Abstract
The endemic diplommatinid land snails (Caenogastropoda: Mollusca) of Belau (Republic of Palau, Micronesia) are an exceptionally diverse group of largely undescribed species distributed among rock and leaf litter habitats on most of Belau's 586 islands. Diplommatinid shell morphology (e.g. shell sculpture) reflects habitat type. In this study, I analysed a subset of the 90 diplommatinid species representing a broad geographical spread of islands in order to reveal the species' phylogenetic relationships and biogeography within the Belau archipelago. Diplommatinid species from the islands of Yap, Pohnpei, Kosrae and Guam are also included in the analysis. One nuclear (28S rRNA) and two mitochondrial (16S rRNA, COI) gene regions comprising 1906 bp were used for phylogenetic reconstruction. Results show that (i) the Belau Diplommatinidae are not monophyletic, as Guam and Yap species should be included as part of the radiation, (ii) Pohnpei and Kosrae species are highly divergent from Belau diplommatinids, (iii) there is little evidence for in situ radiation within individual Belau islands, (iv) spined and heavily calcified rock-dwelling species form a well-supported clade, and (v) Belau diplommatinid genera are in need of revision.
Keywords: molecular phylogenetics, Diplommatinidae, Mollusca, Pacific, Palau, Micronesia
1. Introduction
Wallace (1881, p. 10) suggested that some of the most interesting aspects of the evolution and distribution of species were best studied on islands. Pacific island terrestrial invertebrates in particular have provided important ground for testing evolutionary and biogeographic theory (Miller 1996), including the seminal works of Gulick (1872, 1873) on stochastic evolution in Hawaiian achatinelline land snails (Wright 1977, p. 447), Wilson (1961) on the taxon cycle in Melanesian ants, Clarke and colleagues on partulid land snail speciation (e.g. Clarke & Murray 1969; Cowie 1992) and Carson on Hawaiian drosophilid evolution (Carson et al. 1970, 1990). Low vagility of many terrestrial invertebrates, such as land snails, has not only contributed to the spectacular evolutionary radiations of species on oceanic Pacific archipelagos, but also makes them excellent subjects for biogeographic study. Despite the influence of the works noted above, research on many invertebrate groups has been hampered by the lack of detailed survey work on many island groups, and of adequate species lists or catalogues (Cowie 1996). Micronesian islands, such as those of the Belau archipelago, therefore are a frontier for terrestrial invertebrate research and will provide important insights into evolutionary and biogeographic theory for many years to come.
Belau (the independent Republic of Palau) is an isolated archipelago comprising 586 small islands (total land area 415 km2) at the western edge of Micronesia's Caroline Islands (figure 1). Most of Belau's terrestrial biodiversity awaits evolutionary and ecological study. This includes a rich, yet hardly known group of land snails. Belau is biogeographically interesting owing to its position at the juncture between the Philippines, Borneo, New Guinea and the rest of the western Pacific, allowing for potential colonization from any of these areas and from Southeast Asia (Crombie & Pregill 1999). The propinquity, abundance and varied geology of the Belau islands (as described below) also make them a fertile, if challenging, setting for biogeographic research. Because much of Belau's lowland rainforest is still intact, particularly on the rugged, nearly inaccessible Chelbacheb (Rock Islands; figure 2), these islands provide an unparalleled opportunity to uncover diversity. Land snail species richness and endemism is extraordinarily high on many Pacific islands (Solem 1976, 1983; Cowie 1992; Cowie et al. 1995; Rundell et al. 2004). Unfortunately, Pacific terrestrial gastropods have also suffered massive amounts of human-induced extinction (Hadfield 1986; Solem 1990; Cowie 1992, 2001; Hadfield et al. 1993; Abdou & Bouchet 2000; Lydeard et al. 2004).
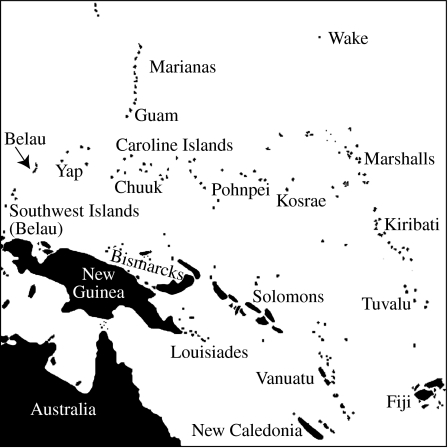
Figure 1.
The western Pacific islands. The Caroline Islands include Belau, Yap (Waqab), Chuuk, Pohnpei and Kosrae. Yap, Chuuk, Pohnpei and Kosrae are states in the Federated States of Micronesia.
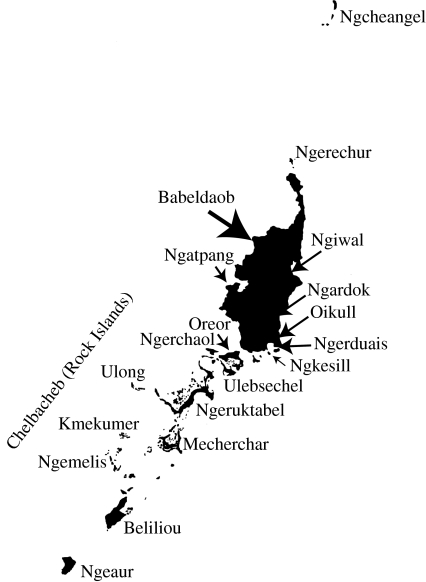
Figure 2.
The main islands of Belau. The distance from Ngcheangel to Ngeaur is 160 km.
Micronesian land snails are poorly known, relative to Polynesian and Melanesian land snails (Cowie 1996). Sixty-five indigenous Belau land snail species have been described (B. D. Smith 1993, unpublished data), but based on my recent surveys there are approximately 200 species, most of which are endemic. Many of these land snails, including the Diplommatinidae, are caenogastropods or ‘prosobranchs’, which have an operculum used for closing their shell opening (as opposed to non-operculate pulmonates, such as the Achatinellidae and Partulidae, which have been the focus of much land snail research in Polynesia). The Belau diplommatinids are extraordinarily species rich relative to island area: according to my surveys, there are approximately 90 diplommatinid species (including the 25 described species; B. D. Smith 1993, unpublished data), all of which are endemic to Belau, and many of which may be single-island endemics. This high species richness occurs throughout the Belau islands, despite the lack of high-elevation cloud forest (maximum elevation is 242 m, though most islands are much lower), which appears to be important for other taxa (e.g. Gillespie et al. 2008). The question of whether Belau's endemic diplommatinids represent a single radiation is of significant interest. Colonization history among other endemic Pacific island faunas has proved more complex than previously thought (Gillespie et al. 1994; Robinson & Sattler 2001; Rundell et al. 2004), and so I use molecular data to begin to understand the relationships within Belau diplommatinids in the context of the western Pacific.
Belau diplommatinid land snails are an excellent group with which to explore the biogeography of island radiations because their habitat preferences (limestone rock or leaf litter) contribute to their low vagility (Solem 1968), thus potentially allowing them to retain a better historical signal of population structure (e.g. relative to some volant vertebrates). The rock and leaf litter habitats occur on nearly all Belau islands, with the exception of Babeldaob (the largest island, at 333 km2) which is mostly volcanic. Within a single island, limestone cliff-face or limestone boulder habitat is adjacent to leaf litter, and there are different suites of diplommatinid species in each habitat type (R. J. Rundell 2007, unpublished data). Though other land snail species occur in these two habitats, diplommatinids are a major part of the fauna. Diplommatinid shell morphology reflects habitat type: rock dwellers are whitish with heavily calcified and/or spined shells, whereas leaf litter dwellers are brown and ovately conical with costae (‘ribs’; figure 3). I investigate whether in situ radiations have occurred repeatedly within these ecologically differentiated island communities, which would reveal a phylogenetic pattern of repeated suites of ecomorphs on different Belau islands, as has occurred within Caribbean Anolis lizards (Losos et al. 1998) and Hawaiian spiders (Gillespie 2004).
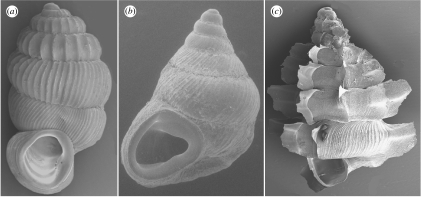
Figure 3.
Scanning electron micrographs (SEM) illustrating the three different general shell morphologies: (a) Palaina albata (note ribs (costae); Beliliou), (b) Diplommatina BG (east Mecherchar) and (c) Hungerfordia U (note spines (lamellae); Eudelchol (Rock Island near Mecherchar)). Palaina albata and Diplommatina BG by R.J.R., using uncoated specimens and an AMRAY 1810 SEM. Hungerfordia U by D. Clarke and R.J.R., using a gold-coated specimen and a LEO EVO 60 SEM. All specimens are approximately 4 mm in shell height.
In this study, I reconstruct the phylogeny of a subset of diplommatinid land snails to address the following: (i) do the Belau Diplommatinidae comprise a monophyletic radiation, (ii) have species with similar morphological traits (i.e. shell heavily calcified and spined or ovately conical with costae) evolved independently on different islands, (iii) what are the biogeographic routes of colonization and diversification within Belau, and (iv) what are the phylogenetic relationships of Belau diplommatinids?
(a) Micronesia and Belau: geology and geography in brief
Micronesia is a vast expanse of more than 2000 Pacific islands and atolls stretching from the Carolines and Marianas in the west to the Marshalls, Nauru and Kiribati in the east (figure 1; island names follow Motteler 2006). Islands within these archipelagos are frequently geologically and ecologically distinct from one another. The Carolines, of which Belau is a part, are no exception; whereas Belau and Yap (Waqab) are crests of arc ridges (Polhemus 1996; Kobayashi 2004), Pohnpei and Kosrae are hot spot islands. Belau, Yap and Guam (in the Marianas) sit on the eastern edge of the Philippine plate at its contact point with the Pacific plate (Ohara et al. 2002) and are relatively old. The oldest organic limestones in Belau are ca 25 Ma old, suggesting that subaerial volcanics were available by 30 Ma (Crombie & Pregill 1999; Kobayashi 2004). Rock formations on Yap are 25–7 Ma old (Ohara et al. 2002). There are no active volcanoes in either the Belau or Yap arcs (Kobayashi 2004). Parts of Guam have been above sea level since at least 20 Ma; Guam rock exposures range in age from 43.8 to 2.3 Ma (Craig et al. 2001; Hall 2002). By contrast, the Caroline Islands of Pohnpei and Kosrae originated on the Pacific plate and are young hot spot islands, 8.6–3.0 and 2.6–1.2 Ma old, respectively (Keating et al. 1984; Craig et al. 2001). The formation of Pohnpei and Kosrae was similar to that of the Hawaiian Islands (i.e. hot spot derived shield volcanoes) and thus the maximum K–Ar dates of subaerial lavas reported by Keating et al. (1984, above) are estimated times during which each island was available for colonization (see Fleischer et al. 1998).
The 160 km chain of Belau's main islands is centred at 7°20′ N and 134° E and is 800 km from Mindanao (Philippines), the Moluccas and New Guinea and 1500 km from Borneo (Crombie & Pregill 1999). Belau's 415 km2 of land (including mangroves) encompasses the following island types: volcanic; high limestone; low limestone; reef or atoll; and a combination of volcanic and limestone (Crombie & Pregill 1999). The 333 km2 volcanic island of Babeldaob constitutes 80 per cent of Belau's land area (figure 2). The remaining islands range in area from less than 1 to 20.2 km2. However, despite their small size, species richness and endemism are also high within the hundreds of limestone karst Chelbacheb (or Rock Islands) south of Babeldaob (R. J. Rundell 2007, unpublished data).
An additional cluster of Belau islands, the low limestone Southwest Islands, lies 320–480 km to the southwest of the main archipelago (Crombie & Pregill 1999). In their herpetological monograph, Crombie & Pregill (1999) suggested that the Southwest Islands, which are part of Belau, but actually closer to New Guinea, may have acted as stepping stones for colonists coming from the greater New Guinea region. My surveys of the Southwest Islands of Pulo Anna, Sonsorol and Hatohobei have yet to reveal diplommatinids, although the main Belau islands could have been colonized from New Guinea directly. Additional source areas are being explored; however, the fact that few thorough surveys have been undertaken in the western Pacific, especially surveys focusing on the smallest members of the land snail fauna, complicates this endeavour.
Belau (figure 2) is part of the Kyushu–Palau Ridge (Kobayashi 2004) and is partially composed of volcanic rocks that emerged above sea level by the Late Oligocene (ca 30 Ma; Kelletat 1991; Crombie & Pregill 1999). Remnants of Miocene reef are also present in southern Babeldaob and Oreor. The Rock Islands, famous for their mushroom shape (bioerosive notches; Kelletat 1991), are composed of Miocene (23–5 Ma) and Pleistocene coral reefs that have been weathered into karst. Pleistocene (and some Miocene) limestone occurs towards the southern end of the island chain (e.g. the uplifted atolls of Beliliou and Ngeaur; Corwin et al. 1956; Kobayashi 2004). Much of Belau is enclosed within a lagoon that is surrounded by a barrier reef to the west and a fringing reef to the east (Kobayashi 2004).
During the Pleistocene and into the Holocene, the Belau islands have experienced substantial sea-level fluctuations (Easton & Ku 1980). Because sea levels were probably depressed by 120 m during the last glacial maximum (18 000 years ago), many islands were presumably connected at times, and, conversely, fragmented when sea level was higher than now (Crombie & Pregill 1999).
(b) Pacific Diplommatinidae
Diplommatinidae are caenogastropods and generally considered as sister to the Cyclophoridae (Ponder & Waren 1988; Ponder & Lindberg 1997; Fretter et al. 1998; Stanisic 1998; Colgan et al. 2007). The higher level relationships between diplommatinids and other families and within the Diplommatinidae are largely unknown. Diplommatinids include species from tropical Asia (Vermeulen 1993, 1994), Melanesia, Micronesia and parts of Polynesia. They have separate sexes and probably feed on fungus, lichens, bacterial films and detritus. Shells are brown or whitish (sometimes with spines (lamellae)), ovately conical or conical, and generally sinistrally coiled (figure 3); most Belau species are less than 5 mm in shell height.
Pacific diplommatinids are found in the Philippines, Melanesia, Micronesia (including the Caroline and Mariana Islands) and in the east through Tonga and Samoa (Solem 1968). Although dispersal mechanisms for Pacific land snails are not well understood or documented, wind transport may play an important, though generally unappreciated, role in the dispersal of tiny snails (Vagvolgyi 1975; Kirchner et al. 1997). Diplommatinid species richness in the Pacific attenuates from west to east, though incomplete knowledge of the Pacific fauna makes quantification of this trend impossible. This trend could be due to the fact that diplommatinids, as caenogastropod land snails, are more physiologically susceptible to dehydration than pulmonate land snails (Little 1990, p. 211; Arad 1993), which may make successful long-distance dispersal across wide oceanic expanses difficult. The fact that diplommatinids have separate sexes may also make them poorer colonizers than the hermaphroditic pulmonate land snails (Cowie 1996).
Most diplommatinid research has focused on taxonomy, with the exception of the studies of Peake (1973), Tillier (1981) and Schilthuizen and colleagues (e.g. Schilthuizen et al. 2002, 2006; Schilthuizen 2003). No taxonomic revision of diplommatinids has been attempted, and there is no catalogue of species.
Three genera are considered in this study: Diplommatina Benson 1849; Palaina Crosse 1866; and Hungerfordia Beddome 1889. The original introduction of Diplommatina by Benson (1849) included two species from the lower western Himalayas in India, neither of which is included in the present study. The type species of Palaina is Diplommatina macgillivrayi Pfeiffer 1855 from Lord Howe Island (by subsequent designation of Iredale 1944; see Solem 1959) and species in this genus have been described from localities throughout the western Pacific, Australasia and Southeast Asia (Thiele 1935). Hungerfordia is a monotypic genus, with the single species from Belau, Hungerfordia pelewensis Beddome 1889 (Thiele 1935; Solem 1959), having a unique shell morphology with small spines. Generic descriptions are brief and based on shell characters (Solem 1959). In the past, Belau species were assigned to each of these genera based on little detailed study. The present study provides preliminary insight into the evolution and biogeography of this little-known group of land snails.
2. Material and methods
(a) Field collection and identification
Land snails were collected on islands throughout the Belau archipelago between June 2003 and May 2007. Snails from Yap, Guam, Pohnpei and Kosrae were collected in October and November 2005. The main islands of Yap and Pohnpei were sampled, but their outer islands were not included in the present study. Kosrae does not have outer islands. Separate searches were undertaken in leaf litter and rock habitats. Both habitats were thoroughly examined by hand, in situ. Snails were located by eye, collected by hand or with forceps, and placed in separate vials according to habitat type. They were killed in 95 per cent ethanol. Ethanol was replaced twice post-killing to ensure proper preservation for identification and DNA analysis. Specimens were sorted according to morphotype and vials were stored in a refrigerator pending shipment to the Field Museum of Natural History (FMNH). All specimens were then stored in 95 per cent ethanol at −80°C pending DNA extraction. Following DNA extraction, they were deposited at FMNH (table 1).
Table 1.
Diplommatinid specimens, with collection localities and Field Museum catalogue numbers, and outgroup taxa, with GenBank accession numbers for all sequences used in this study. (Undescribed species are designated by one or two capital letters, reflecting simply the order in which they were identified.)
| GenBank accession numbers | |||||
|---|---|---|---|---|---|
| species | locality | source | 16S | COI | 28S |
| Hungerfordia T | Oikull, Babeldaob | FMNH310541 | EU742045 | EU742085 | |
| Hungerfordia T | Ngerchaol | FMNH310542 | EU742038 | EU742119 | EU742079 |
| Hungerfordia A | Ngeruktabel | FMNH310543 | EU742027 | EU742108 | EU742068 |
| Hungerfordia K | Ngeruktabel | FMNH310544 | EU742026 | EU742107 | EU742067 |
| Hungerfordia E | Ulong | FMNH310545 | EU742028 | EU742109 | EU742069 |
| Diplommatina CF | Ngeruktabel | FMNH310546 | EU742037 | EU742118 | EU742078 |
| Diplommatina CW | East Mecherchar | FMNH310547 | EU742043 | EU742083 | |
| Diplommatina ringens (Crosse 1866) | Beliliou | FMNH310548 | EU742031 | EU742112 | EU742072 |
| Diplommatinaringens (Crosse 1866) | Ngeaur | FMNH310549 | EU742030 | EU742111 | EU742071 |
| Hungerfordia U | Kmekumer | FMNH310550 | EU742032 | EU742113 | EU742073 |
| Diplommatina BH | Ulong | FMNH310551 | EU742036 | EU742117 | EU742077 |
| Diplommatina AX | Ngemelis | FMNH310552 | EU742034 | EU742115 | EU742075 |
| Diplommatina AL | Omekang, south lagoon | FMNH310553 | EU742044 | EU742084 | |
| Diplommatina CM | West Mecherchar lagoon | FMNH310554 | EU742033 | EU742114 | EU742074 |
| Diplommatina BG | East Mecherchar | FMNH310538 | EU742023 | EU742104 | EU742064 |
| Diplommatina CV | Ngkesill | FMNH310555 | EU742040 | EU742121 | EU742080 |
| Hungerfordia C | Ulong | FMNH310556 | EU742042 | EU742082 | |
| Diplommatina AT | Beliliou | FMNH310557 | EU742029 | EU742110 | EU742070 |
| Diplommatina AF | Ngermid, Oreor | FMNH310558 | EU742025 | EU742106 | EU742066 |
| Diplommatina AF | Ngermid, Oreor | FMNH310582 | EU742024 | EU742105 | EU742065 |
| Palaina CE | Ngeaur | FMNH310559 | EU742014 | EU742095 | EU742055 |
| Palaina albata (Beddome 1889) | Beliliou | FMNH310560 | EU742017 | EU742098 | EU742058 |
| Palaina albata (Beddome 1889) | Ngermid, Oreor | FMNH310561 | EU742016 | EU742097 | EU742057 |
| Palaina dimorpha (Crosse 1866) | Ngeruktabel | FMNH310562 | EU742015 | EU742096 | EU742056 |
| Palaina N | Beliliou | FMNH310563 | EU742041 | EU742081 | |
| Palaina AW | Ulebsechel | FMNH310564 | EU742007 | EU742088 | EU742048 |
| Palaina AS | Ngemelis | FMNH310565 | EU742019 | EU742100 | EU742060 |
| Palaina BM | Ngerechur, north of Babeldaob | FMNH310566 | EU742013 | EU742094 | EU742054 |
| Palaina AU | Ulebsechel | FMNH310567 | EU742018 | EU742099 | EU742059 |
| Palaina AB | Ngatpang, Babeldaob | FMNH310568 | EU742020 | EU742101 | EU742061 |
| Palaina Y | Ngardok, Babeldaob | FMNH310569 | EU742035 | EU742116 | EU742076 |
| Palaina Y | Ngiwal, Babeldaob | FMNH310570 | EU742012 | EU742093 | EU742053 |
| Palaina BR | Babeldaob | FMNH310571 | EU742011 | EU742092 | EU742052 |
| Palaina rubella (Beddome 1889) | Ngerduais, Babeldaob | FMNH310572 | EU742009 | EU742090 | EU742050 |
| Palaina rubella (Beddome 1889) | Oikull, Babeldaob | FMNH310573 | EU742010 | EU742091 | EU742051 |
| Palaina moussoni (Crosse 1866) | Ngcheangel | FMNH310574 | EU742008 | EU742089 | EU742049 |
| Palaina CB | Guam | FMNH310575 | EU742022 | EU742103 | EU742063 |
| Palaina BT | Yap | FMNH310576 | EU742021 | EU742102 | EU742062 |
| Palaina scalarina (von Möllendorff 1897) | Pohnpei | FMNH310577 | EU742039 | EU742120 | |
| Palaina doliolum (von Möllendorff 1897) | Pohnpei | FMNH310578 | EU742006 | EU742087 | EU742047 |
| Palaina CC | Kosrae | FMNH310579 | EU742005 | EU742086 | EU742046 |
| Pomacea bridgesii | GenBank | DQ093480 | DQ916496 | DQ279984 | |
| Conus miles | GenBank | AF108821 | AY588202 | DQ916564 | |
| Conus textile | GenBank | NC008797 | DQ862058 | ||
| Assiminea infima | GenBank | EF667329 | EF667303 | ||
| Opacuincola mete | GenBank | AY634075 | |||
| Opisthostoma concinnum | GenBank | DQ235751 | |||
| Depressigyra globulus | GenBank | AY163400 | |||
| Sepia robsoni | GenBank | AF369957 | |||
| Bellastraea rutidoloma | GenBank | AM403872 | |||
| Katharina tunicata | GenBank | EF201397 | |||
| Aperostoma palmeri | GenBank | DQ279983 | |||
| Biomphalaria glabrata | GenBank | AF435694 | |||
| Lithasiopsis hinkleyi | GenBank | DQ311138 | |||
| Austrolittorina unifasciata | GenBank | DQ916549 | |||
Specimens were identified to species using shell characteristics viewed under a dissecting microscope. Species determinations for described species were based on type material from the Muséum National d'Histoire Naturelle (Paris) and the Bernice P. Bishop Museum (Honolulu).
Undescribed species presented a challenge. All Belau diplommatinids considered herein are endemic to Belau (R. J. Rundell 2007, unpublished data) and it is possible that some species (including undescribed species) may not be referable to the geographically widespread genera Diplommatina and Palaina. But since this is the first detailed systematic study of Belau diplommatinids, I assigned undescribed species to known genera, provisionally, based on their similarity to known Belau species in shell sculpture and shape. For example, undescribed species with shells similar to Diplommatina lutea, as described by Beddome (1889, i.e. heavily calcified whitish shells), were assigned to Diplommatina. Whitish undescribed species with spines were assigned to Hungerfordia. Undescribed species that were brown with different patterns of shell ribbing (costae) and constricted body whorls were assigned to Palaina. Undescribed species are designated by one or two letters, reflecting simply the order in which they were identified. Generic assignments herein are not definitive, but simply used as a tool for understanding the evolution of shell shape in the Diplommatinidae.
(b) Taxon sampling
Belau and other Micronesian Diplommatinidae used in the present analysis were selected from a larger dataset in order to represent a cross section of the geography of the Belau islands. Taxa from all of the largest islands (i.e. Babeldaob, Ngeruktabel, Mecherchar, Oreor, Beliliou, Ngeaur and Ulong) and most distant islands (i.e. the northernmost atoll of Ngcheangel to the southernmost main island of Ngeaur) are represented here (table 1).
Detailed study of the closest relatives of Diplommatinidae has yet to be undertaken, and the higher level relationships of Diplommatinidae to other families are uncertain. Therefore, multiple outgroup taxa were selected based on what was available on GenBank for each of the three gene partitions in this study (table 1); their taxonomic affiliations are as follows: Opisthostoma concinnum (Diplommatinidae); Aperostoma palmeri (Cyclophoridae); Pomacea bridgesii (actually Pomacea diffusa; see Rawlings et al. 2007); Conus miles, Conus textile, Assiminea infima, Opacuincola mete, Lithasiopsis hinkleyi, Austrolittorina unifasciata (various Caenogastropoda); Depressigyra globulus (Neomphaloidea); Biomphalaria glabrata (Pulmonata); Bellastraea rutidoloma (Vetigastropoda); and two non-gastropods, Katharina tunicata (Polyplacophora) and Sepia robsoni (Cephalopoda).
Phylogenetic analyses by Colgan et al. (2007) placed a clade containing Ampullariidae as sister to the Cyclophoridae (probable sister to Diplommatinidae, which were formerly placed within the cyclophorids). Therefore, the ampullariid P. bridgesii (i.e. P. diffusa, see above) was selected as an outgroup in the combined analysis.
(c) DNA extraction, PCR and sequencing
The entire foot muscle tissue was dissected from each specimen under a dissecting microscope, using sterile needles and forceps. A single specimen's foot was used for each extraction and digested in buffer ATL from the Qiagen DNeasy kit (Qiagen, Valencia, CA) and proteinase K for 1–2 days. Following digestion, whole genomic DNA was extracted using a standard phenol–chloroform protocol and Phase Lock Gel (Eppendorf, Hamburg, Germany).
The 16S ribosomal RNA gene region, cytochrome c oxidase subunit I (COI) gene region and a region of the 28S nuclear rRNA gene were amplified for every taxon, with the exceptions of Hungerfordia C, Palaina N and Diplommatina CW and AL, for which only 16S and 28S were amplified, and Palaina scalarina from Kosrae for which only 16S and COI were amplified. Final concentrations for each 25 μl PCR were 1 U Taq (Roche Molecular Systems, Basel, Switzerland), 10× buffer with MgCl2, 2.3 mM extra MgCl2, dNTPs at 200 mM each, 1 μM per primer, 100× bovine serum albumin and 10–100 ng of genomic DNA. A 534 bp region of the 16S rRNA gene was amplified using the primer pair 16Sar and 16Sbr (Palumbi 1996, p. 236) and the following profile: 95°C for 1 min and 39 cycles of 95°C for 30 s, 58°C for 30 s and 72°C for 40 s, followed by 72°C for 2 min. Primers for COI were designed for this study from B. glabrata (GenBank accession no. AY380531) sequences. Primer sequences were H-COIBio1 5′-TGATATAAGATAGGATCACC-3′ and l-COIBio2 5′-CAAACCATAAAGATATTGGTAC-3′. A 596 bp region of COI was amplified using the following profile: 94°C for 12 min and 35 cycles of 94°C for 1 min, 45–61°C for 1 min and 72°C for 1 min, followed by 72°C for 10 min. This temperature profile (with 51–58°C annealing temperature) was also used for amplifying a 776 bp region of the 28S rRNA gene with the primers D6R and D23F (Park & Ó Foighil 2000).
Single-band PCR products were visualized on 1.5 per cent agarose gels containing ethidium bromide to verify fragment size and were purified using a QIAquick PCR purification kit (Qiagen) or ExoSAP-IT enzyme (USB Corp., Cleveland, OH). PCR products were sequenced in both directions by direct double-strand cycle sequencing using PCR primer and BigDye Terminator v. 3.1 chemistry (Applied Biosystems [ABI], Foster City, CA). Following cycle sequencing, samples were precipitated with ethanol, 3 M sodium acetate and 125 mM EDTA and sequenced on an ABI 3730 DNA Analyzer.
(d) Data analysis
Sequences were edited in Sequencher v. 4.8 (Gene Codes, Ann Arbor, MI) and aligned in ClustalX (Thompson et al. 1997) and by eye in MacClade v. 4.05 (Maddison & Maddison 2001), resulting in a 1906 bp long data matrix. Maximum-parsimony (MP) and maximum-likelihood (ML) analyses were undertaken in PAUP* v. 4.0.b10 (Swofford 2002). Additional ML analyses were conducted in Garli v. 0.95 (Zwickl 2006). Gaps were treated as fifth character states.
MP analyses were conducted for each gene partition using the following outgroup taxa for each partition (GenBank accession numbers in table 1): 16S (P. bridgesii, C. miles, C. textile, A. infima, O. concinnum, O. mete, D. globulus, S. robsoni); COI (P. bridgesii, C. miles, C. textile, A. infima, B. rutidoloma, K. tunicata); and 28S (P. bridgesii, C. miles, A. palmeri, B. glabrata, L. hinkleyi, A. unifasciata). All outgroup taxa except for the ampullariid P. bridgesii were removed after tree topologies proved robust to differences in outgroup sampling (i.e. trees were identical and the three major clades were resolved). Sequences were realigned using ClustalX following outgroup pruning. MP analyses combining all three gene partitions (1906 bp total) were then conducted using heuristic searches, the tree-bisection-reconnection branch-swapping algorithm and 1000 random stepwise additions. All characters were unordered and equally weighted. Bootstrap support (Felsenstein 1985) was assessed in PAUP* v. 4.0.b10 (Swofford 2002) based on 1000 pseudoreplicates.
Seven likelihood replicates and non-parametric bootstrap analysis (100 pseudoreplicates) were conducted in Garli v. 0.95 (Zwickl 2006) under the general time-reversible model and using the default settings. Modeltest v. 3.7 (Posada & Crandall 1998) was run using the Akaike information criterion model evaluation approach. The general time-reversible model with gamma distribution and number of invariant sites (GTR+G+I) was the best fit for the data. ML analyses were then conducted in PAUP* v. 4.0.b10 (Swofford 2002) using this model and the same settings described above for MP analyses for 10 replicates. Tree scores from Garli and PAUP* analyses were compared.
3. Results
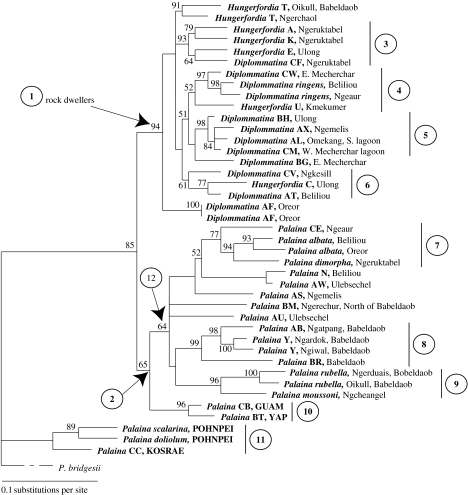
Of the 1906 sites in the analysis, 663 were variable and 404 were parsimony informative. The heuristic search recovered two equally parsimonious trees; the length of the best MP tree was 2274. The strict consensus of these two trees was calculated. The scores of all seven Garli (likelihood) replicates were within 0.02 points of one another, indicating convergence on the most likely tree (across tree space). The score of the best Garli tree (−ln L: −12 738.163) was nearly identical to the score of the ML tree obtained in PAUP* v. 4.0.b10 under the GTR+G+I model and the topologies of the two trees were identical. The ML tree obtained in PAUP* v. 4.0.b10 is shown in figure 4 (−ln L: −12 738.32540) with bootstrap values from the Garli analysis.
Figure 4.
ML phylogram (−ln L: −12 738.32540) based on 1906 bp of 16S rDNA, COI mtDNA and 28S rDNA for selected Belau diplommatinids. Branch for outgroup P. bridgesii (i.e. P. diffusa, see text) is scaled to 1/7. Localities for taxa from the Marianas (Guam) and other Caroline Islands (Yap, Pohnpei and Kosrae) are shown in bold and capital letters. Bootstrap values greater than 50 are indicated at nodes. Circled numbers 1–12 indicate clades described in §3.
The strict consensus of the two MP trees (not shown) was similar to the ML trees with the exception of the following (clade numbers in figure 4): (i) clade 2 does not exist in the MP tree and Palaina is paraphyletic, (ii) in clade 5, Diplommatina species AX and AL are sister (bootstrap 78%), whereas in the ML tree, their relationship is unresolved, (iii) in the MP tree, the relationship of Palaina CE is unresolved with respect to other Palaina species, and (iv) in the MP tree, the Kosrae species Palaina CC is sister to both Pohnpei species (bootstrap 100%, clade 11).
The analyses provide little support for monophyly of the genera Hungerfordia, Diplommatina or Palaina (figure 4). However, because type species of the nominal genera were not included in these analyses, definitive taxonomic assessment of generic placement cannot be made. All Belau Diplommatina and Hungerfordia species (as defined by shell shape) comprise the well-supported clade 1 (ML bootstrap 94%), in which both Diplommatina and Hungerfordia are paraphyletic. Both Diplommatina and Hungerfordia species are limestone rock dwellers.
Palaina species from Belau, Yap and Guam comprise the weakly supported clade 2 (bootstrap 65%), which is not present in the MP strict consensus tree (not shown). Palaina species are more globose than Diplommatina and Hungerfordia species and are generally leaf litter dwellers.
Species in clade 11 are very divergent from the Belau, Guam and Yap ingroup (figure 4; bootstrap support for Pohnpei species 85%), despite the fact that clade 11 species' shell morphology is similar to that of Belau, Guam and Yap ingroup species.
The reconstructed phylogeny does not support the monophyly of Belau Diplommatinidae (figure 4). Yap and Guam species are sister to each other (clade 10; basal within Palaina clade 2) and are part of the Belau radiations (figure 4). Pohnpei and Kosrae species form a single clade (11) basal to the Belau/Yap/Guam ingroup, which is supported in the MP tree by a bootstrap of 100 per cent (not shown).
Species from individual islands do not form monophyletic clades (e.g. all Beliliou species do not form a single clade) and therefore, with few exceptions (e.g. clade 8), there is little evidence for in situ radiation within individual islands.
4. Discussion
(a) Non-monophyly of Belau Diplommatinidae
The close relationship of Belau diplommatinids to Yap and Guam diplommatinids makes sense in light of the geology of these islands. All three island groups are part of Philippine plate arc ridges (Kobayashi 2004), in contrast to the hot spot islands of Pohnpei and Kosrae, both of which are part of the Pacific plate (Kroenke 1996). Although the Belau archipelago (30 Ma to Pleistocene) is geographically distinct from the Guam and the Yap island group (i.e. part of different arc systems: Kobayashi 2004; Guam and Yap are 1295 and 464 km northeast of Belau, respectively), the ages and compositions of these three islands and island groups may support shared evolutionary history. Guam is relatively old (above sea level from at least 20 Ma; Craig et al. 2001; Hall 2002) and limestone deposits are present, though to a lesser degree than in Belau. Yap (the island of Yap itself, not outlying islands) is composed of well-eroded volcanic and metamorphic rocks and has leaf litter habitat suitable for diplommatinids. It is also relatively old (25–7 Ma; Ohara et al. 2002). Yap is 841 km from Guam.
Interestingly, the people of Belau and Yap (and other western Pacific islands; Hezel 1983) have participated in trade at certain points in history. For example, the large round stone money for which Yap is renowned was mined in limestone quarries in Belau and transported by canoe (and later ship) to Yap (Hezel 1983, p. 266). However, given that Belau and Yap species are endemic to their respective island groups, and the earliest human colonization of Belau was only 3000 years ago (Masse et al. 2006), it is unlikely that the phylogenetic pattern shown in figure 4 (with respect to the leaf litter-dwelling Yap Palaina BT (clade 10, figure 4)) resulted from human dispersal of diplommatinids. No diplommatinid species are known to be widespread across the Caroline Islands. Furthermore, diplommatinids are not associated with food crops or ornamental use, as has apparently been the case with some Pacific island partulid tree snails (Lee et al. 2007).
Given the high divergence between Pohnpei/Kosrae and Belau diplommatinids (figure 4), the use of slower evolving genes will be essential for resolving deep relationships within Diplommatinidae. Unfortunately, even though various evolutionary rates have been reported for pulmonate land snails (e.g. Douris et al. 1998; Chiba 1999), little is known about the rates of evolution in caenogastropod land snails.
The evolution of western Pacific diplommatinids may have been complex, as is suggested by Belau/Yap/Guam snails' relationship with Pohnpei and Kosrae snails (as well as Belau diplommatinids' distant relationship with some New Guinea diplommatinid species; R. J. Rundell 2007, unpublished data). Despite the fact that the oldest part of Belau is nearly 30 Ma old (and Guam is greater than 20 Ma old), much older than Pohnpei and Kosrae (8.6–3.0 and 2.6–1.2 Ma, respectively; Keating et al. 1984; Craig et al. 2001), it is possible that other islands have acted as sources of colonists for these eastern Caroline Islands faunas.
The origins of the Belau/Yap/Guam diplommatinid fauna are still unknown and additional sampling, expanding outwards to other possible colonization sources, will be necessary to elucidate them.
(b) Little evidence for in situ radiation on individual islands
The distinct lack of intra-island radiation and a progression rule pattern (i.e. phylogenetic pattern that reflects island age; e.g. Wagner & Funk 1995) in Belau diplommatinids (figure 4) differs from patterns frequently exhibited among taxa in island groups with more sequential and better understood island geologies and ages (e.g. Hawaiian Islands; Funk & Wagner 1995; Rundell et al. 2004). It is perhaps unsurprising that the complex geology of Belau, namely the propinquity of islands, the many island types and the dramatic range in ages of the islands over only 160 km, is reflected in the complex biogeographic pattern among Belau diplommatinids. We will continue to disentangle these patterns as more geological data come to light, including information on the timing and extent of island connectivity.
The low vagility of leaf litter- and rock-dwelling snails may contribute to higher speciation potential (Schilthuizen et al. 2002), and therefore it would not have been surprising to find more evidence of intra-island radiation in the current study, in which only clade 8 Babeldaob snails (figure 4) provided evidence for in situ radiation. Speciation has apparently occurred largely among islands. My data support the notion that speciation rarely occurs between different habitats, and instead occurs within geographically separated similar habitats (Gillespie et al. 2008). For example, rock-dwelling species have not repeatedly given rise to leaf litter species. Rather, substantial evolution has occurred within the rock-dwelling habitat type among many different Belau islands.
In general, there is also a lack of consistent biogeographic pattern among the Belau islands. Vicariance is one possible explanation for some of the phylogenetic pattern. The genetic distances among Belau diplommatinid species suggest that species divergences occurred prior to major sea-level fluctuations in the Pleistocene, but it is possible that sea-level changes pre-dating the Pleistocene were also influential in diplommatinid radiations. Similarly, dispersal cannot be ruled out as having been important in the evolution of Belau diplommatinids. For example, the seemingly random relationships among species from islands relatively distant from one another (e.g. clade 5, figure 4) support dispersal as playing a major role within Belau. Until additional molecular and geological data are brought to bear on this issue, neither vicariance nor dispersal can be discounted as having contributed to these phylogenetic patterns.
Another explanation for the disjunct pattern among Belau diplommatinids is that the ranges of many species were once more extensive, and extinction may have been responsible for seemingly discordant relationships. Fossil evidence could possibly shed light on this issue, although detailed sampling of fossil land shells has yet to be undertaken, and the condition of many of the Belau limestone cave deposits (Steadman 2006, p. 254) may not be ideal for preservation of delicate diplommatinid shells. Fossils in general would be useful for dating these island radiations; however, there is currently no useful fossil calibration for caenogastropod land snails, let alone diplommatinids.
Lack of sampling throughout a species range may also contribute to this problem. However, all of the largest islands across the length of the archipelago have been surveyed, including many neighbouring small Rock Islands. The fact that the majority of the species in this study are endemic to the island locality listed in figure 4 and table 1 or to that island plus nearby subsidiary islands supports the hypothesis that lack of sampling has not played a major role in shaping the biogeographic pattern shown in figure 4.
(c) Evolution and biogeography of spined rock dwellers
The limestone composition of most of Belau's islands may contribute to land snail diversity and abundance, though it is not clear to what extent. Calcium carbonate is important in maintaining shell growth, and Schilthuizen et al. (2002) and Briers (2003) have suggested that more calciphilic snail species have smaller geographical ranges and fragmented population structures that may increase speciation potential.
The present study suggests that the spined or heavily calcified morphotype has not evolved in parallel on multiple islands, but evolved once and species bearing these morphologies have subsequently radiated among the islands, filling the similar limestone cliff-face and limestone boulder niches that are present throughout limestone-bearing Belau islands. Additional sampling in the western Pacific and Southeast Asia will be necessary to address whether multiple colonizations from outside Belau have also contributed to the evolution of this group of rock dwellers. There are interesting phylogenetic patterns among southern lagoon islands rock-dwelling taxa, for example among clade 4 taxa (figure 4), which have apparently dispersed from Kmekumer and Mecherchar southwards to the (younger) Pleistocene low limestone islands of Beliliou and Ngeaur. It is therefore possible that detailed study of suites of nearby islands will be important in revealing colonization patterns and thus evolutionary history in this complex archipelago of more than 500 islands.
(d) Future work on Belau diplommatinid taxonomy needed
No firm taxonomic recommendations are made here, though some insights into Belau diplommatinid evolution have been gained. The rock-dwelling genera Hungerfordia and Diplommatina, as defined by shell morphology, are not monophyletic. Type species of these nominal genera must be included in future analyses in order to delineate genera. It is possible that Belau species currently placed in Diplommatina do not belong to Diplommatina (sensu stricto). This is plausible given that the type species of Diplommatina is probably from the lower western Himalayas, and therefore is probably distantly related to Belau species. Including the type species of Hungerfordia, H. pelewensis, a Belau endemic, will be important to investigate its relationship with other rock dwellers. Regardless of the outcome of such analyses, it is clear that spined species and heavily calcified species of Belau Diplommatina and Hungerfordia are each paraphyletic, and based on present information, should comprise a single taxonomic group.
The genus Palaina also requires revision. For example, among described species, P. albata (clade 7) and P. scalarina (clade 11) group within divergent clades in the phylogeny (figure 4). Species have probably been placed in this genus for lack of detailed study. Most of the taxonomic work on these taxa dates from the mid to late 1800s (e.g. Benson 1849; Pfeiffer 1855; Semper 1865; Crosse 1866; Beddome 1889) and much remains to be done.
The Pohnpei species P. scalarina and Palaina doliolum present additional systematic issues, and further study across all of Cyclophoroidea is needed to better understand their evolutionary affinities. Although P. scalarina and P. doliolum were listed by Kobelt (1902) in his cyclophorid treatise as diplommatinines, the molecular evidence is consistent with family-level divergence from the other Palaina species. Their shell morphology superficially resembles that of other Palaina species (i.e. ovately conical and brown with distinct costae and striae), but it is possible that detailed study will reveal key anatomical features that distinguish them from true Palaina. To date, little work has been done on internal morphology of these animals and taxonomy has relied almost entirely on shell characters.
This molecular study points to key directions for future work on the Belau diplommatinids. First, the rock-dwelling taxa may represent a single radiation within Belau. This group warrants more detailed work to untangle potential cryptic diversity, as well as evolutionary patterns among the hundreds of limestone islands of Belau. Second, further sampling of diplommatinids (and presumed diplommatinids) from outside Belau will assist in determining the origins of the Belau/Guam/Yap Diplommatinidae, as well as the directions of colonization within the western Pacific. Finally, biogeographic patterns within the Belau islands are complex, and additional sampling, as well as the addition of slower evolving genes, may shed light on the biogeography of species within this fascinating archipelago.
Acknowledgments
I thank the National and State Governments of the Republic of Palau and the Palau Bureau of Agriculture for providing research permits, and the Republic of Palau's Office of Environmental Response and Coordination, Palau Conservation Society, Belau Cares, Inc., Belau National Museum, Ibedul Y. Gibbons, Yap Historical Preservation Office, the Federated States of Micronesia, Coral Reef Research Foundation, Yap Community Action Program, Yap Forestry, the Conservation Society of Pohnpei and the Kosrae Island Resources Management Authority for supporting my field research and/or providing facilities. Special thanks are owed to B. Sakuma, and to J. Miles, A. Eledui, T. Holm, L. Colin, P. Colin, R. Crombie, A. Olsen, M. Etpison, A. Kitalong, the Koror State Rangers (Belau) and to my field assistants J. Czekanski-Moir, A. Gawel, D. Mulroney, R. Orben, S. Wilkinson, R. Brewer and C. Carroll. Thanks to D. Ngirkelau, V. Fread and additional field personnel too numerous to list. R. Kawamoto and A. Suzumoto (Bishop Museum), V. Héros and P. Bouchet (Muséum National d'Histoire Naturelle), J. Slapcinsky and F. Thompson (Florida Museum of Natural History) provided access to specimens. J. Gerber, M. Pryzdia, R. Cowie, C. Christensen, B. Holland, M. Gawel, I. Gawel and B. Smith provided additional support. I thank S. Reddy and S. Hackett for their analysis advice; K. Feldheim, B. Stuart, B. Strack, T. Lee, K. Pitz, J. Markel and D. Clarke for their laboratory/SEM advice; and R. Bieler, T. Price, S. Reddy and J. Czekanski-Moir for their comments on a draft. I also thank my dissertation committee: R. Bieler, P. Goldstein, S. Hackett, D. Jablonski and T. Price. Funding was provided by the National Geographic Society Committee for Research and Exploration (NGS CRE grant 7972-06), American Malacological Society, Unitas Malacologica, Conchologists of America, FMNH Zoology Department Marshall Field Fund and the University of Chicago Hinds Fund.
Footnotes
One contribution of 15 to a Theme Issue ‘Evolution on Pacific islands: Darwin's legacy’.
References
- Abdou A, Bouchet P. Nouveaux gastéropodes Endodontidae et Punctidae (Mollusca, Pulmonata) récemment éteints de l'archipel des Gambier (Polynésie) Zoosystema. 2000;22:689–707. [Google Scholar]
- Arad Z. Effect of desiccation on the water economy of terrestrial gastropods of different phylogenetic origins: a prosobranch (Pomatias glaucus) and two pulmonates (Sphincterochila cariosa and Helix engaddensis) Isr. J. Zool. 1993;39:95–104. [Google Scholar]
- Beddome R.H. Descriptions of land-shells from the island of Koror, Pelew Group. Proc. Zool. Soc. Lond. 1889;1889:112–116. [Google Scholar]
- Benson W.H. Characters of Diplommatina, a new genus of terrestrial mollusks belonging to the family of Carychiadae, and of a second species contained in it; also of a new species of Carychium inhabiting Western Himalaya. Ann. Mag. Nat. Hist. 1849;4:193–194. [Google Scholar]
- Briers R.A. Range size and environmental calcium requirements of British freshwater gastropods. Glob. Ecol. Biogeogr. 2003;12:47–51. doi:10.1046/j.1466-822X.2003.00316.x [Google Scholar]
- Carson H.L, Hardy D.E, Speith H.T, Stone W.S. The evolutionary biology of the Hawaiian Drosophilidae. In: Hecht M.K, Steere W.C, editors. Essays in evolution and genetics in honor of Theodosius Dobzhansky. Appleton-Century-Crofts; New York, NY: 1970. pp. 437–543. [Google Scholar]
- Carson H.L, Lockwood J.P, Craddock E.M. Extinction and recolonization of local populations on a growing shield volcano. Proc. Natl Acad. Sci. USA. 1990;87:7055–7057. doi: 10.1073/pnas.87.18.7055. doi:10.1073/pnas.87.18.7055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S. Accelerated evolution of land snails Mandarina in the oceanic Bonin Islands: evidence from mitochondrial DNA sequences. Evolution. 1999;53:460–471. doi: 10.1111/j.1558-5646.1999.tb03781.x. doi:10.2307/2640782 [DOI] [PubMed] [Google Scholar]
- Clarke B.C, Murray J.J. Ecological genetics and speciation in land snails of the genus Partula. Biol. J. Linn. Soc. 1969;1:31–42. doi:10.1111/j.1095-8312.1969.tb01810.x [Google Scholar]
- Colgan D.J, Ponder W.F, Beacham E, Macaranas J. Molecular phylogenetics of Caenogastropoda (Gastropoda: Mollusca) Mol. Phylogenet. Evol. 2007;42:717–737. doi: 10.1016/j.ympev.2006.10.009. doi:10.1016/j.ympev.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Corwin C.G, Rogers C.L, Elmquist P.O. Intelligence Division, Office of the Engineer, Headquarters US Army Forces Far East, Honolulu; Honolulu, HI: 1956. Military geology of Palau Islands, Caroline Islands. [Google Scholar]
- Cowie R.H. Evolution and extinction of Partulidae, endemic Pacific island land snails. Phil. Trans. R. Soc. B. 1992;335:167–191. doi:10.1098/rstb.1992.0017 [Google Scholar]
- Cowie R.H. Pacific island land snails: relationships, origins and determinants of diversity. In: Keast A, Miller S.E, editors. The origin and evolution of Pacific Island biotas, New Guinea to eastern Polynesia: patterns and processes. SPB Academic Publishing; Amsterdam, The Netherlands: 1996. pp. 347–372. [Google Scholar]
- Cowie R.H. Can snails ever be effective biocontrol agents? Int. J. Pest Manage. 2001;47:23–40. doi:10.1080/09670870150215577 [Google Scholar]
- Cowie R.H, Evenhuis N.L, Christensen C.C. Backhuys Publishers; Leiden, The Netherlands: 1995. Catalog of the native land and freshwater molluscs of the Hawaiian Islands. [Google Scholar]
- Craig D.A, Currie D.C, Joy D.A. Geographical history of the central-western Pacific black fly subgenus Inseliellum (Diptera: Simuliidae: Simulium) based on a reconstructed phylogeny of the species, hot-spot archipelagoes and hydrological considerations. J. Biogeogr. 2001;28:1101–1127. doi:10.1046/j.1365-2699.2001.00619.x [Google Scholar]
- Crombie R.I, Pregill G.K. A checklist of the herpetofauna of the Palau Islands (Republic of Belau), Oceania. Herpetol. Monogr. 1999;13:29–80. doi:10.2307/1467060 [Google Scholar]
- Crosse H. Note sur les Mollusques opercules terrestres des iles Pelew ou Palaos. J. Conchyliol. 1866;14:346–350. [Google Scholar]
- Douris V, Cameron R.A.D, Rodakis G.C, Lecanidou R. Mitochondrial phylogeography of the land snail Albinaria in Crete: long-term geological and short-term vicariance effects. Evolution. 1998;52:116–125. doi: 10.1111/j.1558-5646.1998.tb05144.x. doi:10.2307/2410926 [DOI] [PubMed] [Google Scholar]
- Easton W.H, Ku T.-L. Holocene sea-level changes in Palau, west Caroline Islands. Quat. Res. 1980;14:199–209. doi:10.1016/0033-5894(80)90048-4 [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. doi:10.2307/2408678 [DOI] [PubMed] [Google Scholar]
- Fleischer R.C, McIntosh C.E, Tarr C.L. Evolution on a volcanic conveyor belt: using phylogeographic reconstruction and K–Ar based ages of the Hawaiian Islands to estimate molecular evolutionary rates. Mol. Ecol. 1998;7:533–545. doi: 10.1046/j.1365-294x.1998.00364.x. doi:10.1046/j.1365-294x.1998.00364.x [DOI] [PubMed] [Google Scholar]
- Fretter, V. Graham, A., Ponder, W. F. & Lindberg, D. R. 1998 Prosobranchia introduction. In Mollusca: the southern synthesis, vol. 5 (eds P. L. Beesley G. J. B. Ross & A. Wells). Fauna of Australia, pp. 605–638. Melbourne, Australia: CSIRO Publishing.
- Funk V.A, Wagner W.L. Biogeographic patterns in the Hawaiian Islands. In: Wagner W.L, Funk V.A, editors. Hawaiian biogeography. Evolution on a hot spot archipelago. Smithsonian Institution; Washington, DC: 1995. pp. 379–419. [Google Scholar]
- Gillespie R.G. Community assembly through adaptive radiation in Hawaiian spiders. Science. 2004;303:356–359. doi: 10.1126/science.1091875. doi:10.1126/science.1091875 [DOI] [PubMed] [Google Scholar]
- Gillespie R.G, Croom H.B, Palumbi S.R. Multiple origins of a spider radiation in Hawaii. Proc. Natl Acad. Sci. USA. 1994;91:2290–2294. doi: 10.1073/pnas.91.6.2290. doi:10.1073/pnas.91.6.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie R.G, Claridge E.M, Roderick G.K. Biodiversity dynamics in isolated island communities: interaction between natural and human-mediated processes. Mol. Ecol. 2008;17:45–57. doi: 10.1111/j.1365-294X.2007.03466.x. doi:10.1111/j.1365-294x.2007.03466.x [DOI] [PubMed] [Google Scholar]
- Gulick J.T. On the variation of species as related to their geographical distribution, illustrated by Achatinellinae. Nature. 1872;6:222–224. doi:10.1038/006222b0 [Google Scholar]
- Gulick J.T. On diversity of evolution under one set of external conditions. J. Linn. Soc. Zool. 1873;11:496–505. doi:10.1111/j.1096-3642.1873.tb01670.x [Google Scholar]
- Hadfield M.G. Extinction in Hawaiian achatinelline snails. Malacologia. 1986;27:67–81. [Google Scholar]
- Hadfield M.G, Miller S.E, Carwile A.H. The decimation of endemic Hawai‘ian [sic] tree snails by alien predators. Am. Zool. 1993;33:610–622. [Google Scholar]
- Hall R. Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: computer-based reconstructions, model and animations. J. Asian Earth Sci. 2002;20:353–431. doi:10.1016/S1367-9120(01)00069-4 [Google Scholar]
- Hezel F.X. University of Hawaii Press; Honolulu, HI: 1983. The first taint of civilization. [Google Scholar]
- Iredale T. The land Mollusca of Lord Howe Island. Aust. Zool. 1944;10(Pt 17–20):299–334. [Google Scholar]
- Keating B.H, Mattey D.P, Helsey C.E, Naughton J.J, Epp D, Lazarewicz D. Evidence for a hot spot origin of the Caroline Islands. J. Geophys. Res. 1984;89:9937–9948. doi:10.1029/JB089iB12p09937 [Google Scholar]
- Kelletat D. Main trends of Palau Islands' coastal evolution, identified by air and ground truthing. GeoJournal. 1991;24:77–85. doi:10.1007/BF00213059 [Google Scholar]
- Kirchner C, Kratzner R, Wetter-Schultes F.W. Flying snails: how far can Truncatellina (Pulmonata: Vertiginidae) be blown over the sea? J. Mollusc. Stud. 1997;63:479–487. doi:10.1093/mollus/63.4.479 [Google Scholar]
- Kobayashi K. Origin of the Palau and Yap trench-arc systems. Geophys. J. Int. 2004;157:1303–1315. doi:10.1111/j.1365-246X.2003.02244.x [Google Scholar]
- Kobelt W. Verlag von R. Friedlander und Sohn; Berlin, Germany: 1902. Tierreich: Cyclophoridae. [Google Scholar]
- Kroenke L.W. Plate tectonic development of the western and southwestern Pacific: Mesozoic to the present. In: Keast A, Miller S.E, editors. The origin and evolution of Pacific Island biotas, New Guinea to Eastern Polynesia: patterns and processes. SPB Academic Publishing; Amsterdam, The Netherlands: 1996. pp. 19–34. [Google Scholar]
- Lee T, Burch J.B, Coote T, Fontaine B, Gargominy O, Pearce-Kelly P, Ó Foighil D. Prehistoric inter-archipelago trading of Polynesian tree snails leaves a conservation legacy. Proc. R. Soc. B. 2007;274:2907–2914. doi: 10.1098/rspb.2007.1009. doi:10.1098/rspb.2007.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. Cambridge University Press; Cambridge, UK: 1990. The terrestrial invasion: an ecophysiological approach to the origins of land animals. [Google Scholar]
- Losos J.B, Jackman T.R, Larson A, deQueiroz K, Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiation of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. doi:10.1126/science.279.5359.2115 [DOI] [PubMed] [Google Scholar]
- Lydeard C, et al. The global decline of non-marine molluscs. BioScience. 2004;54:321–330. doi:10.1641/0006-3568(2004)054[0321:TGDONM]2.0.CO;2 [Google Scholar]
- Maddison D.R, Maddison W.P. Sinauer Associates, Inc; Sunderland, MA: 2001. MacClade 4: analysis of phylogeny and character evolution. [DOI] [PubMed] [Google Scholar]
- Masse W.B, Liston J, Carucci J, Athens J.S. Evaluating effects of climate change on environment, resource depletion, and culture in the Palau Islands between AD 1200 and AD 1600. Quat. Int. 2006;151:106–132. doi:10.1016/j.quaint.2006.01.017 [Google Scholar]
- Miller S.E. Biogeography of Pacific insects and other terrestrial invertebrates: a status report. In: Keast A, Miller S.E, editors. The origin and evolution of Pacific island biotas, New Guinea to Eastern Polynesia: patterns and processes. SPB Academic Publishing; Amsterdam, The Netherlands: 1996. pp. 463–475. [Google Scholar]
- Motteler L.S. Bishop Museum Press; Honolulu, HI: 2006. Pacific island names. [Google Scholar]
- Ohara Y, Fujioka K, Ishizuka O, Ishii T. Peridotites and volcanics from the Yap arc system: implications for tectonics of the southern Philippine Sea Plate. Chem. Geol. 2002;189:35–53. doi:10.1016/S0009-2541(02)00062-1 [Google Scholar]
- Palumbi S.R. Nucleic acids II: the polymerase chain reaction. In: Hillis D.M, Moritz C, Mable B.K, editors. Molecular systematics. Sinauer Associates; Sunderland, MA: 1996. pp. 205–247. [Google Scholar]
- Park J.-K, Ó Foighil D. Sphaeriid and corbiculid clams represent separate heterodont bivalve radiations into freshwater environments. Mol. Phylogenet. Evol. 2000;14:75–88. doi: 10.1006/mpev.1999.0691. doi:10.1006/mpev.1999.0691 [DOI] [PubMed] [Google Scholar]
- Peake J.F. Species isolation in sympatric populations of the genus Diplommatina (Gastropoda, Prosobranchia, Cyclophoridae, Diplommatininae) Malacologia. 1973;14:303–312. [Google Scholar]
- Pfeiffer L. Descriptions of eighteen new species of Cyclostomacea, from Mr. Cuming's collections. Proc. Zool. Soc. Lond. 1855;1854:299–303. [Google Scholar]
- Polhemus D.A. Island arcs, and their influence on Indo-Pacific biogeography. In: Keast A, Miller S.E, editors. The origin and evolution of Pacific Island biotas, New Guinea to Eastern Polynesia: patterns and processes. SPB Academic Publishing; Amsterdam, The Netherlands: 1996. pp. 51–66. [Google Scholar]
- Ponder W.F, Lindberg D.R. Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zool. J. Linn. Soc. 1997;119:83–265. [Google Scholar]
- Ponder, W. F. Waren, A. 1988 Classification of the Caenogastropoda and Heterostropha: a list of the family-group and higher category names. In Prosobranch phylogeny (ed. W. F. Ponder), pp. 288–326 (sup. 4). Malacological review
- Posada D, Crandall K.A. ModelTest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. doi:10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Rawlings T.A, Hayes K.A, Cowie R.H, Collins T.M. The identity, distribution, and impacts of non-native apple snails in the continental United States. BMC Evol. Biol. 2007;7:97. doi: 10.1186/1471-2148-7-97. doi:10.1186/1471-2148-7-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G.S, Sattler K. Plutella in the Hawaiian Islands: relatives and host-races. Bishop Mus. Occas. Pap. 2001;67:1–27. [Google Scholar]
- Rundell R.J, Holland B.S, Cowie R.H. Molecular phylogeny and biogeography of the endemic Hawaiian Succineidae (Gastropoda: Pulmonata) Mol. Phylogenet. Evol. 2004;31:246–255. doi: 10.1016/j.ympev.2003.07.014. doi:10.1016/j.ympev.2003.07.014 [DOI] [PubMed] [Google Scholar]
- Schilthuizen M. Sexual selection on land snail shell ornamentation: a hypothesis that may explain shell diversity. BMC Evol. Biol. 2003;3:13. doi: 10.1186/1471-2148-3-13. doi:10.1186/1471-2148-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilthuizen M, et al. Microsnails at microscales in Borneo: distributions of Prosobranchia versus Pulmonata. J. Mollusc. Stud. 2002;68:255–258. doi:10.1093/mollus/68.3.255 [Google Scholar]
- Schilthuizen M, van Til A, Salverda M, Liew T.-S, James S.S, Bin Elahan B, Vermeulen J.J. Microgeographic evolution of snail shell shape and predator behavior. Evolution. 2006;60:1851–1858. doi:10.1554/06-114.1 [PubMed] [Google Scholar]
- Semper O. Notice preliminaire sur la famille des Diplommatinacées. J. Conchyl. 1865;13:289–294. [Google Scholar]
- Solem A. Systematics and zoogeography of the land and fresh-water Mollusca of the New Hebrides. Field Zool. 1959;43(Pt 34):1–359. [Google Scholar]
- Solem, A. 1968 Basic distribution of non-marine mollusks. In Proc. Symp. Mollusca, Mar. Biol. Assoc. India, vol. I (ed. R. W. Dexter), pp.231–247. Bangalore, India: Bangalore Press.
- Solem A. Field Museum of Natural History; Chicago, IL: 1976. Endodontoid land snails from Pacific islands (Mollusca: Pulmonata: Sigmurethra). Part I, family Endodontidae. [Google Scholar]
- Solem A. Field Museum of Natural History; Chicago, IL: 1983. Endodontoid land snails from Pacific islands (Mollusca: Pulmonata: Sigmurethra), Part II, families Punctidae and Charopidae, Zoogeography. [Google Scholar]
- Solem A. How many Hawaiian land snail species are left? And what we can do for them. Bishop Mus. Occas. Pap. 1990;30:27–40. [Google Scholar]
- Stanisic J. Superfamily Cyclophoroidea. In: Beesley P.L, Ross G.J.B, Wells A, editors. Mollusca: the southern synthesis. Fauna of Australia. Part B. vol. 5. CSIRO Publishing; Melbourne, Australia: 1998. pp. 703–706. [Google Scholar]
- Steadman D.W. University of Chicago Press; Chicago, IL: 2006. Extinction and biogeography of tropical Pacific birds. [Google Scholar]
- Swofford, D.L. 2002 PAUP*: phylogenetic analysis using parsimony (*and other methods), v. 4.0.b10. Sunderland, MA: Sinauer Associates.
- Thiele J. Gustav Fischer Verlag; Stuttgart, Germany: 1935. Handbook of systematic malacology, Part I. [Google Scholar]
- Thompson J.D, Gibson T.J, Plewniak F, Jeanmougin F, Higgins D.G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. doi:10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillier S. Clines, convergence and character displacement in New Caledonian diplommatinids (land prosobranchs) Malacologia. 1981;21:177–208. [Google Scholar]
- Vagvolgyi J. Body size, aerial dispersal, and origin of the Pacific land snail fauna. Syst. Zool. 1975;24:465–488. doi:10.2307/2412906 [Google Scholar]
- Vermeulen J.J. Notes on the non-marine molluscs of the island of Borneo 5. The genus Diplommatina (Gastropoda Prosobranchia: Diplommatinidae) Basteria. 1993;57:3–69. [Google Scholar]
- Vermeulen J.J. Notes on the non-marine molluscs of the island of Borneo 6. The genus Opisthostoma (Gastropoda Prosobranchia: Diplommatinidae), part 2. Basteria. 1994;58:75–191. [Google Scholar]
- von Möllendorff O.F. Diagnosen neuer und kritischer Landdeckelschnecken. Nachrichtsbl. Deutsch. Malakozool. Gesell. 1897;3:31–45. [Google Scholar]
- Wagner W.L, Funk V.A, editors. Hawaiian biogeography. Evolution on a hot spot archipelago. Smithsonian Institution; Washington, DC: 1995. [Google Scholar]
- Wallace A.R. Harper & Bros; New York, NY: 1881. Island life. [Google Scholar]
- Wilson E.O. The nature of the taxon cycle in the Melanesian ant fauna. Am. Nat. 1961;95:169–193. doi:10.1086/282174 [Google Scholar]
- Wright S. Experimental results and evolutionary deductions. vol. 3. University of Chicago Press; Chicago, IL: 1977. Evolution and genetics of populations, a treatise. [Google Scholar]
- Zwickl, D. J. 2006 Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD dissertation, The University of Texas at Austin. See www.bio.utexas.edu/faculty/antisense/garli/Garli.html