Abstract
In humans, exposure to environmental contexts previously associated with drug intake often provokes relapse to drug use, but the mechanisms mediating this relapse are unknown. Based on early studies by Bouton & Bolles on context-induced ‘renewal’ of learned behaviours, we developed a procedure to study context-induced relapse to drug seeking. In this procedure, rats are first trained to self-administer drug in one context. Next, drug-reinforced lever responding is extinguished in a different (non-drug) context. Subsequently, context-induced reinstatement of drug seeking is assessed by re-exposing rats to the drug-associated context. Using variations of this procedure, we and others reported reliable context-induced reinstatement in rats with a history of heroin, cocaine, heroin–cocaine combination, alcohol and nicotine self-administration. Here, we first discuss potential psychological mechanisms of context-induced reinstatement, including excitatory and inhibitory Pavlovian conditioning, and occasion setting. We then summarize results from pharmacological and neuroanatomical studies on the role of several neurotransmitter systems (dopamine, glutamate, serotonin and opioids) and brain areas (ventral tegmental area, accumbens shell, dorsal striatum, basolateral amygdala, prefrontal cortex, dorsal hippocampus and lateral hypothalamus) in context-induced reinstatement. We conclude by discussing the clinical implications of rat studies on context-induced reinstatement of drug seeking.
Keywords: conditioned cues, drug self-administration, extinction, reinstatement, renewal, relapse
1. Introduction
The seminal studies of Wikler (1973) and subsequent investigations (O'Brien et al. 1992) indicate that environmental contexts previously associated with drug intake can provoke relapse to drug use in humans. Despite this evidence, the role of contextual cues in preclinical models of relapse to drug use has until recently been largely ignored (Shalev et al. 2002). This issue is important for understanding drug relapse, because contexts strongly influence extinction and resumption of learned behaviours (Bouton 2002).
We adapted an ABA renewal procedure (Bouton & Bolles 1979) to study the role of the drug environmental context in reinstatement of drug seeking (Crombag & Shaham 2002). In this procedure, rats are first trained to self-administer drugs in one context (A); each drug infusion is paired with an explicit discrete drug cue (a light cue or a compound tone–light cue). Next, drug-reinforced lever responding in the presence of the discrete drug cue is extinguished in a different (non-drug) context (B), which is distinct from the drug-associated context in its tactile, visual, auditory and olfactory or circadian (time of day) features. Subsequently, context-induced reinstatement of drug seeking is assessed by re-exposing rats to the drug-associated (A) context. During the reinstatement tests under extinction conditions, responding on the previously active lever leads to contingent presentations of the discrete drug cue but not the drug. Using variations of this procedure, we and others found context-induced reinstatement of heroin (Bossert et al. 2004, 2006, 2007), cocaine (Crombag et al. 2002a; Fuchs et al. 2005–2007; Kearns & Weiss 2007; Fletcher et al. 2008; Hamlin et al. 2008), speedball (Crombag & Shaham 2002), alcohol (Burattini et al. 2006; Zironi et al. 2006; Hamlin et al. 2007; Marinelli et al. 2007; Chaudhri et al. 2008) and nicotine (Diergaarde et al. in press) seeking (figure 1). In these studies, the magnitude of lever (or nose-poke) responding during tests for context-induced reinstatement after extinction was similar to that observed on the first extinction session. Thus, an attractive feature of our ‘adapted’ renewal procedure is its reliability across different training conditions, context manipulations, test conditions and drug classes, making it suitable for studying context-induced reinstatement of drug seeking.
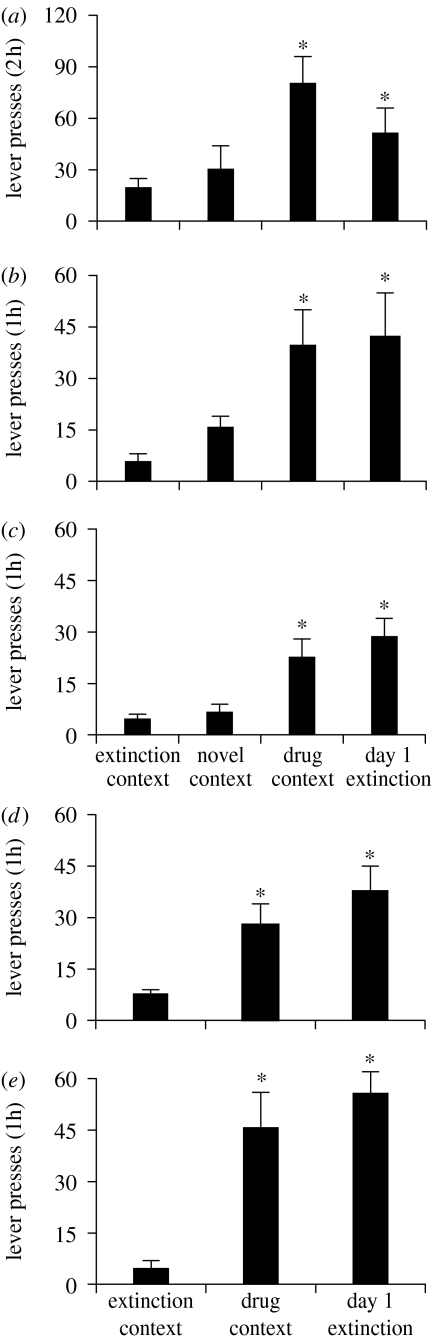
Figure 1.
Context-induced reinstatement of drug seeking. Data are mean±s.e.m. number of non-reinforced lever or nose-poke responses on the previously active manipulandum (previously paired with drug delivery) during tests for context-induced reinstatement of drug seeking; non-reinforced responses on the previously active manipulandum serve as the operational measure of drug seeking during testing. The rats were previously trained to self-administer (a) speedball (a heroin–cocaine combination), (b) heroin, (c) nicotine, (d) cocaine or (e) alcohol. The rats were trained in one context (drug context). Next, lever or nose-poke responding in the presence of a discrete cue was extinguished in a second non-drug context (extinction context). The rats were then tested either in the drug context or in the extinction context. For comparison purposes, the mean±s.e.m. number of non-reinforced responding during the first extinction session is also depicted. Data were adapted from Crombag & Shaham (2002), Bossert et al. (2004), Marinelli et al. (2007), Diergaarde et al. (in press) and Hamlin et al. (2008) for (a–e), respectively. *Different from the extinction context, p<0.05.
Here we summarize recent results (2002–2008) by us and others on psychological, pharmacological and neuroanatomical mechanisms of context-induced reinstatement of drug seeking in rats. We also briefly discuss clinical implications. Table 1 provides a glossary of terms, which appear in italics in the text.
Table 1.
Glossary of terminology.
| Active lever. Responses on this lever lead to drug infusions during drug self-administration training. During extinction training and tests for reinstatement, responses on this lever are not reinforced by the drug. In studies using the reinstatement procedure, non-reinforced responding on the active lever during testing serves as the operational measure of reinstatement of drug-taking behaviour (often refers to as reinstatement of drug seeking; Shaham et al. 2003). |
| Conditioned reinforcer. A previously neutral stimulus (tone and light), which has acquired reinforcing effects through its prior association with a primary or unconditioned reinforcer (food, drug; Mackintosh 1974). |
| Context. Refers to a configuration of diffuse cues providing the background setting of learning. Investigations on context effects in learning indicate that many stimuli can function as contexts, including external cues such as smells and physical environments, interceptive drug states, mood or hormonal states and time of day (Bouton 1993). |
| Discrete drug cue. A neutral stimulus (e.g. light, tone, sound of infusion pump) that during self-administration training becomes a conditioned reinforcer following repeated temporal pairing with drug infusions and effects (Goldberg 1976). In studies on discrete-cue-induced reinstatement, rats are trained to self-administer a drug; each reward delivery is temporally paired with a discrete cue (e.g. tone and light). Lever pressing is then extinguished in the absence of the discrete cue. During reinstatement testing, exposure to the discrete cue, which is earned contingently by responding on the drug-associated lever, reinstates drug seeking (See 2002). |
| Discriminative drug cue. An environmental stimulus that after discrimination training signals whether instrumental performance is reinforced. During training this stimulus, termed the S+ (or SD), is presented just before the drug becomes available or throughout the period of self-administration; a different stimulus, termed the S− (or SΔ), is presented when the drug is not available on alternate days or sessions. Investigators have used discrimination procedures to study the role of discriminative drug cues on reinstatement of drug seeking (McFarland & Ettenberg 1997; Ciccocioppo et al. 2001). |
| Extinction. The decrease in the frequency or intensity of learned responses after the removal of the unconditioned stimulus (e.g. food, drug) that has reinforced the learning (Catania 1992). |
| Excitatory conditioning. A form of Pavlovian conditioning during which pairing of a neutral stimulus (a conditioned stimulus, CS) with reinforcement results in the CS eliciting a conditioned response that often resembles the unconditioned response (Pavlov 1927). |
| Inhibitory conditioning. A Pavlovian CS becomes inhibitory when the probability that the unconditioned stimulus (US) will occur in the presence of the CS is lesser than the probability that the US will occur in the absence of the CS (Rescorla 1969). |
| Occasion setter. In Pavlovian conditioning, occasion setter cues signal whether another conditioned cue (CS) is to be reinforced or not reinforced. In contrast to traditional excitatory of inhibitory Pavlovian CSs, occasion setter cues typically do not affect behaviour directly but modulate behaviour elicited by other Pavlovian CSs (Holland 1992). |
| Pavlovian-instrumental transfer (PIT). Refers to the ability of a Pavlovian conditioned stimulus (CS) to influence instrumental (operant) responding (e.g. lever pressing) for reinforcement. In the PIT procedure, a rat learns to lever press for a reinforcer; the rat also learns in different sessions a Pavlovian association between the CS and the reinforcer. Subsequently, lever pressing is assessed in extinction tests in the presence or absence of the Pavlovian CS; the CS is presented non-contingently during testing. Altered responding in the presence of the CS is referred to as Pavlovian–instrumental transfer and is thought to reflect the general motivating effect of the Pavlovian cue (Lovibond 1983). |
| Reinstatement. In the learning literature, reinstatement refers to the recovery of a learned response (e.g. lever-pressing behaviour) that occurs when a subject is exposed non-contingently to the unconditioned stimulus (e.g. food) after extinction. In studies of reinstatement of drug seeking, reinstatement typically refers to the resumption of drug seeking after extinction following exposure to drugs (de Wit & Stewart 1981; Self et al. 1996; Spealman et al. 1999), different types of drug cues (Meil & See 1996; Weiss et al. 2000; Crombag & Shaham 2002) or different stressors (Shaham & Stewart 1995; Shalev et al. 2001; Shepard et al. 2004). |
| Relapse. A term used to describe the resumption of drug-taking behaviour during periods of self-imposed or forced abstinence in humans. |
| Renewal. Refers to the recovery of extinguished conditioned behaviour, which can occur when the context is changed after extinction; renewal often occurs when the subject returns to the learning (training) environment after extinction of the conditioned response in a different environment (Bouton & Swartzentruber 1991). |
2. Psychological mechanisms of context-induced reinstatement
In discussing psychological mechanisms of context-induced reinstatement, it is important to highlight a procedural difference in the published studies which has implications for the underlying mechanisms. Based on Bouton & Bolles' (1979) work, our original intention was to test the ability of the drug-associated context to renew the rat's conditioned response to discrete injection-paired cues after extinction (Crombag & Shaham 2002). Thus, drug infusions during self-administration training were explicitly paired with a discrete light cue, and during extinction training and reinstatement tests, this cue is presented contingent on lever responding. By contrast, in Fuchs et al. studies, explicit drug-paired cues were not presented during acquisition, extinction and reinstatement tests (Fuchs et al. 2005). This procedural difference is important, because the presence or absence of discrete drug-paired cues during training, extinction and reinstatement can determine whether contexts directly induce drug seeking by acquiring Pavlovian conditioned stimulus (CS) properties, or indirectly by modulating the effects of discrete infusion cues on drug seeking by serving as occasion setters (Rescorla et al. 1985; Holland 1992). Although these two mechanisms are not mutually exclusive (contexts may serve as both traditional Pavlovian CSs and occasion setters), they probably involve different neurobiological substrates (Holland & Bouton 1999).
(a) Excitatory Pavlovian conditioning
One account of context-induced reinstatement is that contexts cause reinstatement by functioning as traditional excitatory Pavlovian CSs. According to this view, because contexts reliably signal drug availability during training, they acquire excitatory conditioned stimulus (CS+) properties, and as extinction occurs in a different context, drug-associated contexts retain their motivational properties and reinstate drug seeking. In agreement with this notion, Fuchs et al. (2005) reported that contexts reinstate cocaine seeking in the absence of any explicit discrete cocaine-paired cues.
An excitatory Pavlovian conditioning mechanism potentially involved in context-induced reinstatement is Pavlovian-to-instrumental transfer (PIT). PIT is inferred from the observations that discrete Pavlovian CSs, previously paired with reward in a non-operant setting, increase non-reinforced instrumental responding for the same reward (Mackintosh 1974; Lovibond 1983). Results from PIT studies are often interpreted to suggest that appetitive Pavlovian CSs become endowed with incentive motivational properties that directly potentiate instrumental goal-directed behaviours (Berridge 2004). There is evidence that psychostimulants enhance PIT in rats trained to lever press for sucrose (Wyvell & Berridge 2000). However, the degree to which PIT contributes to drug relapse remains unknown, because non-contingent presentations of drug-paired discrete cues fail to reinstate cocaine seeking (Grimm et al. 2000; Kruzich et al. 2001). These studies, however, were not designed to assess PIT and, even in non-drug settings, PIT is often difficult to reproduce because this phenomenon is critically dependent on subtle experimental parameters (Lovibond 1981; Crombag et al. 2008). Thus, the degree to which PIT contributes to context-induced reinstatement is a subject for future research.
(b) Inhibitory Pavlovian conditioning
Context-induced reinstatement may also occur because contextual stimuli acquire conditioned inhibitory properties (Rescorla et al. 1985). As context (B) is explicitly paired with non-reinforcement during extinction in the ABA renewal condition, contextual cues may acquire inhibitory CS properties, which actively inhibit drug seeking. On test day, rats are removed from the inhibitory control of context B, resulting in reinstatement of drug seeking in the drug (A) context. Consistent with this view, exposure to a third (novel) context in an ABC condition often reinstates instrumental performance (Gunther et al. 1998). These findings may suggest that the critical manipulation to renew drug seeking is removal from the inhibitory influence of the extinction context, rather than re-exposure to the excitatory influence of the drug context. However, renewal of conditioned responses in the ABC condition is often much weaker than that observed in the ABA condition (Bouton 2002), and ABC renewal has yet to be demonstrated in a drug setting. Thus, in our view it seems unlikely that inhibitory Pavlovian conditioning processes alone can fully account for context-induced reinstatement of drug seeking.
(c) Contexts as occasion setters
Contexts may reinstate drug seeking indirectly by functioning as occasion setters (Holland 1992). According to this view, contexts function as retrieval cues in cases where the meaning of the discrete drug-infusion cues (the CSs) is ambiguous, because these cues have been paired with drug taking in one context and no drug (extinction) in a different context (CS→drug AND CS→no drug). Because the occurrence of drug versus no drug is reliably signalled by contextual cues, responding to the discrete cues is determined by whether the background context retrieves the conditioning (training) or the extinction experience (Bouton 2002).
The notion that contexts serve as retrieval cues to ‘disambiguate’ the meaning and impact of other discrete cues is in line with the phenomenon of occasion setting that was extensively studied by Holland and colleagues while exploring the nature of feature-positive and feature-negative discrimination learning (for review, see Holland 1992). In feature-positive discrimination procedures, one cue (a target CS) is sometimes followed by a reinforcing unconditioned stimulus (UCS) and sometimes not, depending on the presence or absence of another cue called feature cue. Thus, if feature cue A is present, the target CS is reinforced, but if feature cue A is absent, the target CS is not reinforced. In feature-negative discriminations, these contingencies are reversed such that the target CS is reinforced in the absence of feature cue A, while the presence of the feature cue signals non-reinforcement.
Holland and colleagues (1992) reported that feature-positive and feature-negative discrimination procedures often result in the feature cue acquiring occasion setter properties, leading to powerful modulating effects on behaviours induced by the target CS, in the absence of detectable direct conditioned excitatory or inhibitory effects by the feature cue itself. Based on these studies, they concluded that stimuli are more likely to acquire ‘occasion setting’ properties when the stimulus elements are presented sequentially (Feature cue followed by the CS) rather than simultaneously, and that these occasion setting effects depend on the modulation of direct discrete CS–UCS associations (Holland 1992). Importantly, several authors argued that contextual cues, owing to their temporal nature and associative relationship with other stimuli (i.e. preceding and predicting when discrete stimuli are followed by reward), often act in ways similar to that of feature-positive or feature-negative occasion setters (Holland 1992; Bouton 1993; Pearce & Bouton 2001), and evidence for this notion was provided by Swartzentruber (1991).
Finally, Kearns & Weiss (2007) assessed whether cocaine-associated contexts reinstate (renew) drug seeking by serving as discriminative drug cues. They reported that exposure to the cocaine context selectively reinstates the ability of a discriminative tone cue to control nose-poke responding after extinction of the tone's discriminated response in a non-drug context. These data are consistent with the notion that occasion setting properties of contexts contribute to context-induced reinstatement by modulating the conditioned responses to other drug cues.
(d) Conclusions
We outlined several potential mechanisms by which contexts could affect relapse to drug seeking. At present, little empirical evidence exists to determine which of these different learning mechanisms are more likely to contribute to context-induced reinstatement of drug seeking. Additionally, the mechanisms described above (direct excitatory and inhibitory Pavlovian conditioning, and occasion setting) are not mutually exclusive and thus may operate simultaneously to mediate context-induced reinstatement. Studies on the different psychological mechanisms of context-induced reinstatement are important, because they can have implications for relapse prevention in humans. For example, one of the cardinal features of occasion setting cues, which distinguishes them from traditional Pavlovian CSs, is that extended exposure to these cues alone (extinction) has little effect on their ability to affect responding to discrete CSs (Holland 1992). Thus, if contexts modulate drug seeking by serving as occasion setters, extinction-based therapeutic approaches (cue-exposure therapies) may be of little consequence in preventing context-induced relapse.
3. Pharmacology and neuroanatomy of context-induced reinstatement
Results from pharmacological and neuroanatomical studies on context-induced reinstatement of drug seeking are summarized in tables 2–4. In these studies, the effects of pharmacological agents on context-induced reinstatement were observed at doses that had minimal effects on extinction responding in non-drug or novel contexts, inactive lever or nose-poke hole responding or operant responding for non-drug rewards.
Table 2.
Effect of systemic injections of pharmacological agents on context-induced reinstatement of drug seeking.
| cocaine | heroin | alcohol | nicotine | |
|---|---|---|---|---|
| dopamine D1 receptor antagonist: SCH 23390 | Crombag et al. (2002a) | Bossert et al. (2007) | Hamlin et al. (2007) | |
| dopamine D2 receptor antagonist: raclopride | Crombag et al. (2002a) | |||
| glutamate mGluR2/3 agonist: LY379268 | Bossert et al. (2004) | |||
| preferential mu-opiate receptor antagonist: naltrexone | Burattini et al. (2006) and Marinelli et al. (2007) | |||
| Serotonin 5H-T2C receptor agonist: Ro 60-0175 | Fletcher et al. (2008) | |||
| CB1 receptor antagonist: SR141716A | Diergaarde et al. (in press) |
Table 3.
Effect of intracranial injections of pharmacological agents on context-induced reinstatement of drug seeking. (BLA, basolateral amygdala; CPu, caudate putamen; DH, dorsal hippocampus; PFC, prefrontal cortex; VTA, ventral tegmental area.)
| cocaine | heroin | anatomical specificity | |
|---|---|---|---|
| VTA: LY379268 | Bossert et al. (2004) | no effect in substantia nigra | |
| accumbens shell: LY379268, SCH 23390 | Bossert et al. (2006, 2007) | no effect in accumbens core (SCH 23390) and CPu (LY379268) | |
| DH: tetrodotoxin | Fuchs et al. (2005) | no effect in trunk of somatosensory cortex | |
| BLA: tetrodotoxin | Fuchs et al. (2005) | no effect in barrel field of somatosensory cortex | |
| dorsal medial PFC: tetrodotoxin | Fuchs et al. (2005) | no effect in ventral medial PFC | |
| dorsolateral CPu: muscimol+baclofen | Fuchs et al. (2006) | no effect in somatosensory cortex | |
| BLA–DH disconnection: muscimol +baclofen | Fuchs et al. (2007) | note: contralateral but not ipsilateral injections were effective | |
| BLA-dorsal medial PFC disconnection: muscimol+baclofen | Fuchs et al. (2007) | note: both contralateral and ipsilateral injections were effective |
Table 4.
Summary of Fos immediate early gene neuroanatomical mapping studies on context-induced reinstatement of drug seeking. (Under ‘cocaine’ or ‘alcohol’, we list brain sites where there are significant differences in Fos expression between groups of rats exposed to the training context (ABA) versus the distinct (ABB) or recent (AAA) extinction context. Under ‘anatomical specificity’, we provide a partial list of brain sites where group differences in Fos expression were not observed. BLA, basolateral amygdala; BNST, bed nucleus of stria terminalis; CPu, caudate putamen; PFC, prefrontal cortex; VTA, ventral tegmental area.)
| cocaine | alcohol | anatomical specificity | |
|---|---|---|---|
| Marinelli et al. (2007); c-fos mRNA | BLA | accumbens core and shell | |
| lateral amygdala | CPu | ||
| hippocampus CA3 area | septum | ||
| (non-significant trend in CA1 and CA2) | BNSTVTAcentral amygdala | ||
| Hamlin et al. (2007); Fos protein | BLA | accumbens core and rostral pole | |
| lateral hypothalamus | accumbens dorsomedial shell | ||
| ventral accumbens shell | dorsomedial and perifornical hypothalamus | ||
| ventral and dorsal medial PFC | |||
| VTA | |||
| substantia nigra | |||
| Hamlin et al. (2008); Fos protein | BLA | Dorsal medial PFC | |
| lateral hypothalamus | accumbens core and shell | ||
| ventral medial PFC | CPu | ||
| central amygdala | |||
| lateral amygdala | |||
| BNST | |||
| dorsomedial and perifornical hypothalamus | |||
| VTA | |||
| substantia nigra |
(a) Role of dopamine and glutamate
Dopamine and glutamate transmissions in ventral tegmental area (VTA) and its projection areas—accumbens and dorsal medial prefrontal cortex (PFC)—contribute to the reinstatement of drug seeking (Kalivas & McFarland 2003; Bossert et al. 2005; Schmidt et al. 2005). Based on these studies, we initially assessed the effect of systemic injections of SCH 23390 or raclopride (dopamine D1- and D2-family receptor antagonists) or of LY379268, a group II metabotropic glutamate receptor (mGluR2/3), which decreases evoked glutamate release, on context-induced reinstatement. We found that SCH 23390 or raclopride injections attenuate context-induced reinstatement of cocaine seeking (Crombag et al. 2002a), and that SCH 23390 or LY379268 injections attenuate context-induced reinstatement of heroin seeking (Bossert et al. 2004, 2007). Hamlin et al. (2006, 2007) reported that systemic SCH 23390 injections attenuate context-induced reinstatement of alcohol and sucrose seeking.
The VTA and accumbens shell contribute to the effect of systemic injections of SCH 23390 and LY379268 on context-induced reinstatement. We found that medial and lateral accumbens shell (but not core) SCH 23390 injections attenuate context-induced reinstatement of heroin seeking (Bossert et al. 2007). These findings are consistent with those of Hamlin et al. (2007): context-induced reinstatement of alcohol seeking is associated with increased Fos (a neuronal activity marker) expression in accumbens shell, an effect reversed by systemic SCH 23390 injections. Additionally, we found that VTA and medial accumbens shell injections of LY379268 attenuate context-induced reinstatement of heroin seeking (Bossert et al. 2004, 2006). These effects were anatomically specific: substantia nigra and caudate–putamen LY379268 injections were ineffective, and the effective LY379268 dose in accumbens core was 10 times higher than the effective dose in medial accumbens shell.
Because accumbens shell SCH 23390 injections attenuate context-induced reinstatement, the effect of LY379268 injections in VTA on context-induced reinstatement is probably due to decreases in VTA dopamine transmission; this would result in decreases in accumbens shell dopamine release, and consequently decreased stimulation of local D1-family receptors. In VTA, dopamine transmission is controlled in part by excitatory glutamate projections from several brain areas (Geisler et al. 2007), and based on electrophysiological and anatomical studies (Manzoni & Williams 1999; Rouse et al. 2000), LY379268 VTA injections should activate local presynaptic inhibitory mGluR2, resulting in decreased glutamate transmission, which leads to decreased dopamine transmission.
The effect of accumbens shell LY379268 injections on context-induced reinstatement may also be due to decreased glutamate and dopamine transmission. In accumbens, agonist activation of mGluR2/3 receptors decreases glutamate transmission (Xi et al. 2002) via presynaptic mechanisms (Manzoni et al. 1997). Additionally, local perfusion of LY379268 decreases dopamine levels in accumbens shell (Greenslade & Mitchell 2004). Based on findings that accumbens neuronal activity is dependent on glutamate and dopamine D1 receptor-mediated transmission (O'Donnell 2003), we propose that local dopamine–glutamate interaction mediates context-induced reinstatement. Below, we speculate on the nature of this interaction (see figure 2).
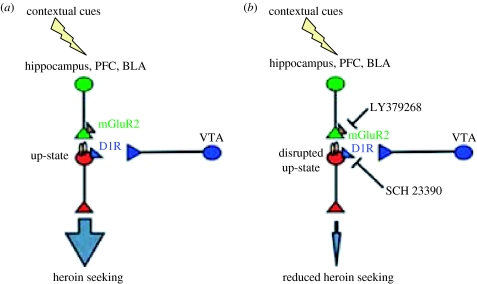
Figure 2.
A model of dopamine–glutamate interaction in the accumbens shell that mediates context-induced reinstatement of heroin seeking. (a) Exposure to heroin-associated contexts activates both dopamine and glutamate neurons that project to accumbens shell. Glutamate activation of shell medium spiny neurons increases the number of up-state neurons, which is further potentiated via D1 receptor (D1R) activation by dopamine, resulting in the activation of downstream targets, which leads to the reinstatement of drug seeking. (b) Blockade of either glutamate transmission in accumbens shell by LY379268 (an agonist of inhibitory mGluR2 located on presynaptic glutamatergic neurons) or dopamine transmission by SCH 23390 (an antagonist of postsynaptic D1 receptors) prevents context-induced transition to the neuronal up-state, resulting in decreased activity of accumbens shell medium spiny output neurons, which in turn leads to the inhibition of context-induced reinstatement of heroin seeking. Blue: dopamine neurons; green: glutamate neurons; red: medium spiny accumbens projection neurons.
Under normal conditions, accumbens neurons exhibit negative resting membrane potentials, referred to as a ‘down-state’. Excitatory glutamate inputs from PFC, hippocampus and amygdala (Voorn et al. 2004) can drive quiescent down-state neurons into an ‘up-state’ and action-potential neuronal firing can occur (Wilson & Kawaguchi 1996). Once neurons are in the up-state, additional neuronal excitation is provided by dopamine-mediated D1 receptor activation, which further enhances the up-state (O'Donnell 2003). LY379268 decreases glutamate release, while SCH 23390 blocks D1-family receptors; each of these effects can interfere with the up-state excitation of accumbens medium spiny neurons. We propose that exposure to drug contexts activates both dopamine and glutamate neurons that project to accumbens shell. Glutamate activation of shell medium spiny neurons causes a shift from a down-state to an up-state, which is further enhanced via dopamine-mediated D1 receptor activation, resulting in the activation of downstream targets (Meredith 1999), and consequently to drug seeking. Within this framework, accumbens shell injections of LY379268 or SCH 23390 prevent context-induced transition to the neuronal up-state, resulting in decreased activity of shell medium spiny output neurons, which in turn leads to attenuation of context-induced reinstatement.
(b) Role of opioid, serotonin and endocannabinoid systems
The preferential mu-opioid receptor antagonist naltrexone attenuates reinstatement of alcohol seeking induced by alcohol priming (Le et al. 1999), and discrete (Liu & Weiss 2002) and discriminative (Katner & Weiss 1999) alcohol cues. Burattini et al. (2006) and Marinelli et al. (2007) reported that systemic injections of naltrexone decrease context-induced reinstatement of alcohol seeking. Marinelli et al. (2007) also found that systemic naltrexone injections decrease context-induced increases in c-fos mRNA expression in basolateral amygdala (BLA) and dorsal hippocampus, suggesting a potential role of mu-opioid receptors in these brain areas in this reinstatement. This suggestion should be confirmed in future studies on the effect of site-specific injections of mu-opiate receptor antagonists on context-induced reinstatement.
Systemic injections of Ro 60-0175 (an agonist of 5-HT2C serotonin receptors) or SR141716A (an antagonist of cannabinoid receptor 1, CB1) decrease drug priming- and discrete cue-induced reinstatement of drug seeking (Grottick et al. 2000; De Vries et al. 2001, 2003; Burbassi & Cervo 2008). Based on these studies, Fletcher et al. (2008) examined the effect of systemic injections of Ro 60-0175 on context-induced reinstatement of cocaine seeking, and Diergaarde et al. (in press) examined the effect of systemic injections of SR141716A on context-induced reinstatement of nicotine seeking. They found that these injections decrease context-induced reinstatement. A question for future research from the findings with Ro 60-0175 and SR141716A is which brain sites mediate their effects on this reinstatement.
(c) Role of BLA, dorsal medial PFC, dorsal hippocampus, dorsal striatum and lateral hypothalamus
Using reversible inactivation methods, Fuchs, See and colleagues provided evidence for a role of BLA, dorsal medial PFC, dorsal hippocampus and dorsolateral striatum in context-induced reinstatement of cocaine seeking. Fuchs et al. (2005) found that tetrodotoxin (a sodium channel blocker) injections into BLA, dorsal hippocampus and dorsal medial (but not ventral medial) PFC attenuate context-induced reinstatement. An issue to consider in interpreting these data is that tetrodotoxin inactivates both cell bodies in the target area and fibres of passage. Thus, tetrodotoxin's behavioural effects may be due to inactivation of fibres that pass through the target area rather than inactivation of cell bodies in the target area. Fuchs et al. (2006) also reported that inactivation of dorsolateral striatum by a mixture of GABAa and GABAb agonists (muscimol+baclofen, which inactivate cell bodies but not fibres of passage) attenuates context-induced reinstatement of cocaine seeking.
Fuchs et al. (2007) used an asymmetric lesion/inactivation procedure to assess whether a BLA–dorsal medial PFC pathway or a BLA–dorsal hippocampus pathway interacts sequentially to control context-induced reinstatement of cocaine seeking. In this procedure, the role a neuronal pathway plays in a given behaviour is inferred from the observation that lesion (permanent or reversible) or receptor blockade of one brain site in one hemisphere together with lesion/receptor blockade of a second brain site in the contralateral hemisphere disrupts the behaviour of interest (Gaffan et al. 1993; Di Ciano & Everitt 2004). Because most learned behaviours can be maintained by an intact single hemisphere (but see Christakou et al. (2005) for data inconsistent with this view), and most neuronal projections are ipsilateral, a main requirement for interpreting results from ‘disconnection’ studies is that the target behaviour remains largely intact after ipsilateral lesion/inactivation of the two brain sites (Setlow et al. 2002). Fuchs et al. found that while contralateral but not ipsilateral inactivation (muscimol+baclofen) of the BLA–dorsal hippocampus attenuates context-induced reinstatement, both contralateral and ipsilateral inactivation of BLA–dorsal medial PFC decrease this reinstatement.
These findings potentially suggest that a serial interaction between BLA and dorsal hippocampus, but not BLA and dorsal medial PFC, mediates context-induced reinstatement. However, before accepting this conclusion, several issues should be considered, especially regarding the similar effects of ipsilateral and contralateral BLA–dorsal medial PFC inactivation. Anatomical studies indicate strong reciprocal connections between the BLA and PFC (McDonald 1998; Pitkanen 2000), including bilateral PFC projections to amygdala (McDonald et al. 1996). Electrophysiology data also suggest that conditioned neuronal responses in PFC depend on amygdala input (Grace 2006). Based on these anatomical and electrophysiology findings, the similar effects of ipsilateral and contralateral BLA–PFC inactivation on context-induced reinstatement may not reflect independence of BLA and PFC in the control of context-induced reinstatement, but rather occur because BLA–PFC unilateral input is not sufficient to maintain normal responding for contexts during reinstatement testing.
In agreement with the results of Fuchs et al., studies using the neuronal activity marker Fos suggest that BLA and dorsal hippocampus neuronal activities contribute to context-induced reinstatement. In both alcohol- and cocaine-trained rats, context-induced reinstatement is associated with selective Fos (or c-fos) induction in BLA (Hamlin et al. 2007, 2008; Marinelli et al. 2007). Context-induced reinstatement of alcohol seeking is also associated with consistent (though modest) increases in c-fos in dorsal hippocampal areas (Marinelli et al. 2007). However, the findings of Hamlin et al. (2008) that context-induced reinstatement of cocaine seeking is associated with Fos induction in ventral medial but not dorsal medial PFC are inconsistent with the results of Fuchs et al. (2005) that tetrodotoxin inactivation of the dorsal medial but not ventral medial PFC attenuates this reinstatement. However, findings with Fos should be interpreted with caution. For example, the accumbens mediates many behavioural effects of cocaine, yet acute cocaine injections have minimal effect on accumbens Fos induction (Crombag et al. 2002,b; Hope et al. 2006). Also, while our data suggest a role of VTA in context-induced reinstatement, this reinstatement is not associated with Fos induction in this brain area (table 4).
An interesting finding emerging from the studies of Hamlin et al. is the association between context-induced reinstatement and Fos induction in lateral hypothalamus. This is a consistent finding that occurs not only in cocaine- and alcohol-trained rats, but also in sucrose-trained rats (Hamlin et al. 2006), suggesting a general role of lateral hypothalamus in context-induced reward seeking. Hamlin et al. (2008) also attempted to determine the afferent areas to lateral hypothalamus that are recruited during tests for context-induced reinstatement. This was achieved by injecting the retrograde tracer cholera toxin into lateral hypothalamus prior to cocaine self-administration training, and subsequently double-labelling the cholera toxin-labelled neurons in the target areas (e.g. PFC, accumbens, VTA) with Fos (induced during reinstatement testing). Surprisingly, very little double-labelling of Fos–cholera toxin was found in the projection areas, suggesting that context-induced lateral hypothalamus neuronal activity is largely independent of neuronal activity in VTA, accumbens and PFC. This unexpected finding suggests that the role of lateral hypothalamus in context-induced reinstatement is independent of the role of VTA, accumbens shell and dorsal medial PFC in this reinstatement. However, based on the limitations of interpreting Fos mapping studies mentioned above, it is important to further test this interesting hypothesis in studies using the disconnection approach.
(d) Conclusions
Studies using systemic drug injections indicate a role of D1- and D2-family, mGluR2/3, mu-opioid, 5-HT2C and CB1 receptors in context-induced reinstatement of drug seeking. Our studies indicate that dopamine and glutamate transmissions in VTA and accumbens shell mediate context-induced reinstatement (figure 2). From a circuitry perspective, questions for future research are the sources of the glutamatergic projections to the VTA and accumbens shell that are activated during the tests for context-induced reinstatement, and the accumbens shell downstream brain sites involved in this reinstatement. There is also evidence for a role of the dorsal hippocampus, BLA, dorsal striatum and dorsal medial PFC in context-induced reinstatement. A question for future research is the neurotransmitters involved in this reinstatement in these brain areas. Fos mapping studies suggest a role of lateral hypothalamus in context-induced reinstatement of drug seeking. However, these data are correlational and should be confirmed in studies using local drug injections.
An unresolved issue for future research, which has begun to be addressed by the laboratories of McNally and Fuchs, is which brain pathways mediate context-induced reinstatement. Fuchs's studies led to the surprising result, based on limited anatomical connectivity (Pitkanen 2000), that a serial interaction between BLA and dorsal hippocampus contributes to context-induced reinstatement. McNally's studies potentially suggest that lateral hypothalamus' role in context-induced reinstatement is independent of its afferents from VTA, accumbens shell and PFC. Another issue for future research, which has begun to be addressed by McNally laboratory, is whether similar or different neuronal mechanisms underlie context-induced reinstatement across drug classes. The studies by Hamilin, McNally and colleagues suggest that these mechanisms are not identical. For example, context-induced reinstatement of cocaine seeking, but not alcohol seeking, is associated with Fos induction in ventral medial PFC, while context-induced reinstatement of alcohol seeking, but not cocaine seeking, is associated with the activation of hypocretin (orexin) neurons in lateral hypothalamus (Hamlin et al. 2007, 2008).
4. Clinical implications
We reviewed studies on context-induced reinstatement of drug seeking in rats. We suggest that to the degree that our preclinical model is relevant to understanding human drug relapse (Epstein et al. 2006), the results of the studies reviewed may have implications for the understanding of the psychological processes underlying this relapse. The reliable effect of context exposure on reinstatement after extinction of the response to the discrete cues in a different context can explain results from ‘cue exposure’ studies demonstrating that most human drug users relapse when they return to their home environment after successful extinction of the physiological and psychological responses to drug-associated discrete cues in the clinic (Carter & Tiffany 1999). More generally, the phenomenon of context-induced reinstatement in laboratory rats can also explain in part why many drug addicts who after having successfully undergone inpatient detoxification (with or without cue-exposure therapy) return to drug use upon returning to their home environment (Hunt et al. 1971).
Finally, the studies reviewed, together with previous studies using non-drug reinforcers (Bouton 2002), may have implications for behavioural treatment of drug addiction. We suggest that for drug addiction interventions to succeed, it is critical that contextual cues are considered, and that treatment strategies aimed at extinguishing the complex association between contextual cues, discrete cues and drug-taking behaviour may improve long-term drug abstinence. In this regard, two recent studies in rats self-administering alcohol or cocaine suggest alternative strategies that could be more effective at altering contextual influences on relapse to drug seeking. These include exposure to alternative reinforcers during extinction (Kearns & Weiss 2007), and extinction in multiple contexts (Chaudhri et al. 2008).
Acknowledgments
Part of the research described in this review was supported by the Intramural Research Program of the National Institute on Drug Abuse. We thank Taco De Vries, Gavan McNally and Peter Marinelli for providing data depicted in figure 1.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘The neurobiology of addiction: new vistas’.
References
- Berridge K.C. Motivation concepts in behavioral neuroscience. Physiol. Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. doi:10.1016/j.physbeh.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Bossert J.M, Liu S.Y, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J. Neurosci. 2004;24:10 726–10 730. doi: 10.1523/JNEUROSCI.3207-04.2004. doi:10.1523/JNEUROSCI.3207-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert J.M, Ghitza U.E, Lu L, Epstein D.H, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur. J. Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. doi:10.1016/j.ejphar.2005.09.030 [DOI] [PubMed] [Google Scholar]
- Bossert J.M, Gray S.M, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. doi:10.1038/sj.npp.1300977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert J.M, Poles G.C, Wihbey K.A, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J. Neurosci. 2007;27:12 655–12 663. doi: 10.1523/JNEUROSCI.3926-07.2007. doi:10.1523/JNEUROSCI.3926-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton M.E. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. doi:10.1037/0033-2909.114.1.80 [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. doi:10.1016/S0006-3223(02)01546-9 [DOI] [PubMed] [Google Scholar]
- Bouton M.E, Bolles R.C. Contextual control of the extinction of conditioned fear. Learn. Motiv. 1979;10:445–466. doi:10.1016/0023-9690(79)90057-2 [Google Scholar]
- Bouton M.E, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clin. Psychol. Rev. 1991;11:123–140. doi:10.1016/0272-7358(91)90091-8 [Google Scholar]
- Burattini C, Gill T.M, Aicardi G, Janak P.H. The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience. 2006;139:877–887. doi: 10.1016/j.neuroscience.2006.01.009. doi:10.1016/j.neuroscience.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology. 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. doi:10.1007/s00213-007-0916-7 [DOI] [PubMed] [Google Scholar]
- Carter B.L, Tiffany S.T. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. doi:10.1046/j.1360-0443.1999.9433273.x [PubMed] [Google Scholar]
- Catania C.A. 3rd edn. Prentice-Hall; Englewood Cliffs, NJ: 1992. Learning. [Google Scholar]
- Chaudhri N, Sahuque L.L, Janak P.H. Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol. Psychiatry. 2008;64:203–210. doi: 10.1016/j.biopsych.2008.03.007. doi:10.1016/j.biopsych.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Robbins T.W, Everitt B.J. Prolonged neglect following unilateral disruption of a prefrontal cortical-dorsal striatal system. Eur. J. Neurosci. 2005;21:782–792. doi: 10.1111/j.1460-9568.2005.03892.x. doi:10.1111/j.1460-9568.2005.03892.x [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna P.P, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc. Natl Acad. Sci. USA. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. doi:10.1073/pnas.98.4.1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag H.S, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav. Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. doi:10.1037/0735-7044.116.1.169 [DOI] [PubMed] [Google Scholar]
- Crombag H, Grimm J.W, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002a;27:1007–1016. doi: 10.1016/S0893-133X(02)00356-1. doi:10.1016/S0893-133X(02)00356-1 [DOI] [PubMed] [Google Scholar]
- Crombag H.S, Jedynak J.P, Redmond K, Robinson T.E, Hope B.T. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav. Brain Res. 2002b;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. doi:10.1016/S0166-4328(02)00196-1 [DOI] [PubMed] [Google Scholar]
- Crombag H.S, Galarce E.M, Holland P.C. Pavlovian influences on goal-directed behavior in mice: the role of cue-reinforcer temporal relations. Learn. Mem. 2008;15:299–303. doi: 10.1101/lm.762508. doi:10.1101/lm.762508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries T.J, Shaham Y, Homberg J.R, Crombag H, Schuurman K, Dieben J, Vanderschuren L.J, Schoffelmeer A.N. A cannabinoid mechanism in relapse to cocaine seeking. Nat. Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. doi:10.1038/nm1001-1151 [DOI] [PubMed] [Google Scholar]
- De Vries T.J, Homberg J.R, Binnekade R, Raaso H, Schoffelmeer A.N. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology. 2003;168:164–169. doi: 10.1007/s00213-003-1422-1. doi:10.1007/s00213-003-1422-1 [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. doi:10.1007/BF00432175 [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt B.J. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J. Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. doi:10.1523/JNEUROSCI.1581-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde, L., de Vries, W., Raasø, H., Schoffelmeer, A. N. M. & de Vries, T. J. In press. Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid-1 receptor antagonist Rimonabant (SR141716A). (doi:10.1016/j.neuropharm.2008.06.003) [DOI] [PubMed]
- Epstein D.H, Preston K.L, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. doi:10.1007/s00213-006-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.J, Rizos Z, Sinyard J, Tampakeras M, Higgins G.A. The 5-HT(2C) receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. doi:10.1038/sj.npp.1301509 [DOI] [PubMed] [Google Scholar]
- Fuchs R.A, Evans K.A, Ledford C.C, Parker M.P, Case J.M, Mehta R.H, See R.E. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. doi:10.1038/sj.npp.1300579 [DOI] [PubMed] [Google Scholar]
- Fuchs R.A, Branham R.K, See R.E. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate–putamen. J. Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. doi:10.1523/JNEUROSCI.5146-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R.A, Eaddy J.L, Su Z.I, Bell G.H. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur. J. Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. doi:10.1111/j.1460-9568.2007.05674.x [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray E.A, Fabre-Thorpe M. Interaction of the amygdala with the frontal lobe in reward memory. Eur. J. Neurosci. 1993;5:968–975. doi: 10.1111/j.1460-9568.1993.tb00948.x. doi:10.1111/j.1460-9568.1993.tb00948.x [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh R.W, Zahm D.S. Glutamatergic afferents of the ventral tegmental area in the rat. J. Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. doi:10.1523/JNEUROSCI.0012-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S.R. Stimuli associated with drug injections as events that control behavior. Pharmacol. Rev. 1976;27:325–340. [PubMed] [Google Scholar]
- Grace A.A. Disruption of cortical-limbic interaction as a substrate for comorbidity. Neurotox. Res. 2006;10:93–101. doi: 10.1007/BF03033238. [DOI] [PubMed] [Google Scholar]
- Greenslade R.G, Mitchell S.N. Selective action of (−)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate ( LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell. Neuropharmacology. 2004;47:1–8. doi: 10.1016/j.neuropharm.2004.02.015. doi:10.1016/j.neuropharm.2004.02.015 [DOI] [PubMed] [Google Scholar]
- Grimm J.W, Kruzich P.J, See R.E. Contingent access to stimuli associated with cocaine self-administration is required for reinstatement of drug-seeking behavior. Psychobiology. 2000;28:383–386. [Google Scholar]
- Grottick A.J, Fletcher P.J, Higgins G.A. Studies to investigate the role of 5-HT2C receptors on cocaine and food maintained behaviour. J. Pharmacol. Exp. Ther. 2000;295:1183–1191. [PubMed] [Google Scholar]
- Gunther L.M, Denniston J.C, Miller R.R. Conducting exposure treatment in multiple contexts can prevent relapse. Behav. Res. Ther. 1998;36:75–91. doi: 10.1016/s0005-7967(97)10019-5. doi:10.1016/S0005-7967(97)10019-5 [DOI] [PubMed] [Google Scholar]
- Hamlin A.S, Blatchford K.E, McNally G.P. Renewal of an extinguished instrumental response: neural correlates and the role of D1 dopamine receptors. Neuroscience. 2006;143:25–38. doi: 10.1016/j.neuroscience.2006.07.035. doi:10.1016/j.neuroscience.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Hamlin A.S, Newby J, McNally G.P. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. doi:10.1016/j.neuroscience.2007.01.063 [DOI] [PubMed] [Google Scholar]
- Hamlin A.S, Clemens K.J, McNally G.P. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. doi:10.1016/j.neuroscience.2007.11.018 [DOI] [PubMed] [Google Scholar]
- Holland P.C. Occasion setting in Pavlovian conditioning. In: Medlin D.L, editor. The psychology of learning and motivation. Academic Press; San Diego, CA: 1992. pp. 69–125. [Google Scholar]
- Holland P.C, Bouton M.E. Hippocampus and context in classical conditioning. Curr. Opin. Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. doi:10.1016/S0959-4388(99)80027-0 [DOI] [PubMed] [Google Scholar]
- Hope B.T, Simmons D.E, Mitchell T.B, Kreuter J.D, Mattson B.J. Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur. J. Neurosci. 2006;24:867–875. doi: 10.1111/j.1460-9568.2006.04969.x. doi:10.1111/j.1460-9568.2006.04969.x [DOI] [PubMed] [Google Scholar]
- Hunt W.A, Barnett L.W, Branch L.G. Relapse rates in addiciton programs. J. Clin. Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. doi:10.1002/1097-4679(197110)27:4<455::AID-JCLP2270270412>3.0.CO;2-R [DOI] [PubMed] [Google Scholar]
- Kalivas P.W, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. doi:10.1007/s00213-003-1393-2 [DOI] [PubMed] [Google Scholar]
- Katner S.N, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin. Exp. Res. 1999;23:1751–1760. [PubMed] [Google Scholar]
- Kearns D.N, Weiss S.J. Contextual renewal of cocaine seeking in rats and its attenuation by the conditioned effects of an alternative reinforcer. Drug Alcohol Depend. 2007;90:193–202. doi: 10.1016/j.drugalcdep.2007.03.006. doi:10.1016/j.drugalcdep.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Kruzich P.J, Congleton K.M, See R.E. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav. Neurosci. 2001;115:1086–1092. doi: 10.1037//0735-7044.115.5.1086. doi:10.1037/0735-7044.115.5.1086 [DOI] [PubMed] [Google Scholar]
- Le A.D, Poulos C.X, Harding S, Watchus W, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress in rats. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. doi:10.1016/S0893-133X(99)00024-X [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J. Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond P.F. Appetitive Pavlovian–instrumental interactions: effects of inter-stimulus interval and baseline reinforcement conditions. Q. J. Exp. Psychol. B. 1981;33:257–269. doi: 10.1080/14640748108400811. [DOI] [PubMed] [Google Scholar]
- Lovibond P.F. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process. 1983;9:225–247. doi:10.1037/0097-7403.9.3.225 [PubMed] [Google Scholar]
- Mackintosh N.J. Academic Press; London, UK: 1974. The psychology of animal learning. [Google Scholar]
- Manzoni O.J, Williams J.T. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J. Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni O, Michel J.M, Bockaert J. Metabotropic glutamate receptors in the rat nucleus accumbens. Eur. J. Neurosci. 1997;9:1514–1523. doi: 10.1111/j.1460-9568.1997.tb01506.x. doi:10.1111/j.1460-9568.1997.tb01506.x [DOI] [PubMed] [Google Scholar]
- Marinelli P.W, Funk D, Juzytsch W, Li Z, Le A.D. Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur. J. Neurosci. 2007;26:2815–2823. doi: 10.1111/j.1460-9568.2007.05898.x. doi:10.1111/j.1460-9568.2007.05898.x [DOI] [PubMed] [Google Scholar]
- McDonald A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. doi:10.1016/S0301-0082(98)00003-3 [DOI] [PubMed] [Google Scholar]
- McDonald A.J, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. doi:10.1016/0306-4522(95)00417-3 [DOI] [PubMed] [Google Scholar]
- McFarland K, Ettenberg A. Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology. 1997;131:86–92. doi: 10.1007/s002130050269. doi:10.1007/s002130050269 [DOI] [PubMed] [Google Scholar]
- Meil W.M, See R.E. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav. Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Meredith G.E. The synaptic framework for chemical signaling in nucleus accumbens. Ann. N. Y. Acad. Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. doi:10.1111/j.1749-6632.1999.tb09266.x [DOI] [PubMed] [Google Scholar]
- O'Brien C.P, Childress A.R, Mclellan T.A, Ehrman R. Classical conditioning in drug dependent humans. Ann. N. Y. Acad. Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. doi:10.1111/j.1749-6632.1992.tb25984.x [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Dopamine gating of forebrain neural ensembles. Eur. J. Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. doi:10.1046/j.1460-9568.2003.02463.x [DOI] [PubMed] [Google Scholar]
- Pavlov I.P. Oxford University Press; Oxford, UK: 1927. Conditioned reflexes. [Google Scholar]
- Pearce J, Bouton M.E. Theories of associative learning in animals. Annu. Rev. Psychol. 2001;52:111–139. doi: 10.1146/annurev.psych.52.1.111. doi:10.1146/annurev.psych.52.1.111 [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton J.P, editor. The amygdala: a functional analysis. Oxford University Press; Oxford, UK: 2000. pp. 31–115. [Google Scholar]
- Rescorla R.A. Conditioned inhibition of fear resulting from negative CS–US contingencies. J. Comp. Physiol. Psychol. 1969;67:504–509. doi: 10.1037/h0027313. doi:10.1037/h0027313 [DOI] [PubMed] [Google Scholar]
- Rescorla R.A, Durlach P.J, Grau J.W. Contextual learning in Pavlovian conditioning. In: Balsam P, Tomie A, editors. Context and learning. Erlbaum; Hillsdale, NJ: 1985. pp. 23–56. [Google Scholar]
- Rouse S.T, Marino M.J, Bradley S.R, Awad H, Wittmann M, Conn P.J. Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: implications for treatment of Parkinson's disease and related disorders. Pharmacol. Ther. 2000;88:427–435. doi: 10.1016/s0163-7258(00)00098-x. doi:10.1016/S0163-7258(00)00098-X [DOI] [PubMed] [Google Scholar]
- Schmidt H.D, Anderson S.M, Famous K.R, Kumaresan V, Pierce R.C. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur. J. Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. doi:10.1016/j.ejphar.2005.09.068 [DOI] [PubMed] [Google Scholar]
- See R.E. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol. Biochem. Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. doi:10.1016/S0091-3057(01)00682-7 [DOI] [PubMed] [Google Scholar]
- Self D.W, Barnhart W.J, Lehman D.A, Nestler E.J. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. doi:10.1126/science.271.5255.1586 [DOI] [PubMed] [Google Scholar]
- Setlow B, Holland P.C, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive Pavlovian second-order conditioned responses. Behav. Neurosci. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. doi:10.1037/0735-7044.116.2.267 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin self-administration behavior in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. doi:10.1007/BF02246300 [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. doi:10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- Shalev U, Yap J, Shaham Y. Leptin attenuates food deprivation-induced relapse to heroin seeking. J. Neurosci. 2001;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm J.W, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol. Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. doi:10.1124/pr.54.1.1 [DOI] [PubMed] [Google Scholar]
- Shepard J.D, Bossert J.M, Liu S.Y, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. doi:10.1016/j.biopsych.2004.02.032 [DOI] [PubMed] [Google Scholar]
- Spealman R.D, Barrett-Larimore R.L, Rowlett J.K, Platt D.M, Khroyan T.V. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol. Biochem. Behav. 1999;64:327–336. doi: 10.1016/s0091-3057(99)00049-0. doi:10.1016/S0091-3057(99)00049-0 [DOI] [PubMed] [Google Scholar]
- Swartzentruber D. Blocking between occasion setters and contextual stimuli. J. Exp. Psychol. Anim. Behav. Processes. 1991;17:163–173. doi: 10.1037//0097-7403.17.2.163. doi:10.1037/0097-7403.17.2.163 [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren L.J, Groenewegen H.J, Robbins T.W, Pennartz C.M. Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. doi:10.1016/j.tins.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar C.S, Parsons L.H, Kerr T.M, Smit D.L, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc. Natl Acad. Sci. USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. doi:10.1073/pnas.97.8.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence, implication of a conditioning theory for research and treatment. Arch. Gen. Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wilson C.J, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell C.L, Berridge K.C. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J. Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z.X, Baker D.A, Shen H, Carson D.S, Kalivas P.W. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J. Pharmacol. Exp. Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. doi:10.1124/jpet.300.1.162 [DOI] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak P.H. Context is a trigger for relapse to alcohol. Behav. Brain Res. 2006;167:150–155. doi: 10.1016/j.bbr.2005.09.007. doi:10.1016/j.bbr.2005.09.007 [DOI] [PubMed] [Google Scholar]