Abstract
Nicotine is a psychoactive ingredient in tobacco that significantly contributes to the harmful tobacco smoking habit. Nicotine dependence is more prevalent than dependence on any other substance. Preclinical research in animal models of the various aspects of nicotine dependence suggests a critical role of glutamate, γ-aminobutyric acid (GABA), cholinergic and dopamine neurotransmitter interactions in the ventral tegmental area and possibly other brain sites, such as the central nucleus of the amygdala and the prefrontal cortex, in the effects of nicotine. Specifically, decreasing glutamate transmission or increasing GABA transmission with pharmacological manipulations decreased the rewarding effects of nicotine and cue-induced reinstatement of nicotine seeking. Furthermore, early nicotine withdrawal is characterized by decreased function of presynaptic inhibitory metabotropic glutamate 2/3 receptors and increased expression of postsynaptic glutamate receptor subunits in limbic and frontal brain sites, while protracted abstinence may be associated with increased glutamate response to stimuli associated with nicotine administration. Finally, adaptations in nicotinic acetylcholine receptor function are also involved in nicotine dependence. These neuroadaptations probably develop to counteract the decreased glutamate and cholinergic transmission that is hypothesized to characterize early nicotine withdrawal. In conclusion, glutamate, GABA and cholinergic transmission in limbic and frontal brain sites are critically involved in nicotine dependence.
Keywords: nicotine, dependence, withdrawal, glutamate, γ-aminobutyric acid, reinstatement
1. Tobacco, nicotine and neuronal nicotinic acetylcholine receptors
Nicotine is one of the main psychoactive ingredients in tobacco that contributes to the harmful tobacco smoking habit (Stolerman & Jarvis 1995; Royal College of Physicians of London 2000) leading to high morbidity and mortality throughout the world (Murray & Lopez 1997). Nicotine dependence is more prevalent than dependence on any other substance of abuse (Anthony et al. 1994). Unfortunately, quit rates remain low despite the availability of several pharmacological treatments aimed at cessation of tobacco smoking (Haas et al. 2004). This article will summarize our current knowledge of the neurobiology of nicotine dependence, with emphasis on the glutamate and γ-aminobutyric acid (GABA) neurotransmitter systems. Although other ingredients in tobacco smoke and metabolites, such as monoamine oxidase inhibitors and nornicotine, are recognized to contribute to tobacco addiction (DeNoble & Mele 1983; Crooks & Dwoskin 1997; Bardo et al. 1999; Belluzzi et al. 2005; Guillem et al. 2006), most neurobiological research has focused on delineating the neurobiology of nicotine dependence as a first step towards discovering the neurosubstrates of tobacco addiction.
Nicotine is an alkaloid that acts as an agonist at brain and peripheral nicotinic acetylcholine receptors (nAChRs). Neuronal nAChRs are ligand-gated ion channels comprising five membrane-spanning subunits that combine to form a functional receptor (Changeux & Taly 2008) and include nine isoforms of the neuronal α-subunit (α2–α10) and three isoforms of the neuronal β-subunit (β2–β4). These subunits combine with a stoichiometry of two α- and three β-, or five α7-subunits to form nAChRs with distinct pharmacologic and kinetic properties. Acetylcholine is the endogenous neurotransmitter that binds and activates nAChRs. Many nAChRs are situated on presynaptic terminals and modulate neurotransmitter release (Wonnacott 1997). Nevertheless, nAChRs also are located at somatodendritic, axonal and postsynaptic sites. Owing to the wide distribution of nAChRs, administration of nicotine stimulates the release of most neurotransmitters throughout the brain (McGehee & Role 1995). Therefore, as discussed below, various transmitter systems are probably involved in the rewarding effects of nicotine and the adaptations that occur in response to chronic nicotine exposure that gives rise to dependence and withdrawal responses.
2. Behavioural effects of acute nicotine and nicotine withdrawal
In humans, acute nicotine administration produces positive effects, including mild euphoria and mildly enhanced cognition. Such subjective positive effects support reliable intravenous nicotine self-administration behaviour in a variety of species, including rats, mice, non-human primates and humans (e.g. Markou & Paterson 2001; for a review, see Picciotto & Corrigall 2002). Persistent nicotine use leads to tolerance that is mediated by neuroadaptations occurring in response to chronic nicotine exposure. Thus, within hours upon cessation of nicotine exposure, a nicotine withdrawal syndrome emerges characterized by depressed mood, irritability, mild cognitive deficits and physiological symptoms (Shiffman et al. 2004).
The avoidance of these withdrawal syndromes, as well as the positive subjective effects of nicotine, motivates nicotine use (Kenny & Markou 2001). In addition, similar to other psychomotor stimulants, nicotine enhances the reward value of other stimuli (Harrison et al. 2002). Owing to the relatively mild euphorigenic properties of nicotine, the contribution of the reward-enhancing effects to maintaining dependence is hypothesized to be greater for nicotine than other drugs of abuse (Chaudhri et al. 2006; Kenny & Markou 2006). Finally, learning processes also contribute to nicotine dependence. Environmental stimuli predictively associated with either the positive subjective effects of nicotine or the induction of nicotine withdrawal motivate nicotine seeking and eventually drug consumption (Paterson et al. 2004; Kenny et al. 2006).
In rats, nicotine withdrawal is characterized by both increases in somatic signs of withdrawal and affective changes analogous to the effects observed in humans. These affective changes include increased anxiety-like behaviour and reward deficits. A method that is used to assess the effects of stress in rats is the light-potentiated startle procedure. In this procedure, baseline startle responding to a loud startling stimulus in the dark is compared with startle responding when the chamber is brightly lit. Bright illumination is aversive for rats and potentiates the startle response, reflecting increased reactivity to a noxious environmental stimulus under stressful conditions. Hence, this procedure allows comparisons of withdrawal effects on startle reactivity between relatively neutral and stressful contexts. Spontaneous withdrawal from chronic nicotine exposure enhanced light-potentiated startle compared with control vehicle-treated rats, while baseline startle reactivity remained unaffected (Jonkman et al. 2007). These results suggest that spontaneous nicotine withdrawal selectively potentiates responses to anxiogenic stimuli but does not by itself produce a strong anxiogenic-like effect.
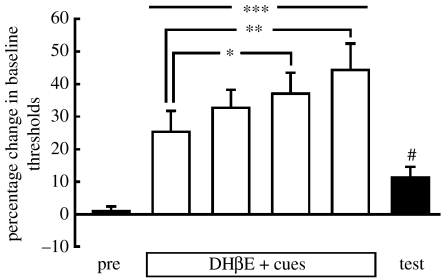
A procedure that assesses brain reward function and potentially depression-like reward deficits characterizing nicotine withdrawal is the intracranial self-stimulation (ICSS) procedure. Brief electrical stimulation of brain reward sites, commonly applied along the medial forebrain bundle, is extremely rewarding. Rats will readily perform an operant task to self-stimulate specific brain reward sites. Nicotine enhances the reward value of such electrical stimulation, resulting in the rats requiring lower current intensities than under baseline conditions to perceive the stimulation as rewarding after the administration of nicotine (Harrison et al. 2002). By contrast, withdrawal from chronic nicotine exposure results in elevations in brain reward thresholds, reflecting a reward deficit and indicating that the rats require higher current intensities to perceive the stimulation as rewarding (Epping-Jordan et al. 1998). Similar reward deficits are seen when nAChR antagonists are administered to rats that are chronically treated with nicotine, while the same doses of the nAChR antagonists have no effect in control vehicle-treated rats (Epping-Jordan et al. 1998; Watkins et al. 2000). Such reward deficits can also be associated through classical conditioning processes to environmental stimuli and come to elicit a conditioned nicotine withdrawal state. Pairing of environmental stimuli, such as a flashing light, with the effects of the nAChR antagonist dihydro-β-erythroidine (DHβE) in nicotine-dependent rats resulted in the presentation of the light alone leading to small, but statistically significant and reliable, elevations in brain reward thresholds (Kenny & Markou 2006; figure 1). Such effects were not seen in rats that had equal exposure to the light, nicotine and the nAChR antagonist but without explicit pairings of the effects of the nAChR antagonist and the cue lights. These findings are highly relevant to the learning mechanisms that contribute to the maintenance of drug dependence because they indicate that environmental stimuli predictively paired with drug withdrawal may lead to a conditioned drug withdrawal state (Kenny & Markou 2006) that can motivate increased drug use (Kenny et al. 2006).
Figure 1.
Reward thresholds in nicotine-dependent rats treated with nAChR antagonist+cues. Conditioned nicotine withdrawal decreased the activity of brain reward systems. Rats trained in the ICSS procedure were prepared with subcutaneous osmotic minipumps containing nicotine (3.16 mg kg−1 per day base) or saline. Several days were allowed to elapse so that nicotine dependence would develop in the nicotine-treated rats. Then, all rats were treated with the nAChR antagonist DHβE and allowed to perform in the ICSS procedure again while a flashing light was on for the duration of the ICSS session. In this graph, only data from rats that were nicotine dependent and were treated with DHβE paired with the flashing lights are presented (n=8). The results indicated a gradual increase in nAChR antagonist precipitated nicotine withdrawal with repeated pairings of the cue flashing lights with DHβE administration. On the test day, rats were injected with saline and presented with the cue lights. The presentation of the cue light alone was sufficient to induce a small but statistically significant elevation in brain reward thresholds, similar in direction but smaller in magnitude than nicotine withdrawal precipitated by administration of the nAChR antagonist. Data from three control groups, including a group that was not nicotine dependent and did not experience explicit pairings of the cue lights with the nAChR antagonist injection, did not show such conditioned effects. Data are shown as per cent change from baseline ICSS thresholds (+s.e.m.) in paired rats on the preconditioning day (pre), during the cue/injection pairings (DHβE+cues), and on the test day when rats were presented with the cues and injected with saline. ***p<0.001, **p<0.01, *p<0.05, compared with thresholds obtained on the preconditioning day (post hoc test after significant one-way analysis of variance); #p<0.05, compared with preconditioning day (paired t-test). Adapted from Kenny & Markou (2005).
3. Neurosubstrates of nicotine reward, dependence and withdrawal
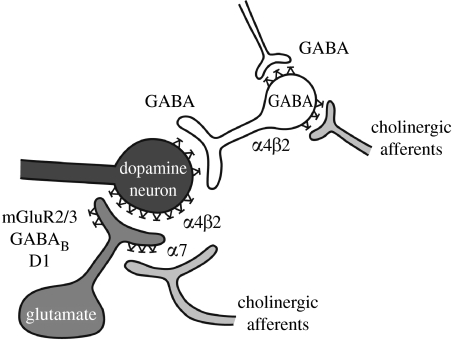
In the midbrain of mammals, interconnected brain structures are referred to as the mesocorticolimbic brain system. This system has been shown to be critically involved in the effects of several drugs of abuse. A component of this system is the dopaminergic projection from the ventral tegmental area (VTA) to the nucleus accumbens, amygdala and frontal cortex. The activity of these VTA dopamine neurons is regulated by the release of the excitatory neurotransmitter glutamate that is released from projections originating from several sites, including the nucleus accumbens and the frontal cortex. Other inputs that also regulate mesolimbic system activity are GABA inhibitory interneurons located within the VTA and the nucleus accumbens, and cholinergic projections from brainstem nuclei to the VTA. The latter projections release the endogenous neurotransmitter acetylcholine that acts on excitatory nAChRs located on glutamate and GABA neuronal terminals in the VTA (figure 2). Extensive research findings demonstrate a critical role of glutamate–GABA–dopamine–acetylcholine interactions, particularly in the VTA (a brain region that has been studied extensively), in several behavioural and affective responses to nicotine. Nevertheless, other brain sites not researched as extensively as the VTA are likely to also contribute to nicotine dependence.
Figure 2.
Nicotine–acetylcholine–glutamate–GABA–dopamine interactions in the VTA. Schematic depicting neurotransmitter interactions in the VTA, which are hypothesized to be critically involved in mediating various effects of nicotine with relevance to nicotine dependence and withdrawal. Neuroadaptations have been shown to develop to several of these receptor and transmitter systems with the development of nicotine dependence. Adapted from Mansvelder & McGehee (2002); see text for details.
Exogenously administered nicotine increases dopamine transmission by direct stimulation of nAChRs, primarily α4β2-containing and α7 homomeric nAChRs within the VTA. Nicotine stimulates nAChRs on glutamatergic terminals that release glutamate, an excitatory neurotransmitter, which results in increased dopamine release in the nucleus accumbens and the frontal cortex. Nicotine also excites nAChRs on GABA-releasing terminals. Thus, the levels of GABA, an inhibitory neurotransmitter, are increased by nicotine as well. However, the interplay between the quick desensitization of nAChRs on the GABA neuron and the higher doses of nicotine required to desensitize the α7 homomeric nAChRs on the glutamate neuron results in a greater overall increase in dopamine levels (figure 2). Interestingly, nAChRs in the VTA play a more important role than those in the nucleus accumbens in the effects of nicotine on the release of nucleus accumbens dopamine (Nisell et al. 1997). Consistent with these neurochemical data, behavioural data indicated that injections of the competitive nAChR antagonist DHβE into the VTA but not into the nucleus accumbens (Corrigall et al. 1994), or lesions of the mesolimbic dopaminergic projections from the VTA to the nucleus accumbens (Corrigall et al. 1992), or cholinergic lesions of the brainstem pedunculopontine nucleus that projects to the VTA (Lança et al. 2000), or systemic administration of dopamine receptor antagonists (Corrigall & Coen 1991) decreased intravenous nicotine self-administration in rats. In terms of nAChR subtypes, studies suggest an involvement of α4β2-containing nAChR subtypes in both the nicotine-induced release of dopamine and nicotine reinforcement (Picciotto et al. 1998). In addition, mutant mice with hypersensitive α4-containing nAChRs show a 50-fold increase in sensitivity to the reinforcing effects of nicotine measured by a conditioned place preference procedure (Tapper et al. 2004). The role of α7 homomeric receptors in the reinforcing effects of nicotine remains unclear, with conflicting data provided by studies in mutant mice lacking the α7 receptor and rats injected with the relatively selective α7 nAChR antagonist methyllycaconitine (Markou & Paterson 2001; Besson et al. 2007).
(a) Glutamate
Glutamate is the major excitatory neurotransmitter in the brain, which plays a critical role in the acute and long-term effects of nicotine. The actions of glutamate are regulated by ionotropic and metabotropic glutamate (mGlu) receptors. Ionotropic glutamate receptors are primarily located postsynaptically, are glutamate-gated ion channels that when activated increase cellular excitability, and comprise the following receptor subtypes: N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) and kainate. Eight mammalian mGlu receptor subtypes have been identified and classified into three groups (I, II and III) based on sequence homology, signal transduction pathways and pharmacological selectivity (for review, Kenny & Markou 2004). Group I (mGlu1 and mGlu5) receptors are predominately located postsynaptically where they couple to Gq-proteins to activate phospholipase C. In addition, group I receptors couple to intracellular Homer proteins that play an important role in trafficking mGlu receptors in and out of the synapses and functionally connect metabotropic to ionotropic glutamate receptors. Group II (mGlu2 and mGlu3) receptors are primarily found presynaptically and also on glial cells and couple to Gi/o proteins to negatively regulate adenylyl cyclase activity. Group III (mGlu4, mGlu6, mGlu7 and mGlu8) receptors are predominately presynaptic autoreceptors coupled to Gi/o-proteins to decrease adenylyl cyclase activity. Thus, mGlu receptors are slow acting and modulate glutamate transmission. They are also widely, but differentially, expressed in the brain. Hence, these receptors offer unique opportunities to alter in pharmacologically subtle ways glutamate transmission, and thus affect motivated behaviour and affective processes without producing gross undesirable or toxic side effects (Markou 2007).
As discussed above, nicotine increases glutamate release by agonist actions at excitatory presynaptic nAChRs on glutamatergic terminals in various brain sites, including the VTA, nucleus accumbens, prefrontal cortex and hippocampus (for reviews, see Mansvelder & McGehee 2002; Kenny & Markou 2004). Furthermore, glutamatergic afferents project from areas such as the frontal cortex, amygdala and hippocampus to brain site that contain dopaminergic cell bodies or terminals, such as the VTA and the nucleus accumbens (for a review, see Kenny & Markou 2004). Indeed, experimenter-administered nicotine has been shown to increase glutamate levels in the VTA (Fu et al. 2000), which presumably acts at metabotropic and ionotropic glutamate receptors on postsynaptic dopamine neurons and increases their bursting activity and neurotransmitter release. Considerable evidence suggests that these actions partly mediate the reinforcing effects of acute nicotine.
Specifically, the blockade of postsynaptic mGlu5 receptors with 2-methyl-6-(phenylethynyl)-pyridine (MPEP) decreased intravenous nicotine self-administration in rats and mice (Paterson et al. 2003) and also decreased the motivation to self-administer nicotine assessed by the progressive ratio schedule of reinforcement (Paterson & Markou 2005). Furthermore, the blockade of postsynaptic NMDA receptors either globally via systemic administration of an NMDA receptor antagonist or via injections of the NMDA receptor antagonist directly into the VTA or the central nucleus of the amygdala decreased intravenous nicotine self-administration in rats (Kenny et al. in press). The effects of mGlu5 and NMDA receptor antagonists on intravenous nicotine self-administration are both seen at doses that have no effects on responding for food reinforcement under similar schedules of reinforcement or reinforcement schedules that equate overall rates of responding for nicotine and food. The effects of the glutamate compounds on nicotine self-administration are likely to be mediated by attenuation of nicotine-stimulated glutamate transmission in the mesolimbic system via the blockade of postsynaptic mGlu5 or NMDA receptors. Finally, the administration of LY379268, an mGlu2/3 receptor agonist, systemically or directly into the posterior VTA or the nucleus accumbens shell dose-dependently decreased nicotine self-administration at doses that had no effect on responding for food. Considering that mGlu2/3 receptors are located presynaptically and negatively modulate glutamate release, the latter data are consistent with the NMDA and mGlu5 receptor data that indicate that decreasing glutamate transmission blocks the rewarding effects of nicotine, thus leading to decreases in nicotine self-administration. Unfortunately, however, rapid tolerance occurred to the LY379268-induced decreases in nicotine self-administration, possibly attributable to the rapid adaptations in function that these receptors have been shown to exhibit (see below). This potential tolerance is unfortunate and may limit the potential usefulness of mGlu2/3 receptors as targets for the treatment of nicotine dependence. Medications for smoking cessation, therefore, would need to be administered chronically to humans to aid in smoking cessation. The chronic effects of repeatedly administering other glutamate compounds that have been shown to decrease nicotine self-administration in rats have not yet been investigated.
As discussed previously, in addition to the primary rewarding effects of nicotine, it also enhances the reward value of other rewarding stimuli (Harrison et al. 2002). Nicotine enhances the reward value of brain stimulation reward so that rats require lower current intensities than under baseline conditions to perceive the stimulation as rewarding (Harrison et al. 2002). Similarly to the primary rewarding effects of nicotine, the reward-enhancing effects of nicotine were also blocked by the administration of an mGlu5 receptor antagonist or an mGlu2/3 receptor agonist, compounds that both decrease glutamate transmission (Harrison et al. 2002). However, this ‘blockade’ of the reward-enhancing effects of nicotine may be a ‘non-specific effect’. The effects of nicotine and glutamatergic compounds are additive. That is, nicotine enhances brain reward function, and the glutamate compounds decrease brain reward function, resulting in an apparent blockade of the reward-enhancing effects of nicotine. Although these effects appear to be pharmacologically additive coupled with the ability of these compounds to decrease nicotine self-administration, these additive effects on nicotine-induced enhancement of reward may be clinically relevant. These compounds may block the ability of tobacco smoking to enhance the reward value of environmental stimuli and thus remove a source of motivation to smoke tobacco.
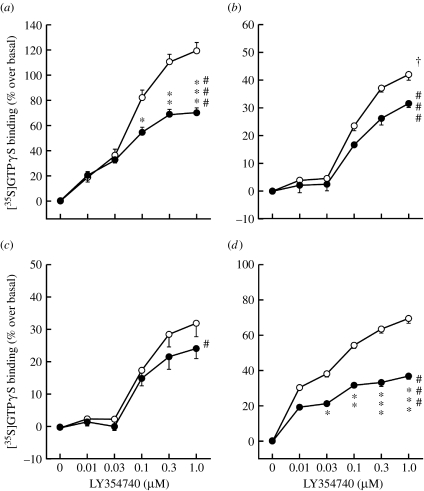
With chronic exposure to nicotine and the development of nicotine dependence, adaptations in glutamate neurotransmission occur, which are likely to mediate the behavioural signs of nicotine withdrawal. In a series of studies, we investigated adaptations in glutamate receptors that may mediate the deficits in brain reward function measured by elevations in ICSS thresholds associated with nicotine withdrawal in rats. The mGlu2/3 receptor agonist LY314582 precipitated withdrawal-like elevations in ICSS thresholds, a sensitive measure of reward function, in nicotine-dependent but not in control rats. LY314582 did not affect response latencies, a measure of performance in the ICSS paradigm. These effects were seen either when LY314582 was injected systemically or when microinfused directly into the VTA (Kenny et al. 2003). These behavioural data suggest that altered function/number of mGlu2/3 receptors, particularly in the VTA, after chronic exposure to nicotine renders them more sensitive to the effects of an agonist. This interpretation is corroborated by recent findings demonstrating that 24 hours after the last daily intravenous nicotine self-administration session, mGlu2/3 receptor function was downregulated, demonstrated by decreased coupling of mGlu2/3 receptors to G-proteins in the [35S]GTPγS-binding assay in all brain sites assessed, including the VTA, prefrontal cortex, nucleus accumbens, amygdala, hippocampus and hypothalamus (figure 3). Because mGlu2/3 receptors mainly function as inhibitory autoreceptors to control glutamate release, this decrease in mGlu2/3 receptor function indicates impaired negative feedback control on glutamatergic terminals, possibly to counteract the decreased glutamate transmission that probably characterizes early nicotine withdrawal. Consistent with this interpretation, a single injection of the Glu2/3 receptor antagonist LY341495 attenuated the threshold elevations observed in rats undergoing spontaneous nicotine withdrawal (Kenny et al. 2003).
Figure 3.
Effects of 24-hour withdrawal from nicotine or food self-administration on functional coupling of mGlu2/3 receptors to G-proteins. Stimulation of [35S]GTPγS binding by the mGlu2/3 receptor agonist LY354740 was significantly decreased in rats self-administering nicotine (filled circles) compared with animals responding for food (open circles), suggesting mGlu2/3 receptor downregulation in all assessed brain areas (LY354740×reward interactions, #p<0.05, ###p<0.001, *p<0.05, **p<0.01, ***p<0.001, difference from food). Data are expressed as mean±s.e.m., n=6. Adapted from Liechti et al. (2007). (a) Prefrontal cortex, (b) nucleus accumbens, (c) VTA, (d) amygdala.
To further explore whether alterations in mGlu2/3 receptor function contribute to nicotine withdrawal by decreasing glutamatergic transmission, we examined whether direct blockade of postsynaptic glutamate receptors precipitated withdrawal-like reward deficits in nicotine-dependent rats. Indeed, the AMPA/kainate receptor antagonist NBQX precipitated withdrawal-like threshold elevations in nicotine-dependent but not in control rats, indicating that decreased glutamate neurotransmission through these receptors contributes to the reward deficits associated with nicotine withdrawal. This conclusion is corroborated by findings showing that 24 hours after nicotine self-administration, increased postsynaptic expression levels of the following were observed: (i) NMDA receptor NR2A subunit in the VTA, (ii) NR1, NR2A and NR2B subunits in the central nucleus of the amygdala, (iii) AMPA receptor GluR1 and GluR2 subunits in the central nucleus of the amygdale, and (iv) GluR1 subunit in the nucleus accumbens (Kenny et al. in press). Additionally, decreased expression levels of the NR2A, NR2B and Glu2 subunits were observed in the prefrontal cortex, and these effects were similar to those seen previously in rat brains immediately after prolonged nicotine self-administration sessions (Wang et al. 2007). This increased expression of ionotropic glutamate receptor subunits may reflect compensatory changes in the glutamatergic system to counteract the effects of decreased glutamate transmission associated with the nicotine withdrawal state. Interestingly, the opposite effects (i.e. decreases rather than increases) in the expression of postsynaptic glutamate receptor subunits were seen in the prefrontal cortex in these same subjects, indicating that different brain sites may mediate different aspects of nicotine dependence. For example, increased activity of glutamatergic projections from the prefrontal cortex to the nucleus accumbens has been shown to play an important role in cocaine seeking (Moran et al. 2005) and the same appears to be true for nicotine (Liechti et al. 2007). Thus, increased glutamate neurotransmission appears to occur during nicotine self-administration and perhaps during nicotine seeking, while decreased glutamate transmission occurs during early nicotine withdrawal that probably mediates the reward deficits, and perhaps other behavioural effects, associated with nicotine withdrawal and low nicotine seeking. Interestingly, however, MPEP or dizocilpine, antagonists at mGlu5 and NMDA receptors, respectively, did not precipitate reward deficits in nicotine-dependent rats. Overall, these data demonstrate that mGlu2/3 receptors play an important role in generating the reward deficits associated with nicotine withdrawal, in part by decreasing glutamate transmission at AMPA/kainate receptors (Markou 2007). Nevertheless, although antagonists at mGlu5 receptors decrease the rewarding effects of nicotine self-administration (Paterson et al. 2003; see above) and block cue-induced reinstatement (Bespalov et al. 2005; see below), these receptors do not appear to show adaptations in function/number with the development of nicotine dependence.
Finally, glutamate appears to play an important role in the reinstatement of nicotine-seeking behaviour seen during protracted abstinence. Systemic administration of the mGlu5 receptor antagonist MPEP (Bespalov et al. 2005) or the mGlu2/3 receptor agonist LY379268 (Liechti et al. 2007) decreased cue-induced reinstatement of nicotine seeking in rats. In the case of MPEP, no effects on cue-induced reinstatement of food were observed. In the case of LY379268, similar doses decreased cue-induced reinstatement of either nicotine or food seeking, suggesting that stimulatory actions at presynaptic inhibitory mGlu2/3 receptors have general effects on the motivational impact of conditioned reinforcers. Thus, protracted abstinence may be associated with an enhanced glutamate response to environmental stimuli that have motivational significance, and this response may be blocked by compounds that directly (e.g. antagonists at postsynaptic glutamate receptors) or indirectly (agonists at presynaptic inhibitory glutamate receptors) diminish the glutamate response to the stimuli (Moran et al. 2005).
(b) γ-Aminobutyric acid
GABA is the major inhibitory transmitter in the brain and another transmitter system critically involved in the reinforcing effects of acute nicotine administration. GABAergic afferents to the VTA from the pedunculopontine tegmental nucleus, ventral pallidum and nucleus accumbens, as well as GABA interneurons within the VTA and medium spiny GABA neurons in the nucleus accumbens, inhibit dopaminergic mesocorticolimbic activity (Klitenick et al. 1992). As discussed previously and depicted in figure 1, complex interactions occur between the GABA, dopaminergic and glutamatergic systems in the VTA (Mansvelder & McGehee 2002). Increased GABAergic transmission abolishes both the nicotine-induced dopamine increases in the nucleus accumbens and the reinforcing effects of nicotine (Dewey et al. 1999; Brebner et al. 2002). γ-Vinyl GABA (GVG; also referred to as vigabatrin) is an irreversible inhibitor of GABA transaminase, the primary enzyme involved in GABA metabolism. Thus, administration of GVG increases GABA levels, and accordingly decreases nicotine self-administration (Paterson & Markou 2002) and abolishes both the acquisition and the expression of conditioned place preference (Dewey et al. 1998). GVG administration also dose dependently lowered nicotine-induced increases in nucleus accumbens dopamine in both naive and chronically nicotine-treated rats and blocked nicotine-induced increases in striatal dopamine in primates as measured by positron emission tomography (Brebner et al. 2002).
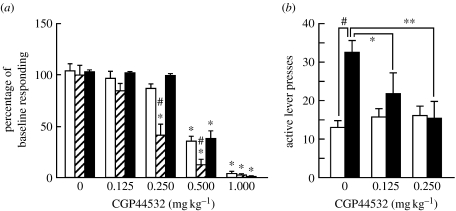
The use of receptor-selective agonists suggests the involvement of GABAB receptors in these effects. Two receptors bind the endogenous neurotransmitter GABA: GABAA ionotropic receptors and GABAB metabotropic receptors. Both GABAA and GABAB receptors are inhibitory and both are found presynaptically and postsynaptically. Systemic injections or microinjections of baclofen or CGP44532 [(3-amino-2[S]-hydroxypropyl)-methylphosphonic acid], two GABAB receptor agonists, into the nucleus accumbens shell, VTA or pedunculopontine tegmental nucleus that sends cholinergic, GABAergic and glutamatergic projections to the VTA (but not injections into the caudate–putamen) decreased the reinforcing effects of nicotine (Shoaib et al. 1998; Corrigall et al. 2000, 2001; Fattore et al. 2002; Paterson et al. 2004; figure 4a). These decreases in nicotine self-administration persisted even after chronic administration of CGP44532 for 14 days, indicating little tolerance to this effect of the GABAB receptor agonist with this length of treatment (Paterson et al. 2005b). The issue of tolerance is important because drug therapies currently need to be administered chronically to humans for smoking cessation. However, in studies of rats, GVG and GABAB receptor agonists also decreased responding for food, although at higher doses than the threshold doses for inducing decreases in nicotine self-administration (Paterson & Markou 2002; Paterson et al. 2004, 2005b; figure 4a). These effects on responding for food may reflect non-specific performance effects of the GABAergic compounds or specific effects on food intake. The latter possibility is intriguing because abstinence-associated weight gain is often a concern for smokers, especially women, who wish to quit smoking cigarettes. Finally, the GABAB receptor agonist CGP44532 also blocked cue-induced reinstatement of nicotine-seeking behaviour (figure 4b). Thus, increased GABA transmission through GABAB receptor activation blocks the reinforcing effects of nicotine.
Figure 4.
Acute administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking. (a) Primary rewarding effects: nicotine was available for self-administration at either of two doses (0.01 or 0.03 mg kg−1 per infusion base). Rats were trained to respond for nicotine or food under a fixed ratio 5, time out 20 s schedule of reinforcement. CGP44532 administration decreased both nicotine and food self-administration but affected nicotine self-administration at doses lower than those that decreased food self-administration. Data are expressed as percentage of baseline responding (mean+s.e.m.). *p<0.05, difference from vehicle pretreatment condition. #p<0.05, difference from the group that responded for food and received the same CGP44532 dose. Adapted from Paterson et al. (2004). Open bars, 0.03 mg kg−1 per infusions of nicotine (n=10); hatched bars, 0.01 mg kg−1 per infusions of nicotine (n=8); filled bars, food (n=12). (b) CGP44532 administration blocked cue-induced reinstatement of nicotine seeking in rats. A within-subjects design was used where all rats (n=8) received all doses of CGP44532. Between drug treatments, rats were returned to extinction conditions. *p<0.05, **p<0.01, difference from saline pretreatment. #p<0.05, difference between the test day and the preceding 3-day baseline. Data are expressed as mean number of lever presses +s.e.m. Adapted from Paterson et al. (2005b). Open bars, extinction conditions (n=8); filled bars, conditioned stimulus presentation.
Nonetheless, GABAB receptor agonists have undesirable side effects, including disruption of performance on the rotarod test, a measure of locomotor impairment (Cryan et al. 2004), and decreased responding for non-drug rewards, such as food and electrical brain stimulation (Macey et al. 2001; Paterson et al. 2005a; Slattery et al. 2005). Interestingly, GABAB receptor-positive allosteric modulator compounds, such as GS39783 and the more recently synthesized BHF177, are likely to have more subtle effects than GABAB receptor agonists, due to their modulatory, rather than full agonist, properties at the receptors (Guery et al. 2007). Accordingly, such compounds do not impair performance in the rotarod test when administered alone (Cryan et al. 2004). In a series of recent studies, we demonstrated that several GABAB receptor positive allosteric modulators decreased nicotine self-administration, decreased break points for nicotine under a progressive ratio schedule of reinforcement and blocked the reward-enhancing effects of nicotine (Paterson et al. in press). These effects were seen at a range of doses that did not affect behaviours reinforced by food. Thus, GABAB receptor-positive allosteric modulators may decrease the rewarding and reward-enhancing effects of nicotine with a better side effect profile than full GABAB receptor agonists.
(c) Adaptations in nAChR function
Chronic nicotine administration leads to an interesting paradoxical change in the function of nAChRs, which consists of receptor desensitization leading to receptor upregulation (Marks et al. 1983; Schwartz & Kellar 1983; Changeux et al. 1984; Wonnacott 1990; Flores et al. 1997; Perry et al. 1999; Mansvelder et al. 2002). Complex theoretical speculations have been put forth about the role of nAChR desensitization and upregulation in the subjective effects of acute nicotine and in the development and maintenance of nicotine dependence (Marks et al. 1992; Dani & Heinemann 1996; Buisson & Bertrand 2002; Quick & Lester 2002). Long-term exposure to nicotine induces an increase in the number (Wonnacott 1990; Marks et al. 1992; Buisson & Bertrand 2001) and function (Rowell & Wonnacott 1990) of nAChRs. Nevertheless, this finding is not consistently demonstrated; others have observed a decrease in nAChR number (Gentry et al. 2003) and function (Marks et al. 1993) with chronic exposure to nicotine. Most studies reporting changes in nAChR number and function have been conducted in in vitro experimental designs, and the functional significance of these changes in vivo is unknown (Buisson & Bertrand 2002) and needs to be explored (Kellar et al. 1999). Behavioural findings are most readily explained by decreased number and/or function of nAChRs with the development of nicotine dependence that counteract the continuous agonist actions of nicotine on the receptors. Specifically, administration of a variety of nAChR antagonists induces withdrawal-like changes in rats exposed to nicotine at doses that have no effect in saline-treated subjects (Epping-Jordan et al. 1998; Watkins et al. 2000; Markou & Paterson 2001; Skjei & Markou 2003). Furthermore, different subtypes of nAChRs desensitize and upregulate at different rates, which may explain the seemingly opposite effects seen in some studies (Kellar et al. 1999; Buisson & Bertrand 2002; Levin 2002; Mansvelder et al. 2002). For example, Mansvelder & McGehee (2002) have shown rapid desensitization of the α4β2 nAChR located on GABAergic terminals and no changes in α7 nAChR function located on glutamatergic terminals in the VTA. Behaviourally, we observe the net effect of these complex adaptations in different receptor types and brain sites. Thus, adaptations in nAChR number and function probably contribute to the various aspects of nicotine dependence and the difficulty in achieving and sustaining nicotine abstinence.
4. Conclusions
Ample evidence indicates that cholinergic–glutamatergic–GABAergic–dopaminergic interactions in limbic brain sites, such as the VTA (figure 2), are critically involved in the primary rewarding effects of nicotine, and possibly the reward-enhancing effects of nicotine. Although many investigations have been conducted that focus on the VTA, it should be emphasized that other brain sites, such as the central nucleus of the amygdala and the frontal cortex, are probably involved in the behavioural effects of nicotine with relevance to dependence. Nicotine-induced changes in these brain sites have started to be investigated, as well as how manipulations of these brain sites may impact on the effects of nicotine (e.g. Liechti et al. 2007; Wang et al. 2007; Kenny et al. in press). Accordingly, compounds that decrease glutamate transmission either through presynaptic or postsynaptic action, or enhance GABA function through GABAB receptors, decrease the primary rewarding effects of nicotine, the motivation to self-administer nicotine and the reward-enhancing effects of nicotine, as well as the motivational impact of stimuli previously associated with nicotine administration. Furthermore, neurochemical and behavioural evidence demonstrates profound changes in glutamate transmission in limbic brain sites, such as the VTA, nucleus accumbens, amygdala and frontal cortex, which are likely to be critically involved in the development of dependence and the expression of affective signs of nicotine withdrawal upon cessation of drug administration. As anticipated, the changes in nAChR receptor number and function are also observed with the development of nicotine dependence, with different receptors showing differential changes in response to chronic nicotine exposure. By contrast, no evidence has shown adaptations in GABA function with the development of nicotine dependence, although this system is critically involved in the acute effects of nicotine. Thus, compounds that modulate glutamate and/or GABA transmission are likely to have therapeutic potential for treating various aspects of nicotine dependence and withdrawal.
Acknowledgements
This work was supported by research grants (R01)DA11946, (R01)DA023209 and (U01)MH69062 from the National Institutes of Health, USA, and research grant 15RT-0022 from the Tobacco-Related Disease Research Program from the State of California, USA. The author thanks Mike Arends for outstanding editorial assistance.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘The neurobiology of addiction: new vistas’.
References
- Anthony J.C, Warner L.A, Kessler R.C. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 1994;2:244–268. doi:10.1037/1064-1297.2.3.244 [Google Scholar]
- Bardo M.T, Green T.A, Crooks P.A, Dwoskin L.P. Nornicotine is self-administered intravenously by rats. Psychopharmacologia. 1999;146:290–296. doi: 10.1007/s002130051119. doi:10.1007/s002130051119 [DOI] [PubMed] [Google Scholar]
- Belluzzi J.D, Wang R, Leslie F.M. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropschopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. doi:10.1038/sj.npp.1300586 [DOI] [PubMed] [Google Scholar]
- Bespalov A.Y, Dravolina O.A, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(Suppl. 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. doi:10.1016/j.neuropharm.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Besson M, et al. Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc. Natl Acad. Sci. USA. 2007;104:8155–8160. doi: 10.1073/pnas.0702698104. doi:10.1073/pnas.0702698104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Childress A.R, Roberts D.C. A potential role for GABAB agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 2002;37:478–484. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J. Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol. Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. doi:10.1016/S0165-6147(00)01979-9 [DOI] [PubMed] [Google Scholar]
- Changeux, J.-P. & Taly, A. 2008 Nicotinic receptors, allosteric proteins and medicine. Trends Mol. Med.14, 93–102. (doi:10.1016/j.molmed.2008.01.001) [DOI] [PubMed]
- Changeux J.-P, Devillers-Thiery A, Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984;225:1335–1345. doi: 10.1126/science.6382611. doi:10.1126/science.6382611 [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula A.R, Donny E.C, Palmatier M.I, Liu X, Sved A.F. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. doi:10.1007/s00213-005-0178-1 [DOI] [PubMed] [Google Scholar]
- Corrigall W.A, Coen K.M. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology. 1991;104:171–176. doi: 10.1007/BF02244174. doi:10.1007/BF02244174 [DOI] [PubMed] [Google Scholar]
- Corrigall W.A, Franklin K.B.J, Coen K.M, Clarke P.B.S. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. doi:10.1007/BF02245149 [DOI] [PubMed] [Google Scholar]
- Corrigall W.A, Coen K.M, Adamson K.L. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. doi:10.1016/0006-8993(94)90401-4 [DOI] [PubMed] [Google Scholar]
- Corrigall W.A, Coen K.M, Adamson K.L, Chow B.L.C, Zhang J. Response of nicotine self-administration in the rat to manipulations of mu-opioid and γ-aminobutyric acid receptors in the ventral tegmental area. Psychopharmacology. 2000;149:107–114. doi: 10.1007/s002139900355. doi:10.1007/s002139900355 [DOI] [PubMed] [Google Scholar]
- Corrigall W.A, Coen K.M, Zhang J, Adamson K.L. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology. 2001;158:190–197. doi: 10.1007/s002130100869. doi:10.1007/s002130100869 [DOI] [PubMed] [Google Scholar]
- Crooks P.A, Dwoskin L.P. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem. Pharmacol. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. doi:10.1016/S0006-2952(97)00117-2 [DOI] [PubMed] [Google Scholar]
- Cryan J.F, et al. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J. Pharmacol. Exp. Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. doi:10.1124/jpet.104.066753 [DOI] [PubMed] [Google Scholar]
- Dani J.A, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. doi:10.1016/S0896-6273(00)80112-9 [DOI] [PubMed] [Google Scholar]
- DeNoble, V. J. & Mele, P. C. 1983 Behavioral pharmacology annual report. Philip Morris collection. Bates no. 1003060364/0441. See http://legacy.library.ucsf.edu/tid/wot74e00
- Dewey S.L, et al. A novel strategy for the treatment of cocaine addiction. Synapse. 1998;30:119–129. doi: 10.1002/(SICI)1098-2396(199810)30:2<119::AID-SYN1>3.0.CO;2-F. doi:10.1002/(SICI)1098-2396(199810)30:2<119::AID-SYN1>3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- Dewey S.L, Brodie J.D, Gerasimov M, Horan B, Gardner E.L, Ashby C.R., Jr A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. doi:10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y [DOI] [PubMed] [Google Scholar]
- Epping-Jordan M.P, Watkins S.S, Koob G.F, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. doi:10.1038/30001 [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta M.C, Fratta W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 2002;37:495–498. doi: 10.1093/alcalc/37.5.495. [DOI] [PubMed] [Google Scholar]
- Flores C.M, Davila-Garcia M.I, Ulrich Y.M, Kellar K.J. Differential regulation of neuronal nicotinic receptor binding sites following chronic nicotine administration. J. Neurochem. 1997;69:2216–2219. doi: 10.1046/j.1471-4159.1997.69052216.x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta S.G, Gao W, Brower V.G, Sharp B.M. Systemic nicotine stimulates dopamine release in nucleus accumbens: re-evaluation of the role of N-methyl-d-aspartate receptors in the ventral tegmental area. J. Pharmacol. Exp. Ther. 2000;294:458–465. [PubMed] [Google Scholar]
- Gentry C.L, Wilkins L.H, Jr, Lukas R.J. Effects of prolonged nicotinic ligand exposure on function of heterologously expressed, human α4β2- and α4β4-nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2003;304:206–216. doi: 10.1124/jpet.102.041756. doi:10.1124/jpet.102.041756 [DOI] [PubMed] [Google Scholar]
- Guery S, Floersheim P, Kaupmann K, Froestl W. Syntheses and optimization of new GS39783 analogues as positive allosteric modulators of GABAB receptors. Bioorg. Med. Chem. Lett. 2007;17:6206–6211. doi: 10.1016/j.bmcl.2007.09.023. doi:10.1016/j.bmcl.2007.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar M.R, Parsons L.H, Koob G.F, Cador M, Stinus L. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur. J. Neurosci. 2006;24:3532–3540. doi: 10.1111/j.1460-9568.2006.05217.x. doi:10.1111/j.1460-9568.2006.05217.x [DOI] [PubMed] [Google Scholar]
- Haas A.L, Munoz R.F, Humfleet G.L, Reus V.I, Hall S.M. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. J. Consult. Clin. Psychol. 2004;72:563–570. doi: 10.1037/0022-006X.72.4.563. doi:10.1037/0022-006X.72.4.563 [DOI] [PubMed] [Google Scholar]
- Harrison A.A, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DHβE and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology. 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. doi:10.1007/s00213-001-0953-6 [DOI] [PubMed] [Google Scholar]
- Jonkman S, Risbrough V.B, Geyer M.A, Markou A. Spontaneous nicotine withdrawal potentiates the effects of stress in rats. Neuropsychopharmacology. 2007;33:2131–2138. doi: 10.1038/sj.npp.1301607. doi:10.1038/sj.npp.1301607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellar, K. J., Da´vila-García, M. I. & Xiao, Y. 1999 Pharmacology of neuronal nicotinic acetylcholine receptors: effect of acute and chronic nicotine. Nicotine Tob. Res 1(Suppl. 1), S117–S120; discussion S139–S140. (doi:10.1080/14622299050011921) [DOI] [PubMed]
- Kenny P.J, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol. Biochem. Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. doi:10.1016/S0091-3057(01)00651-7 [DOI] [PubMed] [Google Scholar]
- Kenny P.J, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol. Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. doi:10.1016/j.tips.2004.03.009 [DOI] [PubMed] [Google Scholar]
- Kenny P.J, Markou A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J. Neurosci. 2005;29:6208–6212. doi: 10.1523/JNEUROSCI.4785-04.2005. doi:10.1523/JNEUROSCI.4785-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny P.J, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kenny P.J, Gasparini F, Markou A. Group II metabotropic and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J. Pharmacol. Exp. Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. doi:10.1124/jpet.103.052027 [DOI] [PubMed] [Google Scholar]
- Kenny P.J, Chen S.A, Kitamura O, Markou A, Koob G.F. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J. Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. doi:10.1523/JNEUROSCI.0740-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, P. J., Chartoff, E., Roberto, M., Carlezon Jr, W. A. & Markou, A. In press. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology [DOI] [PMC free article] [PubMed]
- Klitenick M.A, DeWitte P, Kalivas P.W. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J. Neurosci. 1992;12:2623–2632. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lança A.J, Adamson K.L, Coen K.M, Chow B.L.C, Corrigall W.A. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 2000;96:735–742. doi: 10.1016/s0306-4522(99)00607-7. doi:10.1016/S0306-4522(99)00607-7 [DOI] [PubMed] [Google Scholar]
- Levin E.D. Nicotinic receptor subtypes and cognitive function. J. Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. doi:10.1002/neu.10151 [DOI] [PubMed] [Google Scholar]
- Liechti M.E, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J. Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. doi:10.1523/JNEUROSCI.1766-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey D.J, Froestl W, Koob G.F, Markou A. Both GABAB receptor agonist and antagonists decreased brain stimulation reward in the rat. Neuropharmacology. 2001;40:676–685. doi: 10.1016/s0028-3908(00)00204-5. doi:10.1016/S0028-3908(00)00204-5 [DOI] [PubMed] [Google Scholar]
- Mansvelder H.D, McGehee D.S. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. doi:10.1002/neu.10148 [DOI] [PubMed] [Google Scholar]
- Mansvelder H.D, Keath J.R, McGehee D.S. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. doi:10.1016/S0896-6273(02)00625-6 [DOI] [PubMed] [Google Scholar]
- Markou A. Metabotropic glutamate receptor antagonists: novel therapeutics for nicotine dependence and depression? Biol. Psychiatry. 2007;61:17–22. doi: 10.1016/j.biopsych.2006.03.053. doi:10.1016/j.biopsych.2006.03.053 [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson N.E. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob. Res. 2001;3:361–373. doi: 10.1080/14622200110073380. doi:10.1080/14622200110073380 [DOI] [PubMed] [Google Scholar]
- Marks M.J, Burch J.B, Collins A.C. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J. Pharmacol. Exp. Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks M.J, Pauly J.R, Gross S.D, Deneris E.S, Hermans-Borgmeyer I, Heinemann S.F, Collins A.C. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J. Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M.J, Grady S.R, Collins A.C. Downregulation of nicotinic receptor function after chronic nicotine infusion. J. Pharmacol. Exp. Ther. 1993;266:1268–1276. [PubMed] [Google Scholar]
- McGehee D.S, Role L.W. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. doi:10.1146/annurev.ph.57.030195.002513 [DOI] [PubMed] [Google Scholar]
- Moran M.M, McFarland K, Melendez R.I, Kalivas P.W, Seamans J.K. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J. Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. doi:10.1523/JNEUROSCI.1007-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J, Lopez A.D. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. doi:10.1016/S0140-6736(96)07492-2 [DOI] [PubMed] [Google Scholar]
- Nisell M, Marcus M, Nomikos G.G, Svensson T.H. Differential effects of acute and chronic nicotine on dopamine output in the core and shell of the rat nucleus accumbens. J. Neural Transm. 1997;104:1–10. doi: 10.1007/BF01271290. doi:10.1007/BF01271290 [DOI] [PubMed] [Google Scholar]
- Paterson N.E, Markou A. Increased GABA neurotransmission via administration of gamma-vinyl GABA decreased nicotine self-administration in the rat. Synapse. 2002;44:252–253. doi: 10.1002/syn.10073. doi:10.1002/syn.10073 [DOI] [PubMed] [Google Scholar]
- Paterson N.E, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. doi:10.1007/s00213-004-2070-9 [DOI] [PubMed] [Google Scholar]
- Paterson N.E, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology. 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Paterson N.E, Froestl W, Markou A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology. 2004;172:179–186. doi: 10.1007/s00213-003-1637-1. doi:10.1007/s00213-003-1637-1 [DOI] [PubMed] [Google Scholar]
- Paterson N.E, Bruijnzeel A.W, Kenny P.J, Wright C.D, Froestl W, Markou A. Prolonged nicotine exposure does not alter GABAB receptor-mediated regulation of brain reward function. Neuropharmacology. 2005a;49:953–962. doi: 10.1016/j.neuropharm.2005.04.031. doi:10.1016/j.neuropharm.2005.04.031 [DOI] [PubMed] [Google Scholar]
- Paterson N.E, Froestl W, Markou A. Repeated administration of the GABAB receptor agonist CGP44532 decreased nicotine self-administration, and acute administration decreased cue-reinstatement of nicotine-seeking in rats. Neuropsychopharmacology. 2005b;30:119–128. doi: 10.1038/sj.npp.1300524. doi:10.1038/sj.npp.1300524 [DOI] [PubMed] [Google Scholar]
- Paterson, N. E., Vlachou, S., Guery, S., Kaupmann, K., Froestl, W. & Markou, A. In press. Positive modulation of GABAB receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J. Pharmacol. Exp. Ther. [DOI] [PMC free article] [PubMed]
- Perry D.C, Davila-Garcia M.I, Stockmeier C.A, Kellar K.J. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharmacol. Exp. Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Picciotto M.R, Corrigall W.A. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J. Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto M.R, Zoli M, Rimondini R, Léna C, Marubio L.M, Pich E.M, Fuxe K, Changeux J.P. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. doi:10.1038/34413 [DOI] [PubMed] [Google Scholar]
- Quick M.W, Lester R.A. Desensitization of neuronal nicotinic receptors. J. Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. doi:10.1002/neu.10109 [DOI] [PubMed] [Google Scholar]
- Rowell P.P, Wonnacott S. Evidence for functional activity of up-regulated nicotine binding sites in rat striatal synaptosomes. J. Neurochem. 1990;55:2105–2110. doi: 10.1111/j.1471-4159.1990.tb05802.x. doi:10.1111/j.1471-4159.1990.tb05802.x [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians of London. Royal College of Physicians of London; London, UK: 2000. Nicotine addiction in Britain: a report of the Tobacco Advisory Group of the Royal College of Physicians. [Google Scholar]
- Schwartz R.D, Kellar K.J. Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. doi:10.1126/science.6828889 [DOI] [PubMed] [Google Scholar]
- Shiffman S, West R.J, Gilbert D.G. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob. Res. 2004;6:599–614. doi: 10.1080/14622200410001734067. doi:10.1080/14622200410001734067 [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner L.S, Beyer C.E, Goldberg S.R, Schindler C.W. The GABAB agonist baclofen modifies cocaine self-administration in rats. Behav. Pharmacol. 1998;9:195–206. [PubMed] [Google Scholar]
- Skjei K.L, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology. 2003;168:280–292. doi: 10.1007/s00213-003-1414-1. doi:10.1007/s00213-003-1414-1 [DOI] [PubMed] [Google Scholar]
- Slattery D.A, Markou A, Froestl W, Cryan J.F. The GABAB receptor-positive modulator GS39783 and the GABAB receptor agonist baclofen attenuate the reward-facilitating effects of cocaine: intracranial self-stimulation studies in the rat. Neuropsychopharmacology. 2005;30:2065–2072. doi: 10.1038/sj.npp.1300734. doi:10.1038/sj.npp.1300734 [DOI] [PubMed] [Google Scholar]
- Stolerman I.P, Jarvis M.J. The scientific case that nicotine is addictive. Psychopharmacology. 1995;117:2–10. doi: 10.1007/BF02245088. doi:10.1007/BF02245088 [DOI] [PubMed] [Google Scholar]
- Tapper A.R, et al. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. doi:10.1126/science.1099420 [DOI] [PubMed] [Google Scholar]
- Wang F, Chen H, Steketee J.D, Sharp B.M. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology. 2007;32:103–109. doi: 10.1038/sj.npp.1301033. doi:10.1038/sj.npp.1301033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S.S, Stinus L, Koob G.F, Markou A. Reward and somatic changes during precipitated nicotine withdrawal in rats: centrally and peripherally mediated effects. J. Pharmacol. Exp. Ther. 2000;292:1053–1064. [PubMed] [Google Scholar]
- Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol. Sci. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. doi:10.1016/0165-6147(90)90242-Z [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. doi:10.1016/S0166-2236(96)10073-4 [DOI] [PubMed] [Google Scholar]