Abstract
New Zealand biogeography has been dominated by the knowledge that its geophysical history is continental in nature. The continental crust (Zealandia) from which New Zealand is formed broke from Gondwanaland ca 80 Ma, and there has existed a pervading view that the native biota is primarily a product of this long isolation. However, molecular studies of terrestrial animals and plants in New Zealand indicate that many taxa arrived since isolation of the land, and that diversification in most groups is relatively recent. This is consistent with evidence for species turnover from the fossil record, taxonomic affinity, tectonic evidence and observations of biological composition and interactions. Extinction, colonization and speciation have yielded a biota in New Zealand which is, in most respects, more like that of an oceanic archipelago than a continent.
Keywords: New Zealand, Gondwanan, Zealandia, biogeography, dispersal, phylogeography
1. Introduction
New Zealand is a group of islands (270 534 km2) isolated by more than 1500 km of ocean from any other significant land area, but is continental in stratigraphic composition (see box 1). Unlike most island systems in the Pacific, understanding the evolution of the New Zealand biota is significantly influenced by continental biogeography (Cowie & Holland 2006) and has specifically and popularly been described as ‘Moa's ark’1 (Bellamy et al. 1990), essentially captive and evolved in isolation for up to 80 Ma. However, New Zealand has also been described as an endemism hot spot (Daugherty et al. 1993) with a biota comparable to oceanic islands such as Hawaii and the Galápagos (Gibbs 2006). It harbours a distinctive biota including ‘relict’ animal taxa such as tuatara (Sphenodon), leiopelmatid frogs, and a Gondwanan element including weta (Orthoptera), peripatus (Onychophora), southern beech (Nothofagus) and kauri (Agathis australis; Cooper & Millener 1993), but it is also home to many recent colonists (Falla 1953; Fleming 1979). Though frequently referred to as a Gondwanan element, the relevance of this term has been increasingly questioned (e.g. McGlone 2005). The term ‘Gondwanan’ can be misleading when applied to indicate historical process rather than simply distribution pattern. In the case of the New Zealand biota, three alternative meanings can be identified. First, the most restrictive meaning implies that a taxon is Gondwanan if its lineage has been continuously present in New Zealand since rifting from the rest of Gondwanaland. Second, a taxon might also be considered Gondwanan if it is descended from a lineage that was present in Gondwanaland prior to break-up but arrived in New Zealand since Zealandia (see box 1 and figure 1) rifted away from Gondwanaland and sank. Third, Gondwanan might describe a particular type of distribution of lineages in the Southern Hemisphere with no expectation of a common historical process to explain different instances of this pattern. For example, penguins are found in New Zealand, Australia, southern Africa, South America and Antarctica and certainly demonstrate a Gondwanan distribution (third meaning). Penguins have a fossil history to at least 62 Ma (Slack et al. 2006) and molecular analyses suggest a much earlier origin in the Gondwana landmass (Baker et al. 2006), implying that they might occupy the New Zealand region by virtue of having been in Gondwanaland when Zealandia rifted away (first meaning). But modern extant penguins are monophyletic, and fossil and molecular data suggest they evolved in the Early Oligocene2 (Baker et al. 2006), and extant penguin genera of Eudyptes, Eudyptula and Megadyptes may have colonized New Zealand from other former Gondwanan landmasses since that time (second meaning). Indeed, Eudyptula, the blue penguin, is found in Australia and New Zealand with evidence of recent contact between these populations (Banks et al. 2002). Thus, the modern distribution of penguins could be described as Gondwanan in all three senses. Distributional data are clearly not indicative of historical processes alone and therefore the presence of lineages in New Zealand cannot in itself be taken as evidence of a dominant role of continental drift in the origination of the biota. Unfortunately, it is just this supposition that is widely made. The addition of timing of divergence is necessary to choose between the three meanings of Gondwanan.
Box 1. New Zealand continental origin.
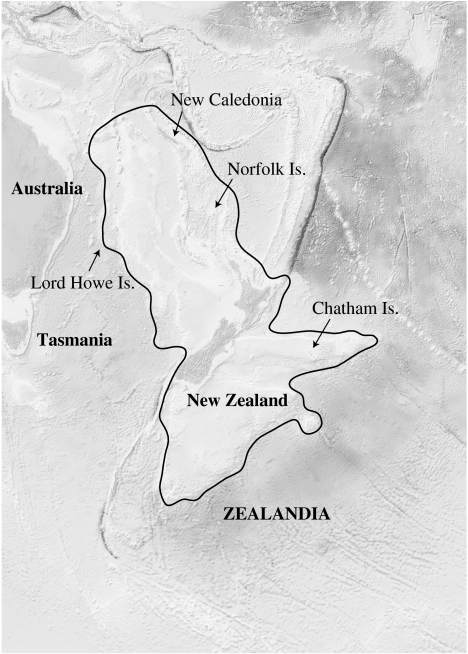
The Tasman Ocean that today separates New Zealand from the nearest continent (Australia) began forming ca 83 Ma. A section of continental crust rotated away from the eastern Australian section of Gondwanaland and this fragment is referred to as Zealandia (Campbell & Hutching 2007; Trewick et al. 2007; Neall & Trewick 2008). In geological terms, ‘continental’ means being composed of continental rather than oceanic crust, but for continental crust to be emergent as land, it has to be thick enough to stand above sea level. Zealandia is much larger than modern New Zealand, about the size of India, and includes the Campbell Plateau, Challenger Plateau, Lord Howe Rise, Norfolk Ridge, Chatham Rise and New Caledonia. Over about 60 million years, Zealandia was stretched and thinned, effectively losing buoyancy, and sinking some 2–3000 m. Today, approximately 93 per cent of Zealandia is beneath the sea, and in the Oligocene the New Zealand sector may have also been entirely submerged (Landis et al. 2008). New Zealand exists above water today owing to a plate boundary collision. It has been vigorous and sustained since its abrupt initiation in latest Oligocene time (Sutherland 1999; Cande & Stock 2004). This activity is prominently expressed along the alpine fault, where tectonic activity has generated 480 km of lateral motion and, since the Pliocene, some 20 km of uplift resulting in the formation of the Southern Alps (Kamp et al. 1989; Kamp 1992; Whitehouse & Pearce 1992). Today, the continental crust of Zealandia, including New Zealand, is in geological terms thin (20–25 km) and New Zealand could be viewed biologically as a comparatively old oceanic island group (Trewick et al. 2007). The geological evidence for the survival of any ancient terrestrial parts of Zealandia is unexpectedly weak, due in part to the destructive impact of later tectonics, questioning the assumptions of the ancient origin of New Zealand's biota (Landis et al. 2008).
Figure 1.
The continent of Zealandia (modified from Stagpoole 2002).
In recent years, it has become increasingly apparent that diversification and, in many cases, origin of New Zealand lineages substantially post-date the break-up of Gondwanaland (Pole 1994; McGlone 2005; McDowall 2008). The major impetus for the resurgence of dispersal as an accepted contributing factor in the formation of the biota comes from molecular studies. Owing to the interesting biotic assemblage present in New Zealand, the question of whether New Zealand should be treated as an island or a micro-continent (Daugherty et al. 1993) has led to many studies on speciation and colonization which focus on New Zealand's biota (Gillespie & Roderick 2002) and its relationship to other close landmasses (i.e. Australia, New Caledonia; see Sanmartin & Ronquist 2004; Cook & Crisp 2005).
(a) Illogical juxtaposition
So attractive has the notion of an ancient (Gondwanan) insular biota been, that it is frequently, though illogically, juxtaposed with observations of the composition of the fauna and flora, which have in the past been recognized as consistent with island biota subjected to dispersal (Wallace 1876; Falla 1953; Darlington 1957, 1965; Fleming 1962a, 1963a; Caughley 1964; Gaskin 1970, 1975; Raven 1973; Cracraft 1974, 1975).
Several influential though not necessarily meaningful observations have been made about New Zealand and its biota: (i) it is isolated from other landmasses; (ii) the biota is unique; (iii) it has high endemicity; (iv) it includes behaviourally or morphologically strange and distinctive taxa; and (v) the composition of New Zealand biota is disharmonic (Gibbs 2006; McDowall 2008).
…its whole biota is anomalous, depauperate, and rather different from that of Australia.
(Keast 1971, p. 359).
…Although New Zealand enjoys a rich, unique biodiversity, it can equally well be described as ‘naturally depauperate’, meaning that many of the types of animals and plants that one might expect to find here are absent.
(Gibbs 2006, p. 20)
Since the rise of vicariance biogeography and acceptance of continental drift (e.g. Skipworth 1974; and see Waters & Craw 2006 and references therein), observations of this type have been interpreted as evidence of an old island with a biota that has been isolated for a very long time (i.e. 80 Ma). However, they are actually what might be expected from the fauna of a young oceanic island and/or a high level of extinction. Isolation ensures that only a subset of nearby continental faunas will be represented on the islands and the lack of great age of the island would account for the low diversity levels. Very commonly, the New Zealand biota is described as ‘unique’ and unlike anything elsewhere. Diamond (1997) described the biology as ‘the nearest approach to life on another planet’. In fact, the nature of the biota is inconsistent with the process to which it is frequently ascribed because,
physical isolation does not equate to biological isolation,
all biotas are unique,
Islands resemble one another in that each is unique
species endemicity is usually high on oceanic islands,
New Zealand ranks alongside island groups like Hawaii and the Galápagos Islands for its levels of endemism.
(Gibbs 2006, p. 12)
distinctive taxa are common products of evolution on islands, and
disharmonic biotas are best and usually explained as resulting from stochastic colonization and extinction (Carlquist 1965).
Trans-oceanic dispersal by air and water from neighbouring continental areas and islands was thought to have played quite an important role at all times in New Zealand's history in assembly of the disharmonic fauna and flora of the Archipelago
(Gaskin 1975, p. 87).
If New Zealand was isolated since 80 Ma, we would expect it to support the descendants of a Zealandian biota with high diversity, complex coevolutionary associations, endemicity at deeper taxonomic/phylogenetic levels and a more complete faunal composition.
(b) Ancient lineages and living fossils
Several ‘ancient’ lineages have been identified within the New Zealand biota. For example, the tuatara (Sphenodon) is a relict of the sister group to the squamate reptiles and has an independent history of over 250 million years (Hugall et al. 2007). Other ancient endemic lineages are the leiopelmatid frogs (Estes & Reig 1973; Roelants & Bossuyt 2005) and acanthisittid wrens (Ericson et al. 2002). The presence of these lineages in New Zealand, but not elsewhere, is tantalizing evidence that Gondwanan lineages have persisted in the New Zealand region since the break-up of Gondwanaland (Gibbs 2006). However, on their own, ancient lineages tell us very little about their longevity in the region as endemism on islands can arise in several ways (Emerson & Kolm 2005). Lineages now endemic to New Zealand might have been present in Australia, for instance, but have gone extinct there, or they may have speciated within either Australia or New Zealand. Neither of these scenarios is age dependent in that they could have occurred in lineages present since the break-up of Gondwanaland or after more recent colonization of New Zealand. For example, the tuatara lineage may have been present in New Zealand since the break-up but subsequently gone extinct in Australia (and elsewhere) or it might have colonized New Zealand any time over the past 80 Ma and subsequently gone extinct in Australia. ‘Ancient lineages’ tend to be recognized as such by the absence of close relatives, but this presents a problem when inferring their history. The tuatara and its closest living relatives have a common ancestor over 100 Ma before Zealandia broke away from Gondwanaland (Hugall et al. 2007), but this clearly does not inform us about their biogeographical history (Crisp & Cook 2005). The absence of fossils, or lack of reliably time-constrained or taxonomically precise fossils, also limits inferences.
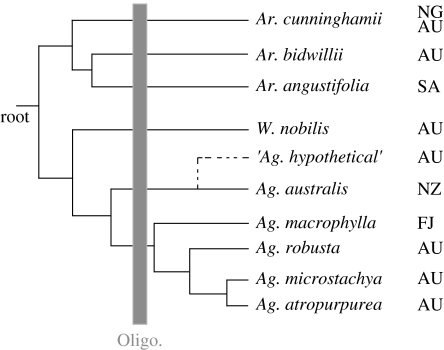
A New Zealand lineage might be found, after careful calibration of genetic divergences, to have a common ancestor with a nearest relative in Australia dating to ca 80 Ma. This would imply the lineage has been present in New Zealand since break-up, but a more closely related lineage in Australia might have been extinguished. If so, the evidence for a post-break-up dispersal history would have been lost. For instance, morphological (Parrish et al. 1998) and DNA (Stöckler et al. 2002; Knapp et al. 2007) studies are consistent with the conifer genus Agathis (Araucariaceae), having been continuously present in New Zealand since Cretaceous time, and living New Zealand kauri, A. australis appears to be sister to other living Agathis in New Guinea, Australia and New Caledonia (Landis et al. 2008). This pattern is consistent with an ancient vicariant origin in New Zealand, and is probably the best example of any ancient element supported by molecular evidence. However, a single sister species of A. australis in Australia would falsify that inference; such a sister lineage might have existed until recently but now be extinct and unavailable for analysis (figure 2). In fact, the oldest fossils of Agathis in New Zealand are Late Oligocene/Early Miocene (Lee et al. 2007) and the oldest pollen fossils of A. australis date to the Pliocene/Pleistocene and there are no records of similar Cenozoic araucarian fossils younger than Early Miocene (Pole 2001); evidence that is consistent with both a post-Gondwanan colonization scenario and a vicariant Gondwanaland history (Waters & Craw 2006). The issue of whether fossil absences are real or not is, therefore, crucial in interpreting the biogeographical history of extant lineages. So do relict taxa tell us anything about the biogeographical history of New Zealand? Individually, each taxon adds just one datum. An overwhelming number of relict taxa might be compelling. However, there is no research examining how many relictual taxa we might reasonably expect to see after 80 million years for us to assess whether the number that we identify in New Zealand is more or less than this expected number.
Figure 2.
Historical inference from phylogenetic trees is sensitive to tree shape and taxon sampling (see Crisp & Cook 2005). For instance, a single hypothetical undiscovered or recently extinct Australian lineage closely related to the extant New Zealand species A. australis (‘Ag. hypothetical’, dashed line) would yield a significantly different inference of the group's history (tree modified from Knapp et al. 2007) and falsify the inference that A. australis is ‘the sole representative of an early diverged lineage within the genus’. (NG, New Guinea; NZ, New Zealand; AU, Australia; SA, South America; FJ, Tropical Australasia; Oligo., Oligocene).
New fossil evidence of a Miocene mouse-size mammal from South Island, New Zealand has important implications for the biogeography of New Zealand (Worthy et al. 2006), as the Holocene biota lacks native terrestrial mammals except three bat species. This find clearly illustrates the importance of ‘missing’ fossils. Prior to 2006, the biogeographical history of New Zealand had been presented as one without mammals, with many inferences that the flora and fauna had evolved over 80 million years in response to this absence (e.g. Wilson 2004). Now it is evident that there was a mammalian fauna for at least part of New Zealand's history (Worthy et al. 2006). The subsequent (prehuman) extinction of mammals and other lineages since the Miocene reveals that, whatever the origin of New Zealand biota, it had been subjected to major extinction and replacement events (e.g. Lee et al. 2001; Pole 2001). This alone demonstrates that to attribute the extant biota primarily to an ancient vicariant event (Gondwanan break-up) is fraught with difficulties.
2. Spatial paradigms in New Zealand biogeography: molecular evidence
Here, we examine how molecular studies of New Zealand taxa have shed light on the origins and development of the biota. For convenience, we approach this at six spatial/ecological levels, although some studies inform at more than one. At each level, we identify one or more taxonomic exemplars that illustrate available evidence, focusing our attention on the terrestrial fauna but referring also to additional relevant studies for comparison, including plants and freshwater taxa where this makes a pertinent contribution. An important feature of molecular studies is their capacity, with appropriate calibration, to provide estimates for the timing of phylogenetic events. However, molecular clocks have their limitations. The strict molecular method (using a fixed rate of molecular evolution) is often employed when fossils or geographical calibrations are not available, and researchers generally treat resulting date estimates cautiously. A strict clock does not take into account the variation in rates of molecular evolution that exist among genes, taxonomic groups and across time (Avise 2004). Relaxed molecular clock methods attempt to accommodate minor rate variation over time and among lineages (Kishino et al. 2001). Here, we report inferences made by the original authors and treat conservatively the timing of phylogenetic events indicated by level of DNA sequence divergence.
(a) New Zealand and ‘Gondwana’
The New Zealand landscape is essentially the product of tectonic activity initiated ca 25 Ma. It is far from certain how much land persisted in the region prior to this time as the continental crust (called Zealandia, box 1) thinned and submerged beneath the sea surface after the separation from Gondwanaland starting ca 83 Ma (Campbell & Hutching 2007; Trewick et al. 2007; Neall & Trewick 2008). It is clear that extensive land reduction took place (Landis et al. 2006, 2008) and this period in New Zealand's natural history is thought to have had a major influence on the subsequent composition of the biota (Cooper & Cooper 1995).
Despite the fact that several molecular studies now implicate colonization of New Zealand by a diverse range of animals and plants (e.g. bowerbirds: Christidis et al. 1996; Sophora: Hurr et al. 1999; insects: Trewick 2000a; freshwater fishes: Waters et al. 2000; parakeets: Boon et al. 2001a,b; cicadas: Buckley et al. 2002, Arensburger et al. 2004b; hebe: alpine buttercups, forget-me-nots, Winkworth et al. 2002; short-tailed bats: Teeling et al. 2003; southern beech: Knapp et al. 2005; plants: De Queiroz 2005; ferns and other plants: Perrie & Brownsey 2007), there is still a prevailing sense that the most important components of the New Zealand biota are of ancient vicariant origin and that New Zealand's biota is very different from other island biota due to this ancient origin.
(i) Ratites
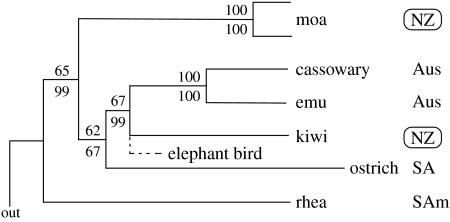
Among large vertebrates, which are rare in New Zealand, the ratites (Palaeognaths) have long been seen as having a classic Gondwanan distribution (Craw et al. 1999), with representatives in Australia, New Zealand, Africa, South America, but interestingly not India or New Caledonia. New Zealand is unusual in having had two quite distinct groups of ratites (moa and kiwi), although the fossil record for these extends no further than Late Pliocene (Worthy & Holdaway 2002). Despite being extinct, molecular data have been gleaned from numerous Holocene fossil bones of moa. Analysis indicates that moa form a monophyletic group among ratites (Cooper et al. 2001; Haddrath & Baker 2001; Worthy & Holdaway 2002) and that much of the morphological diversity that at one time was attributed to 64 species (in 20 genera), actually represents sexual dimorphism among 11 species (Bunce et al. 2003; Huynen et al. 2003). Moa are, however, not monophyletic with kiwi, which appear to share a closer common ancestor with the Australian emu, leading to the inference that at least some ratites may have migrated to New Zealand after separation of Zealandia (Cooper et al. 2001; figure 3). Currently, moa do not appear to have close allies among any extant ratites, and this pattern could be consistent with a vicariant origin. However, the species radiations of moa (Baker et al. 2005) and kiwi are relatively young (Baker et al. 1995; Burbidge et al. 2003). Moa are inferred as speciating after the Oligocene, even though calibration assumed that moa ancestors have been in New Zealand since isolation from Australia (Baker et al. 2005).
Figure 3.
Phylogeny of the ratites (redrawn from Cooper et al. 2001) emphasizing that New Zealand taxa (moa and kiwi) are not monophyletic (NZ=New Zealand; Aus=Australia; SA=South Africa; SAm=South America).
(ii) Harvestmen
The Pettalidae family of morphologically conserved harvestmen are found in leaf litter and have a classic Gondwanan distribution (Boyer & Giribet 2007). Despite finding that most groups within this family form monophyletic continental clades, New Zealand is home to three different lineages represented by the genera Neopurcellia, Rakaia and Aoraki (Boyer & Giribet 2007). Contrary to the inference of a vicariant history to explain this pattern, the levels of molecular divergence among the pettalid lineages are too low to be consistent with an ancient origin, unless there has been a very substantial taxon specific change in mutation rate. Diversity and spatial structuring on South Island mountains that are ca 5 Ma old suggest an arrival within the past few million years.
(iii) Other examples
In recent years, other examples of purported ‘ancient Gondwanan lineages’ have been shown to have dispersed and speciated after the break-up, including animal groups, such as galaxiid fishes (Waters et al. 2000) and wattlebirds (Shepherd & Lambert 2007), as well as plants, such as southern beech (Knapp et al. 2005). The implication of these findings is that the Gondwanan element of the New Zealand biota is primarily type 2 (see above, i.e. of southern distribution but not vicariant origin).
(b) New Zealand and Australia
Linkages between the fauna of New Zealand and Australia have long been recognized (Falla 1953; Fleming 1962a,b, 1979) and key taxa include weta (Orthoptera), peripatus (Onychophora), plus iconic plant taxa such as southern beech (Nothofagus) and kauri (Agathis australis). The extinct giant New Zealand eagle (Harpagornis), which once hunted moa, was closely related to one of the smallest extant Australian eagles (Hieraaetus). Genetic distances measured from DNA of Holocene fossil bones indicate morphogenesis of this eagle lineage after colonization of New Zealand during the Pleistocene, ca 0.7–1.8 Ma (Bunce et al. 2005).
(i) Hepialid moths
The moth family Hepialidae is found in Australia, New Guinea and New Zealand. Within New Zealand there has been a radiation of this forest group into grasslands, particularly in the sub-alpine zone and recently into exotic pasture where it has become an important pest species. Brown et al. (1999) found that at least two hepialid lineages dispersed from Australia to New Zealand successfully over the past 4–5 Ma coinciding with uplift along the alpine fault.
(ii) Spiders
Latrodectus widow spiders have a global distribution and New Zealand and Australia have the endemic katipo (Latrodectus katipo) and redback (Latrodectus hasselti) species, respectively. Griffith et al. (2005) found that genetic divergence between katipo and redbacks was equivalent to that of two very closely related species and that katipo are relatively recent arrivals into New Zealand. Likewise, the diverse Lycosid wolf spiders of New Zealand share a close relationship to Australian relatives and show a New Zealand species radiation (approx. 20 species) no older than 5 Ma (Vink & Paterson 2003).
(iii) Other examples
Other examples of the strong and often recent connection of the New Zealand biota to Australia are many bird species (Falla 1953). The silvereye (Zosterops lateralis) was self-introduced from Australia in the early 1800s and has since colonized the surrounding islands (Clegg et al. 2002). Interestingly, range expansion of silvereye within New Zealand has resulted in gradual reduction in allelic diversity, whereas the initial overseas colonization brought high genetic diversity consistent with the arrival of a flock rather than few individuals (Clegg et al. 2002). Slightly earlier arrivals include the Petroicidae (Australasian robins), which appear to have colonized New Zealand from Australia in two separate events in relatively recent times (H. C. Miller 2003, unpublished data). Intriguingly, even the iconic Onychophora (peripatus or velvet worms) show an unexpected phylogenetic pattern. Although based on rather few data, the New Zealand onychophoran fauna appears to be more closely allied to that of Tasmania than either are to Australian species, despite the closer proximity and recent connection of Australia and Tasmania (Gleeson et al. 1998).
(c) New Zealand in the Pacific
A range of New Zealand animal taxa including snails, land birds, seabirds as well as plants such as Metrosideros have their closest living relatives on islands of the Pacific (Fleming 1979). Molecular analysis of Metrosideros indicates dispersal and speciation since the Pliocene (Wright et al. 2000; Percy et al. 2008), and complex patterns indicative of multiple dispersal events have been found in several other plant groups in the region (e.g. Bartish et al. 2005; Harbaugh & Baldwin 2007).
(i) Land snails
Pulmonate land snails of the genus Placostylus are found only in the Western Pacific, in northern New Zealand and on islands between New Zealand and Melanesia (Solomon Islands, Fiji, New Caledonia, Vanuatu, Papua New Guinea and Lord Howe) (Suter 1916; Ponder et al. 2003). Despite the fact that most of these islands emerged from beneath the ocean, this distribution has been considered by some researchers to be consistent with an ancient Gondwanan origin (Stanisic 1981). The large size of these taxa and their intolerance of seawater have generally led to the assumption that Placostylus are unlikely to disperse across the ocean. However, Placostylus arrived on Lord Howe after its formation (less than 7 Ma; McDougall et al. 1981), and New Caledonian Placostylus have dispersed to the nearby Loyalty Islands since 2 Ma. Comparison of sequence data from New Caledonian Placostylus and representatives of the genus from New Zealand and Lord Howe Island indicates that the New Caledonian radiation might have originated by dispersal from these southern locations (Ponder et al. 2003; Trewick et al. in press).
(ii) Cormorants and shags
The Phalacrocoracidae (cormorants and shags) are a prominent component of New Zealand's seabird fauna. There are two main lineages of shags in New Zealand, the king shag species complex and the cliff shags. Both lineages have close links into the Pacific and even further afield. The king shags, including Phalacrocorax chalconotus from Stewart Island and several sub-Antarctic species, are, on the basis of DNA sequence divergence, very closely related to Phalacrocorax bougainvillii from southern South America (Kennedy et al. 2000). The New Zealand spotted shag, Stictocarbo punctatus, is closely related to other species from Australia, Japan, Africa and Europe (Kennedy et al. 2000).
(iii) Other examples
Further examples for Pacific connections, post-dating Gondwanaland, are found in many animal lineages. Among cicada there is a strong and recent affinity of the New Zealand cicada fauna to taxa in other parts of the Pacific region including Norfolk Island, Chatham Islands, Australia and New Caledonia (Arensburger et al. 2004a,b). The freshwater shrimp (Paratya) has colonized islands from Japan to New Zealand since 19 Ma (Page et al. 2005). Lizards including skinks (Smith et al. 2007) and geckos (Chambers et al. 2001), and birds such as parakeets (Boon et al. 2001a,b; Chambers et al. 2001) and robins (H. C. Miller 2003, unpublished data) show similar Pacific connection, especially to New Caledonia. The wandering albatross complex that includes species in New Zealand and islands in the region shows extremely low levels of genetic diversity (Burg & Croxall 2004). Though many are extinct, the Rallidae (Gallirallus and Porphyrio) are represented in New Zealand and Chatham Islands (as on most oceanic islands, Steadman 2006) by numerous flightless endemics, each having evolved following colonization (Trewick 1997a,b). New Zealand weta (crickets) are allied to biotas of Australia and New Caledonia (see Pratt et al. 2008), and the New Zealand stick insects form a monophyletic group with relatives in the same region (Trewick et al. 2008a).
(d) New Zealand: Chatham Islands
The Chathams Islands are a small archipelago approximately 850 km to the east of New Zealand (comprising five islands and several small islets, 970 km2 land area in total; figure 4a). Like New Zealand, it is formed from continental crust supplemented locally by volcanic sediments, and some have assumed it to be an ancient Gondwanan land surface. Craw (1988) presented a panbiogeographic thesis that found an explanation for the assemblage of the biota in the perceived composite nature of geological terrains (northern and southern elements).
Figure 4.
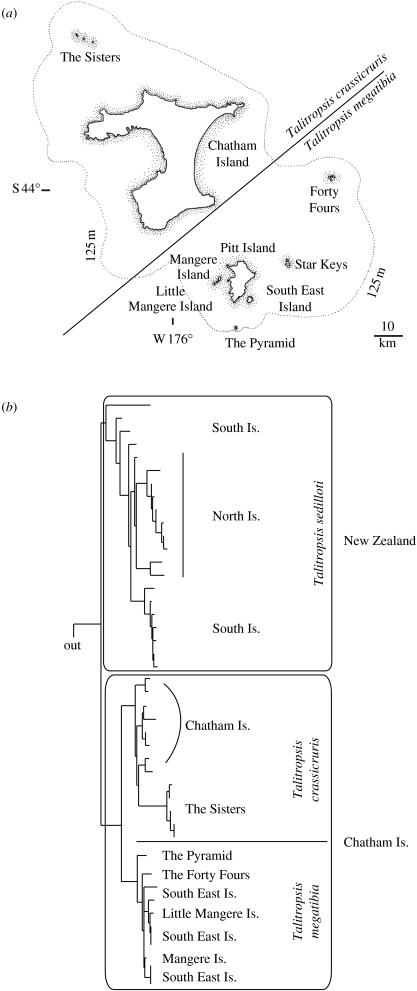
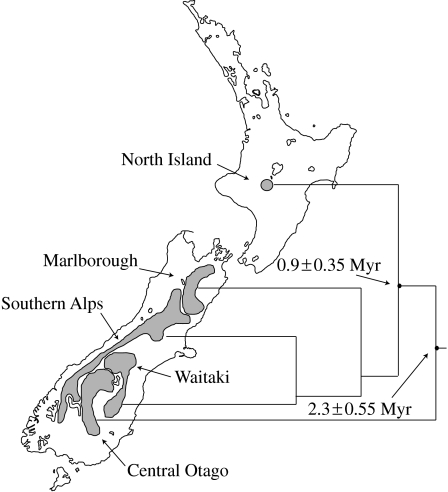
(a) Map of the Chatham Islands with the distribution of two species of the cave weta (cricket) genus Talitropsis on the archipelago. The broken line indicates the inferred land area above sea level during the Last Glacial Maximum. (b) The phylogeny of Talitropsis in New Zealand and the Chatham Islands, highlighting the haplotype diversity on the geologically young Chatham Islands (max. 4 Ma; Campbell et al. 2006). Low levels of variation in mitochondrial COI data between New Zealand and Chatham species and within the Chatham Islands (J. Goldberg 2007, unpublished data) corroborate earlier results for Talitropsis (Trewick 2000a) and emphasize that speciation can be rapid even in a small, young landscape.
Geological evidence indicates that the Chatham archipelago emerged from the sea since 4 Ma (Campbell 1998; Campbell et al. 2006). Genetic evidence from invertebrate taxa so far studied are consistent with this inference (Trewick 2000a; Chinn & Gemmell 2004; Arensburger et al. 2004b; Hill et al. 2005; Trewick et al. 2005; Paterson et al. 2006). A relatively young biota is also evident from studies of plants (Wagstaff & Garnock-Jones 1998) and vertebrates such as rails (Trewick 1997b), parakeets (Boon et al. 2001a,b), pigeons (Millener & Powlesland 2001; Liggins et al. 2008) and robins (H. C. Miller 2003, unpublished data). Endemism is almost entirely confined to species level or below. Naturally, the biota of the Chathams is unique, a particular assemblage of lineages including a wide variety of endemic taxa that includes large flightless beetles and crickets, freshwater fishes and flightless birds. Despite a recent origin, the level of speciation and genetic structure on the islands can be impressive, as can be seen in the cave weta genus Talitropsis (Raphidophoridae; figure 4b). It comprises three species, two of which are endemic to the Chatham Islands (Talitropsis megatibia and Talitropsis crassicruris) and one that is widespread throughout both islands of New Zealand (Talitropsis sedilloti). Despite the low genetic divergences within the islands and between New Zealand and the Chatham Islands, the species on the Chathams still show a high level of haplotype diversity and cladogenesis consistent with the two endemic species (Trewick 1999, 2000a; J. Goldberg 2008, unpublished data). Other invertebrates such as the coastal earwig Anisolabis littorea show no morphological and minimal genetic differentiation from New Zealand populations.
The Chatham Islands provide an important lesson in how rapidly a unique biota can evolve from dispersal in the New Zealand region. Clearly, evidence from species endemicity is no justification for thinking that much of the native fauna of New Zealand could not have originated since 25 Ma after the peak of marine inundation, to occupy free niche space.
(e) Alpine New Zealand
The mountains of New Zealand are very young in comparison with the time since isolation of Zealandia. The alpine ranges of the South Island developed by extreme crustal uplift and orogenesis since the Pliocene (Kamp 1992), resulting in a substantial area of mountainous and ecologically diverse habitat. These South Island ranges, in particular, have a diverse and extensive alpine biota (e.g. Fleming 1963b; Gibbs 2006), including insect taxa that are freeze tolerant (Sinclair et al. 1999). Their presence poses the question of the origin of this biota. Fleming (1963b) extended two alternatives as follows: (i) colonization of New Zealand by cold-adapted taxa from elsewhere (e.g. Australia (Raven 1973), Antarctica (Hooker 1860), or northern boreal habitats) and (ii) radiation and adaptation in New Zealand during the Pleistocene glacial epoch. A third alternative can be added: evolution in New Zealand in response to the development of an alpine zone on mountain ranges that emerged during the Pliocene. In the North Island, the ranges are less extensive and even younger (ca 1 Ma) than those in South Island. The Northern alpine biota is less diverse and primarily a subset of its southern counterpart. It is becoming increasingly evident from molecular studies that the alpine biota has evolved in response to mountain habitat development and cyclic expansion of the alpine zones during the Pleistocene.
(i) Scree weta
The alpine scree weta (Deinacrida connectens) is one of the most striking alpine insects of New Zealand. It is a large, flightless species belonging to the Anostostomatidae (Orthoptera), which has a classic Gondwanan distribution. Deinacrida connectens lives only above the treeline in alpine scree slopes of the Southern Alps (South Island). Mitochondrial COI sequence data revealed comparatively high genetic distances among populations of D. connectens (Trewick et al. 2000), although nuclear markers indicate this does not reflect the presence of cryptic species (Morgan-Richards & Gibbs 1996). Population structure appears instead to represent restricted gene flow among mountain ranges and may date back to Pliocene uplift of the Southern Alps (Trewick et al. 2000; Trewick 2001a,b).
(ii) Cicadas
The endemic cicada genus Maoricicada is an alpine specialist with the majority of described species occupying alpine or sub-alpine habitats. Comparison of sequence data and relaxed molecular clock dating reveals that speciation of alpine Maoricicada falls within the timeframe of the Pliocene uplift of the Southern Alps (ca 5 Ma; Buckley & Simon 2007). Additionally, the phylogeographic structure within the alpine species M. campbelli in the Southern Alps and North Island mountains dates to the Pleistocene (Buckley et al. 2001; figure 5).
Figure 5.
Phylogeographic structure of the alpine New Zealand cicada Maoricicada campbelli based on mitochondrial sequences. The inferred timing of lineage formation in the mountain ranges of the Southern Alps correlates with Pleistocene/Pliocene orogenics (modified from Buckley et al. 2001).
(iii) Other examples
Other examples of terrestrial species radiations (adaptive or otherwise) in New Zealand are associated with alpine habitats, mostly in South Island. These include invertebrates (tree and giant weta: Morgan-Richards & Gibbs 2001, Trewick & Morgan-Richards 2005; Brachaspis and Sigaus grasshoppers: Trewick 2001a, 2007; Celatoblatta cockroaches: Trewick & Wallis 2001, Chinn & Gemmell 2004; Powelliphanta snails: Trewick et al. in press b), vertebrates (skinks: Hickson et al. 2000, see also Greaves et al. 2007, 2008; moa: Baker et al. 2005) and many alpine plants (Hebe: Wagstaff & Garnock-Jones 1998; Ranunculus buttercups: Lockhart et al. 2001; Pachycladon: Heenan & Mitchell 2003, Winkworth et al. 2005; Ourisia: Meudt & Simpson 2006).
(f) Lowland New Zealand
Prehuman New Zealand in the Holocene was dominated by mixed temperate to subtropical forests (Trewick & Morgan-Richards in press), most of which have been cleared since human settlement. Palaeoecological reconstructions reveal that during Pleistocene glacials forest was reduced and largely restricted to northern New Zealand (McGlone et al. 2001). The extent, composition and number of other forest remnants during the glacials are unclear, but it is likely that there were some, most probably in the northwest of South Island of New Zealand (Alloway et al. 2007). During glacials, lowland New Zealand was dominated by grass and scrub, so it is reasonable to expect that populations of forest animals experienced severe reduction during those episodes (Trewick & Wallis 2001). Prior to the Pleistocene, the configuration of islands in the New Zealand archipelago was quite different from that of today, with, in particular, numerous small islands in the north during the Pliocene (Fleming 1979; Isaac et al. 1994; Ballance & Williams 1992). A prominent biogeographic feature in South Island is the north–south disjunction of many species distributions and this pattern, referred to as the ‘beech gap’, has been attributed to vicariant separation by movement along the Alpine fault that extends through the island (Heads 1998; Wallis & Trewick 2001). However, molecular evidence from a wide range of invertebrate taxa reveal phylogenetic patterns and levels of genetic diversity that are inconsistent with this inference (Trewick & Wallis 2001). Disjunctions are better explained by local extinction and range expansion during and since the Pleistocene (e.g. Trewick & Wallis 2001; Leschen et al. 2008; Hill et al. in press).
(i) Kauri snails
Fleming (1979) proposed that taxa in northern New Zealand may have subdivided and speciated in response to the presence of Pliocene islands that subsequently united to form Northland, New Zealand. Carnivorous snails of the Rhytididae have an intriguing distribution that encompasses Gondwanan landmasses as well as Pacific archipelagos. The subfamily Paryphantinae contains four genera, including the kauri snails, Paryphanta, is almost entirely limited to Northland and some offshore islands in this region and is among taxa that might have experienced population subdivision during the Pleistocene (Fleming 1979). Spencer et al. (2006) found that speciation probably did happen in the Pliocene, but they failed to find spatial patterns consistent with Pliocene vicariance events. A similar complex pattern, developed since the Pliocene, exists among Oligosoma skinks in the region (Hare et al. 2008).
(ii) Tree weta
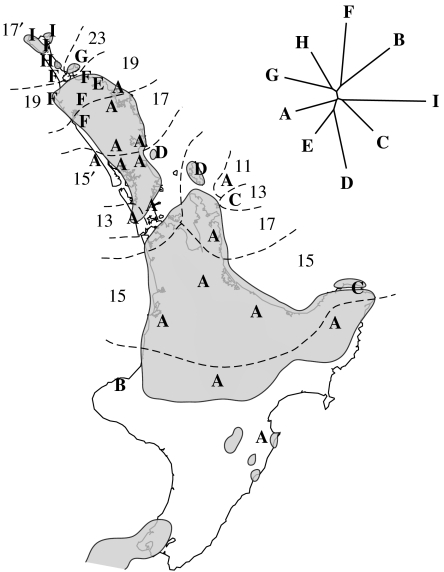
The New Zealand tree weta Hemideina thoracica is an arboreal herbivore. It is flightless like all other Anostostomatidae (Orthoptera) species in New Zealand, and it is confined to North Island. Analysis of mitochondrial DNA sequences revealed higher genetic diversity in northern populations of H. thoracica than in the south of the island (Morgan-Richards et al. 2001) and indicated that genetic diversity, genetic distances, spatial distribution of mitochondrial lineages and chromosome races are consistent with simultaneous formation of at least five isolated populations on Pliocene islands in northern New Zealand (figure 6).
Figure 6.
The estimated distribution of land in North Island, New Zealand during the Pliocene (grey areas). The distribution (ranges indicated by dashed lines) of eight chromosome races of the tree weta Hemideina thoracica. Values 11, 13, 15, etc. are male diploid chromosome numbers. The distribution of nine well-differentiated mitochondrial (COI) lineages (A–I) is also indicated and supports differentiation of chromosome races during the Pliocene (Morgan-Richards et al. 2001).
(iii) Other examples
Several New Zealand insect groups have distributions that extend from North Island into northern South Island, which are indicative of recent range expansion (e.g. Clitarchus stick insects: Trewick et al. 2005; tree weta: Trewick & Morgan-Richards 2005). Such range shifts probably reflect shifting habitat availability following the last Pleistocene glaciation (i.e. warming climate and southwards expansion of forests), although some appear to be earlier than Late Pleistocene (freshwater crayfish; Apte et al. 2007). Flying forest birds (e.g. parakeets: Boon et al. 2001a; Australasian robins: H. C. Miller 2003, unpublished data; kokako: Murphy et al. 2006; wood pigeons: J. Goldberg 2008, unpublished data) and the endemic short-tailed bat (Lloyd 2003) also have low levels of genetic variation among populations, suggesting they too suffered intense bottlenecking during the Mid- to Late Pleistocene. Cicadas of the genus Kikihia are found throughout New Zealand in many lowland habitats and several species have moved into the sub-alpine region, but none are alpine. Relaxed molecular clock dating indicates that the major radiation in Kikihia species happened ca 4 Ma (Arensburger et al. 2004b; Marshall et al. in press). However, some invertebrates, including peripatus (Trewick 2000b) and giant spring-tails (Holacanthella; Stevens et al. 2007), have more complex and often sympatric distributions over the same landscape, or have high within-group genetic diversity (mite harvestmen: Boyer et al. 2007). This suggests an older history with local survival of populations through climate cycles, and this pattern may reflect the distinctive reproductive systems and the population structure of these invertebrates that have narrow habitat requirements. The genetic diversity of the flightless brown kiwi (Apteryx) is more like these latter invertebrates, with five main spatially partitioned lineages indicating local persistence through climate and thus vegetation cycles of the Pleistocene (Baker et al. 1995; Burbidge et al. 2003).
3. Discussion
Biogeographic interpretations based only on observations of animal distributions are often misleading, but the inclusion of molecular evidence has added a vital temporal dimension to biogeographic analysis and evolutionary study in general. The temporal dimension enables the likelihood of alternative explanations for a given topology of species–area relationships to be assessed. For instance, the alternative inferences of Gondwanan distributions discussed earlier could be distinguished in this way. A long-standing debate has focused on the dichotomy of vicariance and dispersal influences on New Zealand's biology, but a much more productive approach is to explore data on a case by case basis and to recognize that some, and perhaps many, instances yield equivocal results (McGlone 2005). In the search for unifying concepts, biogeographers have tended to oversimplify the history of New Zealand, which is more complicated and less well understood than that of typical continents and typical oceanic islands. This complexity, with a combination of geophysical features characteristic of both continents and oceanic islands (Daugherty et al. 1993), is very probably a major source of the distinct quality of New Zealand's biota.
While the final separation of Zealandia from Gondwanaland could be viewed as a finite event in time, biological exchange would not have ceased at this point. Opportunities for gene flow would have been reduced, but Zealandia would have been subjected to an ongoing rain of propagules and individuals. As it moved further from Australia, it is likely that the intensity of this rain diminished, but the effects would be regionally variable with northern Zealandia (New Caledonia) remaining closer to Australia than the south (New Zealand), offering different potential colonization rates. Dispersal rates are likely to have been dependent on changing wind and ocean currents (e.g. initiation of the circumpolar current following the separation of Antarctica from Australia (ca 35 Ma; Veevers 1991; Sanmartin & Ronquist 2004), and Antarctica from South America (ca 28 Ma; Sanmartin et al. 2007), and colonization rates would be affected by habitat availability and ecological competition (Whittaker & Fernández-Palacios 2006).
Although far from perfectly described, our understanding of the physical processes influencing the biota of New Zealand continues to improve. What is clear is that the current landscape (its size, shape and topography) is primarily the product of tectonic activity since ca 25 Ma. The biota too must have developed primarily after that time, whether derived from relict Zealandian lineages or from colonists. It is therefore unreasonable to expect an ancient (Zealandian) biota in New Zealand developed over more than 80 million years like that of Australia. Furthermore, if any substantial Zealandian biota, or indeed even a Miocene (since 23 Ma) biota, had survived intact to modern times, we would expect not only within-group phylogenetic signals consistent with this but also other ecological attributes, including clear adaptive radiations and at least some derived interactions (mutualisms and coevolved traits), especially through coevolutionary escalation (Thompson 2005). But neither of these features of biological complexity is well represented in New Zealand (Didham 2005), to the extent that even specialized insect pollinators are scarce, with most pollination attended to by flies.
Meagre examples of biological complexity include parasitism (scale insects on southern beech trees; Harris et al. 2007), animal–plant mutualisms such as the foraging behaviour of the tui honeyeater (Prosthemadera novaeseelandiae) on native mistletoes (Loranthaceae; Robertson et al. 1999), and the pollination of the wood rose (Dactylanthus taylorii) by the largely terrestrial short-tailed bat (Mystacina tuberculata; Ecroyd 1996). However, the apparent ornithophilous syndrome of New Zealand mistletoes does not restrict their exploitation (and pollination) to tui, or even birds, as native bees are also important pollinators (Robertson et al. 2005), which indicates a rather more generalised interaction. A proposal that some weta (anostostomatid orthopterans) may have a mutualistic relationship with native fruiting plants (Duthie et al. 2006) has been extended to the suggestion that general features of New Zealand fruiting shrubs might be products of coevolution with weta (Burns 2006). However, this notion is unsubstantiated and numerous lines of reasoning indicate it is unconvincing (Morgan-Richards et al. 2008).
This lack of biological complexity in New Zealand may reflect a lack of observations in some cases, one or more episodes of major biotic disruption or turnover, or simply a young New Zealand biota. Significantly, the pre-Holocene terrestrial fossil record in New Zealand (mostly plants until recently, but see Worthy et al. 2006) indicates a substantial turnover of the biota after the Mid-Miocene (Pole 1994, 2001; Lee et al. 2001). Clearly, from this perspective alone, there is little justification in viewing the biota as ancient.
Nevertheless the New Zealand biota does have many peculiarities when viewed from a global perspective. Is this evidence for ancient interactions? One of the most striking is the unusual ‘divaricate’ growth form typical of many New Zealand woody plants. Divaricate plants, which typically have a wide branching angle, closely interlaced branches and small leaves concentrated to the interior of the plant, are rare elsewhere in the world but are represented in New Zealand by more than 50 species in 27 genera from 22 families or around 10 per cent of native woody plant species (Wardle 1991). Alternative hypotheses advanced to explain this growth form include adaptation to herbivory by moa, and response to cold, dry Pleistocene climate. Regardless, many divaricates have closely related large leaved relatives, which suggests divarication has evolved during recent geological time rather than over 80 million years (Greenwood & Atkinson 1977; Howell et al. 2002; Lusk 2002).
Similarly, the existence until recently in New Zealand of the world's largest eagle (Harpagornis moorei) indicates adaption to large prey (moa) in the absence of other large (terrestrial mammal) predators. The giant eagle–moa relationship could reasonably be assumed to attest to an ancient adaptive history, but instead it appears to have evolved during the Pleistocene (1.8 Ma) from a small Australian colonist (Bunce et al. 2005). Evolution of Harpagornis therefore also nicely demonstrates the rapidity and extent of morphological evolution on an oceanic island rather than testifying to the ancient isolation of New Zealand's fauna. Indeed, from the perspective of the moa, there is as yet no direct evidence (oldest moa bones are Late Pliocene, Worthy et al. 1991) that their pre-Pleistocene ancestors were giants before the emergence of Harpagornis.
Molecular evidence for diversification after the Oligocene ‘crisis’ (Cooper & Cooper 1995) is not extensive. Early analysis of moa (and wren) diversification indicated that their radiations might date to Early Miocene time (Cooper et al. 2001), but more recent analyses indicate the moa radiation is younger and primarily Pliocene (Baker et al. 2005). Perhaps this too reflects a Late Miocene climate-related assemblage change, as indicated by the plant fossil record. If an extensive extinction phase occurred in the Late Miocene in response to rather subtle climate change, we must accept that earlier and more intense environmental perturbations (e.g. submergence of Zealandia) would have had equal or greater impact on the biota. The fact that the signal from earlier extinctions (and colonizations) is obscured by later events should not prevent us from seeking evidence for them. Biologists frequently refer to New Zealand's ‘turbulent geological history’ (e.g. McDowall 2000; Trewick 2000b; Apte et al. 2007; Stevens et al. 2007), but our knowledge of the major phases of Zealandian and New Zealand geophysical history ought to provide the basis for rather more sophisticated hypotheses about which periods were most evolutionarily influential. Major events since separation of Zealandia include K/T asteroid impact, Oligocene submergence, Miocene and Plio-Pleistocene climate change, Pliocene orogenics and Pleistocene volcanics.
4. Conclusion
To advance our understanding of the evolutionary history of New Zealand, we especially need more molecular studies, in an appropriate taxonomic framework linking New Zealand fauna to their counterparts in other parts of Australasia, the Pacific and the world. There are relatively few such studies of terrestrial animals but rather more to date on plant taxa. The presumption that the New Zealand fauna is captive and thus monophyletic is untenable and inappropriate as a starting point, if meaningful inferences of biological history are to be made, but suitable sampling allows the prediction of New Zealand monophyly to be examined (e.g. Breitwieser & Ward 2003; Arensburger et al. 2004a; Shepherd & Lambert 2007; Pratt et al. 2008; Trewick et al. 2008a). Important obstacles for understanding processes in the formation of the New Zealand biota are the patchy fossil record in New Zealand and Australia and the fact that some taxa are not amenable to biogeographic inference where they are represented by one or few extant lineages (e.g. hihi; Driskell et al. 2007). In some cases, it might be impossible to prove ancient origin or disprove recent arrival where a group is underepresented in one or more geographic areas: missing lineages have a major effect on interpretation of phylogenetic trees (Crisp & Cook 2005). Research should consider the potential perturbations of the New Zealand biota, its gains and losses over the past 80 million years; much more sophisticated approaches to modelling biological and geophysical process need to be developed to better document New Zealand's past (e.g. Alloway et al. 2007). Biologists are increasingly recognizing that the New Zealand biota is not a museum of relicts but a dynamic and relatively young evolutionary system (Didham 2005; McGlone 2005). New Zealand should not be set apart as a ‘continental island’ (Cowie & Holland 2006), but at the same time, New Zealand is certainly no more a ‘fly paper of the Pacific’ (McGlone 2005) than oceanic islands such as Hawaii. The challenge for students of New Zealand biogeography is to explore the evolution of its biota in the context of an improving understanding of the history of Zealandia.
Acknowledgments
We would like to thank Mary Morgan-Richards, Leon Perrie, Chris Simon, Frank Wieland and anonymous referees who provided valuable comments and suggestions to improve this manuscript. Additionally, we would like to acknowledge the Marsden Fund of the Royal Society of New Zealand (no. 03-GNS-302) and the Allan Wilson Centre for support of this study.
One contribution of 15 to a Theme Issue ‘Evolution on Pacific islands: Darwin's legacy’.
Endnotes
Moa's ark alludes to the iconic formally dominant but now extinct radiation of palaeognathus birds (moa) endemic to New Zealand.
For geological timescale, see Cooper (2004).
References
- Alloway B.V, et al. Towards a climate event stratigraphy for New Zealand over the past 30 000 years (NZ-INTIMATE project) J. Quat. Sci. 2007;22:9–35. doi:10.1002/jqs.1079 [Google Scholar]
- Apte S, Smith P.J, Wallis G. Mitochondrial phylogeography of New Zealand freshwater crayfishes, Paranephrops spp. Mol. Ecol. 2007;16:1897–1908. doi: 10.1111/j.1365-294X.2007.03224.x. doi:10.1111/j.1365-294X.2007.03224.x [DOI] [PubMed] [Google Scholar]
- Arensburger P, Buckley T.R, Simon C, Moulds M, Holsinger K.E. Biogeography and phylogeny of the New Zealand cicada genera (Hemiptera: Cicadidae) based on nuclear and mitochondrial DNA data. J. Biogeogr. 2004a;31:557–569. doi:10.1046/j.1365-2699.2003.01012.x [Google Scholar]
- Arensburger P, Simon C, Holsinger K. Evolution and phylogeny of the New Zealand cicada genus Kikihia Dugdale (Homoptera: Auchenorrhyncha: Cicadidae) with special reference to the origin of the Kermadec and Norfolk Islands' species. J. Biogeogr. 2004b;31:1769–1783. doi:10.1111/j.1365-2699.2004.01098.x [Google Scholar]
- Avise J.C. 2nd edn. Sinauer Associates; Sunderland, MA: 2004. Molecular markers, natural history, and evolution. [Google Scholar]
- Baker A.J, Daugherty C.H, Colbourne R, McLennan J.L. Flightless brown kiwis of New Zealand possess extremely subdivided population structure and cryptic species like small mammals. Proc. Natl Acad. Sci. USA. 1995;92:8254–8258. doi: 10.1073/pnas.92.18.8254. doi:10.1073/pnas.92.18.8254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A.J, Huynen L.J, Haddrath O, Millar C.D, Lambert D.M. Reconstructing the tempo and mode of evolution in an extinct clade of birds with ancient DNA: the giant moas of New Zealand. Proc. Natl Acad. Sci. USA. 2005;102:8257–8262. doi: 10.1073/pnas.0409435102. doi:10.1073/pnas.0409435102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A.J, Pereira S.L, Haddrath O.P, Edge K.-A. Multiple gene evidence for expansion of extant penguins out of Antarctica due to global cooling. Proc. R. Soc. B. 2006;273:11–17. doi: 10.1098/rspb.2005.3260. doi:10.1098/rspb.2005.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballance P.F, Williams P.W. The geomorphology of Auckland and Northland. In: Soons J.M, Selby M.J, editors. Landforms of New Zealand. Longman Paul; Hong Kong: 1992. pp. 210–232. [Google Scholar]
- Banks J.C, Mitchell A.D, Waas J.R, Paterson A.M. An unexpected pattern of molecular divergence within the blue penguin (Eudyptula minor) complex. Notornis. 2002;49:29–38. [Google Scholar]
- Bartish I.V, Swenson U, Munzinger J, Anderberg A.A. Phylogenetic relationships among New Caledonian Sapotaceae (Ericales): molecular evidence for generic polyphyly and repeated dispersal. Am. J. Bot. 2005;92:667–673. doi: 10.3732/ajb.92.4.667. doi:10.3732/ajb.92.4.667 [DOI] [PubMed] [Google Scholar]
- Bellamy D, Springett B, Hayden P. Viking; New York, NY: 1990. Moa's Ark, the voyage of New Zealand. [Google Scholar]
- Boon W.M, Kearvell J.C, Daugherty C.H, Chambers G.K. Molecular systematics and conservation of kakariki (Cyanoramphus spp.) Sci. Conserv. (Wellington, N.Z.) 2001a;176:1–46. [Google Scholar]
- Boon W.M, Daugherty C.H, Chambers G.K. The Norfolk Island green parrot and New Caledonian red-crowned parakeet are distinct species. Emu. 2001b;101:113–121. doi:10.1071/MU00001 [Google Scholar]
- Boyer S.L, Giribet G. A new model Gondwanan taxon: systematics and biogeography of the harvestman family Pettalidae (Arachnida, Opiliones, Cyphophthalmi), with a taxonomic revision of genera from Australia and New Zealand. Cladistics. 2007;23:337–361. doi: 10.1111/j.1096-0031.2007.00149.x. doi:10.1111/j.1096-0031.2007.00149.x [DOI] [PubMed] [Google Scholar]
- Boyer S.L, Baker J.M, Giribet G. Deep genetic divergences in Aoraki denticulata (Arachnida, Opiliones, Cyphophthalmi): a widespread ‘mite harvestman’ defies DNA taxonomy. Mol. Ecol. 2007;16:4999–5016. doi: 10.1111/j.1365-294X.2007.03555.x. doi:10.1111/j.1365-294X.2007.03555.x [DOI] [PubMed] [Google Scholar]
- Breitwieser I, Ward J.M. Phylogenetic relationships and character evolution in New Zealand and selected Australian Gnaphalieae (Compositae) inferred from morphological and anatomical data. Bot. J. Linn. Soc. 2003;141:183–203. doi:10.1046/j.1095-8339.2003.00141.x [Google Scholar]
- Brown B, Emberson R.M, Paterson A.M. Phylogeny of “Oxycanus” lineages of hepialid moths from New Zealand inferred from sequence variation in the mtDNA COI and II gene regions. Mol. Phylogenet. Evol. 1999;13:463–473. doi: 10.1006/mpev.1999.0662. doi:10.1006/mpev.1999.0662 [DOI] [PubMed] [Google Scholar]
- Buckley T.R, Simon C. Evolutionary radiation of the cicada genus Maoricicada Dugdale (Hemiptera: Cicadoidea) and the origins of the New Zealand alpine biota. Biol. J. Linn. Soc. 2007;91:419–435. doi:10.1111/j.1095-8312.2007.00807.x [Google Scholar]
- Buckley T.R, Simon C, Chambers G.K. Phylogeography of the New Zealand cicada Maoricicada campbelli based on mitochondrial DNA sequences: ancient clades associated with Cenozoic environmental change. Evolution. 2001;55:1395–1407. doi: 10.1111/j.0014-3820.2001.tb00661.x. doi:10.1111/j.0014-3820.2001.tb00661.x [DOI] [PubMed] [Google Scholar]
- Buckley T.R, Arensbruger P, Simon C, Chambers G.K. Combined data. Bayesian phylogenetics, and the origin of the New Zealand cicada genera. Syst. Biol. 2002;51:4–18. doi: 10.1080/106351502753475844. doi:10.1080/106351502753475844 [DOI] [PubMed] [Google Scholar]
- Bunce M, Worthy T.H, Ford T, Hoppitt W, Willerslev E, Drummond A, Cooper A. Extreme reversed sexual size dimorphism in the extinct New Zealand moa Dinornis. Nature. 2003;425:172–175. doi: 10.1038/nature01871. doi:10.1038/nature01871 [DOI] [PubMed] [Google Scholar]
- Bunce M, Szulkin M, Lerner H.R.L, Barnes I, Shapiro B, Cooper A, Holdaway R.N. Ancient DNA provides new insights into the evolutionary history of New Zealand's extinct Giant Eagle. PLoS Biol. 2005;3:44–46. doi: 10.1371/journal.pbio.0030009. doi:10.1371/journal.pbio.0030009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbidge M.L, Colbourne R.M, Robertson H.A, Baker A.J. Molecular and other biological evidence supports the recognition of at least three species of brown kiwi. Conserv. Genet. 2003;4:167–177. doi:10.1023/A:1023386506067 [Google Scholar]
- Burg T.M, Croxall J.P. Global population structure and taxonomy of the wandering albatross species complex. Mol. Ecol. 2004;13:2345–2355. doi: 10.1111/j.1365-294X.2004.02232.x. doi:10.1111/j.1365-294X.2004.02232.x [DOI] [PubMed] [Google Scholar]
- Burns K.C. Weta and the evolution of fleshy-fruits in New Zealand. New Zeal. J. Ecol. 2006;30:405–406. [Google Scholar]
- Campbell, H. J. 1998 Fauna and flora of the Chatham Islands: less than 4 MY old? (eds R. A. Cooper & C. Jones). Geol. Soc. New Zeal. Misc. Publ.97, pp. 15–16.
- Campbell H.J, Hutching G. Penguin, Auckland & GNS Science; Lower Hutt, New Zealand: 2007. In search of ancient New Zealand. [Google Scholar]
- Campbell, H. J., Begg, J. G., Beu, A. G., Carter, R. M., Davies, G., Holt, K., Landis, C. & Wallace, C. 2006 On the turn of a scallop (eds S. A. Trewick & M. J. Phillips). Geol. Soc. New Zeal. Misc. Publ 121, p. 9.
- Cande S.C, Stock J.M. Pacific–Antarctic–Australia motion and the formation of the Macquarie Plate. Geophys. J. Int. 2004;157:399–414. doi:10.1111/j.1365-246X.2004.02224.x [Google Scholar]
- Carlquist, S. H. 1965 Island life: a natural history of the islands of the world, p. 451. Garden City, NY: Natural History Press.
- Caughley G. Does the New Zealand vertebrate fauna conform to zoogeographic principles? Tuatara. 1964;12:50–57. [Google Scholar]
- Chambers G.K, Boon W.M, Buckley T.R, Hitchmough R.A. Using molecular methods to understand the Gondwanan affinities of the New Zealand biota: three case studies. Aust. J. Bot. 2001;49:377–387. doi:10.1071/BT00021 [Google Scholar]
- Chinn W.G, Gemmell N.J. Adaptive radiation within New Zealand endemic species of the cockroach genus Celatoblatta Johns (Blattidae): a response to Plio-Pleistocene mountain building and climate change. Mol. Ecol. 2004;13:1507–1518. doi: 10.1111/j.1365-294X.2004.02160.x. doi:10.1111/j.1365-294x.2004.02160.x [DOI] [PubMed] [Google Scholar]
- Christidis L, Leeton P.R, Westerman M. Were bowerbirds part of the New Zealand fauna? Proc. Natl Acad. Sci. USA. 1996;93:3898–3901. doi: 10.1073/pnas.93.9.3898. doi:10.1073/pnas.93.9.3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg S.L, Degnan S.M, Kikkawa J, Moritz C, Estoup A, Owens I.P.F. Genetic consequences of sequential founder events by an island-colonizing bird. Proc. Natl Acad. Sci. USA. 2002;99:8127–8132. doi: 10.1073/pnas.102583399. doi:10.1073/pnas.102583399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook L.G, Crisp M.D. Directional asymmetry of long-distance dispersal and colonization could mislead reconstructions of biogeography. J. Biogeogr. 2005;32:741–754. doi:10.1111/j.1365-2699.2005.01261.x [Google Scholar]
- Cooper R.A. The New Zealand geological timescale. Inst. Geol. Nucl. Sci. Monogr. 2004;22:43–53. [Google Scholar]
- Cooper A, Cooper R.A. The Oligocene bottleneck and New Zealand biota: genetic record of a past environmental crisis. Proc. R. Soc. B. 1995;261:293–302. doi: 10.1098/rspb.1995.0150. doi:10.1098/rspb.1995.0150 [DOI] [PubMed] [Google Scholar]
- Cooper R.A, Millener P.R. The New Zealand biota: historical background and new research. Trends Ecol. Evol. 1993;8:429–433. doi: 10.1016/0169-5347(93)90004-9. doi:10.1016/0169-5347(93)90004-9 [DOI] [PubMed] [Google Scholar]
- Cooper A, Lalueza-Fox C, Anderson S, Rambaut A, Austin J, Ward R. Complete mitochondrial genome sequences of two extinct moas clarify ratite evolution. Nature. 2001;409:704–707. doi: 10.1038/35055536. doi:10.1038/35055536 [DOI] [PubMed] [Google Scholar]
- Cowie R.H, Holland B.S. Dispersal is fundamental to biogeography and the evolution of biodiversity on oceanic islands. J. Biogeogr. 2006;33:193–198. doi:10.1111/j.1365-2699.2005.01383.x [Google Scholar]
- Cracraft J. Continental drift and vertebrate distribution. Annu. Rev. Ecol. Syst. 1974;5:215–261. doi:10.1146/annurev.es.05.110174.001243 [Google Scholar]
- Cracraft J. Mesozoic dispersal of terrestrial faunas around the southern end of the world. Mém. Mus. nat. Hist. nat. A. 1975;88:29–54. [Google Scholar]
- Craw R. Continuing the synthesis between panbiogeography, phylogenetic systematics and geology as illustrated by empirical studies on the biogeography of New Zealand and the Chatham Islands. Syst. Zool. 1988;37:291–310. doi:10.2307/2992374 [Google Scholar]
- Craw R.C, Grehan J.R, Heads M.J. Panbiogeography—tracking the history of life. In: Hallam A, Rosen B.R, Whitmore T.C, editors. Oxford biogeography series. Oxford University Press; Oxford, UK: 1999. p. 229. [Google Scholar]
- Crisp M.D, Cook L.G. Do early branching lineages signify ancestral traits? Trends Ecol. Evol. 2005;20:122–128. doi: 10.1016/j.tree.2004.11.010. doi:10.1016/j.tree.2004.11.010 [DOI] [PubMed] [Google Scholar]
- Darlington P.J. Wiley; New York, NY: 1957. Zoogeography: the geographical distribution of animals. [Google Scholar]
- Darlington P.J. Harvard University Press; Cambridge, MA: 1965. Biogeography of the southern end of the world. [Google Scholar]
- Daugherty C.H, Gibbs G.W, Hitchmough R.A. Mega-island or micro-continent? New Zealand and its fauna. Trends Ecol. Evol. 1993;8:437–442. doi: 10.1016/0169-5347(93)90006-B. doi:10.1016/0169-5347(93)90006-B [DOI] [PubMed] [Google Scholar]
- De Queiroz A. The resurrection of oceanic dispersal in historical biogeography. Trends Ecol. Evol. 2005;20:68–73. doi: 10.1016/j.tree.2004.11.006. doi:10.1016/j.tree.2004.11.006 [DOI] [PubMed] [Google Scholar]
- Diamond J. Random House; London, UK: 1997. Guns, germs and steel: the fates of human societies. [Google Scholar]
- Didham R.K. New Zealand: ‘fly-paper of the Pacific?’. The Weta. 2005;29:1–5. [Google Scholar]
- Driskell A.C, Christidis L, Gill B.J, Boles W.E, Barker F.K, Longmore N.W. A new endemic family of New Zealand passerine birds: adding heat to a biodiversity hotspot. Aust. J. Zool. 2007;55:73–78. doi:10.1071/ZO07007 [Google Scholar]
- Duthie C, Gibbs G, Burns K.C. Seed dispersal by weta. Science. 2006;311:1575. doi: 10.1126/science.1123544. doi:10.1126/science.1123544 [DOI] [PubMed] [Google Scholar]
- Ecroyd C.E. The ecology of Dactylanthus taylorii and threats to its survival. New Zeal. J. Ecol. 1996;20:81–100. [Google Scholar]
- Emerson B.C, Kolm N. Species diversity can drive speciation. Nature. 2005;434:1015–1017. doi: 10.1038/nature03450. doi:10.1038/nature03450 [DOI] [PubMed] [Google Scholar]
- Ericson P.G.P, Christidis L, Cooper A, Irestedt M, Jackson J, Johansson U.S, Norman J.A. A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proc. R. Soc. B. 2002;269:235–241. doi: 10.1098/rspb.2001.1877. doi:10.1098/rspb.2001.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes R, Reig O.A. The early fossil record of frogs: a review of the evidence. In: Vial J.L, editor. Evolutionary biology of the anurans. University of Missouri Press; Columbia, MO: 1973. pp. 11–63. [Google Scholar]
- Falla R.A. The Australian element in the avifauna of New Zealand. Emu. 1953;53:36–46. [Google Scholar]
- Fleming C.A. New Zealand biogeography—a palaeontologist's approach. Tuatara. 1962a;10:53–108. [Google Scholar]
- Fleming C.A. History of the New Zealand land bird fauna. Notornis. 1962b;9:270–274. [Google Scholar]
- Fleming C.A. The nomenclature of biogeographic elements in the New Zealand biota. Trans. R. Soc. New Zeal. 1963a;1:14–22. [Google Scholar]
- Fleming C.A. Age of the alpine biota. Proc. New Zeal. Ecol. Soc. 1963b;10:15–18. [Google Scholar]
- Fleming C.A. Auckland University Press; Auckland, New Zealand: 1979. The geological history of New Zealand and its life. [Google Scholar]
- Gaskin D.E. The origins of the New Zealand fauna and flora: a review. Geogr. Rev. 1970;60:414–434. doi:10.2307/214041 [Google Scholar]
- Gaskin D.E. Reconsideration of New Zealand biogeographical problems, with special reference to the Mesozoic. Mem. Mus. nat. Hist. nat. A. 1975;88:87–97. [Google Scholar]
- Gibbs G. Craig Potton; Nelson, New Zealand: 2006. Ghosts of Gondwana—the history of life in New Zealand. [Google Scholar]
- Gillespie R.G, Roderick G.K. Arthropods on islands: colonization, speciation, and conservation. Annu. Rev. Entomol. 2002;47:595–632. doi: 10.1146/annurev.ento.47.091201.145244. doi:10.1146/annurev.ento.47.091201.145244 [DOI] [PubMed] [Google Scholar]
- Gleeson D.M, Rowell D.M, Tait N.N, Briscoe D.A, Higgins A.V. Phylogenetic relationships among Onychophora from Australasia inferred from the mitochondrial cytochrome oxidase subunit i gene. Mol. Phylogenet. Evol. 1998;10:237–248. doi: 10.1006/mpev.1998.0512. doi:10.1006/mpev.1998.0512 [DOI] [PubMed] [Google Scholar]
- Greaves S.N.J, Chapple D.G, Gleeson D.M, Daugherty C.H, Ritchie P.A. Phylogeography of the spotted skink (Oligosoma lineoocellatum) and green skink (O. chloronoton) species complex (Lacertilia: Scincidae) in New Zealand reveals pre-Pleistocene divergence. Mol. Phylogenet. Evol. 2007;45:729–739. doi: 10.1016/j.ympev.2007.06.008. doi:10.1016/j.ympev.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Greaves S.N.J, Chapple D.G, Daugherty C.H, Gleeson D.F, Ritchie P.A. Genetic divergences pre-date Pleistocene glacial cycles in the New Zealand speckled skink Oligosoma infrapunctatum. J. Biogeogr. 2008;35:853–864. doi:10.1111/j.1365-2699.2007.01848.x [Google Scholar]
- Greenwood R.M, Atkinson I.A.E. Evolution of divaricating plants in New Zealand in relation to moa browsing. Proc. New Zeal. Ecol. 1977;24:21–33. [Google Scholar]
- Griffith J.W, Paterson A.M, Vink C.J. Molecular insights into the biogeography and species status of New Zealand's endemic Latrodectus spider species; L. katipo and L. atritus (Araneae, Theridiidae) J. Arachnol. 2005;33:776–784. doi:10.1636/S04-11.1 [Google Scholar]
- Haddrath O, Baker A.J. Complete mitochondrial DNA genome sequences of extinct birds: ratite phylogenetics and the vicariance biogeography hypothesis. Proc. R. Soc. B. 2001;268:939–945. doi: 10.1098/rspb.2001.1587. doi:10.1098/rspb.2001.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbaugh D.T, Baldwin B.G. Phylogeny and biogeography of the sandalwoods (Santalum, Santalaceae): repeated dispersals throughout the Pacific. Am. J. Bot. 2007;94:1028–1040. doi: 10.3732/ajb.94.6.1028. doi:10.3732/ajb.94.6.1028 [DOI] [PubMed] [Google Scholar]
- Hare K.M, Daugherty C.H, Chapple D.G. Comparative phylogeography of three skink species (Oligosoma moco, O. smithi, O. suteri; Reptilia: Scincidae) in northeastern New Zealand. Mol. Phylogenet. Evol. 2008;46:303–315. doi: 10.1016/j.ympev.2007.08.012. doi:10.1016/j.ympev.2007.08.012 [DOI] [PubMed] [Google Scholar]
- Harris A.C, Bannister J.M, Lee D.E. Fossil scale insects (Hemiptera, Coccoidea, Diaspididae) in life position on an angiosperm leaf from an Early Miocene lake deposit, Otago, New Zealand. J. R. Soc. New Zeal. 2007;37:1–13. [Google Scholar]
- Heads M. Biogeographic disjunction along the Alpine fault, New Zealand. Biol. J. Linn. Soc. 1998;63:161–176. doi:10.1111/j.1095-8312.1998.tb01512.x [Google Scholar]
- Heenan P.B, Mitchell A.D. Phylogeny, biogeography and adaptive radiation of Pachycladon (Brassicaceae) in the mountains of South Island, New Zealand. J. Biogeogr. 2003;30:1737–1749. doi:10.1046/j.1365-2699.2003.00941.x [Google Scholar]
- Hickson R.E, Slack K.E, Lockhart P. Phylogeny recapitulates geography, or why New Zealand has so many species of skinks. Biol. J. Linn. Soc. 2000;70:415–433. doi:10.1006/bijl.1999.0411 [Google Scholar]
- Hill K.B.R, Marshall D.C, Cooley J.R. Crossing Cook Strait: possible human transportation and establishment of two New Zealand cicadas from North Island to South Island (Kikihia scutellaris and K. ochrina, Hemiptera: Cicadidae) New Zeal. Entomol. 2005;28:71–80. [Google Scholar]
- Hill, K. B. R., Simon, C., Marshall, D. C. & Chambers, G. K. In press. Surviving glacial ages within the Biotic Gap: phylogeography of the New Zealand cicada Maoricicada campbelli J. Biogeogr
- Hooker J.D. Part III. Flora Tasmaniae. vol. 1. Lovell Reeve; London, UK: 1860. The botany of the Antarctic voyage of H.M. Discovery ships Erebus and Terror in the years 1839–1843. [Google Scholar]
- Howell C.J, Kelly D, Turnbull M.H. Moa ghosts exorcised? New Zealand's divaricate shrubs avoid photoinhibition. Funct. Ecol. 2002;16:232–240. doi:10.1046/j.1365-2435.2002.00613.x [Google Scholar]
- Hugall A.F, Foster R, Lee M.S.Y. Calibration choice, rate smoothing, and the pattern of tetrapod diversification according to the long nuclear gene RAG-1. Syst. Biol. 2007;56:543–563. doi: 10.1080/10635150701477825. doi:10.1080/10635150701477825 [DOI] [PubMed] [Google Scholar]
- Hurr K.A, Lockhart P.J, Heenan P.B, Penny D. Evidence for the recent dispersal of Sophora (Leguminosae) around the Southern Oceans: molecular data. J. Biogeogr. 1999;26:565–577. doi:10.1046/j.1365-2699.1999.00302.x [Google Scholar]
- Huynen L, Millar C.D, Scofield R.P, Lambert D.M. Nuclear DNA sequences detect species limits in ancient moa. Nature. 2003;425:175–178. doi: 10.1038/nature01838. doi:10.1038/nature01838 [DOI] [PubMed] [Google Scholar]
- Isaac M.J, Herzer R.H, Brook F.J, Hayward B.W. Cretaceous and Cenozoic sedimentary basins of Northland, New Zealand. Inst. Geol. Nucl. Sci. 1994;8:203. [Google Scholar]
- Kamp, P. J. J. 1992 Tectonic architecture of New Zealand. Landforms of New Zealand, pp. 1–31, 2nd edn. New Zealand: Longman Paul.
- Kamp P.J.J, Green P.F, White S.H. Fission track analysis reveals character of collisional tectonics in New Zealand. Tectonics. 1989;8:169–196. doi:10.1029/TC008i002p00169 [Google Scholar]
- Keast A. Continental drift and the evolution of the biota on southern continents. Quart. Rev. Biol. 1971;46:359. [Google Scholar]
- Kennedy M, Gray R.D, Spencer H.G. The phylogenetic relationships of the shags and cormorants: can sequence data resolve a disagreement between behavior and morphology? Mol. Phylogenet. Evol. 2000;17:345–359. doi: 10.1006/mpev.2000.0840. doi:10.1006/mpev.2000.0840 [DOI] [PubMed] [Google Scholar]
- Kishino H, Thorne J.L, Bruno W. Performance of a divergence time estimation method under a probabilistic model of rate evolution. Mol. Biol. Evol. 2001;18:352–361. doi: 10.1093/oxfordjournals.molbev.a003811. [DOI] [PubMed] [Google Scholar]
- Knapp M, Stöckler K, Havell D, Delsuc F, Sebastiani F, Lockhart P.J. Relaxed molecular clock provides evidence for long-distance dispersal of Nothofagus (southern beech) PLoS Biol. 2005;3:e14. doi: 10.1371/journal.pbio.0030014. doi:10.1371/journal.pbio.0030014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M, Mudaliar R, Havell D, Wagstaff S.J, Lockhart P.J. The drowning of New Zealand and the problem of Agathis. Syst. Biol. 2007;56:862–870. doi: 10.1080/10635150701636412. doi:10.1080/10635150701636412 [DOI] [PubMed] [Google Scholar]
- Landis, C. A., Campbell, H. J., Begg, J. G., Paterson, A. M. & Trewick, S. A. 2006 The drowning of Zealandia: evidence and implications (eds S. A. Trewick & M. J. Phillips). Geol. Soc. New Zeal. Misc. Publ 121, p. 21.
- Landis C.A, Campbell H.J, Begg J.G, Mildenhall D.C, Paterson A.M, Trewick S.A. The Waipounamu erosion surface: questioning the antiquity of the New Zealand land surface and terrestrial fauna and flora. Geol. Mag. 2008;145:1–25. doi:10.1017/S0016756807004268 [Google Scholar]
- Lee D.E, Lee W.G, Mortimer N. Where and why have all the flowers gone? Depletion and turnover in the New Zealand Cenozoic angiosperm flora in relation to palaeogeography and climate. Aust. J. Bot. 2001;49:341–356. doi:10.1071/BT00031 [Google Scholar]
- Lee D.E, Bannister J.M, Lindqvist J.K. Late Oligocene–Early Miocene leaf macrofossils confirm a long history of Agathis in New Zealand. New Zeal. J. Bot. 2007;45:565–578. [Google Scholar]
- Leschen R.A.B, Buckley T.R, Harman H.M, Shulmeister J. Determining the origin and age of the Westland beech (Nothofagus) gap, New Zealand, using fungus beetle genetics. Mol. Ecol. 2008;17:1256–1276. doi: 10.1111/j.1365-294X.2007.03630.x. doi:10.1111/j.1365-294X.2007.03630.x [DOI] [PubMed] [Google Scholar]
- Liggins L, Chapple D.G, Daugherty C.H, Ritchie P.A. Origin and post-colonization evolution of the Chatham Islands skink (Oligosoma nigriplantare nigriplantare) Mol. Ecol. 2008;17:3290–3305. doi: 10.1111/j.1365-294X.2008.03832.x. doi:10.1111/j.1365-294X.2008.03832.x [DOI] [PubMed] [Google Scholar]
- Lloyd B.D. Intraspecific phylogeny of the New Zealand short-tailed bat Mystacina tuberculata inferred from multiple mitochondrial gene sequences. Syst. Biol. 2003;52:460–476. doi: 10.1080/10635150390218187. doi:10.1080/10635150390218187 [DOI] [PubMed] [Google Scholar]
- Lockhart P.J, McLenachan P.A, Havell D, Glenny D, Huson D, Jensen U. Phylogeny, radiation, and transoceanic dispersal of New Zealand alpine buttercups: molecular evidence under split decomposition. Ann. Miss. Bot. Gard. 2001;88:458–477. doi:10.2307/3298586 [Google Scholar]
- Lusk C.H. Does photoinhibition avoidance explain divarication in the New Zealand flora? Funct. Ecol. 2002;16:858–860. doi:10.1046/j.1365-2435.2002.06821.x [Google Scholar]
- Marshall, D. C., Slon, K., Cooley, J. R., Hill, K. & Simon, C. In press. Steady Plio-Pleistocene diversification and a 2-million-year sympatry threshold in a New Zealand cicada radiation. Mol. Phylogenet. Evol [DOI] [PubMed]
- McDougall I, Embleton B.J.J, Stone D.B. Origin and evolution of Lord Howe Island, southwest Pacific Ocean. J. Geol. Soc. Aust. 1981;28:155–176. [Google Scholar]
- McDowall R.M. Biogeography of the New Zealand torrentfish, Cheimarrichthys fosteri (Teleostei: Pinguipedidae): a distribution driven mostly by ecology and behaviour. Environ. Biol. Fish. 2000;58:119–131. doi:10.1023/A:1007666014842 [Google Scholar]
- McDowall R.M. Process and pattern in the biogeography of New Zealand—a global microcosm? J. Biogeogr. 2008;35:197–212. doi:10.1111/j.1365-2699.2007.01830.x [Google Scholar]
- McGlone M.S. Goodbye Gondwana. J. Biogeogr. 2005;32:739–740. doi:10.1111/j.1365-2699.2005.01278.x [Google Scholar]
- McGlone M.S, Duncan R.P, Heenan P.B. Endemism, species selection and the origin and distribution of the vascular plant flora of New Zealand. J. Biogeogr. 2001;28:199–216. doi:10.1046/j.1365-2699.2001.00525.x [Google Scholar]
- Meudt H.M, Simpson B.B. The biogeography of the austral, subalpine genus Ourisia (Plantaginaceae) based on molecular phylogenetic evidence: South American origin and dispersal to New Zealand and Tasmania. Biol. J. Linn. Soc. 2006;87:479–513. doi:10.1111/j.1095-8312.2006.00584.x [Google Scholar]
- Millener P.R, Powlesland R.G. The Chatham Islands pigeon (parea) deserves full species status; Hemiphaga chathamensis (Rothschild 1891); Aves: Columbidae. J. R. Soc. New Zeal. 2001;31:365–383. [Google Scholar]
- Morgan-Richards M, Gibbs G.W. Colour, allozyme and karyotype variation in the New Zealand giant scree weta Deinacrida connectens (Orthoptera: Stenopelmatidae) Hereditas. 1996;125:265–276. doi:10.1111/j.1601-5223.1996.00265.x [Google Scholar]
- Morgan-Richards M, Gibbs G.W. A phylogenetic analysis of New Zealand giant and tree weta (Orthoptera: Anostostomatidae: Deinacrida and Hemideina) using morphological and genetic characters. Invert. Taxon. 2001;15:1–12. doi:10.1071/IT99022 [Google Scholar]
- Morgan-Richards M, Trewick S.A, Wallis G. Chromosome races with pliocene origins: evidence from mtDNA. Heredity. 2001;86:303–312. doi: 10.1046/j.1365-2540.2001.00828.x. doi:10.1046/j.1365-2540.2001.00828.x [DOI] [PubMed] [Google Scholar]
- Morgan-Richards M, Trewick S.A, Dunavan S. When is it coevolution? The case of ground weta and fleshey fruits in New Zealand. New Zeal. J. Ecol. 2008;32:108–114. [Google Scholar]
- Murphy S.A, Flux I.A, Double M.C. Recent evolutionary history of New Zealand's North and South Island kokako (Callaeas cinerea) inferred from mitochondrial DNA sequences. Emu. 2006;106:41–48. doi:10.1071/MU05007 [Google Scholar]
- Neall V.E, Trewick S.A. The age and origin of the Pacific Islands: a geological overview. Phil. Trans. R. Soc. B. 2008;363:3293–3308. doi: 10.1098/rstb.2008.0119. doi:10.1098/rstb.2008.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T.J, Baker A.M, Cook B.D, Hughes J.M. Historical transoceanic dispersal of a freshwater shrimp: the colonization of the South Pacific by the genus Paratya (Atyidae) J. Biogeogr. 2005;32:581–593. doi:10.1111/j.1365-2699.2004.01226.x [Google Scholar]
- Parrish J.T, Daniel I.L, Kennedy E.M, Spicer R.A. Paleoclimatic significance of Mid-Cretaceous floras from the Middle Clarence Valley, New Zealand. PALAIOS. 1998;13:149–159. doi:10.2307/3515486 [Google Scholar]
- Paterson, A. M., Trewick, S. A., Armstrong, K., Goldberg, J. & Mitchell, A. 2006 Recent and emergent: molecular anaylsis of the biota supports a young Chatham Islands. (eds S. A. Trewick & M. J. Phillips). Geol. Soc. New Zeal. Misc. Publ 121, pp. 27–29.
- Percy, D. M., Garver, A. M., Wagner, W. L., James, H. F., Cunningham, C. W., Miller, S. E. & Fleischer, R. C. 2008 Progressive island colonization and ancient origin of Hawaiian Metrosideros (Myrtaceae). Proc. R. Soc. B275, 1479–1490. (doi:10.1098/rspb.2008.0191) [DOI] [PMC free article] [PubMed]
- Perrie L, Brownsey P. Molecular evidence for long-distance dispersal in the New Zealand pteridophyte flora. J. Biogeogr. 2007;34:2028–2038. doi:10.1111/j.1365-2699.2007.01748.x [Google Scholar]
- Pole M. The New Zealand flora—entirely long-distance dispersal? J. Biogeogr. 1994;21:625–635. doi:10.2307/2846036 [Google Scholar]
- Pole M.S. Can long-distancs dispersal be inferred from the New Zealand plant fossil record? Aust. J. Bot. 2001;49:357–366. doi:10.1071/BT00022 [Google Scholar]
- Ponder W.F, Colgan D.J, Gleeson D.M, Sherley G.H. Relationships of Placostylus from Lord Howe Island: an investigation using the mitochondrial cytochrome c oxidase 1 gene. Mollusc. Res. 2003;23:159–178. doi:10.1071/MR03001 [Google Scholar]
- Pratt R.C, Morgan-Richards M, Trewick S.A. Diversification of New Zealand weta (Orthoptera: Ensifera: Anostostomatidae) and their relationships in Australasia. Phil. Trans. R. Soc. B. 2008;363:3427–3437. doi: 10.1098/rstb.2008.0112. doi:10.1098/rstb.2008.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quammen D. Scribner; New York, NY: 1996. The song of the dodo: island biogeography in an age of extinctions. [Google Scholar]
- Raven P.H. Evolution of subalpine and alpine plant groups in New Zealand. New Zeal. J. Bot. 1973;11:177–200. [Google Scholar]
- Robertson A.W, Kelly D, Ladley J.J, Sparrow A.D. Effects of pollinator loss on endemic New Zealand mistletoes (Loranthaceae) Conserv. Biol. 1999;13:499–508. doi:10.1046/j.1523-1739.1999.97471.x [Google Scholar]
- Robertson A.W, Ladley J.J, Kelly D. Effectiveness of short-tongued bees as pollinators of apparently ornithophilous New Zealand mistletoes. Austral Ecology. 2005;30:298–309. doi:10.1111/j.1442-9993.2005.01474.x [Google Scholar]
- Roelants K, Bossuyt F. Archaeobatrachian paraphyly and Pangaean diversification of crown-group frogs. Syst. Biol. 2005;54:111–126. doi: 10.1080/10635150590905894. doi:10.1080/10635150590905894 [DOI] [PubMed] [Google Scholar]
- Sanmartin I, Ronquist F. Southern hemisphere biogeography inferred by event-based models: plant versus animal patterns. Syst. Biol. 2004;53:216–243. doi: 10.1080/10635150490423430. doi:10.1080/10635150490423430 [DOI] [PubMed] [Google Scholar]
- Sanmartin I, Wanntorp L, Winkworth R.C. West Wind Drift revisited: testing for directional dispersal in the Southern Hemisphere using event-based tree fitting. J. Biogeogr. 2007;34:398–416. doi:10.1111/j.1365-2699.2006.01655.x [Google Scholar]
- Shepherd L.D, Lambert D.M. The relationships and origins of the New Zealand wattlebirds (Passeriformes, Callaeatidae) from DNA sequence analyses. Mol. Phylogenet. Evol. 2007;43:480–492. doi: 10.1016/j.ympev.2006.12.008. doi:10.1016/j.ympev.2006.12.008 [DOI] [PubMed] [Google Scholar]
- Sinclair B.J, Worland S.M, Wharton D.A. Ice nucleation and freezing tolerance in New Zealand alpine and lowland weta, Hemideina spp. (Orthoptera; Stenopelmatidae) Physiol. Entomol. 1999;24:56–63. doi:10.1046/j.1365-3032.1999.00112.x [Google Scholar]
- Skipworth J.P. Continental drift and the New Zealand biota. New Zeal. J. Geogr. 1974;57:1–13. [Google Scholar]
- Slack K.E, Jones C.M, Ando T, Harrison G.L, Fordyce R.E, Arnason U, Penny D. Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution. Mol. Biol. Evol. 2006;23:1144–1155. doi: 10.1093/molbev/msj124. doi:10.1093/molbev/msj124 [DOI] [PubMed] [Google Scholar]
- Smith S.A, Sadlier R.A, Bauer A.M, Austin C.C, Jackman T. Molecular phylogeny of the scincid lizards of New Caledonia and adjacent areas: evidence for a single origin of the endemic skinks of Tasmantis. Mol. Phylogenet. Evol. 2007;43:1151–1166. doi: 10.1016/j.ympev.2007.02.007. doi:10.1016/j.ympev.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Spencer H.G, Brook F.J, Kennedy M. Phylogeography of kauri snails and their allies from Northland, New Zealand (Mollusca: Gastropoda: Rhytididae: Paryphantinae) Mol. Phylogenet. Evol. 2006;38:835–842. doi: 10.1016/j.ympev.2005.10.015. doi:10.1016/j.ympev.2005.10.015 [DOI] [PubMed] [Google Scholar]
- Stagpoole V.M. Institute of Geological and Nuclear Sciences; Lower Hutt, Wellington, New Zealand: 2002. The New Zealand Continent, 1:7500000. Institute of Geological, Nuclear Sciences Ltd Geophysical map GPM15. [Google Scholar]
- Stanisic J. Occasional reports of the Australian Museum No. 1. Australian Museum; Sydney, Australia: 1981. Land Mollusca in Lord Howe Island. A summary of current and projected scientific and environmental activities. [Google Scholar]
- Steadman D.W. University of Chicago Press; Chicago, IL: 2006. Extinction and biogeography of tropical Pacific birds. [Google Scholar]
- Stevens M.I, Winter D.J, Morris R, McCartney J, Greenslade P. New Zealand's giant collembola: new information on distribution and morphology for Holacanthella Börner, 1906 (Neanuridae: Uchidanurinae) New Zeal. J. Zool. 2007;34:63–78. [Google Scholar]
- Stöckler K, Daniel I.L, Lockhart P.J. New Zealand kauri (Agathis australis (D. don) Lindl., Araucariaceae) survives Oligocene drowning. Syst. Biol. 2002;51:827–832. doi: 10.1080/10635150290102474. doi:10.1080/10635150290102474 [DOI] [PubMed] [Google Scholar]
- Suter H. On the origin of a new species by isolation. Trans. Proc. R. Soc. New Zeal. 1916;49:279–283. [Google Scholar]
- Sutherland R. Basement geology and tectonic development of the greater New Zealand region: an interpretation from regional magnetic data. Tectonophysics. 1999;308:341–362. doi:10.1016/S0040-1951(99)00108-0 [Google Scholar]
- Teeling E.C, Madsen O, Murphy W.J, Springer M.S, O'Brien S.J. Nuclear gene sequences confirm an ancient link between New Zealand's short-tailed bat and South American noctilionoid bats. Mol. Phylogenet. Evol. 2003;28:308–319. doi: 10.1016/s1055-7903(03)00117-9. doi:10.1016/S1055-7903(03)00117-9 [DOI] [PubMed] [Google Scholar]
- Thompson J.N. University of Chicago Press; Chicago, IL: 2005. The geographic mosaic of coevolution. [Google Scholar]
- Trewick S.A. Flightlessness and phylogeny amongst endemic rails (Aves: Rallidae) of the New Zealand region. Phil. Trans. R. Soc. B. 1997a;352:429–446. doi: 10.1098/rstb.1997.0031. doi:10.1098/rstb.1997.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewick S.A. Sympatric flightless rails Gallirallus dieffenbachii and G. modestus on the Chatham Islands, New Zealand; morphometrics and alternative evolutionary scenarios. J. R. Soc. New Zeal. 1997b;27:451–464. [Google Scholar]
- Trewick S.A. A new weta from the Chatham Islands (Orthoptera: Raphidophoridae) J. R. Soc. New Zeal. 1999;29:165–173. [Google Scholar]
- Trewick S.A. Molecular evidence for dispersal rather than vicariance as the origin of flightless insect species on the Chatham Islands, New Zealand. J. Biogeogr. 2000a;27:1189–1200. doi:10.1046/j.1365-2699.2000.00492.x [Google Scholar]
- Trewick S.A. Mitochondrial DNA sequences support allozyme evidence for cryptic radiation of New Zealand Peripatoides (Onychophora) Mol. Ecol. 2000b;9:269–281. doi: 10.1046/j.1365-294x.2000.00873.x. doi:10.1046/j.1365-294x.2000.00873.x [DOI] [PubMed] [Google Scholar]
- Trewick S.A. Identity of an endangered grasshopper (Acrididae: Brachaspis): taxonomy, molecules and conservation. Conserv. Genet. 2001a;2:233–243. doi:10.1023/A:1012263717279 [Google Scholar]
- Trewick S.A. Scree weta phylogeography: surviving glaciation and implications for Pleistocene biogeography in New Zealand. New Zeal. J. Zool. 2001b;28:291–298. [Google Scholar]
- Trewick S.A. DNA barcoding is not enough: mismatch of taxonomy and genealogy in New Zealand grasshoppers (Orthoptera: Acrididae) Cladistics. 2007;23:1–15. doi:10.1111/j.1096-0031.2007.00174.x [Google Scholar]
- Trewick S.A, Morgan-Richards M. After the deluge: mitochondrial DNA indicates Miocene radiation and Pliocene adaptation of tree and giant weta (Orthoptera: Anostostomatidae) J. Biogeogr. 2005;32:295–309. 10.1111/j.1365-2699.2004.01179.x [Google Scholar]
- Trewick, S. A. & Morgan-Richards, M. In press. Biology of New Zealand (Aotearoa) Encyclopedia of Islands Berkeley, CA: University of California Press.
- Trewick S.A, Wallis G.P. Bridging the “beech-gap”: New Zealand invertebrate phylogeography implicates Pleistocene glaciation and Pliocene isolation. Evolution. 2001;55:2170–2180. doi: 10.1111/j.0014-3820.2001.tb00733.x. doi:10.1554/0014-3820(2001)055[2170:BTBGNZ]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Trewick S.A, Wallis G.P, Morgan-Richards M. Phylogeographical pattern correlates with Pliocene mountain building in the alpine scree weta (Orthoptera, Anostostomatidae) Mol. Ecol. 2000;9:657–666. doi: 10.1046/j.1365-294x.2000.00905.x. doi:10.1046/j.1365-294x.2000.00905.x [DOI] [PubMed] [Google Scholar]
- Trewick S.A, Goldberg J, Morgan-Richards M. Fewer species of Argosarchus and Clitarchus stick insects (Phasmida, Phasmatinae): evidence from nuclear and mitochondrial DNA sequence data. Zool. Scr. 2005;34:438–491. doi:10.1111/j.1463-6409.2005.00204.x [Google Scholar]
- Trewick S.A, Paterson A.M, Campbell H.J. Hello New Zealand. J. Biogeogr. 2007;34:1–6. doi:10.1111/j.1365-2699.2006.01643.x [Google Scholar]
- Trewick, S. A., Morgan-Richards, M. & Collins, L. J. 2008a Are you my mother? Phylogenetic analysis reveals orphan hybrid stick insect genus is part of a monophyletic New Zealand clade. Mol. Phylogenet. Evol 48, 799–808. (doi:10.1016/j.ympev.2008.05.025) [DOI] [PubMed]
- Trewick, S. A., Walker, K. J. & Jordan, C. J. 2008b Taxonomic and conservation status of a newly discovered giant landsnail from Mount Augustus, New Zealand. Conserv. Genet Online First (doi:10.1007/s10592-007-9495-8)
- Trewick, S. A., Brescia, F. & Jordan, C. In press. Diversity and phylogeny of New Caledonian Placostylus land snails; evidence from mitochondrial DNA. Zoologia Neocaledonica 6. Biodiversity studies in New Caledonia. (ed. P. Grandcolas). Mém. Mus. nat. Hist. nat.196
- Veevers J.J. Phanerozoic Australia in the changing configuration of proto-Pangea through Gondwanaland and Pangea to the present dispersed continents. Aust. Syst. Bot. 1991;4:121–161. [Google Scholar]
- Vink C.J, Paterson A.M. Combined molecular and morphological phylogenetic analyses of the New Zealand wolf spider genus Anoteropsis (Araneae: Lycosidae) Mol. Phylogenet. Evol. 2003;28:576–587. doi: 10.1016/s1055-7903(03)00219-7. doi:10.1016/S1055-7903(03)00219-7 [DOI] [PubMed] [Google Scholar]
- Wagstaff S.J, Garnock-Jones P.J. Evolution and biogeography of the Hebe complex (Scrophulariaceae) inferred from ITS sequences. New Zeal. J. Bot. 1998;36:425–437. [Google Scholar]
- Wallace A.R. The geographical distribution of animals: with a study of the relations of living and extinct faunas. vol. 1–2. MacMillan and Co; London, UK: 1876. [Google Scholar]
- Wallis G.P, Trewick S.A. Finding fault with vicariance: a critique of Heads (1998) Syst. Biol. 2001;50:602–609. doi:10.1080/106351501750435130 [PubMed] [Google Scholar]
- Wardle P. Cambridge University Press; Cambridge, UK: 1991. Vegetation of New Zealand. [Google Scholar]
- Waters J.M, Craw D. Goodbye Gondwana: New Zealand biogeography, geology and the problem of circularity. Syst. Biol. 2006;55:351–356. doi: 10.1080/10635150600681659. doi:10.1080/10635150600681659 [DOI] [PubMed] [Google Scholar]
- Waters J.M, Dijkstra L.H, Wallis G.P. Biogeography of a southern hemisphere freshwater fish: how important is marine dispersal? Mol. Ecol. 2000;9:1815–1821. doi: 10.1046/j.1365-294x.2000.01082.x. doi:10.1046/j.1365-294x.2000.01082.x [DOI] [PubMed] [Google Scholar]
- Whitehouse I.E, Pearce A.J. Shaping the mountains of New Zealand. In: Soons J.M, Selby M.J, editors. Landforms of New Zealand. 2nd edn. Longman Paul; Auckland, New Zealand: 1992. pp. 144–160. [Google Scholar]
- Whittaker R.J, Fernández-Palacios J.M. 2nd edn. Oxford University Press; Oxford, UK: 2006. Island biogeography: ecology, evolution, and conservation. [Google Scholar]
- Wilson K.-J. Canterbury University Press; Christchurch, New Zealand: 2004. Flight of the Huia. Ecology and conservation of New Zealand's frogs, reptiles, birds and mammals. [Google Scholar]
- Winkworth R.C, Wagstaff S.J, Glenny D, Lockhart P.J. Plant dispersal N.E.W.S from New Zealand. Trends Ecol. Evol. 2002;17:514–520. doi:10.1016/S0169-5347(02)02590-9 [Google Scholar]
- Winkworth R.C, Wagstaff S.J, Glenny D, Lockhart P.J. Evolution of the New Zealand mountain flora: origins, diversification and dispersal. ODE. 2005;5:237–247. doi:10.1016/j.ode.2004.12.001 [Google Scholar]
- Worthy T.H, Holdaway R.N. Canterbury University Press; Christchurch, New Zealand: 2002. The lost world of the moa: prehistoric life of New Zealand. [Google Scholar]
- Worthy T.H, Edwards A.R, Millener P.R. The fossil record of moas (Aves: Dinornithiformes) older than the Otira (last) glaciation. J. R. Soc. New Zeal. 1991;21:101–118. [Google Scholar]
- Worthy T.H, Tennyson A.J, Archer M, Musser A.M, Hand S.J, Jones C, Douglas B.J, McNamara J.A, Beck R.M.D. Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific. Proc. Natl Acad. Sci. USA. 2006;103:19 419–19 423. doi: 10.1073/pnas.0605684103. doi:10.1073/pnas.0605684103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.D, Jong C.G, Dawson J.W, Whittaker D.J, Gardner R.C. Riding the ice age El Niño? Pacific biogeography and evolution of Metrosideros subg. Metrosideros (Myrtaceae) inferred from nuclear ribosomal DNA. Proc. Natl Acad. Sci. USA. 2000;97:4118–4123. doi: 10.1073/pnas.050351197. doi:10.1073/pnas.050351197 [DOI] [PMC free article] [PubMed] [Google Scholar]