Abstract
Binge drinking is an increasingly recognized problem within the UK. We have studied the relationship of binge drinking to cognitive and emotional functioning in young adults, and have found evidence for increased impulsivity, impairments in spatial working memory and impaired emotional learning. Since in human studies it is difficult to understand whether such behavioural changes pre-date or are a consequence of binge drinking, we have also studied parallel behaviours in a rodent model, in which rats are exposed to intermittent episodes of alcohol consumption and withdrawal. In this model, and in parallel with our findings in human binge drinkers, and alcoholic patients who have undergone multiple episodes of detoxification, we have found evidence for impairments in aversive conditioning as well as increased impulsivity. These behavioural changes are accompanied by facilitated excitatory neurotransmission and reduced plasticity (long-term potentiation (LTP)) in amygdala and hippocampus. The impaired LTP is accompanied by both impaired associative learning and inappropriate generalization of previously learned associations to irrelevant stimuli. We propose that repeated episodes of withdrawal from alcohol induce aberrant neuronal plasticity that results in altered cognitive and emotional competences.
Keywords: alcoholism, withdrawal, conditioning, aberrant plasticity, executive function, anxiety
1. Introduction
Alcohol abuse and dependence are increasingly recognized problems of Western societies. The UK, in particular, has a high incidence of binge drinking, defined as consumption of twice the recommended daily limit of alcohol. According to UK government recommendations, this amount corresponds to eight units (a unit equals 7.9 g alcohol) for men (equivalent to four pints of 5% beer) and six units for women. A similar definition has been used in the USA (five or more drinks (a drink containing 14 g alcohol) per occasion for men, and four or more drinks for women (Wechsler et al. 1994)). Using these definitions, in the UK, men binge drink on 40% of occasions on which they consume alcohol, and women on 22% of such occasions (Drummond et al. 2004), with approximately 5.9 million UK residents drinking at these levels on at least one occasion per annum. Those aged 16–24 are more likely to engage in binge drinking, with 36 and 27% of men and women, respectively, in this age group reporting that they binge drink at least once a week.
Concerns that these definitions of bingeing ignore duration of consumption and blood alcohol concentration (BAC), which are associated with intoxication, led the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to approve the following definition: ‘A ‘binge’ is a pattern of drinking alcohol that brings BAC to about 0.08 gram-per cent or above. For the typical adult, this pattern corresponds to consuming 5 or more drinks (male), or 4 or more drinks (female), in about 2 hours’ (NIAAA 2004). In our own studies of binge drinking, we have used a more behavioural and potentially more conservative approach based on the Alcohol Use Questionnaire (Mehrabian & Russell 1978), which incorporates speed of drinking, and the behavioural measures, ‘numbers of times being drunk in the last six months’ (with drunkenness defined as loss of coordination, nausea and/or the inability to speak clearly, or blackout) and the percentage of times getting drunk when drinking (Townshend & Duka 2002). Although differences in definition of binge drinking may give rise to some confusion both in the scientific literature and among the general public, it is likely that the multiple definitions tap into closely related phenomena, albeit with different sensitivity (Cranford et al. 2006).
An additional characteristic of binge drinking is not only the consumption of large amounts of alcohol within a limited time period, but also the fact that drinking is followed by a period of abstinence (as opposed to regular drinking in which a person might consume similar weekly amounts of alcohol but without the extremes of alcohol intoxication and withdrawal). This pattern of cycles of alcohol intoxication followed by acute episodes of withdrawal may be analogous to a common clinical experience, in which alcoholic patients undergo cycles of alcohol abuse, followed by detoxification, a period of abstinence (that may be very short), followed by relapse, a further period of abuse, and further detoxification treatment. It has long been recognized that such repeated episodes of alcohol abuse and detoxification lead to increased risk of withdrawal-induced seizures (Ballenger & Post 1978), and more recently we, and others, have demonstrated a wide range of cognitive deficits in such patients (Duka et al. 2004). Several of the cognitive deficits we have observed in repeatedly detoxified alcoholic patients are also to be found in young adult binge drinkers (Duka et al. 2004). In studies of alcoholic patients and binge drinkers, it is difficult to determine whether the cognitive and behavioural differences observed are consequences of the drinking patterns, or pre-date excessive consumption. However, by imposing periods of alcohol consumption and withdrawal, we have been able to model several aspects of the cognitive deficit in rodents. These experiments suggest that binge patterns of alcohol consumption in both humans and rats lead to altered function of amygdala and frontal cortices.
2. Evidence of altered cognitive function in binge drinkers
Alcohol itself is known to have long-term effects on prefrontal cortex function (Moselhy et al. 2001; Tarter et al. 2004), while studies of alcoholic patients who have undergone multiple withdrawals suggest that previous experience of detoxification is also associated with prefrontal cortex dysfunction (Duka et al. 2003). We have compared prefrontal cortex function between binge drinkers and non-binge drinkers among heavy social drinkers who were matched for age and IQ. Binge drinkers were impaired in the vigilance task from the Gordon Diagnostic System, a task that challenges the ability to withhold a prepotent response and is thus a measure of impulsivity. Female binge drinkers were particularly impaired in this task, being unable to inhibit their response to the alerting stimulus, suggesting a lack of inhibitory control from the frontal lobes (Townshend & Duka 2005). Age at which heavy drinking started also appeared to play a role in this impairment. Previous studies have also shown impairments in cognitive function associated with heavy drinking during early adolescence (Brown et al. 2000) and that early exposure to binge drinking is associated with frontal lobe damage (Crews et al. 2007).
Increased impulsivity is not always deleterious, and in the same study (Townshend & Duka 2005) we found binge drinkers to be faster on the visual search matching task, a task from the CANTAB test battery that allows a separation between choice and movement time. In this task, participants are required to search among eight similar shapes to match one of them to an identical target shape displayed simultaneously. Binge drinkers showed faster movement time, rather than thinking time, suggestive of a motor impulsivity. Such impulsivity is associated with altered functioning of prefrontal–subcortical circuits, particularly the orbitofrontal circuit (Spinella 2004).
Binge drinking was also found to be associated with impairment in a spatial working memory task from the CANTAB, which is also dependent on prefrontal function (Weissenborn & Duka 2003). We have recently replicated this finding, but in our more recent studies, the deficit was limited to females (Townshend & Duka 2005; J. Scaife & T. Duka 2008, unpublished data; table 1). In our studies, although male binge drinkers are usually found to drink more alcohol than female binge drinkers, their binge scores are lower. Presumably, this reflects a lower tolerance of females, so that female drinkers, although consuming less, may become drunk more often, thus achieving a higher binge score in the Alcohol Use Questionnaire. Thus, it may be less the amount of alcohol consumed than the magnitude of its effect on individuals that predicts impairment of cognitive function. In agreement with our observations, in a study that compared student social drinkers to teetotallers (Randall et al. 2004), high alcohol consumers (especially females) were worse in performing a colour STROOP task, indicating an inability to inhibit a prepotent response, an executive function controlled by prefrontal cortex. A similar conclusion of a relationship between harmful drinking and neurocognitive deficits was derived from Zeigler et al.'s (2005) review of articles identified in a MEDLINE search for articles addressing neurotoxic and neurocognitive effects of harmful drinking among young adolescents and college students.
Table 1.
Binge scores for male and female social drinkers and the number of errors they make when performing in the spatial working memory task from the CANTAB test battery. (Binge drinkers (only females in studies 2 and 3) make more errors than non-bingers. Subjects commit this type of error when, in the process of searching through a spatial array of boxes to collect tokens hidden inside, they return to a box in which a token was previously found.)
| non-bingers | bingers | |||
|---|---|---|---|---|
| males | females | males | females | |
| study 1 (Weissenborn & Duka 2003) | ||||
| binge score | 12.4±0.7 | 12.5±1.0 | 35.8±3.6 | 28.0±2.6 |
| between search errors | 9.6±1.4a | 14.9±3.8a | 15.2±2.5 | 19.0±3.0 |
| study 2 (Townshend & Duka 2005) | ||||
| binge score | 11.3±0.7 | 10.3±0.8 | 37.1±2.9 | 45.5±4.7 |
| between search errors | 11.0±1.6 | 8.5±1.7b | 6.9±1.5 | 14.5±2.4 |
| study 3 (J. Scaife & T. Duka 2008, unpublished data) | ||||
| binge score | 16.6±2.3 | 20.2±1.8 | 56.7±4.1 | 52.1±4.5 |
| between search errors | 8.8±2.9 | 7.25±1.5b | 6.33±1.6 | 14.0±2.5 |
main effect of binge drinking; p<0.05.
p<0.05 compared to the same sex in the bingers group.
Many studies have suggested that prefrontal dysfunction is a predisposing factor to heavy drinking. For instance, in young adult social drinkers, a relationship was found between impaired executive function and both the frequency of drinking to ‘get high’ and ‘get drunk’ (Deckel et al. 1995) and the severity of drinking consequences (Giancola et al. 1996). This consideration makes it difficult to know from our own studies whether the cognitive effects we observe in binge drinkers may have been premorbid. Although impairment in certain cognitive tasks might be the cause of extreme drinking patterns (including binge drinking), data from animals suggest that binge patterns of consumption can also induce cortical damage and aberrant plasticity, and lead to related cognitive deficits (see below). Only a prospective study investigating cognitive performance in adolescents before and after starting binge drinking would clarify these questions.
3. Evidence of altered emotional reactivity in binge drinkers
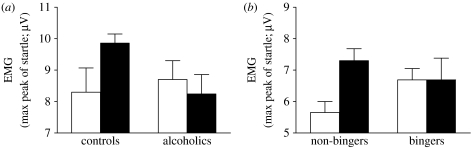
In addition to altered cognitive ability, binge drinking is also associated with changes in emotional competence. Increased negative emotional sensitivity has been recognized in patients following multiple detoxifications for some years (Adinoff et al. 1994; Duka et al. 2002). Related effects can be seen in binge drinkers who also show a lowered positive mood state in their subjective ratings obtained via the Profile of Mood Scale compared with their non-binge drinking counterparts (Townshend & Duka 2005). Deficits in emotional behaviour can also be found in laboratory settings. A recent study has examined conditioned fear. Following training trials, in which participants learned to discriminate an auditory stimulus that predicted an aversive white noise (S+) from a stimulus (S−) that was unpaired with the aversive noise, the ability of the S+ and S− to influence the startle response to an aversive stimulus was assessed. While social drinkers showed the anticipated potentiation of startle in the presence of the S+, there was no differential conditioned response to the S+ and S− in bingers (Stephens et al. 2005). We have seen a similar deficit in patients with a history of multiple detoxifications (figure 1; see table 2 for demographics). Again, although these deficits in learning about an aversive conditioned stimulus (CS) may have preceded the onset of binge drinking, this possibility is made less likely by the fact that similar impairments in aversive conditioning of discrete cues are also found in rats exposed to multiple episodes of high alcohol intake and withdrawal (see below).
Figure 1.
Conditioned fear in (a) alcoholic patients and their control counterparts and (b) human bingeing and non-bingeing social drinkers; groups were matched for age, gender and verbal IQ. Electromyographic activity of the orbicularis oculi muscle (EMG) to an aversive white noise (97 dB) in the presence of an auditory CS+ (filled bars) and CS− (open bars) stimulus of the same intensity (63 dB) but different frequency (900 or 1700 Hz). During training sessions, CS+ was followed by aversive white noise (US) and CS− by nothing. Testing took place in the presence of CS stimuli without reinforcement (test of CS effects) and also when each stimulus (CS+ and CS−) was followed by the white noise startle stimulus (test of CS-induced potentiation of startle). A group×stimulus interaction was found in the comparison between bingers and non-bingers and also between alcoholic patients and controls (F2,32=6.98; p=0.003 and F2,48=4.31; p=0.02, respectively). This interaction was attributable to a higher response to the CS+ compared with the CS− in non-binger and control groups, but not in binger and alcoholic patient groups.
Table 2.
Gender distribution, age, verbal IQ and alcohol history of the group of alcoholic patients and their control counterparts. (The two groups were compared in the potentiated startle response (figure 1). SADQ (Stockwell et al. 1983): Severity of Alcohol Dependence Questionnaire is a 20-item questionnaire for the assessment of the severity of dependence.)
| variables | controls | alcohol patients |
|---|---|---|
| gender (M/F) | 8/5 | 8/5 |
| age | 45 (27–63) | 47 (26–66) |
| SADQ | 1.5 (0–11) | 33.8 (9–65) |
| units of alcohol/week (1 unit=8 g) | 13.9 (0–53) | 253 (126–354) |
| starting age of drinking (years) | 15.5 (14–17) | 17 (14–32) |
| verbal IQ | 111.7 | 109.9 |
Further evidence of altered emotional competence following repeated detoxification is seen in the ability of alcoholic patients to interpret emotions in the facial expressions of others. Thus, when alcoholic patients were presented with a series of emotional facial expressions, they overestimated the amount of fear present if they had already undergone several detoxifications (Townshend & Duka 2003). Perception of fear in facial expressions is associated with activation of the amygdala in functional magnetic resonance imaging studies, and patients who have amygdala lesions show an impaired perception of fear in emotional facial expression (Adolphs et al. 1999; Calder et al. 2001; Morris et al. 1998). Given the similarities between the consequences of amygdala kindling and multiple alcohol detoxifications (Pinel & Van Oot 1975; Pinel et al. 1975; Pinel 1980; Carrington et al. 1984), the increased perception of fear in emotional expressions by alcoholic patients with multiple detoxifications may be the result of a facilitated neurotransmission within the amygdala (Townshend & Duka 2003). Our animal studies would support such an interpretation (see below).
4. Prefrontal–amygdala interactions in alcohol abuse
Many of the behavioural impairments seen in binge drinkers can be ascribed to alterations in the function of amygdala and prefrontal cortical areas (Duka et al. 2003, 2004). Human imaging studies indicate that activity in prefrontal cortex and amygdala is inversely correlated, suggesting that prefrontal cortex may be involved in suppressing amygdala-mediated responses (Hariri et al. 2000). We have speculated that if repeated episodes of withdrawal impair prefrontal function, a consequence might be that such alcoholic patients may be predisposed to recall aversive experiences that are normally suppressed (Stephens et al. 2005).
The loss of the ability of prefrontal cortex to inhibit behaviours mediated by subcortical systems (such as amygdala) is also a major contributor to the loss of control of drug taking in addicts (Volkow et al. 2003), as executive functions, such as the ability to plan and to inhibit habitual tendencies, reflect virtues that are essential for controlling excessive consumption. Thus, impairment of frontal function as a consequence of repeated detoxifications (cycles of high intake followed by periods of withdrawal) or binge drinking (which also leads to frequent high amounts of alcohol in the brain followed by withdrawal) may predispose to uncontrolled consumption and impair resistance to relapse in the abstaining alcoholic, as well as having long-term effects on emotional behaviour.
Clinical experience, as well as animal laboratory experimental studies, indicates that repeated experience of detoxification results in profound behavioural changes associated with neurobiological changes in several brain regions. The best documented of such changes is the increased propensity to seizures experienced following multiple withdrawals. This so-called kindling of convulsant activity has been suggested to reflect changes in the efficiency of nervous transmission in the amygdala (Pinel & Van Oot 1975; Pinel et al. 1975; Pinel 1980; Carrington et al. 1984). The amygdala is crucially implicated in the formation of associations between discrete environmental events and aversive stimuli, and the expression of fear reactions through its projections to brainstem structures governing behavioural, autonomic and endocrine responses to threat. It is thus important whether repeated periods of alcohol exposure and withdrawal also affect emotional competence and Pavlovian conditioning of emotional events.
5. Rodent model of binge patterns of alcohol intake
Many of the behavioural changes seen in binge drinkers can be modelled in the rodent (indeed, some of the deficits we have subsequently described in alcoholics were predicted on the basis of our prior rat studies (Stephens et al. 2001, 2005)). Thus, the increased propensity to show seizures following several episodes of alcohol withdrawal has been routinely demonstrated (Becker & Veatch 2002). Since prior electrical kindling of the amygdala predisposes to withdrawal-induced seizures (Pinel et al. 1975), while repeated episodes of alcohol withdrawal facilitate the development of electrical kindling of the amygdala (Ulrichsen et al. 1998), facilitation of transmission in the amygdala has been viewed as an important consequence of ethanol withdrawal. In our studies, high alcohol intakes are induced in rats by providing them with an alcohol-containing diet as their sole source of nutrition for either 24 days continuously, followed by a two-week withdrawal period (single withdrawal (SWD) group), or with the treatment interrupted by two additional withdrawal periods (repeated withdrawal group). A third, control, group pair fed to the SWD group receives a non-alcoholic diet. As well as increasing seizure sensitivity, such repeated periods of alcohol exposure and withdrawal (compared to equal alcohol intake but a SWD episode) increase the degree of withdrawal-induced neuronal excitability, as measured by c-fos expression, in several brain areas including amygdala, hippocampus, ventral striatum, periaqueductal grey (Borlikova et al. 2006b) and frontal cortical areas (L. Hoang & D. N. Stephens 2008, unpublished data).
6. Effects of ethanol withdrawal on amygdala function
Consistent with altered amygdala function, repeated experience of withdrawal results in impairment, several weeks after cessation of the alcohol treatment, in acquiring a conditioned emotional response (CER), in which, in control animals, presentation of tone or flashing light conditioned stimuli (CS+) that predicted mild footshock resulted in the suppression of ongoing instrumental behaviour (Stephens et al. 2001). When the shock intensity was increased in steps over a period of five weeks, the repeatedly withdrawn (RWD) rats eventually showed some evidence of behavioural suppression in response to the CS+. Whether this eventual acquisition reflected the higher shock levels or the prolonged training period or recovery of function is not clear. However, it is unlikely that the deficit in learning the CS-shock association reflected insensitivity to shock, as no differences were seen between RWD and control rats in the acquisition of contextual fear conditioning (Borlikova et al. 2006a), which depends upon intact processing within the hippocampus (Selden et al. 1991; Fendt & Fanselow 1999; Bannerman et al. 2001), while the formation of associations between shock and discrete cues such as tones or lights is processed within the amygdala (Selden et al. 1991; Killcross et al. 1997; Fanselow & LeDoux 1999). Furthermore, if training on the conditional emotional response task took place prior to alcohol exposure and withdrawal, then the repeated withdrawal rats were not impaired in expression of the CER, suggesting that the effects of withdrawal are in learning the relationship between the CS+ and the shock, rather than in them having blunted fear responses (Ripley et al. 2003). Interestingly, however, the repeated withdrawal animals were impaired in extinguishing the CS+-shock association when the CS+ was presented repeatedly in the absence of the shock reinforcer (Ripley et al. 2003), and in a reversal experiment, in which the CS+ and CS− stimuli, trained prior to alcohol exposure, were switched and retrained following repeated alcohol withdrawal treatment. This series of experiments suggests that the repeated periods of alcohol exposure and withdrawal impair the learning of new associations, but that, if the associations have been learned prior to alcohol exposure, there is no impairment in the expression of the conditioned response.
According to one model, fear conditioning depends upon information processing in the amygdala; as a result of conditioning, the CS+ gains access to the lateral amygdala's outflow to the central nucleus (Fanselow & LeDoux 1999), which in turn induces activity in output pathways eliciting diverse symptoms of fear and anxiety. In keeping with this model, an acoustic signal, previously conditioned to shock, increased the number of neurons showing c-fos immunoreactivity in the central and basal nuclei of the amygdala (Beck & Fibiger 1995; Hall et al. 2001b). Consistent with those findings, high levels of c-fos expression were seen in both control and SWD animals in the core and shell of the accumbens and in the basolateral and central nuclei of the amygdala after exposure of rats to a tone CS+ previously paired with shock, but c-fos was expressed in fewer neurons in the RWD group (Stephens et al. 2005). Thus, repeated periods of alcohol exposure and withdrawal (but not simply an equivalent amount of alcohol exposure) impair the formation of associations between a tone stimulus and an aversive event, consistent with the behavioural observations (Stephens et al. 2001; Ripley et al. 2003). That the deficit occurred at the level of conditioned activation of amygdala neurons indicates that the deficit seen in a CER following repeated withdrawal is in forming the CS–shock association, rather than an inability to control the behavioural output.
These observations are commensurate with altered transmission within the amygdala, though there appear to be differences between the consequences of repeated ethanol exposure and withdrawal and electrical kindling. Although there are similarities between electrical kindling of seizures and alcohol withdrawal seizures, in the case of fear conditioning, repeated alcohol withdrawal and electrical kindling of the basolateral amygdala have opposite effects, since electrical kindling facilitates fear conditioning to a discrete cue (Ripley et al. 2003). However, it should be noted that although the lateral part of the amygdala plays an important role in fear conditioning as the area that receives input regarding both aversive events, and associated cues (Fanselow & LeDoux 1999), and then provides inputs to the central nucleus, recent studies suggest that the central nucleus may also function independently of the lateral nuclei, receiving highly processed sensory input from entorhinal cortex and related areas (see Killcross et al. 1997). It thus seems possible that the major effects of repeated withdrawal from alcohol on fear conditioning are mediated by the central amygdala, rather than its lateral aspects.
7. Mechanisms underlying effects of withdrawal
By what mechanism does repeated alcohol withdrawal lead to impaired fear conditioning? Long-term potentiation (LTP) has been proposed as a mechanism whereby synaptic transmission is facilitated as a result of use. In associative LTP, transmission in the pathway carrying information regarding the CS+ is facilitated as a result of it being activated contemporaneously with the pathway signalling the unconditioned stimulus (US; Maren 2005; Sigurdsson et al. 2007). In support of this kind of mechanism underlying fear conditioning, LTP is found in the pathway from the medial geniculate body to the lateral nucleus of the amygdala, which is thought to mediate conditioning of fear responses to acoustic stimuli, and tetanic stimulation of the medial geniculate body also results in a long-lasting potentiation of a field potential in the lateral amygdala elicited by a naturally transduced acoustic stimulus (Rogan & LeDoux 1995; Rogan et al. 1997). The stimulation coincidence parameters that are necessary for induction of LTP in the lateral amygdala closely resemble those required for the formation of associations between CS and US in fear conditioning experiments (Bauer et al. 2001). Taken together, these experiments suggest that LTP-like mechanisms underlie amygdala-mediated fear conditioning (Blair et al. 2001). Why then should alcohol withdrawal affect such a mechanism?
Acute alcohol treatment is associated with the facilitation of GABAergic inhibitory mechanisms (Samson & Harris 1992; Roberto et al. 2004a), while alcohol also acts as an antagonist of glutamatergic N-methyl-d-aspartate (NMDA) receptors (Samson & Harris 1992). During chronic alcohol exposure, transmission in glutamatergic systems is facilitated (to compensate for these two major actions of alcohol), both through increased NMDA receptor sensitivity (Roberto et al. 2004b) and increased glutamate turnover (Dahchour & De Witte 1999), resulting in partial tolerance to alcohol's sedative effects. Following withdrawal from alcohol, the glutamatergic system continues to be overactive (Dahchour & De Witte 1999), while NMDA receptor function remains elevated (Roberto et al. 2004b) but this overactivity is no longer balanced by alcohol's effects on GABAergic systems.
Several pieces of evidence indicate that intermittent exposure to ethanol and withdrawal facilitates glutamatergic synaptic transmission in both central (Roberto et al. 2006) and basolateral amygdala (Floyd et al. 2003; Lack et al. 2007). An increased probability of glutamate release from the presynaptic terminal (Lack et al. 2007), and increased postsynaptic NMDA receptor function (Roberto et al. 2006; Lack et al. 2007) may lead to increased postsynaptic AMPA receptor function (Lack et al. 2007). We suggest that this imbalance towards glutamatergic excitatory transmission might have consequences similar to overactivation of glutamatergic synapses that occur during LTP.
The apparent paradox of heightened seizure sensitivity and exaggerated anxiety responses during withdrawal, but impaired fear conditioning, could then be accounted for if repeated experience of withdrawal induces synaptic plasticity, resulting in facilitated transmission in glutamatergic pathways, but reduced capacity for further plasticity necessary for learning. Information regarding discrete cues, such as the CSs in our experiments, are relayed to the lateral amygdala from sensory cortex and sensory thalamus (Pitkanen et al. 1997). LTP is found in the pathway from the external capsule to the lateral nucleus of the amygdala (Chapman et al. 1990) and high-frequency stimulation of the medial geniculate input to the amygdala also results in a long-lasting potentiation of a field potential in the lateral amygdala elicited by a naturally transduced acoustic stimulus (Rogan et al. 1997).
We therefore compared excitability and plasticity in the amygdala of rats that had undergone repeated, or a single, withdrawal. Field potentials in the lateral amygdala increased monotonically with increased intensity of stimulation of the external capsule accessory pathway, and these input–output curves were shifted to the left in slices from rats that had undergone repeated withdrawal, consistent with increased efficiency of synaptic transmission. Such changes could, in principle, account for increased sensitivity to seizures following repeated withdrawal. Furthermore, such increased efficiency might imply that fear-related stimuli activating these pathways might be more effective in eliciting anxious responses following repeated periods of alcohol exposure and withdrawal, as has been reported in both humans (George et al. 1990; Krystal et al. 1997) and in some (Overstreet et al. 2002), but not all animal models of anxiety (Borlikova et al. 2006b; Ripley et al. 2003).
As well as leftward shifts in the input–output curves, repeated withdrawal reduced the ability to support LTP in the lateral amygdala response to high-frequency stimulation of the external capsule. In the case of the lateral amygdala, both SWD and repeated withdrawal groups showed equally reduced capacity for LTP (Stephens et al. 2005). These observations are consistent with reduced capacity for associative learning following repeated periods of alcohol exposure and withdrawal. However, while both SWD and repeated withdrawal treatments gave rise to similar size reductions in LTP, in our behavioural experiments using fear conditioning, we have found repeated withdrawal treatment to impair conditioning more than SWD treatment (Stephens et al. 2001; Ripley et al. 2003). Nevertheless, these electrophysiological data provide an interesting parallel to the conditioning deficits, and the entire set of electrophysiological and behavioural data might be reconciled by suggesting that repeated withdrawal increases efficiency of synaptic connections, leading to facilitation of synaptic transmission, but reduced capacity for further plasticity. Consistent with that interpretation, while several withdrawal episodes result in increased levels of c-fos expression in central amygdala relative to rats that have undergone only a SWD, in the case of another immediate early gene, zif-268, a marker of synaptic plasticity (Hall et al. 2001a), increases are seen following a SWD, but not if the animals have undergone prior withdrawal experience (Borlikova et al. 2006b).
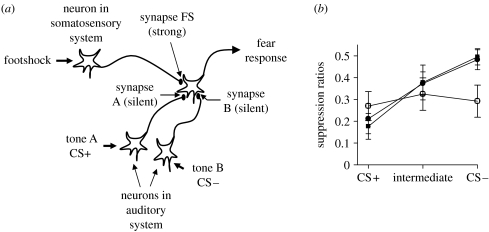
This aberrant plasticity hypothesis gives rise to an interesting prediction. Figure 2a illustrates a conventional account of conditioning. According to this model, activation of a neural system carrying information regarding a strong stimulus (such as a shock) is able to activate pathways leading to an unconditioned behavioural output (such as response suppression), while pathways carrying information regarding a weak biologically neutral stimulus, such as a mild tone, are unable to gain access to neural pathways subserving the behavioural output. However, if the tone pathway is active at the same time as the shock pathway, then as a consequence of associative processes, such as LTP, the connection between the tone pathway and the output pathway will be strengthened so that eventually the tone will itself become capable of eliciting the behavioural output, independent of the shock. Physiological accounts of such associative learning posit that it occurs as a consequence of LTP (Maren 2005). Although the exact mechanisms underlying induction and expression of LTP in amygdala remain ambiguous (Kim & Jung 2006), a conventional model holds that synapses carrying the weak signal are initially ‘silent’ (Liao et al. 1995), possibly because they employ only NMDA receptors that are blocked by the presence of magnesium ions in the channel. However, signalling in the US (shock) pathway synapses is postulated to be mediated by glutamate acting at AMPA receptors (Maren 2005; Sigurdsson et al. 2007) that are not subject to magnesium block. On occasions when both the tone CS and the US pathways are activated concurrently, membrane depolarization elicited by the US pathway will allow the magnesium block in neighbouring NMDA receptors (including those in the CS pathway) to be removed, allowing glutamate release in this pathway to cause postsynaptic depolarization via NMDA receptors that will then initiate processes underlying LTP; subsequently, activation of the tone pathway will be effective in activating the behavioural output. Although the details of the mechanisms underlying amygdala LTP remain to be elucidated, we postulate that during withdrawal, enhanced glutamate release will occur in many synapses, activating processes that serve LTP (e.g. insertion of AMPA receptors into hitherto silent synapses). Presumably, such synaptic strengthening would have at least two consequences; firstly, withdrawal-strengthened synapses would no longer be silent, and will not be available for the formation of new associations; secondly, natural events activating pathways that were already strengthened by withdrawal would gain access to output pathways in the absence of conditioning. The former consequence might explain why RWD rats and binge-drinking humans fail to show evidence of fear conditioning (Stephens et al. 2001, 2005). The second consequence predicts that once conditioning has occurred, then other neutral stimuli might gain access to the output pathways via inappropriately withdrawal-strengthened synapses.
Figure 2.
(a) The aberrant plasticity model of withdrawal-induced deficits in associative conditioning. Prior to learning, activation of synapse FS as a consequence of footshock-induced activity in somatosensory systems leads to activation of an output neuron giving rise to a fear response. Activation of an auditory neuron by tone A is unable to gain access to the output neuron subserving the fear response, as synapse A is ‘silent’ at this stage. During associative learning, if the neuron carrying information about tone A (CS+) is active at the same time as the neuron carrying information regarding the footshock, then as a result of activation of the synapse FS, local depolarization will occur, allowing activity at synapse A also to induce depolarization in the postsynaptic membrane. Consequently, synapse A will be strengthened, so that the activation of the tone A pathway will now gain access to the output pathway, i.e. associative conditioning has occurred. Synapse B remains silent as it is never activated at the same time as synapse FS. However, if alcohol withdrawal induces activation of synapse B as well as of synapse FS, then synapse B should also be strengthened, so that tone B might now gain access to the output pathway, even though it has never been paired with footshock. Furthermore, if withdrawal-induced synaptic strengthening occurs prior to conditioning, then synapse A will already have been strengthened, and will no longer be available for conditioning. (b) Suppression ratios (a measure of conditioned fear) in RWD rats (squares), given the same exposure to alcohol, but only a SWD (filled circles), or rats fed a non-alcohol control diet (CON (open circles)). The rats were trained prior to alcohol treatment to associate a tone CS+ with footshock, so that the CS+ caused a suppression of behaviour (giving suppression ratio values less than 0.5). The CS− was an alternative tone signal, that did not predict shock, and which therefore did not suppress behaviour (giving a suppression ratio of approx. 0.5). Two weeks following the final day of alcohol treatment, the rats were once again presented with the CS+, as well as the CS−, and a novel tone, intermediate between the CS+ and the CS−. The SWD and control rats continued to show suppression to the CS+, but not to the CS−, with an intermediate degree of suppression to the novel tone. By contrast, the RWD rats showed equal suppression to all three tones (adapted from Stephens et al. 2005).
We tested this idea by training the rats to associate one of two tones (CS+) with shock, in a conditioned emotional response test. The other tone (CS−) was not paired with shock. After training, presentation of the CS+, but not the CS−, resulted in the suppression of ongoing instrumental behaviour. The rats were then matched for performance, and allocated to treatment groups with repeated withdrawal episodes, or a SWD episode, or control treatment. Following two weeks recovery, the rats were tested once again in the conditioned emotional response test. As shown in figure 2b, while both the control group and SWD group behaved appropriately in showing suppression to the CS+ but not the CS−, and an intermediate suppression to a novel tone of an intermediate frequency, the RWD rats showed equal suppression to all three tones, consistent with aberrant plasticity having taken place, allowing the CS− access to the behavioural output (Stephens et al. 2005).
The results described here refer to aversive conditioning, but similar mechanisms may underlie appetitive conditioning. Thus, repeated withdrawal experience leads to deficits in aspects of appetitive conditioning, including Pavlovian-to-instrumental transfer (Ripley et al. 2004). Taken together, these findings suggest a mechanism whereby chronic alcohol treatment and withdrawal may lead to a deficit in functioning of the amygdala with consequences for associative learning. Such deficits may have implications for the use of conditioning approaches to behavioural therapies for alcoholics.
8. Effects of ethanol withdrawal on frontal cortical function in rodents
The amygdala is connected with many brain structures, and the extent to which the effects of repeated withdrawal are due to interference with the amygdala itself, or with its connections, is not clear. Of particular interest in the study of cognitive impairments resulting from repeated periods of alcohol exposure and withdrawal are connections to the prefrontal cortex and hippocampus. Although there is good evidence that repeated intermittent ethanol administration, or repeated withdrawal, results in both physiological (Stephens et al. 2005) and pathological (Obernier et al. 2002a,b) changes in hippocampus, the limited evidence available has so far failed to demonstrate a marked impairment in behaviours, such as spatial learning (Borlikova et al. 2006a; Obernier et al. 2002b), or contextual conditioning (Borlikova et al. 2006a), thought to be mediated by hippocampal processes.
Borlikova et al. (2006b), however, did find a marked impairment in a negative patterning task (Bussey et al. 2000), in which rats were required to initiate a response when either a light or a tone stimulus was presented, but to inhibit the response when both stimuli were presented simultaneously. Although initially proposed as a test of the ability of rats to integrate information from different sensory modalities, and thus mediated by hippocampus (Rudy & Sutherland 1989), others (Gallagher & Holland 1992; Davidson et al. 1993; Bussey et al. 2000; Moreira & Bueno 2003) have not found an influence of hippocampal lesions, and it seems that deficits in performing the task may relate to an inability to withhold responding (Whishaw & Tomie 1991; Davidson et al. 1993; Blackburn & Hevenor 1996; Richmond et al. 1997; Papadimitriou & Wynne 1999) rather than by disruption of configural association. We are therefore inclined to interpret our negative patterning data as revealing changes in responsiveness following repeated episodes of withdrawal. This kind of deficit might have more in common with alterations in frontal cortical function than hippocampus. Using a different rat model of binge drinking, Crews et al. (2000) reported that young adolescent rats (approx. 35 days old) show increased levels of amino cupric silver staining (indicating neuronal cell death) in frontal areas following exposure to a binge pattern of alcohol consumption. These results would be consistent with observations in human alcoholics and binge drinkers who show impaired cognitive function in executive control tasks sensitive to dysfunction of prefrontal cortex (Duka et al. 2003, 2004; Weissenborn & Duka 2003; Townshend & Duka 2005).
There are strong interactions between amygdala and prefrontal cortex in determining behavioural output. Recent human imaging studies indicate that activity in prefrontal cortex and amygdala are inversely correlated, so that prefrontal cortex may be involved in suppressing amygdala-mediated fear responses (Hariri et al. 2000). Thus, withdrawal-induced changes in prefrontal cortex function might predispose alcoholics to retain fear experiences that are suppressed in normal people. Glutamatergic projections from the medial prefrontal cortex activate GABAergic interneurons in the amygdala (Grace & Rosenkranz 2002; Rosenkranz & Grace 2002), which leads to a reduction in the firing rate of neurons in the basolateral amygdala. This process is believed to be vital in the control of responsivity to conditioned stimuli, so that a decrease in activity in this inhibitory pathway may lead to overexpression of conditioned behaviours and may underlie some aspects of pathological conditions, such as anxiety and drug abuse. Conversely, stimulation of infralimbic prefrontal cortex neurons results in low levels of conditioned behaviour in animals (Milad & Quirk 2002). Connections between the orbitofrontal cortex and the basolateral amygdala may be vital for assessing the incentive value of cues associated with appetitive or aversive reinforcers, with neurons in these regions firing selectively to cues based on their associative strengths. Due to the time course of the acquisition of firing patterns to these cues, it has been suggested that the basolateral amygdala encodes the motivational significance of the cues and then the orbitofrontal cortex uses this information to select and execute the correct behavioural strategy (Schoenbaum et al. 1999).
Exposure to repeated withdrawal results in excessive activation of several subregions of prefrontal cortex (as seen in our c-fos-expression studies; Hoang & Stephens, unpublished data), though whether such excessive activation would result in excitotoxicity as seen by Crews et al. (2000) in piriform and perirhinal cortices, or facilitated excitatory transmission as we have seen in the lateral amygdala (Stephens et al. 2005) is unclear. Behaviourally, we have found evidence that the RWD rats are impaired in suppressing prepotent responses (Stephens et al. 2001; Borlikova et al. 2006a), showing shorter latencies in initiating inappropriate responses than control rats, even when the controls fail to inhibit the response (Borlikova et al. 2006a). This impairment in suppressing a prepotent response is reminiscent of the poor performance of binge drinkers and multiply detoxified alcoholic patients in the Gordon Diagnostic Adult Vigilance task (Duka et al. 2003; Townshend & Duka 2005).
Impaired frontal function is often associated with the loss of control over drug taking. An interesting speculation is whether such changes as we have observed might predict that binge drinking itself leads to the loss of control over alcohol consumption. In that context, it may be important that we (Brown et al. 1998) and others (Schulteis et al. 1996) have published evidence that previous episodes of ethanol exposure and withdrawal lead to facilitated responding for ethanol rewards, as well as facilitated reinstatement of extinguished responding for ethanol by drug-related cues (Ciccocioppo et al. 2003).
In summary, we have found, both in human binge drinkers and in an animal model of binge patterns of alcohol intake, behavioural evidence for altered function of prefrontal cortex and amygdala. Such changes may reflect aberrant plasticity induced by repeated periods of alcohol exposure and withdrawal in neuronal systems subserving conditioning, and resulting in both hyperactivity of these neural systems and impaired associative learning.
Acknowledgments
Studies with human volunteers and patients were carried out following ethical review by UK institutional ethical committees. Animal studies were carried out under the authority of the UK Animal (Experimental Procedures) Act, 1986.
The research of the authors described in this review was supported by the UK Medical Research Council. We gratefully acknowledge the contributions of our colleagues Tamzin Ripley, Julia Townshend, Gilyana Borlikova, Doris Albrecht, Ruth Weissenborn, Julie LeMerrer, Lee Hogarth, Leigh Hoang and Jess Scaife.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘The neurobiology of addiction: new vistas’.
References
- Adinoff B, O'Neill K, Ballenger J.C. Alcohol withdrawal and limbic kindling. Am. J. Addict. 1994;4:5–17. [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young A.W, Calder A.J, Phelps E.A, Anderson A, Lee G.P, Damasio A.R. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. doi:10.1016/S0028-3932(99)00039-1 [DOI] [PubMed] [Google Scholar]
- Ballenger J.C, Post R.M. Kindling as a model for alcohol withdrawal syndromes. Br. J. Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bannerman D.M, Yee B.K, Lemaire M, Jarrard L, Iversen S.D, Rawlins J.N, Good M.A. Contextual fear conditioning is disrupted by lesions of the subcortical, but not entorhinal, connections to the hippocampus. Exp. Brain Res. 2001;141:304–311. doi: 10.1007/s002210100869. doi:10.1007/s002210100869 [DOI] [PubMed] [Google Scholar]
- Bauer E.P, LeDoux J.E, Nader K. Fear conditioning and LTP in the lateral amygdala are sensitive to the same stimulus contingencies. Nat. Neurosci. 2001;4:687–688. doi: 10.1038/89465. doi:10.1038/89465 [DOI] [PubMed] [Google Scholar]
- Beck C.H, Fibiger H.C. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H.C, Veatch L.M. Effects of lorazepam treatment for multiple ethanol withdrawals in mice. Alcohol. Clin. Exp. Res. 2002;26:371–380. [PubMed] [Google Scholar]
- Blackburn J.R, Hevenor S.J. Amphetamine disrupts negative patterning but does not produce configural association deficits on an alternative task. Behav Brain Res. 1996;80:41–49. doi: 10.1016/0166-4328(96)00017-4. doi:10.1016/0166-4328(96)00017-4 [DOI] [PubMed] [Google Scholar]
- Blair H.T, Schafe G.E, Bauer E.P, Rodrigues S.M, LeDoux J.E. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. doi:10.1101/lm.30901 [DOI] [PubMed] [Google Scholar]
- Borlikova G.G, Elbers N.A, Stephens D.N. Repeated withdrawal from ethanol spares contextual fear conditioning and spatial learning but impairs negative patterning and induces over-responding: evidence for effect on frontal cortical but not hippocampal function? Eur. J. Neurosci. 2006a;24:205–216. doi: 10.1111/j.1460-9568.2006.04901.x. doi:10.1111/j.1460-9568.2006.04901.x [DOI] [PubMed] [Google Scholar]
- Borlikova G.G, Le Merrer J, Stephens D.N. Previous experience of ethanol withdrawal increases withdrawal-induced c-fos expression in limbic areas, but not withdrawal-induced anxiety and prevents withdrawal-induced elevations in plasma corticosterone. Psychopharmacology (Berl.) 2006b;185:188–200. doi: 10.1007/s00213-005-0301-3. doi:10.1007/s00213-005-0301-3 [DOI] [PubMed] [Google Scholar]
- Brown G, Jackson A, Stephens D.N. Effects of repeated withdrawal from chronic ethanol on oral self-administration of ethanol on a progressive ratio schedule. Behav. Pharmacol. 1998;9:149–161. [PubMed] [Google Scholar]
- Brown S.A, Tapert S.F, Granholm E, Delis D.C. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol. Clin. Exp. Res. 2000;24:164–171. doi:10.1111/j.1530-0277.2000.tb04586.x [PubMed] [Google Scholar]
- Bussey T.J, Dias R, Redhead E.S, Pearce J.M, Muir J.L, Aggleton J.P. Intact negative patterning in rats with fornix or combined perirhinal and postrhinal cortex lesions. Exp. Brain Res. 2000;134:506–519. doi: 10.1007/s002210000481. doi:10.1007/s002210000481 [DOI] [PubMed] [Google Scholar]
- Calder A.J, Lawrence A.D, Young A.W. Neuropsychology of fear and loathing. Nat. Rev. Neurosci. 2001;2:352–363. doi: 10.1038/35072584. doi:10.1038/35072584 [DOI] [PubMed] [Google Scholar]
- Carrington C.D, Ellinwood E.H, Jr, Krishnan R.R. Effects of single and repeated alcohol withdrawal on kindling. Biol. Psychiatry. 1984;19:525–537. [PubMed] [Google Scholar]
- Chapman P.F, Kairiss E.W, Keenan C.L, Brown T.H. Long-term synaptic potentiation in the amygdala. Synapse. 1990;6:271–278. doi: 10.1002/syn.890060306. doi:10.1002/syn.890060306 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl.) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. doi:10.1007/s00213-002-1380-z [DOI] [PubMed] [Google Scholar]
- Cranford J.A, McCabe S.E, Boyd C.J. A new measure of binge drinking: prevalence and correlates in a probability sample of undergraduates. Alcohol. Clin. Exp. Res. 2006;30:1896–1905. doi: 10.1111/j.1530-0277.2006.00234.x. doi:10.1111/j.1530-0277.2006.00234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F.T, Braun C.J, Hoplight B, Switzer R.C, III, Knapp D.J. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol. Clin. Exp. Res. 2000;24:1712–1723. doi:10.1111/j.1530-0277.2000.tb01973.x [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol. Biochem. Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. doi:10.1016/j.pbb.2006.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol. Clin. Exp. Res. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. doi:10.1111/j.1530-0277.1999.tb04063.x [DOI] [PubMed] [Google Scholar]
- Davidson T.L, McKernan M.G, Jarrard L.E. Hippocampal lesions do not impair negative patterning: a challenge to configural association theory. Behav. Neurosci. 1993;107:227–234. doi: 10.1037//0735-7044.107.2.227. doi:10.1037/0735-7044.107.2.227 [DOI] [PubMed] [Google Scholar]
- Deckel A.W, Bauer L, Hesselbrock V. Anterior brain dysfunctioning as a risk factor in alcoholic behaviors. Addiction. 1995;90:1323–1334. doi: 10.1046/j.1360-0443.1995.901013234.x. doi:10.1111/j.1360-0443.1995.tb03550.x [DOI] [PubMed] [Google Scholar]
- Drummond C, et al. Department of Health; London, UK: 2004. Alcohol needs assessment project. [Google Scholar]
- Duka T, Townshend J.M, Collier K, Stephens D.N. Kindling of withdrawal: a study of craving and anxiety after multiple detoxifications in alcoholic inpatients. Alcohol. Clin. Exp. Res. 2002;26:785–795. [PubMed] [Google Scholar]
- Duka T, Townshend J.M, Collier K, Stephens D.N. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol. Clin. Exp. Res. 2003;27:1563–1572. doi: 10.1097/01.ALC.0000090142.11260.D7. doi:10.1097/01.ALC.0000090142.11260.D7 [DOI] [PubMed] [Google Scholar]
- Duka T, Gentry J, Malcolm R, Ripley T.L, Borlikova G, Stephens D.N, Veatch L.M, Becker H.C, Crews F.T. Consequences of multiple withdrawals from alcohol. Alcohol. Clin. Exp. Res. 2004;28:233–246. doi: 10.1097/01.alc.0000113780.41701.81. doi:10.1097/01.ALC.0000113780.41701.81 [DOI] [PubMed] [Google Scholar]
- Fanselow M.S, LeDoux J.E. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. doi:10.1016/S0896-6273(00)80775-8 [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow M.S. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. doi:10.1016/S0149-7634(99)00016-0 [DOI] [PubMed] [Google Scholar]
- Floyd D.W, Jung K.Y, McCool B.A. Chronic ethanol ingestion facilitates N-methyl-d-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J. Pharmacol. Exp. Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. doi:10.1124/jpet.103.057505 [DOI] [PubMed] [Google Scholar]
- Gallagher M, Holland P.C. Preserved configural learning and spatial learning impairment in rats with hippocampal damage. Hippocampus. 1992;2:81–88. doi: 10.1002/hipo.450020111. doi:10.1002/hipo.450020111 [DOI] [PubMed] [Google Scholar]
- George D.T, Nutt D.J, Dwyer B.A, Linnoila M. Alcoholism and panic disorder: is the comorbidity more than coincidence? Acta Psychiatr. Scand. 1990;81:97–107. doi: 10.1111/j.1600-0447.1990.tb06460.x. doi:10.1111/j.1600-0447.1990.tb06460.x [DOI] [PubMed] [Google Scholar]
- Giancola P.R, Zeichner A, Yarnell J.E, Dickson K.E. Relation between executive cognitive functioning and the adverse consequences of alcohol use in social drinkers. Alcohol. Clin. Exp. Res. 1996;20:1094–1098. doi: 10.1111/j.1530-0277.1996.tb01952.x. doi:10.1111/j.1530-0277.1996.tb01952.x [DOI] [PubMed] [Google Scholar]
- Grace A.A, Rosenkranz J.A. Regulation of conditioned responses of basolateral amygdala neurons. Physiol. Behav. 2002;77:489–493. doi: 10.1016/s0031-9384(02)00909-5. doi:10.1016/S0031-9384(02)00909-5 [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas K.L, Everitt B.J. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J. Neurosci. 2001a;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas K.L, Everitt B.J. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur. J. Neurosci. 2001b;13:1453–1458. doi: 10.1046/j.0953-816x.2001.01531.x. doi:10.1046/j.0953-816x.2001.01531.x [DOI] [PubMed] [Google Scholar]
- Hariri A.R, Bookheimer S.Y, Mazziotta J.C. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. doi:10.1097/00001756-200001170-00009 [DOI] [PubMed] [Google Scholar]
- Killcross S, Robbins T.W, Everitt B.J. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature. 1997;388:377–380. doi: 10.1038/41097. doi:10.1038/41097 [DOI] [PubMed] [Google Scholar]
- Kim J.J, Jung M.W. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci. Biobehav. Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. doi:10.1016/j.neubiorev.2005.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal J.H, Webb E, Grillon C, Cooney N, Casal L, Morgan C.A, III, Southwick S.M, Davis M, Charney D.S. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP) Psychopharmacology (Berl.) 1997;131:207–215. doi: 10.1007/s002130050285. doi:10.1007/s002130050285 [DOI] [PubMed] [Google Scholar]
- Lack A.K, Diaz M.R, Chappell A, DuBois D.W, McCool B.A. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J. Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. doi:10.1152/jn.00189.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler N.A, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. doi:10.1038/375400a0 [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. doi:10.1016/j.neuron.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Mehrabian A, Russell J.A. A questionnaire measure of habitual alcohol use. Psychol. Rep. 1978;43:803–806. doi: 10.2466/pr0.1978.43.3.803. [DOI] [PubMed] [Google Scholar]
- Milad M.R, Quirk G.J. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. doi:10.1038/nature01138 [DOI] [PubMed] [Google Scholar]
- Moreira R.C, Bueno J.L. Conditional discrimination learning and negative patterning in rats with neonatal hippocampal lesion induced by ionizing radiation. Behav. Brain Res. 2003;138:29–44. doi: 10.1016/s0166-4328(02)00227-9. doi:10.1016/S0166-4328(02)00227-9 [DOI] [PubMed] [Google Scholar]
- Morris J.S, Friston K.J, Buchel C, Frith C.D, Young A.W, Calder A.J, Dolan R.J. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. doi:10.1093/brain/121.1.47 [DOI] [PubMed] [Google Scholar]
- Moselhy H.F, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- NIAAA. Binge drinking defined. NIAAA Newslett. 2004;3:3. [Google Scholar]
- Obernier J.A, Bouldin T.W, Crews F.T. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol. Clin. Exp. Res. 2002a;26:547–557. doi:10.1111/j.1530-0277.2002.tb02573.x [PubMed] [Google Scholar]
- Obernier J.A, White A.M, Swartzwelder H.S, Crews F.T. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol. Biochem. Behav. 2002b;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. doi:10.1016/S0091-3057(02)00715-3 [DOI] [PubMed] [Google Scholar]
- Overstreet D.H, Knapp D.J, Breese G.R. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol. Clin. Exp. Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou A, Wynne C.D. Preserved negative patterning and impaired spatial learning in pigeons (Columba livia) with lesions of the hippocampus. Behav. Neurosci. 1999;113:683–690. doi:10.1037/0735-7044.113.4.683 [PubMed] [Google Scholar]
- Pinel J.P. Alcohol withdrawal seizures: implications of kindling. Pharmacol. Biochem. Behav. 1980;13(Suppl. 1):225–231. doi: 10.1016/s0091-3057(80)80034-7. [DOI] [PubMed] [Google Scholar]
- Pinel J.P, Van Oot P.H. Generality of the kindling phenomenon: some clinical implications. Can. J. Neurol. Sci. 1975;2:467–475. doi: 10.1017/s0317167100020618. [DOI] [PubMed] [Google Scholar]
- Pinel J.P, Van Oot P.H, Mucha R.F. Intensification of the alcohol withdrawal syndrome by repeated brain stimulation. Nature. 1975;254:510–511. doi: 10.1038/254510a0. doi:10.1038/254510a0 [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux J.E. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. doi:10.1016/S0166-2236(97)01125-9 [DOI] [PubMed] [Google Scholar]
- Randall D.C, Elsabagh S.M, Hartley D.E, File S.E. Does drinking have effects on mood and cognition in male and female students? Pharmacol. Biochem. Behav. 2004;78:629–638. doi: 10.1016/j.pbb.2004.04.029. doi:10.1016/j.pbb.2004.04.029 [DOI] [PubMed] [Google Scholar]
- Richmond M.A, Nichols B.P, Deacon R.M, Rawlins J.N. Effects of scopolamine and hippocampal lesions on negative patterning discrimination performance in rats. Behav. Neurosci. 1997;111:1217–1227. doi: 10.1037//0735-7044.111.6.1217. doi:10.1037/0735-7044.111.6.1217 [DOI] [PubMed] [Google Scholar]
- Ripley T.L, O'Shea M, Stephens D.N. Repeated withdrawal from ethanol impairs acquisition but not expression of conditioned fear. Eur. J. Neurosci. 2003;18:441–448. doi: 10.1046/j.1460-9568.2003.02759.x. doi:10.1046/j.1460-9568.2003.02759.x [DOI] [PubMed] [Google Scholar]
- Ripley T.L, Borlikova G, Lyons S, Stephens D.N. Selective deficits in appetitive conditioning as a consequence of ethanol withdrawal. Eur. J. Neurosci. 2004;19:415–425. doi: 10.1111/j.0953-816x.2003.03114.x. doi:10.1111/j.0953-816X.2003.03114.x [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba S.G, Stouffer D.G, Parsons L.H, Siggins G.R. Increased GABA release in the central amygdala of ethanol-dependent rats. J. Neurosci. 2004a;24:10 159–10 166. doi: 10.1523/JNEUROSCI.3004-04.2004. doi:10.1523/JNEUROSCI.3004-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba S.G, Stouffer D.G, Parsons L.H, Siggins G.R. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J. Neurosci. 2004b;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. doi:10.1523/JNEUROSCI.5077-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba S.G, Siggins G.R. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacology. 2006;31:988–996. doi: 10.1038/sj.npp.1300840. doi:10.1038/sj.npp.1300840 [DOI] [PubMed] [Google Scholar]
- Rogan M.T, LeDoux J.E. LTP is accompanied by commensurate enhancement of auditory-evoked responses in a fear conditioning circuit. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. doi:10.1016/0896-6273(95)90070-5 [DOI] [PubMed] [Google Scholar]
- Rogan M.T, Staubli U.V, LeDoux J.E. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. doi:10.1038/37601 [DOI] [PubMed] [Google Scholar]
- Rosenkranz J.A, Grace A.A. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J. Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy J.W, Sutherland R.J. The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behav. Brain Res. 1989;34:97–109. doi: 10.1016/s0166-4328(89)80093-2. doi:10.1016/S0166-4328(89)80093-2 [DOI] [PubMed] [Google Scholar]
- Samson H.H, Harris R.A. Neurobiology of alcohol abuse. Trends Pharmacol. Sci. 1992;13:206–211. doi: 10.1016/0165-6147(92)90065-e. doi:10.1016/0165-6147(92)90065-E [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba A.A, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Hyytia P, Heinrichs S.C, Koob G.F. Effects of chronic ethanol exposure on oral self-administration of ethanol or saccharin by Wistar rats. Alcohol. Clin. Exp. Res. 1996;20:164–171. doi: 10.1111/j.1530-0277.1996.tb01060.x. doi:10.1111/j.1530-0277.1996.tb01060.x [DOI] [PubMed] [Google Scholar]
- Selden N.R, Everitt B.J, Jarrard L.E, Robbins T.W. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–350. doi: 10.1016/0306-4522(91)90379-3. doi:10.1016/0306-4522(91)90379-3 [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain C.K, LeDoux J.E. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. doi:10.1016/j.neuropharm.2006.06.022 [DOI] [PubMed] [Google Scholar]
- Spinella M. Neurobehavioral correlates of impulsivity: evidence of prefrontal involvement. Int. J. Neurosci. 2004;114:95–104. doi: 10.1080/00207450490249347. doi:10.1080/00207450490249347 [DOI] [PubMed] [Google Scholar]
- Stephens D.N, Brown G, Duka T, Ripley T.L. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur. J. Neurosci. 2001;14:2023–2031. doi: 10.1046/j.0953-816x.2001.01824.x. doi:10.1046/j.0953-816x.2001.01824.x [DOI] [PubMed] [Google Scholar]
- Stephens D.N, Ripley T.L, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol. Psychiatry. 2005;58:392–400. doi: 10.1016/j.biopsych.2005.04.025. doi:10.1016/j.biopsych.2005.04.025 [DOI] [PubMed] [Google Scholar]
- Stockwell T, Murphy D, Hodgson R. The severity of alcohol dependence questionnaire: its use, reliability and validity. Br. J. Addict. 1983;78:145–155. doi: 10.1111/j.1360-0443.1983.tb05502.x. [DOI] [PubMed] [Google Scholar]
- Tarter R.E, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug Alcohol Depend. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. doi:10.1016/j.drugalcdep.2003.07.004 [DOI] [PubMed] [Google Scholar]
- Townshend J.M, Duka T. Patterns of alcohol drinking in a population of young social drinkers: a comparison of questionnaire and diary measures. Alcohol Alcohol. 2002;37:187–192. doi: 10.1093/alcalc/37.2.187. [DOI] [PubMed] [Google Scholar]
- Townshend J.M, Duka T. Mixed emotions: alcoholics' impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41:773–782. doi: 10.1016/s0028-3932(02)00284-1. doi:10.1016/S0028-3932(02)00284-1 [DOI] [PubMed] [Google Scholar]
- Townshend J.M, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol. Clin. Exp. Res. 2005;29:317–325. doi: 10.1097/01.alc.0000156453.05028.f5. doi:10.1097/01.ALC.0000156453.05028.F5 [DOI] [PubMed] [Google Scholar]
- Ulrichsen J, Woldbye D.P, Madsen T.M, Clemmesen L, Haugbol S, Olsen C.H, Laursen H, Bolwig T.G, Hemmingsen R. Electrical amygdala kindling in alcohol-withdrawal kindled rats. Alcohol Alcohol. 1998;33:244–254. doi: 10.1093/oxfordjournals.alcalc.a008388. [DOI] [PubMed] [Google Scholar]
- Volkow N.D, Fowler J.S, Wang G.-J. The addicted human brain: insights from imaging studies. J. Clin. Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. doi:10.1172/JCI200318533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S. Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. J. Am. Med. Assoc. 1994;272:1672–1677. doi:10.1001/jama.272.21.1672 [PubMed] [Google Scholar]
- Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology (Berl.) 2003;165:306–312. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- Whishaw I.Q, Tomie J.A. Acquisition and retention by hippocampal rats of simple, conditional, and configural tasks using tactile and olfactory cues: implications for hippocampal function. Behav. Neurosci. 1991;105:787–797. doi: 10.1037//0735-7044.105.6.787. doi:10.1037/0735-7044.105.6.787 [DOI] [PubMed] [Google Scholar]
- Zeigler D.W, Wang C.C, Yoast R.A, Dickinson B.D, McCaffree M.A, Robinowitz C.B, Sterling M.L. The neurocognitive effects of alcohol on adolescents and college students. Prev. Med. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. doi:10.1016/j.ypmed.2004.04.044 [DOI] [PubMed] [Google Scholar]