Abstract
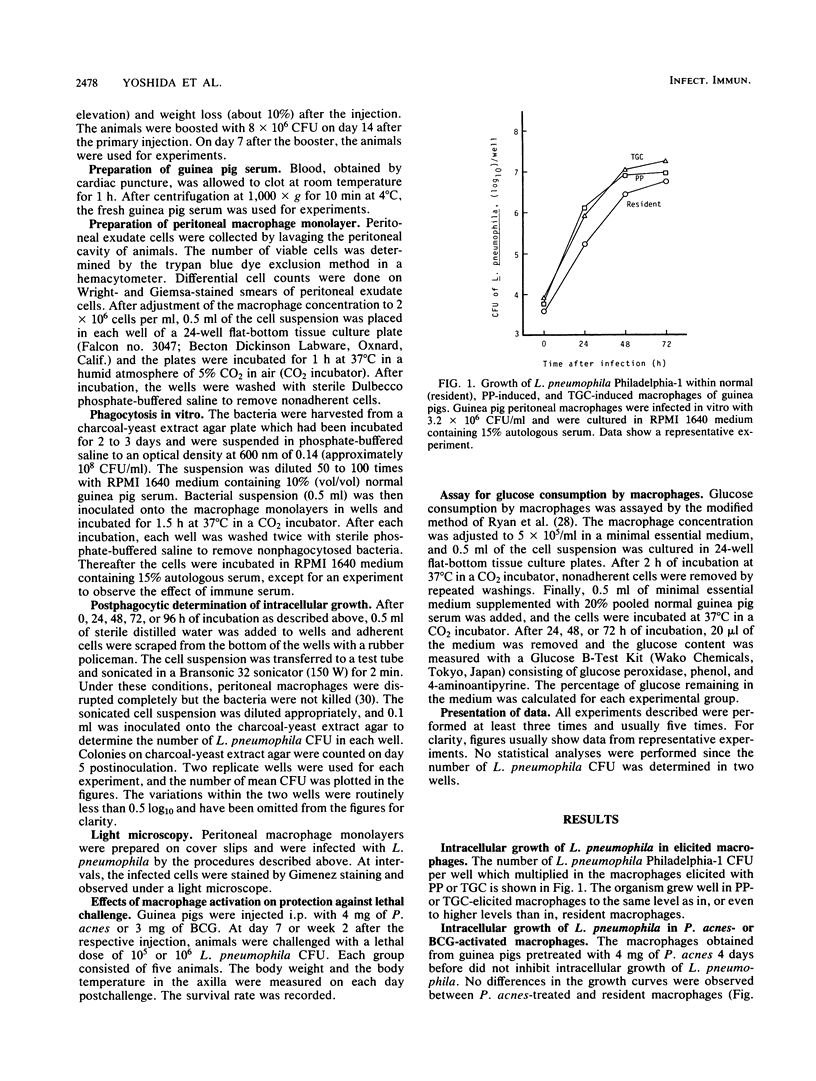
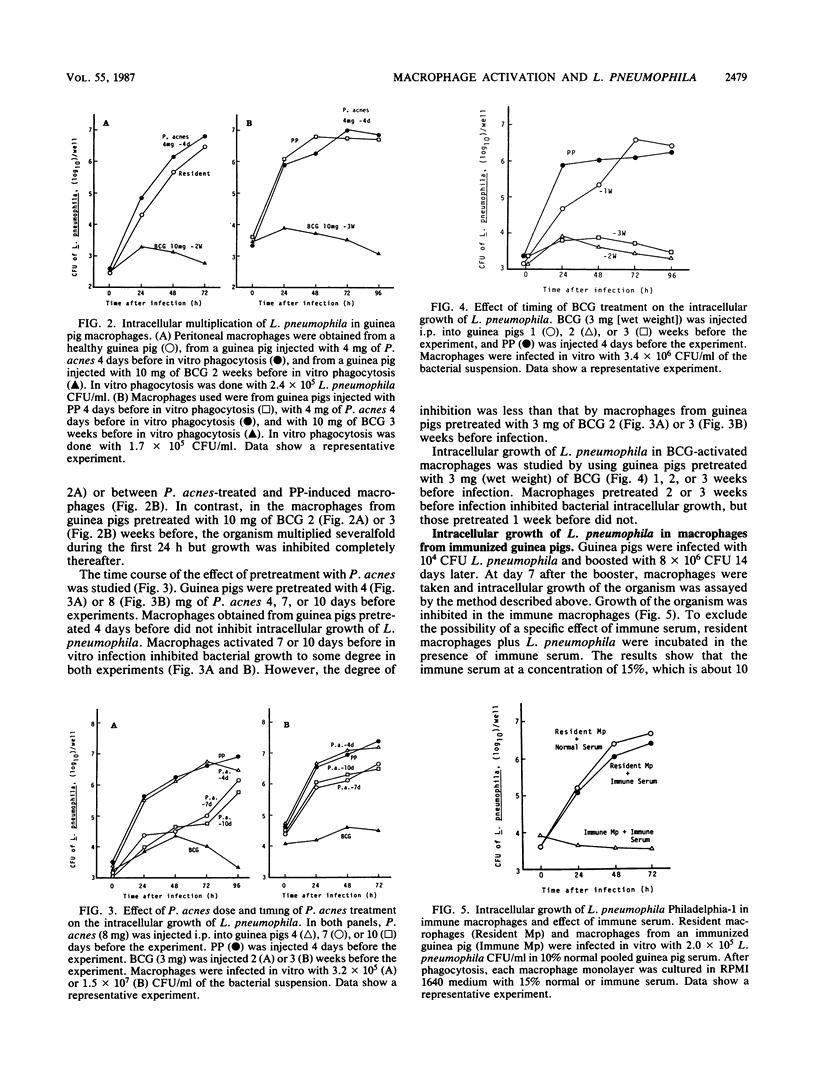
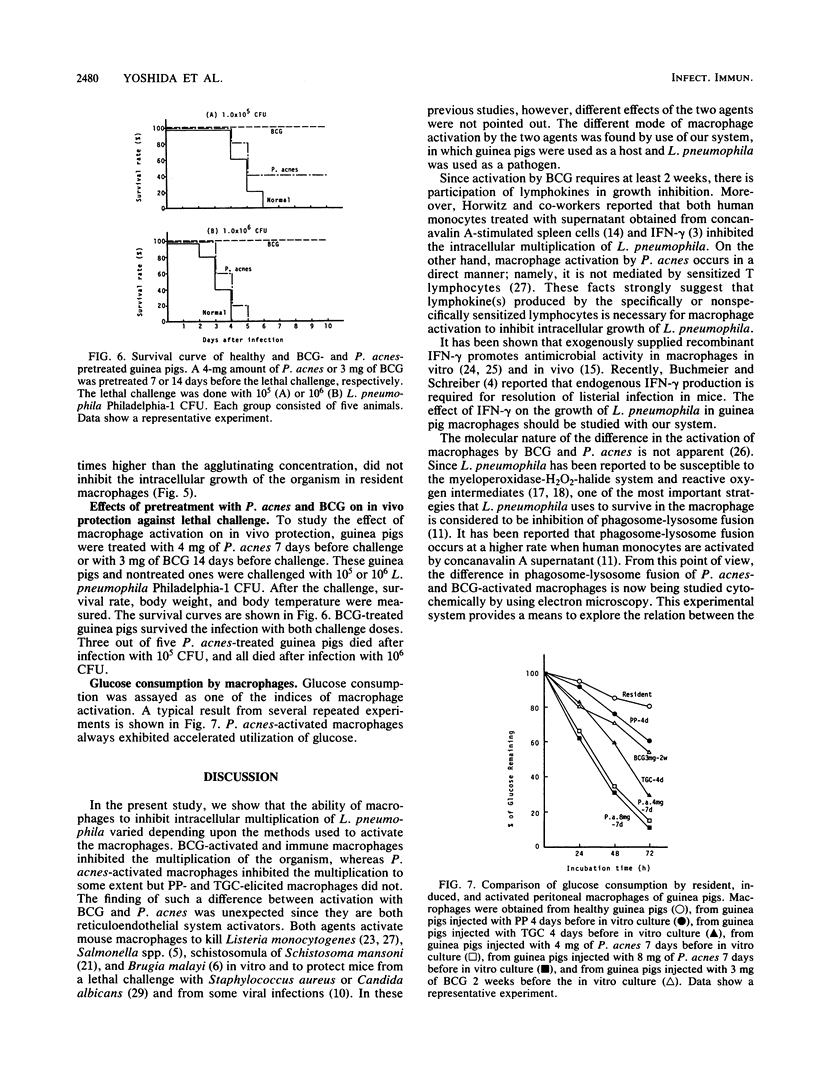
Legionella pneumophila is known to grow intracellularly in resident peritoneal macrophages of guinea pigs. The present study was done to determine what kinds of macrophage stimulants are able to activate guinea pig macrophages to inhibit intracellular growth of the organism. Peritoneal macrophages were harvested from healthy guinea pigs, from guinea pigs injected intraperitoneally with proteose peptone (PP) or thioglycolate medium, from guinea pigs injected intraperitoneally with live Mycobacterium bovis BCG or killed Propionibacterium acnes (Corynebacterium parvum), and from guinea pigs surviving infection with live L. pneumophila. After in vitro phagocytosis, the L. pneumophila CFU in each well were counted on charcoal-yeast extract agar plates. In the macrophages elicited by PP or thioglycolate medium, the organism grew as well as it did in resident macrophages. In BCG-activated and immune macrophages, growth was inhibited almost completely. In P. acnes-activated macrophages, the initial growth of L. pneumophila was inhibited to some extent, but its growth reached the same level as in the resident and PP-induced macrophages after 3 or 4 days of culture. In the lethal challenge experiments in vivo, the superior protection provided by BCG over P. acnes was ascertained and the importance of macrophages in resistance to L. pneumophila was confirmed. Difference of activation by BCG and P. acnes in relation to the inhibition of intracellular growth of L. pneumophila in guinea pig macrophages is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Williams J. C. Partial characterization of peptidoglycan-associated proteins of Legionella pneumophila. J Biochem. 1983 Aug;94(2):601–606. doi: 10.1093/oxfordjournals.jbchem.a134392. [DOI] [PubMed] [Google Scholar]
- Amano K., Williams J. C. Peptidoglycan of Legionella pneumophila: apparent resistance to lysozyme hydrolysis correlates with a high degree of peptide cross-linking. J Bacteriol. 1983 Jan;153(1):520–526. doi: 10.1128/jb.153.1.520-526.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N., Nash T. W., Horwitz M. A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986 Oct 15;137(8):2662–2669. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Scott M. T. Effect of Corynebacterium parvum treatment on the growth of Salmonella enteritidis in mice. Infect Immun. 1974 May;9(5):863–869. doi: 10.1128/iai.9.5.863-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning M. M., Kazura J. W. Lack of biological significance of in vitro Brugia malayi microfilarial cytotoxicity mediated by Propionibacterium acnes ("Corynebacterium parvum")-and Mycobacterium bovis BCG-activated macrophages. Infect Immun. 1986 May;52(2):534–537. doi: 10.1128/iai.52.2.534-537.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeley J. C., Gibson R. J., Gorman G. W., Langford N. C., Rasheed J. K., Mackel D. C., Baine W. B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979 Oct;10(4):437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Iglewski B. H., Miller R. D. Identification of a cytotoxin produced by Legionella pneumophila. Infect Immun. 1980 Jul;29(1):271–274. doi: 10.1128/iai.29.1.271-274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Lochner J. E., Bigley R. H., Iglewski B. H. The effects of Legionella pneumophila toxin on oxidative processes and bacterial killing of human polymorphonuclear leukocytes. J Infect Dis. 1982 Sep;146(3):328–334. doi: 10.1093/infdis/146.3.328. [DOI] [PubMed] [Google Scholar]
- Glasgow L. A., Fischbach J., Bryant S. M., Kern E. R. Immunomodulation of host resistance to experimental viral infections in mice: effects of Corynebacterium acnes, Corynebacterium parvum, and Bacille calmette-guérin. J Infect Dis. 1977 May;135(5):763–770. doi: 10.1093/infdis/135.5.763. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Maxfield F. R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984 Dec;99(6):1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Kishimoto R. A., White J. D., Shirey F. G., McGann V. G., Berendt R. F., Larson E. W., Hedlund K. W. In vitro responses of guinea pig peritoneal macrophages to Legionella pneumophila. Infect Immun. 1981 Mar;31(3):1209–1213. doi: 10.1128/iai.31.3.1209-1213.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner J. E., Friedman R. L., Bigley R. H., Iglewski B. H. Effect of oxygen-dependent antimicrobial systems on Legionella pneumophila. Infect Immun. 1983 Jan;39(1):487–489. doi: 10.1128/iai.39.1.487-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Jacobs R. F., Wilson C. B., Weaver W. M., Klebanoff S. J. Susceptibility of Legionella pneumophila to oxygen-dependent microbicidal systems. J Immunol. 1982 Nov;129(5):2192–2197. [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Peters P. A., Civil R. H., Remington J. S. In vitro killing of schistosomula of Schistosoma mansoni by BCG and C. parvum-activated macrophages. J Immunol. 1979 May;122(5):1655–1657. doi: 10.2196/41502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake S., Takeya K., Matsumoto T., Yoshikai Y., Nomoto K. Relation between bactericidal and phagocytic activities of peritoneal macrophages induced by irritants. J Reticuloendothel Soc. 1980 Apr;27(4):421–427. [PubMed] [Google Scholar]
- Miyata H., Nomoto K., Takeya K. Characteristics of resistance to Listeria monocytogenes enhanced by Corynebacterium parvum in mice. Immunology. 1980 May;40(1):33–39. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozaki K., Hashimoto T. Different mechanisms of macrophage activation with guinea pig macrophage activation factor, lipopolysaccharide and muramyl dipeptide. Int Arch Allergy Appl Immunol. 1985;76(4):296–301. doi: 10.1159/000233710. [DOI] [PubMed] [Google Scholar]
- Ruittenberg E. J., van Noorle Jansen L. M. Effect of Corynebacterium parvum on the course of a Listeria monocytogenes infection in normal and congenitally athymic (nude) mice. Zentralbl Bakteriol Orig A. 1975;231(1-3):197–205. [PubMed] [Google Scholar]
- Ryan J. L., Glode L. M., Rosenstreich D. L. Lack of responsiveness of C3H/HeJ macrophages to lipopolysaccharide: the cellular basis of LPS-stimulated metabolism. J Immunol. 1979 Mar;122(3):932–935. [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Greenberg L. E., Bernard S. Effects of BCG, Corynebacterium parvum, and methanol-extration residue in the reduction of mortality from Staphylococcus aureus and Candida albicans infections in immunosuppressed mice. Infect Immun. 1975 Dec;12(6):1325–1330. doi: 10.1128/iai.12.6.1325-1330.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Mizuguchi Y. Antibiotic susceptibility of Legionella pneumophia Philadelphia-1 in cultured guinea-pig peritoneal macrophages. J Gen Microbiol. 1984 Apr;130(4):901–906. doi: 10.1099/00221287-130-4-901. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Mizuguchi Y. Multiplication of Legionella pneumophila Philadelphia-1 in cultured peritoneal macrophages and its correlation to susceptibility of animals. Can J Microbiol. 1986 May;32(5):438–442. doi: 10.1139/m86-083. [DOI] [PubMed] [Google Scholar]



