOI] [PubMed] [Google Scholar]
- Lê A.D, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl.) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. doi:10.1007/s00213-004-2036-y [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt D.M, Spealman R.D. Pharmacological blockade of α2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. doi:10.1038/sj.npp.1300391 [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced, but not cocaine-induced reinstatement, by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking. A PET/[11C]Raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. doi:10.1016/S0893-133X(02)00366-4 [DOI] [PubMed] [Google Scholar]
- Leyton M, Casey K.F, Delaney J.S, Kolivakis T, Benkelfat C. Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav. Neurosci. 2005;119:1619–1627. doi: 10.1037/0735-7044.119.6.1619. doi:10.1037/0735-7044.119.6.1619 [DOI] [PubMed] [Google Scholar]
- Liechti M.E, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J. Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. doi:10.1523/JNEUROSCI.1766-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J. Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.S, Pu L, Poo M.M. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. doi:10.1038/nature04050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.R, Lu L, Zhu X.G, Gong J.P, Shaham Y, Uhl G.R. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. doi:10.1016/j.brainres.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm J.W, Shaham Y, Hope B.T. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J. Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. doi:10.1046/j.1471-4159.2003.01824.x [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu S.Y, Bossert J.M, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J. Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. doi:10.1523/JNEUROSCI.5124-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey D.J, Smith H.R, Nader M.A, Porrino L.J. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. J. Neurosci. 2003;23:12–16. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau K.S, Mantsch J.R, Abdulhameed O, Hearing M, Grier M.D, Baker D.A. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J. Neurosci. 2007;27:13 968–13 976. doi: 10.1523/JNEUROSCI.2808-07.2007. doi:10.1523/JNEUROSCI.2808-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas P.W. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish C.C, Kalivas P.W. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge S.B, Lapish C.C, Kalivas P.W. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. doi:10.1523/JNEUROSCI.4177-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M.M, McFarland K, Melendez R.I, Kalivas P.W, Seamans J.K. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J. Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. doi:10.1523/JNEUROSCI.1007-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav. Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. doi:10.1016/S0166-4328(00)00239-4 [DOI] [PubMed] [Google Scholar]
- Mueller D, Perdikaris D, Stewart J. Persistence and drug-induced reinstatement of a morphine-induced conditioned place preference. Behav. Brain Res. 2002;136:389–397. doi: 10.1016/s0166-4328(02)00297-8. doi:10.1016/S0166-4328(02)00297-8 [DOI] [PubMed] [Google Scholar]
- Mueller D, Chapman C.A, Stewart J. Amphetamine induces dendritic growth in ventral tegmental area dopaminergic neurons in vivo via basic fibroblast growth factor. Neuroscience. 2006;137:727–735. doi: 10.1016/j.neuroscience.2005.09.038. doi:10.1016/j.neuroscience.2005.09.038 [DOI] [PubMed] [Google Scholar]
- Nestler E.J, Kelz M.B, Chen J. DeltaFosB: a molecular mediator of long-term neural and behavioral plasticity. Brain Res. 1999;835:10–17. doi: 10.1016/s0006-8993(98)01191-3. doi:10.1016/S0006-8993(98)01191-3 [DOI] [PubMed] [Google Scholar]
- Nestler E.J, Barrot M, Self D.W. DeltaFosB: a sustained molecular switch for addiction. Proc. Natl Acad. Sci. USA. 2001;98:11 042–11 046. doi: 10.1073/pnas.191352698. doi:10.1073/pnas.191352698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cabal L, Liu J, Pollandt S, Schmidt K, Shinnick-Gallagher P, Gallagher J.P. Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala-to-medial prefrontal cortex synaptic transmission: functional switch after chronic cocaine administration. J. Neurosci. 2008;28:529–542. doi: 10.1523/JNEUROSCI.2666-07.2008. doi:10.1523/JNEUROSCI.2666-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L.A, McDonald R.V. Reinstatement of both a conditioned place preference and a conditioned place aversion with drug primes. Pharmacol. Biochem. Behav. 2000;66:559–561. doi: 10.1016/s0091-3057(00)00222-7. doi:10.1016/S0091-3057(00)00222-7 [DOI] [PubMed] [Google Scholar]
- Parkinson J.A, Olmstead M.C, Burns L.H, Robbins T.W, Everitt B.J. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by d-amphetamine. J. Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson P.E, Camp D.M, Robinson T.E. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. doi:10.1007/BF02244248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Schulkin J, Berridge K.C. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. doi:10.1186/1741-7007-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza P.V, Deminere J.M, Le Moal M, Simon H. Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. doi:10.1016/0006-8993(90)90431-A [DOI] [PubMed] [Google Scholar]
- Placenza, F. M., Rajabi, H. & Stewart, J. In press. Effects of chronic buprenorphine treatment on levels of nucleus accumbens glutamate and on the expression of cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl.). [DOI] [PubMed]
- Porrino L.J, Daunais J.B, Smith H.R, Nader M.A. The expanding effects of cocaine: studies in a nonhuman primate model of cocaine self-administration. Neurosci. Biobehav. Rev. 2004;27:813–820. doi: 10.1016/j.neubiorev.2003.11.013. doi:10.1016/j.neubiorev.2003.11.013 [DOI] [PubMed] [Google Scholar]
- Pu L, Liu Q.S, Poo M.M. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat. Neurosci. 2006;9:605–607. doi: 10.1038/nn1687. doi:10.1038/nn1687 [DOI] [PubMed] [Google Scholar]
- Robinson T.E, Becker J.B. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. Rev. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. doi:10.1016/0165-0173(86)90002-0 [DOI] [PubMed] [Google Scholar]
- Robinson T.E, Berridge K.C. The psychology and neurobiology of addiction: an incentive- sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. doi:10.1080/09652140050111681 [DOI] [PubMed] [Google Scholar]
- Robinson T.E, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana D.A, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. doi:10.1016/j.neuroscience.2007.09.043 [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka R.C. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. doi:10.1016/S0896-6273(03)00021-7 [DOI] [PubMed] [Google Scholar]
- Sarti F, Borgland S.L, Kharazia V.N, Bonci A. Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. Eur. J. Neurosci. 2007;26:749–756. doi: 10.1111/j.1460-9568.2007.05689.x. doi:10.1111/j.1460-9568.2007.05689.x [DOI] [PubMed] [Google Scholar]
- Schmidt H.D, Anderson S.M, Pierce R.C. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur. J. Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. doi:10.1111/j.1460-9568.2005.04524.x [DOI] [PubMed] [Google Scholar]
- Schulkin J, Morgan M.A, Rosen J.B. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005;28:629–635. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- See R.E. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol. Biochem. Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. doi:10.1016/S0091-3057(01)00682-7 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl.) 1995;119:334–341. doi: 10.1007/BF02246300. doi:10.1007/BF02246300 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology (Berl.) 1996;125:385–391. doi: 10.1007/BF02246022. doi:10.1007/BF02246022 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown T.J, Walker C.-D, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J. Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Rev. 2000a;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. doi:10.1016/S0165-0173(00)00024-2 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur. J. Neurosci. 2000b;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. doi:10.1046/j.1460-9568.2000.00899.x [DOI] [PubMed] [Google Scholar]
- Shalev U, Hope B, Clements A, Morales M, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. doi:10.1007/s002130100748 [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm J.W, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol. Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. doi:10.1124/pr.54.1.1 [DOI] [PubMed] [Google Scholar]
- Shen R.-Y, Altar C.A, Chiodo L.A. Brain-derived neurotrophic factor increases the electrical activity of pars compacta dopamine neurons in vivo. Proc. Natl Acad. Sci. USA. 1994;91:8920–8924. doi: 10.1073/pnas.91.19.8920. doi:10.1073/pnas.91.19.8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard J.D, Bossert J.M, Liu S.Y, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. doi:10.1016/j.biopsych.2004.02.032 [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin L.R, O'Malley S.S. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl.) 2000;152:140–148. doi: 10.1007/s002130000499. doi:10.1007/s002130000499 [DOI] [PubMed] [Google Scholar]
- Sorge R.E, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology (Berl.) 2005;183:210–217. doi: 10.1007/s00213-005-0160-y. doi:10.1007/s00213-005-0160-y [DOI] [PubMed] [Google Scholar]
- Sorge R.E, Stewart J. The effects of chronic buprenorphine on intake of heroin and cocaine in rats and its effects on nucleus accumbens dopamine levels during self-administration. Psychopharmacology (Berl.) 2006;188:28–41. doi: 10.1007/s00213-006-0485-1. doi:10.1007/s00213-006-0485-1 [DOI] [PubMed] [Google Scholar]
- Sorge R.E, Rajabi H, Stewart J. Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology. 2005;30:1681–1692. doi: 10.1038/sj.npp.1300712. doi:10.1038/sj.npp.1300712 [DOI] [PubMed] [Google Scholar]
- Spealman R.D, Lee B, Tiefenbacher S, Platt D.M, Rowlett J.K, Khroyan T.V. Triggers of relapse: nonhuman primate models of reinstated cocaine seeking. Nebr. Symp. Motiv. 2004;50:57–84. [PubMed] [Google Scholar]
- Stewart, J. 1992 Neurobiology of conditioning to drugs of abuse. In The neurobiology of drug and alcohol addiction. Annals of the New York Academy of Sciences, vol. 654 (eds P. W. Kalivas & H. H. Samson), pp. 335–346. New York, NY: New York Academy of Sciences. [DOI] [PubMed]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J. Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: factors controlling the reinitiation of drug seeking after abstinence. Nebr. Symp. Motiv. 2004;50:197–234. [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol. Rev. 1984;91:251–268. doi:10.1037/0033-295X.91.2.251 [PubMed] [Google Scholar]
- Sun W, Akins C.K, Mattingly A.E, Rebec G.V. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology. 2005a;30:2073–2081. doi: 10.1038/sj.npp.1300744. doi:10.1038/sj.npp.1300744 [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf M.E. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J. Neurosci. 2005b;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. doi:10.1523/JNEUROSCI.4603-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.J, Beurrier C, Bonci A, Malenka R.C. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. doi:10.1038/nn757 [DOI] [PubMed] [Google Scholar]
- Ungless M.A, Singh V, Crowder T.L, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. doi:10.1016/S0896-6273(03)00461-6 [DOI] [PubMed] [Google Scholar]
- Ungless M.A, Whistler J.L, Malenka R.C, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. doi:10.1038/35079077 [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. doi:10.1016/j.neubiorev.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Vezina, P. (ed.) 2007 Sensitization, drug addiction and psychophathology in animals and humans. Prog. Neuro-Psychopharmacol. Biol. Psychiatry31, 1553–1555. (doi:10.1016/j.pnpbp.2007.08.030) [DOI] [PMC free article] [PubMed]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise R.A, You Z.B. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J. Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. doi:10.1523/JNEUROSCI.0955-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr. Opin. Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. doi:10.1016/j.coph.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence, implication of a conditioning theory for research and treatment. Arch. Gen. Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wolf M.E. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog. Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. doi:10.1016/S0301-0082(97)00090-7 [DOI] [PubMed] [Google Scholar]
- You Z.B, Wang B, Zitzman D, Azari S, Wise R.A. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J. Neurosci. 2007;27:10 546–10 555. doi: 10.1523/JNEUROSCI.2967-07.2007. doi:10.1523/JNEUROSCI.2967-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dayas C.V, Aujla H, Baptista M.A, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J. Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. doi:10.1523/JNEUROSCI.2384-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas P.W. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol. Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. doi:10.1016/j.biopsych.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]